-
PDF
- Split View
-
Views
-
Cite
Cite
Neha Pathania, Praveen Kumar Dubey, A review on high-frequency sound waves and nutrients: influence of ultra-sonication on nutritional aspect of fruit juice blends, International Journal of Food Science and Technology, Volume 60, Issue 1, January 2025, vvae086, https://doi.org/10.1093/ijfood/vvae086
Close - Share Icon Share
Abstract
Fruit-based blends are gaining popularity due to their tremendous properties like they are rich in vitamins, minerals, antioxidants, flavonoids, carotenoids, and dietary fibres, making them highly able to reduce the risk of certain diseases. Due to high water content, fruit juices are highly vulnerable to microbial contamination and spoilage. Therefore, it is a challenge for food industry to maintain the integrity and reduce the destruction of these compounds in fruit juices. Thermal treatments can destroy the heat sensitive compounds like vitamins and antioxidants. Therefore ultrasound (ultra-sonication [US]) has gained popularity as a sustainable non-thermal technology as high frequency sound waves from ultrasound cause disruption of the matrix and favour the compounds to seep out of the cell. US facilitates the extraction of bioactive components like ascorbic acid, carotenoids, and phenolic compounds as well as nutrients such as carbohydrates, proteins, and lipids and increase their accessibility, enhancing the nutritive value of the fruit juices. The current review’s objective is to provide a brief summary of research findings that focus specifically on the current use of US on various fruit-based juices. However, there are fewer researches evaluating the impact of US on the macro and micronutrients.
Introduction
Rising segment of the food processing industry is the development of functional food items, which aims to satisfy the demands of ever-demanding consumers. Eating patterns have been shifting in recent years due to increased consumer awareness and consciousness worldwide regarding the role nutrition plays in promoting health and preventing certain chronic diseases (such as obesity, diabetes, cardiovascular disease, etc.) (Cassani et al., 2020). Overall, this has led to a constantly rising desire for meals that are thought to be healthier, fresh, no additives, ready to eat, and less processed. However, a major issue with producing nutritious ready-to-eat foods, such those made with fresh fruit and vegetables, that do not last very long. The food industry is faced with the task of prolonging the shelf life of these items through the use of mild processing technologies that have minimum impact on their sensory and nutritional qualities (Putnik et al., 2020).
Thermal treatments are the most commonly used methods, but they have some disadvantages. Due their high prices, high energy consumption, and high water consumption, thermal processing technologies are less sustainable than non-thermal processing technologies. The heat produced results in colour changes, creation of toxic compounds, and loss of some aromatic and nutritional molecules, which negatively affects sensory and nutritional quality of fruit juices (Sebastià et al., 2023). The most commonly used method among thermal technologies in the food business for extending the shelf life of fruit juice is pasteurisation, which deactivates harmful bacteria and enzymes. But there are numerous disadvantages associated with its use due to its negative effect on quality and nutritional content of fruit juice (which decreases consumer desire), but also for consuming a lot of water and energy (Wang et al., 2020a), hence the food sector has been encouraged by the increased awareness of environmental sustainability to adopt more creative, green, and sustainable processing methods, which increase production efficiency by using less water, energy, and time.
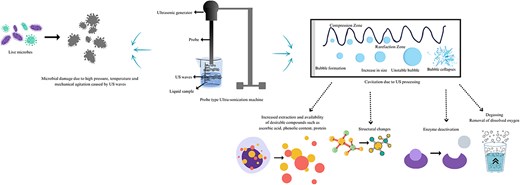
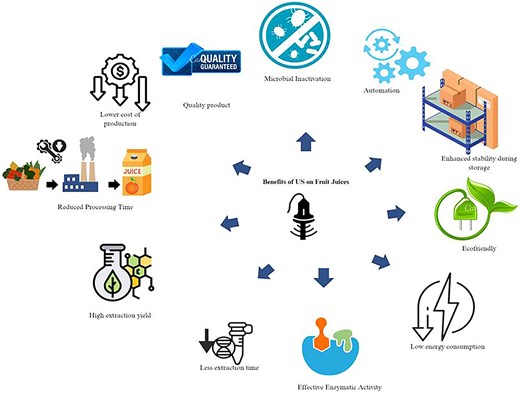
Since ultrasound-assisted processing allows for the eradication of harmful and pathogenic microbes in fruit juices while strengthening their beneficial composition, it has been shown to be a viable substitute for heat pasteurisation (Wang et al., 2019b). Moreover, ultrasound is simple to scale up, dependable, and eco-friendly, indicating that it is a more viable option for commercial use than other expensive non-thermal methods (Nadeem et al., 2018). Figure 1 demonstrates the possible advantages of ultra-sonication (US) in food juice preparation.
Functional plant-based beverages are currently gaining popularity due to the growth of the vegan and vegetarian markets as well as the need for products designed specifically for people who are allergic to milk-based products. Furthermore, the environmental effects of food processing on sustainability and animal welfare are of greater significance to humans. Consequently, the market is becoming more receptive to fruit and vegetable juices mostly due to the link between their consumption and a lower chance of developing chronic illnesses like cancer, diabetes, cardiovascular disease, and hypertension (Guimaraes et al., 2019; Miles & Calder, 2021; Prakoso et al., 2023; Putnik et al., 2020). Juice intake can be influenced by a number of elements, such as its temperature, appearance, flavour, consistency, and colour, also the selection of vegetables and fruits for juice making varies based on availability, accessibility, and cultural norms around the nations (Lepaus et al., 2023).
