-
PDF
- Split View
-
Views
-
Cite
Cite
Laura G González Briceño, Magali Viaud, Jacques Beltrand, Isabelle Flechtner, Yamina Dassa, Dinane Samara-Boustani, Caroline Thalassinos, Christian Pauwels, Kanetee Busiah, Graziella Pinto, Delphine Jaquet, Michel Polak, Improved General and Height-Specific Quality of Life in Children With Short Stature After 1 Year on Growth Hormone, The Journal of Clinical Endocrinology & Metabolism, Volume 104, Issue 6, June 2019, Pages 2103–2111, https://doi.org/10.1210/jc.2018-02523
Close - Share Icon Share
Abstract
Short stature in children and adolescents may lead to social and emotional stress, with negative effects on quality of life (QoL). GH treatment may improve QoL through height normalization. Our objective here was to evaluate general and height-specific QoL after 1 year of GH treatment.
Prospective, single-center, observational cohort study.
Children ≥ 4 years of age starting GH at our center from 2012 to 2015 to treat short stature were studied. Patients with serious diseases, syndromic short stature, or developmental delay were excluded. At treatment initiation and 1 year later, patients and their parents completed the general PedsQL 4.0 and height-specific Quality of Life in Short Stature Youth (QoLiSSY) questionnaires. Correlations between self-report and parent-report scores and between height gain and QoL improvements were assessed based on Pearson correlation coefficients.
Seventy-four children (42 boys, 32 girls), median age (± SD), 10.2 ± 3.0 years (range, 4.1 to 16.6 years), were included. The self-report PedsQL indicated significant improvements in emotional (P = 0.02) and social (P = 0.03) QoL. As assessed by the QoLiSSY, children reported improvement of social QoL (+0.2 SD; P = 0.04), and parents reported improvement of children’s physical (+0.1 SD; P < 0.0001), emotional (+0.3 SD; P < 0.0001), and social (+0.3 SD; P < 0.0001) QoL. Height SD score (SDS) gains showed moderate positive correlations with QoLISSY self-report score gains (R = 0.53, R2 = 0.28; P < 0.001) and QoLISSY parent-report gains (R = 0.60, R2 = 0.41; P < 0.00001).
After 1 year of GH treatment, children had significant gains in emotional and social QoL, as assessed by a general self-report questionnaire and height-specific parent-report questionnaire.
Short stature can generate emotional and social stress in children and adolescents and in their parents (1). The patients and parents often express a strong desire for an accurate diagnosis, treatment of the underlying cause, follow-up, and specific psychological support. Mitigating the adverse effects of short stature on quality of life (QoL) is one of the key treatment objectives.
QoL in children can be evaluated by self-report or proxy report, although agreement between these two methods is sometimes limited (2). Studies of both general and condition-specific QoL have shown lower scores in children with various chronic conditions compared with healthy peers (3). GH deficiency and small-for-gestational age (SGA) status have been associated with impairments in psychological well-being that sometimes persist into late adolescence and early adulthood (1, 4). Compared with healthy normal-stature controls, children with short stature but no GH deficiency exhibited QoL impairments, whereas those of similar short stature with GH deficiency and GH treatment did not (5). This observation suggests that GH treatment may improve QoL, perhaps by inducing height gains (5), as well as the well-documented effect of GH on other physical dimensions (bone mass, body composition, lipid profile, muscle strength) (6–8). The most important improvements in QoL appear in the area of social adjustment (4).
The available studies of associations between short stature and QoL have suffered from the absence of adequate scales for assessing height-specific QoL (1, 4, 9, 10). Another major issue is the need for age-adjusted versions of assessment tools, as experiences and cognitive abilities vary with age (11). PedsQL is a generic pediatric QoL assessment tool that evaluates physical, emotional, and social QoL, as well as school functioning (3). Three age-adjusted versions of the PedsQL are available, for the 5- to 7-, 8- to 12-, and 13- to 18-year age groups. The Quality of Life in Short Stature Youth (QoLISSY) questionnaire was specifically designed by an international group of researchers and pediatricians from France, Germany, Spain, Sweden, and the United Kingdom to measure QoL in children with short stature from the perspectives of both the patient and the parents (12). Age-adjusted versions are available. QoLISSY has been validated in patients with GH deficiency and idiopathic short stature. Investigating the effect of GH on QoL by these two scales used in parallel would provide interesting and original information with respect to an aged-matched reference population on the one hand and an aged-matched short population on the other hand.