Fresh food and its derivatives, like juices, are high in water and nutrients and are also utilised as a substrate by microorganisms like moulds, yeasts, and bacteria that can withstand acidic environments; they are vulnerable to microbial deterioration. Thus, malfunctions in the stages of production and microbial management may adversely affect juices, result in an unpleasant odour, alter turbidity or staining, and produce gas and alcohol as a result of decaying microorganisms, in addition to the possibility of diseases brought on by pathogens (José et al., 2022). In this respect, the food sector has been motivated by the increased awareness of environmental sustainability to embrace more innovative, sustainable, and green processing methods, which have increased production competence by lowering the amount of energy, water, and time used (Cassani et al., 2020).
While a number of studies in the literature have explored the benefits of fruit juices, their safety, and quality and enhancing their shelf life. As of now, only a few review papers have been published on this topic like Roobab et al. (2023) which covers ultrasound technology with other thermal technologies on the effect on microbial decontamination of fruit juices. Whereas, Lepaus et al. (2023) covers the effect of US on fruit and vegetable juices, its effect on the nutritional properties and microbial decontamination. However this review paper focuses specifically on the effect of US treatment on the proteins, lipids, carbohydrates, bioactive compounds, antioxidant activity, etc., of fruit juices. However, this is a comprehensive review focusing on the efficient applications of ultrasound and its effects on various nutritional parameters of the fruit juices and its potential applications in food industries.
Understanding conventional thermal and novel non-thermal techniques
To regulate the safety, stability, and shelf life of food products, various types of thermal processes are used conventionally such as pasteurisation, microwave heating, ohmic heating, and infrared radiation. These conventional techniques produce a large amount of heat when used causing increased temperature of the fruit beverages which leads to loss of volatile or thermo labile nutrients such as ascorbic acid and bioactive compounds. It affects the juices’ nutritional and physicochemical qualities, including their pH, colour, and levels of organic acids, carotenoids, and polyphenols and hence reduce the anti-oxidant activity, which is beneficial for human health (Anaya-Esparza et al., 2017). Moreover, heat treatments alter the structure of endogenous enzymes such as polyphenol oxidase and peroxidase, which play a key role in the deterioration of juices (Wibowo et al., 2019).
However novel non thermal techniques such as US, pulse electric field (PEF), high pressure processing, ultraviolet radiation are environment friendly, scalable and cost-effective (Cassani et al., 2020). They preserve heat labile nutrients and maintain quality by reducing heat generated, preventing microbial contamination and altering taste or smell (Nonglait et al., 2022). They may also improve product quality and account for lesser space, energy and water (Bigi et al., 2023). Despite the numerous advantages, there are hinders for their commercial use, such as irradiation technology has low consumer acceptance due to misunderstanding of the term “irradiation.” To a non-food technologist, it sounds similar to “radiation therapy,” raising concern about production of certain carcinogens in food (Perez & Moreira, 2021). In contrast, PEF has very high equipment expenses, very high energy usage, and very low customer awareness (Dubey et al., 2023). Ozone is used for the disinfection of processing equipment. It is very reactive and reacts with many components in food, leading to undesirable changes. It also induces oxidation in food lipids (Jadhav et al., 2021). It has been shown that ultrasound-assisted processing enhances the composition of beneficial compounds, while inactivating harmful and pathogenic microbes hence is a viable substitute for pasteurisation (Nadeem et al., 2018). Moreover, ultrasound is simple to scale up, dependable, occupies lesser space and eco-friendly, indicating that it is a more viable option for commercial use than other costly non-thermal methods (Wang et al., 2019a).
Ultra-sonication: an overview
US uses sound waves of high frequency of about 20 kHz for treating liquid foods, which is above the human hearing limits (Tahir et al., 2023; Wen et al., 2019). The power, frequency, and treatment duration decides the effectiveness of US. According to Das et al. (2022), longitudinal sound waves generate vibrations resulting in cycles of expansion and compression. The cycle of compression forces molecules away from each other as midsection being under positive pressure; due to the variations in the pressure across the system, the liquid medium’s cycle of compression and expansion promotes the production of bubbles (Lepaus et al., 2023). These bubbles ultimately destroy the microbial cell and mechanical shock at intracellular level aids in the inactivation of enzymes. Figure 2 depicts the phenomenon of cavitation due to US and its effect on microbial cell as well as plant cell.
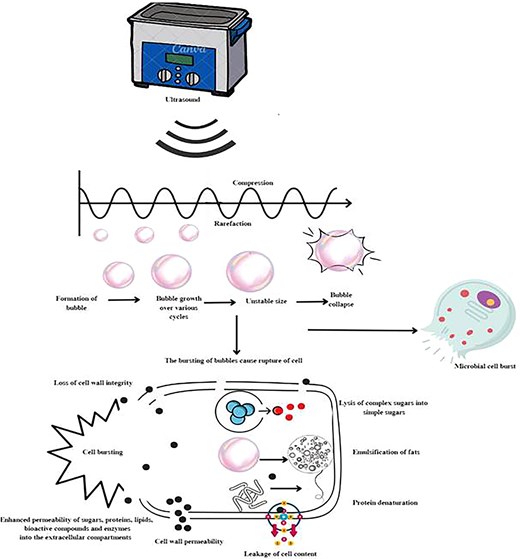
Phenomenon of cavitation due to US and its effect on microbial and plant cell.
US processing is separated as: (a) high power (up to 100 W) and frequency (20–100 kHz) cause extreme cavitation, which may alter the microstructure of the food component, and (b) low power (10 W) and frequency (2–10 MHz) is non-destructive and primarily used in the food processing industry (Pravallika & Chakraborty, 2022). A temperature rise of 5,000 K and pressure 50 MPa is produced by US waves causing strong shearing effect resulting in disruption of the cell wall, cell membrane, and DNA (Bagheri & Abbaszadeh, 2020).
Three primary components make up the US system: generator, transducer, and emitter.
The electrical signal is transformed into a specific frequency by the generator; the transducer then translates this high-frequency electrical signal into mechanical vibrations, which are then emitted by the emitter (Mehta et al., 2022).
Use of US can be risk-free, non-toxic, and suitable for the environment, although its antibacterial activity may be compromised as low microorganism mortality rate was observed at room temperature application of US (Lepaus et al., 2023). Menelli et al. (2021) discovered that contamination was reduced slightly when applying US at 25 °C and more significantly when applying US at higher temperatures. US can be enhanced by combining it with other preservation techniques like pressure (mano-sonication), heat (thermo-sonication), or even a combination of heat and pressure (mano-thermo-sonication) (Dolas et al., 2019).
Effect of US on various nutritional aspects of fruit juices
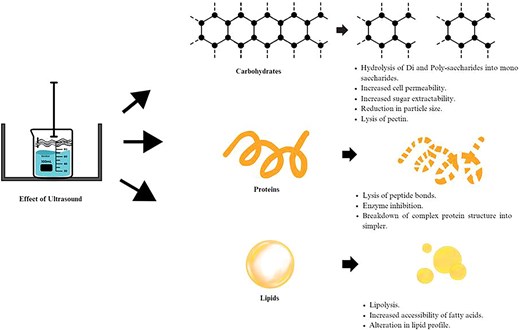
Fruit juices comprises of all the significant nutrients such as macronutrients (proteins, lipids, carbohydrates), micronutrients (minerals and vitamins), and water. Also the organic pigments such as phenolic compounds and carotenoids greatly influence the nutritional quality of the fruit beverages (Pravallika & Chakraborty, 2022). Figure 3 portraits the effect of US on macromolecules present in fruit juices, and the rest is discussed throughout the following parts of the article.
Effect of US on proteins
The building blocks of a protein are its amino acid sequence, which are linked by peptide bonds (Vernes et al., 2019). They are among the most significant and essential nutrients in diet since they promote the growth of muscles and the preservation of cell structure (Vanga et al., 2021). According to the findings (Linhares et al., 2020, Mala et al., 2021; Wang et al., 2019a; 2020a) it was observed that US caused alteration in the protein structure and can damage proteins in the fruits. US can disrupt the peptide links and change the protein structure. Through this procedure, the technology becomes a potentially useful treatment to reduce the allergenicity caused by related allergens present in food (Wang et al., 2020a). Furthermore, due to cell destruction and the protein extraction from within the fruit tissue, greater protein content can be seen after sonication. Thus, it is crucial to comprehend how the US can alter these components in juices.
Different effects on the content of proteins can be observed in juices treated with US (Table 1). The results of US treatment differ in different fruit juices depending on the type of juice used and the type of treatment conditions applied, therefore no alteration in the protein content of strawberry juice was observed in the treated sample (20 kHz, 400 W, 4–16 min) as well as the untreated sample (Wang et al., 2019b) whereas the total protein content of kiwi fruit juice decreased with increase in the US processing time (Wang et al., 2019a). This decline could be linked to the disintegration of peptide chains and hydrogen bonds found in the proteins undergoing ultrasonic therapy, which could change the structure of the proteins (Wang et al., 2019a). It suggests that ultrasonic therapy may be used to change the structure of proteins or break their peptide chains, which may have an impact on the allergenicity of the related allergens.