The primary objective of our study was to assess changes in both general and height-specific QoL in children after 1 year of GH treatment by using the PedsQL and the QoLISSY questionnaires. Our hypothesis was that GH therapy would be associated with QoL improvement.
Particpants and Methods
This prospective, observational, single-center study was conducted at the Necker-Enfants Malades University Hospital in Paris, France. The study was approved by the local ethics committee (Comité de Protection des Personnes; Réf. CPP: 2013-12-02, 17 November 2014). All the study data were collected on paper forms, anonymized, and kept at a secure location accessible only to medical personnel, as required by the French committee on the protection of computerized data (law on Commission Nationale de l’Informatique et des Libertés). Results of the questionnaires were then included in an electronic database, for further analysis.
Study patients
Patients aged 4 to 18 years who started GH therapy for short stature [height ≤ −2 SD score (SDS)] between April 2012 and September 2015 at Necker-Enfants Malades University Hospital, in the Department of Pediatric Endocrinology, Gynecology and Diabetology, were consecutively included. GH deficiency was defined by two growth hormone stimulation tests with a peak GH level < 20 mUI/l. SGA was defined by weight and/or length at birth ≤ −2 SDs for term. Bone dysplasia was diagnosed after consultation between an interdisciplinary staff with the clinical genetics team at Hôpital Necker, and included dyschondrosteosis, hypochondroplasia, pycnodysostosis, and indeterminate bone dysplasia. Exclusion criteria were age < 4 years or > 18 years, height > −2 SDS at GH therapy initiation, moderate-to-severe chronic disease (e.g., brain tumor) or syndromic cause of short stature, and patient and/or parents unable to complete the study questionnaires or unwilling to participate in the study. All patients/parents gave their written informed consent before inclusion.
GH treatment
Each patient was included on the day of the first GH injection. During a therapeutic education session led by a nurse and a clinical research assistant, the child and parents (one or both) completed the age-appropriate PedsQL and QoLISSY questionnaires. If the child was <8 years of age, the QoLISSY questionnaire was completed only by the parents.
During the closest visit performed after 1 year of GH treatment, QoL was reassessed. This interval was chosen because the greatest increase in height velocity is usually observed during the first year of GH therapy. In addition to the routine follow-up assessments, the children and parents completed the same questionnaires.
Tools used to assess QoL
General health-related QoL was assessed by using the PedsQL version 4.0 (3), validated in French by the Mapi Research Institute (http://www.pedsql.org/translations.html). Children <8 years of age completed the questionnaire with their parents. The PedsQL has 23 items that investigate physical, emotional, and social QoL, as well as school functioning. This tool has been validated in the 2- to 18-year age range. The parents complete the questionnaire if the child is <5 years. After that age, the child completes the age-appropriate version of the questionnaire (5 to 7, 8 to 12, or 13 to 18 years). The score is expressed as a percentage, with scores ranging from 0% to 100% (100% is the best score). Questionnaire subscales are considered evaluable if answers are provided for ≥80% of the items.
To assess height-specific QoL, we used the QoLISSY intended for patients aged 4 to 18 years who have GH deficiency or idiopathic short stature and for their parents (12). QoLISSY has been validated in children with short stature (height ≤ 2 SD) (12) in five languages. The French questionnaires were used in the current study. QoLISSY questionnaires include two different forms: parent questionnaire (for parents of children aged 4 to 18 years) and child questionnaire (only for children aged ≥ 8 years). Both questionnaires have 50 items that cover three core domains (physical, emotional, and social) and additional complementary domains (coping in daily life, beliefs about the importance of height, and GH treatment–specific aspects of QoL). The parent questionnaire includes two additional scales (16 items) assessing parental stress related to the child’s short stature and anxiety about the child’s future (13). Scores are converted to SDSs and compared with age- and sex-specific data from a reference population of children and adolescents with short stature. A total score is computed as the mean score on the three core scales (physical, emotional, and social). Questionnaire subscales are considered evaluable if answers are provided for ≥80% of the items.