Effect of various US treatments on protein and lipid content of fruit juices.
| Type of juice . | Engineering operations . | US processing conditions . | Outcomes . | References . |
|---|---|---|---|---|
| Strawberry juice | Peak ripe staged “Seascape” strawberries were harvested → Smashed into double distilled water (1:2) | US type: US probe Frequency: 20 kHz Power: 400 W Time interval: 4–16 min Temperature: ND Other: Pulsed US waves were used. Ice bath used to control the temperature 100 ml of juice sample was dried using freeze drier for 48 hr and rest stored at 4 °C | No alteration in the protein content between treated and untreated product | Wang et al. (2019b) |
| Kiwifruit juice | Fresh ripened kiwi fruit→ Peeled→ Crushed→ Mixed with water (1:2) | US type: US probe Frequency: 20 kHz Power: 400 W Time interval: 4–16 min Temperature: ND Other: 200 ml of sample was treated. Ice bath was applied to control temperature 100 ml of sample was refrigerated at 4° C and rest was freeze dried for FTIR analysis | With increase in the US timing protein content was notably altered | Wang et al. (2020b) |
| Acai juice | Frozen pulp (−18 °C) → Dilution in potable water (1:2) | US type: US Probe with 13 mm titanium tip Frequency: 19 kHz Power: 400 W Time interval: 2–10 min Temperature: 40 °C Other: 150 ml of sample was treated | Betaine content increased during treatment but reduced after 5 min of processing at 372.93 W | Linhares et al. (2020) |
| Pineapple juice | Fresh ripe pineapples → Peeled → Chopped into chunks → Juice obtained through juice extractor | US type: US probe of 13 mm diameters Frequency: 24 kHz Power: 200 W Time interval: 2–10 min Temperature: 25–65 °C Other: Temperature was maintained using digital water bath. Samples stored at 4 °C | Processed sample showed reduced protein content compared to the control Delay in reduction of protein during storage (0–28 days) due to ultra-sonication treatment | Mala et al. (2021) |
| LIPIDS | ||||
| Acai juice | Frozen pulp (−18 °C) → Dilution in potable water (1:2) | US type: US probe with 13 mm titanium tip Frequency: 19 kHz. Power: 400 W. Time interval: 2–10 min Temperature: 40° C Other: 150 ml of sample was treated | Increase in fatty acid content due to treatment | Linhares et al. (2020) |
| Acai juice | Fresh and mature acai fruit → Crushed → Mixed with potable water (1:1) | US type: US probe of 25 mm diameter Frequency: 20 kHz Power: 750 W Time interval: 10 min Other: 100 ml of sample was treated and ice bath was used to control temperature | Increase in oleic acid content at lower energy density (2.7 kJ cm). Linoleic acid showed reduction even at lower levels of energy density but increased when 2.7 and 3.6 kJ cm of energy density was applied | Carvalho et al. (2020) |
| Buriti juice | Fresh and mature buriti fruit → Crushed → Mixed with potable water (1:1) | US type: US probe of 25 mm diameter Frequency: 20 kHz Power: 750 W Time interval: 10 min Other: 100 ml of sample was treated and ice bath was used to control temperature | The concentrations of stearic, palmitic, and myristic acids were increased by sonication. Higher energies decreased the level of fatty acid, although lower energy density treatment enhanced the amount of oleic acid | Carvalho et al. (2020) |
| Type of juice . | Engineering operations . | US processing conditions . | Outcomes . | References . |
|---|---|---|---|---|
| Strawberry juice | Peak ripe staged “Seascape” strawberries were harvested → Smashed into double distilled water (1:2) | US type: US probe Frequency: 20 kHz Power: 400 W Time interval: 4–16 min Temperature: ND Other: Pulsed US waves were used. Ice bath used to control the temperature 100 ml of juice sample was dried using freeze drier for 48 hr and rest stored at 4 °C | No alteration in the protein content between treated and untreated product | Wang et al. (2019b) |
| Kiwifruit juice | Fresh ripened kiwi fruit→ Peeled→ Crushed→ Mixed with water (1:2) | US type: US probe Frequency: 20 kHz Power: 400 W Time interval: 4–16 min Temperature: ND Other: 200 ml of sample was treated. Ice bath was applied to control temperature 100 ml of sample was refrigerated at 4° C and rest was freeze dried for FTIR analysis | With increase in the US timing protein content was notably altered | Wang et al. (2020b) |
| Acai juice | Frozen pulp (−18 °C) → Dilution in potable water (1:2) | US type: US Probe with 13 mm titanium tip Frequency: 19 kHz Power: 400 W Time interval: 2–10 min Temperature: 40 °C Other: 150 ml of sample was treated | Betaine content increased during treatment but reduced after 5 min of processing at 372.93 W | Linhares et al. (2020) |
| Pineapple juice | Fresh ripe pineapples → Peeled → Chopped into chunks → Juice obtained through juice extractor | US type: US probe of 13 mm diameters Frequency: 24 kHz Power: 200 W Time interval: 2–10 min Temperature: 25–65 °C Other: Temperature was maintained using digital water bath. Samples stored at 4 °C | Processed sample showed reduced protein content compared to the control Delay in reduction of protein during storage (0–28 days) due to ultra-sonication treatment | Mala et al. (2021) |
| LIPIDS | ||||
| Acai juice | Frozen pulp (−18 °C) → Dilution in potable water (1:2) | US type: US probe with 13 mm titanium tip Frequency: 19 kHz. Power: 400 W. Time interval: 2–10 min Temperature: 40° C Other: 150 ml of sample was treated | Increase in fatty acid content due to treatment | Linhares et al. (2020) |
| Acai juice | Fresh and mature acai fruit → Crushed → Mixed with potable water (1:1) | US type: US probe of 25 mm diameter Frequency: 20 kHz Power: 750 W Time interval: 10 min Other: 100 ml of sample was treated and ice bath was used to control temperature | Increase in oleic acid content at lower energy density (2.7 kJ cm). Linoleic acid showed reduction even at lower levels of energy density but increased when 2.7 and 3.6 kJ cm of energy density was applied | Carvalho et al. (2020) |
| Buriti juice | Fresh and mature buriti fruit → Crushed → Mixed with potable water (1:1) | US type: US probe of 25 mm diameter Frequency: 20 kHz Power: 750 W Time interval: 10 min Other: 100 ml of sample was treated and ice bath was used to control temperature | The concentrations of stearic, palmitic, and myristic acids were increased by sonication. Higher energies decreased the level of fatty acid, although lower energy density treatment enhanced the amount of oleic acid | Carvalho et al. (2020) |
Note. US = ultra-sonication; ND = not defined.
Effect of various US treatments on protein and lipid content of fruit juices.
| Type of juice . | Engineering operations . | US processing conditions . | Outcomes . | References . |
|---|---|---|---|---|
| Strawberry juice | Peak ripe staged “Seascape” strawberries were harvested → Smashed into double distilled water (1:2) | US type: US probe Frequency: 20 kHz Power: 400 W Time interval: 4–16 min Temperature: ND Other: Pulsed US waves were used. Ice bath used to control the temperature 100 ml of juice sample was dried using freeze drier for 48 hr and rest stored at 4 °C | No alteration in the protein content between treated and untreated product | Wang et al. (2019b) |
| Kiwifruit juice | Fresh ripened kiwi fruit→ Peeled→ Crushed→ Mixed with water (1:2) | US type: US probe Frequency: 20 kHz Power: 400 W Time interval: 4–16 min Temperature: ND Other: 200 ml of sample was treated. Ice bath was applied to control temperature 100 ml of sample was refrigerated at 4° C and rest was freeze dried for FTIR analysis | With increase in the US timing protein content was notably altered | Wang et al. (2020b) |
| Acai juice | Frozen pulp (−18 °C) → Dilution in potable water (1:2) | US type: US Probe with 13 mm titanium tip Frequency: 19 kHz Power: 400 W Time interval: 2–10 min Temperature: 40 °C Other: 150 ml of sample was treated | Betaine content increased during treatment but reduced after 5 min of processing at 372.93 W | Linhares et al. (2020) |
| Pineapple juice | Fresh ripe pineapples → Peeled → Chopped into chunks → Juice obtained through juice extractor | US type: US probe of 13 mm diameters Frequency: 24 kHz Power: 200 W Time interval: 2–10 min Temperature: 25–65 °C Other: Temperature was maintained using digital water bath. Samples stored at 4 °C | Processed sample showed reduced protein content compared to the control Delay in reduction of protein during storage (0–28 days) due to ultra-sonication treatment | Mala et al. (2021) |
| LIPIDS | ||||
| Acai juice | Frozen pulp (−18 °C) → Dilution in potable water (1:2) | US type: US probe with 13 mm titanium tip Frequency: 19 kHz. Power: 400 W. Time interval: 2–10 min Temperature: 40° C Other: 150 ml of sample was treated | Increase in fatty acid content due to treatment | Linhares et al. (2020) |
| Acai juice | Fresh and mature acai fruit → Crushed → Mixed with potable water (1:1) | US type: US probe of 25 mm diameter Frequency: 20 kHz Power: 750 W Time interval: 10 min Other: 100 ml of sample was treated and ice bath was used to control temperature | Increase in oleic acid content at lower energy density (2.7 kJ cm). Linoleic acid showed reduction even at lower levels of energy density but increased when 2.7 and 3.6 kJ cm of energy density was applied | Carvalho et al. (2020) |
| Buriti juice | Fresh and mature buriti fruit → Crushed → Mixed with potable water (1:1) | US type: US probe of 25 mm diameter Frequency: 20 kHz Power: 750 W Time interval: 10 min Other: 100 ml of sample was treated and ice bath was used to control temperature | The concentrations of stearic, palmitic, and myristic acids were increased by sonication. Higher energies decreased the level of fatty acid, although lower energy density treatment enhanced the amount of oleic acid | Carvalho et al. (2020) |
| Type of juice . | Engineering operations . | US processing conditions . | Outcomes . | References . |
|---|---|---|---|---|
| Strawberry juice | Peak ripe staged “Seascape” strawberries were harvested → Smashed into double distilled water (1:2) | US type: US probe Frequency: 20 kHz Power: 400 W Time interval: 4–16 min Temperature: ND Other: Pulsed US waves were used. Ice bath used to control the temperature 100 ml of juice sample was dried using freeze drier for 48 hr and rest stored at 4 °C | No alteration in the protein content between treated and untreated product | Wang et al. (2019b) |
| Kiwifruit juice | Fresh ripened kiwi fruit→ Peeled→ Crushed→ Mixed with water (1:2) | US type: US probe Frequency: 20 kHz Power: 400 W Time interval: 4–16 min Temperature: ND Other: 200 ml of sample was treated. Ice bath was applied to control temperature 100 ml of sample was refrigerated at 4° C and rest was freeze dried for FTIR analysis | With increase in the US timing protein content was notably altered | Wang et al. (2020b) |
| Acai juice | Frozen pulp (−18 °C) → Dilution in potable water (1:2) | US type: US Probe with 13 mm titanium tip Frequency: 19 kHz Power: 400 W Time interval: 2–10 min Temperature: 40 °C Other: 150 ml of sample was treated | Betaine content increased during treatment but reduced after 5 min of processing at 372.93 W | Linhares et al. (2020) |
| Pineapple juice | Fresh ripe pineapples → Peeled → Chopped into chunks → Juice obtained through juice extractor | US type: US probe of 13 mm diameters Frequency: 24 kHz Power: 200 W Time interval: 2–10 min Temperature: 25–65 °C Other: Temperature was maintained using digital water bath. Samples stored at 4 °C | Processed sample showed reduced protein content compared to the control Delay in reduction of protein during storage (0–28 days) due to ultra-sonication treatment | Mala et al. (2021) |