Statistics
Statistical analyses were performed by using the SPSS statistical software 2018 (IBM, Armonk, NY).
Quantitative variables are described by using median (range) and qualitative variables as absolute (n) and relative (%) frequency.
PedsQL and QoLISSY data were described in the whole population and analyzed according to the severity of short stature at baseline. For that purpose, the study population was divided into three subgroups: (i) ≤ −3 SDs, (ii) > −3 SD and ≤ −2.5 SD, and (iii) > −2.5 SDs and ≤ −2 SD. Statistical comparisons between these groups were performed by using the Kruskal-Wallis nonparametric test.
Change in QoL scores was assessed as the relative variation between evaluation at 1 year and baseline [(T12 − T0)/T0] computed for each subscale of each questionnaire. The statistical significance of these changes was further analyzed by using a Wilcoxon rank test.
Correlations between children’s and parents’ QoL scores, and between height gains (T12 − T0 in SD) and change in QoL were assessed by using the Pearson correlation test.
A P value < 0.05 was considered to indicate a statistically significant difference.
Results
Patient population
Of 341 consecutive patients who started on GH therapy during the study period, 80 were eligible for our study and were included (Fig. 1). Among them, 74 completed the follow-up evaluation, at a median of 1.0 year (range, 0.7 to 2.0 years) after baseline. Of these 74 patients, complete PedsQL questionnaires were available at both time points for 71 children and 70 parents, and complete QoLISSY questionnaires were obtained from 57 children and 71 parents. Seventeen patients (23%) were <8 years of age at baseline and therefore had QoLISSY questionnaires completed by the parents only.
Clinical characteristics of the study population according to the severity of short stature are summarized in Table 1.
| . | ≤ −3 SD . | > −3 to ≤ −2,5 SD . | > −2,5 to −2 SD . |
|---|---|---|---|
| Number of patients | 22 | 23 | 29 |
| Girls/boys | 9/13 | 13/10 | 10/19 |
| Median age in years (range) | 9.7 (4.1–16.4) | 11.1 (4.2–14.3) | 10.5 (5.7–16.6) |
| GHD, % | 13.6% | 43.5% | 44.8% |
| SGA, % | 45.5% | 26.1% | 27.6% |
| Bone dysplasia, % | 31.8% | 17.4% | 24.1% |
| ISS, % | 9.1% | 13.0% | 3.5% |
| . | ≤ −3 SD . | > −3 to ≤ −2,5 SD . | > −2,5 to −2 SD . |
|---|---|---|---|
| Number of patients | 22 | 23 | 29 |
| Girls/boys | 9/13 | 13/10 | 10/19 |
| Median age in years (range) | 9.7 (4.1–16.4) | 11.1 (4.2–14.3) | 10.5 (5.7–16.6) |
| GHD, % | 13.6% | 43.5% | 44.8% |
| SGA, % | 45.5% | 26.1% | 27.6% |
| Bone dysplasia, % | 31.8% | 17.4% | 24.1% |
| ISS, % | 9.1% | 13.0% | 3.5% |
Abbreviations: GHD, GH deficiency; ISS, idiopathic short stature.