| LIPIDS | ||||
| Acai juice | Frozen pulp (−18 °C) → Dilution in potable water (1:2) | US type: US probe with 13 mm titanium tip Frequency: 19 kHz. Power: 400 W. Time interval: 2–10 min Temperature: 40° C Other: 150 ml of sample was treated | Increase in fatty acid content due to treatment | Linhares et al. (2020) |
| Acai juice | Fresh and mature acai fruit → Crushed → Mixed with potable water (1:1) | US type: US probe of 25 mm diameter Frequency: 20 kHz Power: 750 W Time interval: 10 min Other: 100 ml of sample was treated and ice bath was used to control temperature | Increase in oleic acid content at lower energy density (2.7 kJ cm). Linoleic acid showed reduction even at lower levels of energy density but increased when 2.7 and 3.6 kJ cm of energy density was applied | Carvalho et al. (2020) |
| Buriti juice | Fresh and mature buriti fruit → Crushed → Mixed with potable water (1:1) | US type: US probe of 25 mm diameter Frequency: 20 kHz Power: 750 W Time interval: 10 min Other: 100 ml of sample was treated and ice bath was used to control temperature | The concentrations of stearic, palmitic, and myristic acids were increased by sonication. Higher energies decreased the level of fatty acid, although lower energy density treatment enhanced the amount of oleic acid | Carvalho et al. (2020) |
Note. US = ultra-sonication; ND = not defined.
According to Linhares et al. (2020), initially an increase in the content of amino acid betaine was observed during the treatment (19 kHz 75.34–372.93 W, 25° C, 2–10 min); however after 5 min of treatment it started reducing. The possible reason of increase in the protein content is due to extraction of protein and cell damage caused by US treatment. However, according to a separate study (Mala et al., 2021), pineapple juice’s protein concentration decreased somewhat on day 0 following sonication (24 kHz, 62 °C, 2 min, amplitude 35%), although bromelain activity stayed constant; while there was a steady decline in bromelain activity across all treatments after a 28-day storage period, the thermosonicated sample exhibited considerably higher enzyme activity than the control.
Therefore it can be concluded from the literature that the protein content of some fruit juices after US treatment increased possibly due to the enhanced extraction of proteins from the intracellular compartments which was earlier bound; however, some contradictory studies have shown a decrease in the protein content which possibly can be due to protein degradation and parameters such as juice type, conditions applied such as frequency, power, temperature, time, etc., also influence the result therefore future studies are required (Linhares et al., 2020).
Effect of US on lipids
Lipids, like proteins, are not the primary constituents of fruits. Additionally, fruits such as avocado, coconut, olives, acai, and buriti are good source of fatty acids. US is a promising technique for the extraction of plant based oils as it improves the oil recovery with shorter extraction time, do not affect the fatty acid composition and thermal behaviour of papaya seed oil (Senrayan & Venkatachalam, 2019). US improves lipid dispersion by converting larger fat globules into smaller forms, resulting in emulsion stability in plant based beverages (Rojas et al., 2022). The oil obtained from seeds while extracting the fruit juice is rich in triglycerides that is an excellent source of fatty acids, such as omega-3, omega-6, PUFA (poly unsaturated fatty acids), MUFA (mono unsaturated fatty acid), EFA (essential fatty acid), and also saturated fatty acids, which are essential for maintaining human health (Kaseke et al., 2020).
The application of ultrasound results in small droplet size and enhances the dispersion phase, both of which support emulsion stability, moreover, sonication and lipolysis events brought on by enzymes like lipases or esterase can result in oxidation reactions in fatty acids (Guimaraes et al., 2019). In contrary to the conventional processes, which transfers the heat from the heating source to the vessel by conduction and then to the solvent by convection, ultrasound-assisted operation uses cavitation events to deliver the acoustic energy directly into the solvent-matrix mixture. The method of indirect transfer is time-consuming and energy-intensive (Leong et al., 2017). Due to ultrasound-induced shear force and micro turbulence cause large droplets to break into smaller ones, US can increase the interfacial area for lipid extraction, increase the degree of emulsification, and thus increase extraction rate (Yatipanthalawa et al., 2021). The lipid extraction is enhanced by US without altering the fatty acid profile (Meena et al., 2024). Capar et al. (2021) stated in his studies on cranberry seeds that US-assisted process gave a yield of 7.44% lipid with an extraction time of 10 min, whereas the conventional process resulted in a lower yield of 3.6% with an extraction time of 8 hr and temperature 100° C.
Given the studies conducted and the literature published, very less is known about the potential effects of ultrasound on the lipid content of juices, mostly since juices does not contain a significant amount of it. Therefore, understanding potential content changes and their potential effects is difficult.
Effect of US on carbohydrates (mono- and poly-saccharides)
Carbohydrates are the most abundant organic molecules found in the nature; termed as hydrates of carbon as the core carbon atom is combined with water with empirical formula Cn (H₂O)n, where n > 3 (Albuquerque et al., 2020). Class of chemically defined compounds with numerous physiological, physical, and health-promoting characteristics make up carbohydrates. The degree of polymerisation and the type of linkage are two structural characteristics that are used to classify carbohydrates including monosaccharide and disaccharides in addition to those made from short-chain pectin, galacto-oligosaccharides, xylan-oligosaccharides, dextrins, and oligofructans (Galanakis, 2021). Fruits have monosaccharides such as fructose and glucose, hence the sweetness, flavour, and scent of juice is all influenced by these simple carbohydrates (Wang et al., 2019a).
Hydrolysis of simple and complex carbohydrates was noted during the US treatment (Table 2). It was observed that the sucrose content decreased after 0–12 min of treatment, but it increased after 16 min (Wang et al., 2020b). US facilitates more mass transfer and increases cell permeability, which enhances the ability to take sugars from cells. This phenomenon is the result of degradation due to the Maillard reaction or effects of the oxidation (Silva et al., 2020). Sugars are removed from intracellular structures by US and added to the fluid as a result of the physical and mechanical impacts of technology shattering vegetative cells (Rojas et al., 2021). Contrary to other studies Silva et al. (2020) and Cheng et al. (2020) observed no alteration in the contents of glucose, fructose, and sucrose in both treated as well as untreated orange (20 kHz, 0–1,200 W, 0–88 °C, 10 min) and mandarin (19 kHz, 750 W, 50 °C, 36 min) juice sample, respectively.
| Type of juice . | Engineering operations . | US processing conditions . | Outcomes . | References . |
|---|---|---|---|---|
| Kiwi juice | Ripe kiwi → Cold centrifugal juicer → Juice refrigerated at 4 °C | US type: US probe Frequency: 20 kHz Power: 400 W Time interval: 4–16 min Temperature: ND Other: 300 ml of sample was capped with polytetrafluoroethylene film and stored at 4 °C. Rest of the samples were freeze dried for 48 hr and stored at −20 °C | 0–12 min of treatment reduced the sucrose content whereas an increase was observed after 16 min of treatment Increase in water soluble pectin content | Wang et al. (2020b) |
| Orange juice | Frozen orange pulp → Mixed with purified water (20 g orange concentrate in 79 g water) | US type: US probe of 10 mm diameter Frequency: 20 kHz Power: 0–1,200 W Time interval: 10 min Temperature: 0–88° C Other: Ice bath at 0 °C was used to avoid overheating | No alteration in the content of glucose, sucrose, and fructose in both treated as well as control sample | Silva et al. (2020) |
| Mandarin juice | Mandarin fruit harvested → Juice extracted in manual juicer → Filtered to remove peels and seeds → Refrigerated at 4 °C | US type: US probe Frequency: 19 kHz Power: 750 W Time interval: 36 min Temperature: 50° C Other: Water bath was used to maintain temperature | Fructose, glucose and sucrose content remained stable | Cheng et al. (2020) |
| Acai juice | Frozen pulp (−18 °C) → Dilution in potable water (1:2) | US type: Stored at 4 °C for analysis Frequency: 19 kHz Power: ND Time interval: 2–10 min Temperature: 40° C Other: 150 ml sample was used | Increase in glucose and fructose content after processing | Linhares et al. (2020) |
| Apple juice | Fresh Fuji apples → Washed → Peeled → Chopped into pieces → Pulped in presence of ascorbic acid → Filtered | US type: US probe with 15 mm diameter Frequency: ND Power:525–1,125 W Time interval: 5–12 min Temperature: 30–70° C Other: 200 ml of sample was treated and stored at 4 °C for analysis | Increase in water soluble pectin content | Shen et al. (2021) |
| Type of juice . | Engineering operations . | US processing conditions . | Outcomes . | References . |
|---|---|---|---|---|
| Kiwi juice | Ripe kiwi → Cold centrifugal juicer → Juice refrigerated at 4 °C | US type: US probe Frequency: 20 kHz Power: 400 W Time interval: 4–16 min Temperature: ND Other: 300 ml of sample was capped with polytetrafluoroethylene film and stored at 4 °C. Rest of the samples were freeze dried for 48 hr and stored at −20 °C | 0–12 min of treatment reduced the sucrose content whereas an increase was observed after 16 min of treatment Increase in water soluble pectin content | Wang et al. (2020b) |
| Orange juice | Frozen orange pulp → Mixed with purified water (20 g orange concentrate in 79 g water) | US type: US probe of 10 mm diameter Frequency: 20 kHz Power: 0–1,200 W Time interval: 10 min Temperature: 0–88° C Other: Ice bath at 0 °C was used to avoid overheating | No alteration in the content of glucose, sucrose, and fructose in both treated as well as control sample | Silva et al. (2020) |
| Mandarin juice | Mandarin fruit harvested → Juice extracted in manual juicer → Filtered to remove peels and seeds → Refrigerated at 4 °C | US type: US probe Frequency: 19 kHz Power: 750 W Time interval: 36 min Temperature: 50° C Other: Water bath was used to maintain temperature | Fructose, glucose and sucrose content remained stable | Cheng et al. (2020) |
| Acai juice | Frozen pulp (−18 °C) → Dilution in potable water (1:2) | US type: Stored at 4 °C for analysis Frequency: 19 kHz Power: ND Time interval: 2–10 min Temperature: 40° C Other: 150 ml sample was used | Increase in glucose and fructose content after processing | Linhares et al. (2020) |
| Apple juice | Fresh Fuji apples → Washed → Peeled → Chopped into pieces → Pulped in presence of ascorbic acid → Filtered | US type: US probe with 15 mm diameter Frequency: ND Power:525–1,125 W Time interval: 5–12 min Temperature: 30–70° C Other: 200 ml of sample was treated and stored at 4 °C for analysis | Increase in water soluble pectin content | Shen et al. (2021) |
Note. US = ultra-sonication; ND = not defined.