| . | ≤ −3 SD . | > −3 to ≤ −2,5 SD . | > −2,5 to −2 SD . |
|---|---|---|---|
| Number of patients | 22 | 23 | 29 |
| Girls/boys | 9/13 | 13/10 | 10/19 |
| Median age in years (range) | 9.7 (4.1–16.4) | 11.1 (4.2–14.3) | 10.5 (5.7–16.6) |
| GHD, % | 13.6% | 43.5% | 44.8% |
| SGA, % | 45.5% | 26.1% | 27.6% |
| Bone dysplasia, % | 31.8% | 17.4% | 24.1% |
| ISS, % | 9.1% | 13.0% | 3.5% |
| . | ≤ −3 SD . | > −3 to ≤ −2,5 SD . | > −2,5 to −2 SD . |
|---|---|---|---|
| Number of patients | 22 | 23 | 29 |
| Girls/boys | 9/13 | 13/10 | 10/19 |
| Median age in years (range) | 9.7 (4.1–16.4) | 11.1 (4.2–14.3) | 10.5 (5.7–16.6) |
| GHD, % | 13.6% | 43.5% | 44.8% |
| SGA, % | 45.5% | 26.1% | 27.6% |
| Bone dysplasia, % | 31.8% | 17.4% | 24.1% |
| ISS, % | 9.1% | 13.0% | 3.5% |
Abbreviations: GHD, GH deficiency; ISS, idiopathic short stature.
The participants consisted of 42 (57%) boys and 32 girls (43%) with a median age at baseline of 10.9 years (range, 4.1 to 16.6 years). Diagnoses were as follows: GH deficiency, n = 26 (35.2%); SGA, n = 24 (32.4%); bone dysplasia, n = 18 (24.3%: 14 patients with dyschondrosteosis, 1 with hypochondroplasia, 1 with pycnodysostosis, and 2 with indeterminate bone dysplasia); and idiopathic short stature, n = 6 (8.1%). All girls starting GH at age 12 years or later (eight girls) had started puberty at T0, except for one girl, who was Tanner stage B1; she had delayed puberty, with menarche at age 15 years. For boys starting GH at age 13 years or later (eight boys), five had started puberty and three were nonpubertal (two of the three had delayed puberty, which started at age 14 years and 8 months for one boy and 15 years and 4 months for the other).
Median height at baseline (T0) was −2.5 SD (range, −5.0 to −2.0 SD)]. Of the 70 children for whom the heights of both parents were available, 53 (76%) had heights −1.5 SD below the target height. At the follow-up evaluation (T12), median height was −1.7 SD (−4.1 to −0.5 SD) and mean height gain was 0.7 SD (−0.2 to 2.0 SD).
Children were separated into three groups according to the severity of short stature at baseline (Table 1). Twenty-two children were in the most severe (≤ −3 SD) group, 23 in the intermediate group (> −3 to ≤ −2.5 SD), and 29 in the less severe group (> −2.5 to ≤ −2 SD). Age did not differ among the three groups. The less severe group had twice as many boys as girls. SGA and bone dysplasia were more represented in the most severe group, whereas GHD was more frequent in the less severe group.
QoL Measures
Figure 2 illustrates the PedsQL scores at baseline and follow-up in children and parents, according to the severity of short stature, in comparison with the mean scores observed in an age-matched general pediatric population and in chronically ill children (3). Figure 3 shows the QoLISSY scores at baseline and follow-up in children and parents, according to the severity of short stature.
![Self-report (A) and parent-report (B) QoL assessed by PedsQL 4.0 according to severity of short stature at baseline and after 1 year of GH treatment. Results are expressed in median. The dashed black lines illustrate the mean QoL scores measured in chronically ill population and the solid gray lines those measured in the general healthy population, using the PedsQL score [Varni et al. (3)]. P values are given for T0 and T12 on the whole population using the Wilcoxon test.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/jcem/104/6/10.1210_jc.2018-02523/2/m_jc.2018-02523f2.jpeg?Expires=1747862008&Signature=FKmadq0rWlBtDYkQaEx-~EWDnlL1ZjO6WnucqV3JJFsiqQiWnlU8166xS7zcM1Nt7~1OzGmU3UBw9RdPeSPHRRorVH1mLVA5k229gz8nlzqH14FoyA06kadYAI7Zpum8JrFsVrs-i630rJWjWIuc0ve~2saOWD3~fKFSzhzoyAQS0eJO~G53wIX8RXuxr78OMO-3TKSWy-rVUpisOy8CyfFhiKsMeTO0G1nXMzSqkAiDbCauUPvtT4kyPZAOOsE5repIsKphayd8Pp4NqmYA7yiXpS3Sa7ldvCQA-rxc-KaRCSk8SB4VPIq12x~Z3djsDvj0OI4jQY2Z2whmiEYNzA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Self-report (A) and parent-report (B) QoL assessed by PedsQL 4.0 according to severity of short stature at baseline and after 1 year of GH treatment. Results are expressed in median. The dashed black lines illustrate the mean QoL scores measured in chronically ill population and the solid gray lines those measured in the general healthy population, using the PedsQL score [Varni et al. (3)]. P values are given for T0 and T12 on the whole population using the Wilcoxon test.
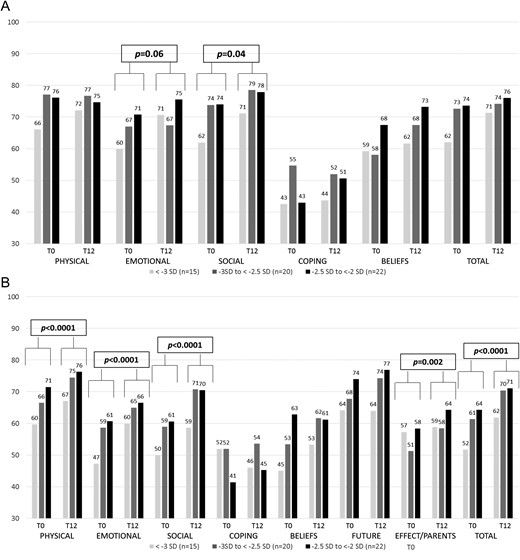
Self-report (A) and parent-report (B) QoL assessed by QoLISSY according to severity of short stature at baseline and after 1 year of GH treatment. Results are expressed in median. P values are given for T0 and T12 on the whole population using the Wilcoxon test.
At baseline
Children with the most severe short stature demonstrated scores similar to those observed in chronically ill children or even lower and a significant alteration in social QOL (P = 0.02) school functioning QoL (P = 0.02) and total QoL (P = 0.03) when compared with the less severe ones (Fig. 2). The intermediate group still showed a visible impact on the emotional scale, with QoL similar to that of chronically ill children, whereas the less severe group showed an average QoL similar to that of the general population (Fig. 2). There was no significant difference according to the severity of short stature in the parents’ subscales. However, parental scores of children with the most severe short stature were similar to or even lower than those observed in parents of chronically ill children in all except physical scores. Emotional parental scores were altered in all three groups (Fig. 2).
As expected, QoLISSY self-report scores at baseline were similar to the values reported in the referent short children population [total score: median, 0.02 (range, −2.0 to 1.3) SD] as well as parent-report scores [total score: median, −-0.2 (range, −2.8 to 1.1) SDS] (13) (Fig. 3).
When analyzed with respect to the severity of short stature, total self-report scores were lower, at the limit of significance (P = 0.05), in children with the most severe short stature (median, −0.3 (range, −2.0 to 0.7) SD] when compared with the intermediate (0.3 (range, −1.4 to 1.1) SD] and the less severe [0.1 (range, −1.8 to 1.3) SD] groups.
Parent-report scores also showed this tendency, with lower scores in the most severe short stature group [−0.7 SD (range, −2.3 to 1.1)] than in the intermediate [−0.1 SD (range, −2.8 to 0.8) SD]) and the less severe [−0.2 SD (range, −1.3 to 0.8) SD; P = 0.06] groups.
Regarding the parent-report subscores, the social score was significantly lower for children with the most severe short stature [−0.6 (range, −2.3 to 1.0) SD] when compared with the intermediate [0.0 (range, −2.7 to 0.7) SD] and less severe -−0.3 (−1.3 to 1.2) SD; P = 0.03] groups.
At follow-up
The median duration of GH treatment at T12 was of 1.0 year (range, 0.7 to 2.0 years). Height improvement at 1 year according to diagnoses is shown in Table 2.
| . | . | ≤ −3 SD . | > −3 to ≤ −2,5 SD . | > −2,5 to −2 SD . |
|---|---|---|---|---|
| GH deficiency (26 patients) | T0 | 3 | 10 | 13 |
| T12 | 0 | 1 | 25 | |
| SGA (24 patients) | T0 | 10 | 6 | 8 |
| T12 | 3 | 4 | 17 | |
| Bone dysplasia (18 patients) | T0 | 7 | 4 | 7 |
| T12 | 4 | 3 | 11 | |
| ISS (6 patients) | T0 | 2 | 3 | 1 |
| T12 | 0 | 1 | 5 |
| . | . | ≤ −3 SD . | > −3 to ≤ −2,5 SD . | > −2,5 to −2 SD . |
|---|---|---|---|---|
| GH deficiency (26 patients) | T0 | 3 | 10 | 13 |
| T12 | 0 | 1 | 25 | |
| SGA (24 patients) | T0 | 10 | 6 | 8 |
| T12 | 3 | 4 | 17 | |
| Bone dysplasia (18 patients) | T0 | 7 | 4 | 7 |
| T12 | 4 | 3 | 11 | |
| ISS (6 patients) | T0 | 2 | 3 | 1 |
| T12 | 0 | 1 | 5 |
Abbreviation: ISS, idiopathic short stature.
| . | . | ≤ −3 SD . | > −3 to ≤ −2,5 SD . | > −2,5 to −2 SD . |
|---|---|---|---|---|
| GH deficiency (26 patients) | T0 | 3 | 10 | 13 |
| T12 | 0 | 1 | 25 | |
| SGA (24 patients) | T0 | 10 | 6 | 8 |
| T12 | 3 | 4 | 17 | |
| Bone dysplasia (18 patients) | T0 | 7 | 4 | 7 |
| T12 | 4 | 3 | 11 | |
| ISS (6 patients) | T0 | 2 | 3 | 1 |
| T12 | 0 | 1 | 5 |
| . | . | ≤ −3 SD . | > −3 to ≤ −2,5 SD . | > −2,5 to −2 SD . |
|---|---|---|---|---|
| GH deficiency (26 patients) | T0 | 3 | 10 | 13 |
| T12 | 0 | 1 | 25 | |
| SGA (24 patients) | T0 | 10 | 6 | 8 |
| T12 | 3 | 4 | 17 | |
| Bone dysplasia (18 patients) | T0 | 7 | 4 | 7 |
| T12 | 4 | 3 | 11 | |
| ISS (6 patients) | T0 | 2 | 3 | 1 |
| T12 | 0 | 1 | 5 |
Abbreviation: ISS, idiopathic short stature.
The PedsQL self-report scores significantly improved in the emotional (P = 0.02) and social (P = 0.03) domains (Fig. 2), and this was particularly true in the shortest group, for which the median increase was 25% (range, −38% to 180%) in the emotional score and the median increase was 17% (range, −6% to 200%) for the social score. No statistically significant changes were observed in the PedsQL parent-report scores over the first year of GH treatment, except for the social domain [5% (range, −83% to 200%); P = 0.03] (Fig. 2).
QoLISSY self-reports significantly improved only in the social score [median change, 0.1 (range, −1.3 to 2.5) SD; P = 0.04], with a tendency toward improvement in the emotional score [0.1 SD (range, −1.0 to 1.8); P = 0.06]. QoLISSY parent-report scores indicated strong statistically significant improvements in perceived physical [0.2 (range, −1.9 to 1.7) SD; P < 0.0001], emotional [0.3 (range, −1.4 to 2.1) SD; P < 0.0001], social [0.4 SD (range, −1.7 to 2.3); P < 0.0001], and total [0.3 (range, −1.3 to 1.6) SD; P < 0.0001] QoL. No significant changes occurred for the other subscale scores, including parental stress related to the child’s short stature.
Self-reported and parent-reported increase in total QoL, as assessed by the QoLISSY questionnaire, were significantly correlated (R = 0.45, R2 = 0.20; P < 0.001).
The QoLISSY score for GH treatment–specific aspects of QoL exhibited a moderate positive correlation with the improvement in total QoL in both children’s (R = 0.559, R2 = 0.3123; P < 0.00001) and parents’ (R = 0.600, R2 = 0.3604; P < 0.00001) questionnaires.
Change in QoLISSY self-report total score (R = 0.532, R2 = 0.283; P < 0.001) and QoLISSY parent-report showed moderate positive correlations with height SDS gains (R = 0.638, R2 = 0.407; P < 0.00001).
Discussion
There have been several studies addressing the impact of short stature on QoL, in adults (14–17) and in children (1, 2, 4, 5, 10), but to our knowledge, this study is the first to report data concerning variation in QoL of short children under GH treatment by using two different pediatric QoL scales: a general scale and a condition-specific (height-specific) scale.
At one year of the GH treatment initiation there is an improvement in emotional and social QoL, in both general PedsQL and height-specific QoLISSY scores, as perceived by children and parents. It is important to note that children did not report a significant improvement in physical scores. However, when compared with normal and chronically ill population, this was not the most affected dimension in these patients except maybe for the shortest ones. Parents reported an improvement in all core scores of the QoLISSY height-specific questionnaire, which is very interesting.
When compared with the general pediatric population and a chronically ill one, QoL scores were substantially lower at baseline, especially in the shortest group, who displays scores that are equivalent to the “chronically ill” population (3). The intermediate height group has also a low emotional baseline score, comparable to chronically ill children. It is important to bear in mind that our study excluded children with comorbidities and other chronic conditions that would potentially lower the QoL by other means than height. This allows us to independently evaluate the influence of short stature on QoL.
Height has been traditionally considered as an important component in well-being and success in adult life, with observations showing an influence on job position (18, 19) and interpersonal attraction, particularly in men (19, 20). Influence of short stature on adult life QoL has not been clearly demonstrated (14–17). However, in the pediatric population, several studies have shown that it may adversely affect behavior, emotional health, and psychosocial well-being, even in the absence of underlying disease (1, 2, 4, 5, 10). Bullying, teasing and victimization, whether real or feared, may be more common in short children (21, 22). It is important to keep in mind that emotional problems that appear during childhood, linked to chronic conditions, may persist beyond childhood into adult life (23). Moreover, short stature during adolescence may have an impact on future labor market disparities, regardless of final height (24), possibly through a decreased participation of short teenagers in social and nonacademic activities, which enable accumulation of productive skills. This observation suggests it is important to address short stature during childhood, as early as possible, to minimize the impact on future QoL.
GH therapy may improve emotional health and self-esteem (2, 5, 25), even though it is important to emphasize that beside the management of short stature itself, appropriate measures remain critical to prevent and manage situations such as bullying, and to adapt to shorter children's needs (school material: chairs, desks, etc.).
Our results show that GH treatment of one year has a significant impact on social and emotional QoL, whereas a physical impact, which could be expected given the visible gain in height, seems to be less important. This may likely be related to the moderate physical impact of short stature at baseline. However, there is a correlation between height gain in SD and change in QoL, which clearly suggests the contribution of statural increase to the QoL improvement.
It is very difficult to rule out a possible placebo effect of treatment in our population, since all children were treated by GH. We were unable to perform a placebo vs. control study, since this was an observational study. However, we notice that there is a correlation between height gain in SD and improvement in QoL, which suggests that height increase is responsible, at least in part, for the QoL improvement. Moreover, we find a different effect according to severity groups: increase in QoL is more important in the most severe group; this seems to be a good argument against placebo effect. Future studies may help clarify this observation.
The PedsQL score allows to compare a specific child’s QoL with that of the general population, and can be used as a static (one questionnaire) or dynamic (pre and post intervention) measure.
Application of the QoLISSY scale compares the child to a reference short stature population, but not the general population. Used at the individual level, it provides a good assessment of QoL. However, it becomes more interesting when used as a dynamic measure to assess how the management of short stature could positively impact the child QoL, and it seems to be more accurate for parental perception of children’s QoL.
The QoLISSY questionnaire provides specific and comprehensive information on height-related QoL. Furthermore, completing the QoLISSY at GH therapy initiation provides valuable opportunities for individual discussion, as several items help the patient and parents to understand and better express their concerns and anxieties. The existence of the QoLISSY questionnaire can help the parents realize that their worries are shared by others and acknowledged as legitimate by the health care community. Thus, it may have a major role to play in therapeutic education. However, the great variability observed in the different dimension and even greater when used as a dynamic tool makes it more difficult to use and to interpret at a population-based level.
The presence of emotional and social score improvements by both the self-report (PedsQL and QoLISSY) and parent-report (QoLISSY) evaluations lends coherence and support to our findings.
It has been stated that QoL gain should be > +0.5 SD to be considered as clinically relevant at an individual level (26). All QoL gains on the QoLISSY score were below this threshold, indicating a need for circumspection. Nevertheless, the kinetics of the treatment response to GH suggest that greater gains may develop after longer treatment durations.
GH therapy is indicated only in specific situations. Impaired QoL related to short stature by itself is not a valid reason to start GH therapy but should be considered when making treatment decisions, especially in very short children. A psychosocial evaluation performed without delaying further diagnostic procedures can be helpful in ruling out emotional or behavioral problems that require simultaneous specific treatment (22). QoL is a dynamic and individual process that varies over time and can improve with a supportive environment. Resilience plays an important role in adapting to various conditions, including short stature, but frequently develops only in early adulthood (15). Furthermore, even resilient children may have QoL impairments, particularly in the emotional domain.
GH treatment requires long-term daily subcutaneous injections, which place a considerable burden on the child. GH treatment-specific aspects of QoL showed a moderate positive correlation with global QoL in our population, in keeping with earlier reports (27, 28). The treatment burden should be discussed openly with the patient and parents when considering GH therapy to treat short stature without GH deficiency.
A possible bias of this study is the fact that it included children referred for short stature only, which implies potential parental and child anxiety regarding height and altered QoL (16, 17). It could be interesting to compare initial QoL questionnaires with a control population of nonreferred and untreated short stature children (as has been done in adults (16) but not in pediatric populations); however, this may prove to be challenging, since they have not been addressed nor evaluated for potential underlying conditions.
One of the strengths of our study is the prospective inclusion of unselected patients. Furthermore, the use of the patients as their own controls adds to the validity of our findings.
In conclusion, QoL is strongly affected by short stature especially when height is less than -2.5 SD, even in patients with no underlying medical conditions. In these patients, emotional and social QoL, assessed by both patients and parents, significantly improves after one year of GH therapy. It is therefore of the utmost importance to refer children with short stature to Pediatric Endocrinology care as early as possible, to limit the severity of short stature and the potential long-term consequences on QoL.
Abbreviations:
- QoL
quality of life
- QoLiSSY
Quality of Life in Short Stature Youth
- SDS
SD score
- SGA
small for gestational age
Acknowledgments
We thank the health care team at the Paediatric Endocrinology, Gynaecology and Diabetology Department of the Necker-Enfants Malades University Hospital, including the nurses (Mireille Bichet, Véronique Elbaz,† Annabelle Voltine, Laetitia Delame), as well as the patients and parents for their patience and willingness to complete the questionnaires. (†Deceased.)
Financial Support: This study was partially supported by an educational grant by Pfizer France. Pfizer employees were not involved in the design, interpretation, and writing of the results.
Author Contributions: Laura González Briceño and Michel Polak designed the study and prepared the manuscript; Magali Viaud and Laura González Briceño delivered the questionnaires at T0, and also T12 when necessary, prepared the Excel data tables, and analyzed these data; Jacques Beltrand, Isabelle Flechtner, Yamina Dassa, Dinane Samara-Boustani, Caroline Thalassinos, Christian Pauwels, Kanetee Busiah, Graziella Pinto, and Michel Polak delivered the questionnaires at T12, participated in several staff discussions concerning the project, and reviewed the manuscript; Delphine Jaquet was consultant for thorough review and analysis of the manuscript.
Disclosure Summary: The authors have nothing to disclose.