| Type of juice . | Engineering operations . | US processing conditions . | Outcomes . | References . |
|---|---|---|---|---|
| Kiwi juice | Ripe kiwi → Cold centrifugal juicer → Juice refrigerated at 4 °C | US type: US probe Frequency: 20 kHz Power: 400 W Time interval: 4–16 min Temperature: ND Other: 300 ml of sample was capped with polytetrafluoroethylene film and stored at 4 °C. Rest of the samples were freeze dried for 48 hr and stored at −20 °C | 0–12 min of treatment reduced the sucrose content whereas an increase was observed after 16 min of treatment Increase in water soluble pectin content | Wang et al. (2020b) |
| Orange juice | Frozen orange pulp → Mixed with purified water (20 g orange concentrate in 79 g water) | US type: US probe of 10 mm diameter Frequency: 20 kHz Power: 0–1,200 W Time interval: 10 min Temperature: 0–88° C Other: Ice bath at 0 °C was used to avoid overheating | No alteration in the content of glucose, sucrose, and fructose in both treated as well as control sample | Silva et al. (2020) |
| Mandarin juice | Mandarin fruit harvested → Juice extracted in manual juicer → Filtered to remove peels and seeds → Refrigerated at 4 °C | US type: US probe Frequency: 19 kHz Power: 750 W Time interval: 36 min Temperature: 50° C Other: Water bath was used to maintain temperature | Fructose, glucose and sucrose content remained stable | Cheng et al. (2020) |
| Acai juice | Frozen pulp (−18 °C) → Dilution in potable water (1:2) | US type: Stored at 4 °C for analysis Frequency: 19 kHz Power: ND Time interval: 2–10 min Temperature: 40° C Other: 150 ml sample was used | Increase in glucose and fructose content after processing | Linhares et al. (2020) |
| Apple juice | Fresh Fuji apples → Washed → Peeled → Chopped into pieces → Pulped in presence of ascorbic acid → Filtered | US type: US probe with 15 mm diameter Frequency: ND Power:525–1,125 W Time interval: 5–12 min Temperature: 30–70° C Other: 200 ml of sample was treated and stored at 4 °C for analysis | Increase in water soluble pectin content | Shen et al. (2021) |
| Type of juice . | Engineering operations . | US processing conditions . | Outcomes . | References . |
|---|---|---|---|---|
| Kiwi juice | Ripe kiwi → Cold centrifugal juicer → Juice refrigerated at 4 °C | US type: US probe Frequency: 20 kHz Power: 400 W Time interval: 4–16 min Temperature: ND Other: 300 ml of sample was capped with polytetrafluoroethylene film and stored at 4 °C. Rest of the samples were freeze dried for 48 hr and stored at −20 °C | 0–12 min of treatment reduced the sucrose content whereas an increase was observed after 16 min of treatment Increase in water soluble pectin content | Wang et al. (2020b) |
| Orange juice | Frozen orange pulp → Mixed with purified water (20 g orange concentrate in 79 g water) | US type: US probe of 10 mm diameter Frequency: 20 kHz Power: 0–1,200 W Time interval: 10 min Temperature: 0–88° C Other: Ice bath at 0 °C was used to avoid overheating | No alteration in the content of glucose, sucrose, and fructose in both treated as well as control sample | Silva et al. (2020) |
| Mandarin juice | Mandarin fruit harvested → Juice extracted in manual juicer → Filtered to remove peels and seeds → Refrigerated at 4 °C | US type: US probe Frequency: 19 kHz Power: 750 W Time interval: 36 min Temperature: 50° C Other: Water bath was used to maintain temperature | Fructose, glucose and sucrose content remained stable | Cheng et al. (2020) |
| Acai juice | Frozen pulp (−18 °C) → Dilution in potable water (1:2) | US type: Stored at 4 °C for analysis Frequency: 19 kHz Power: ND Time interval: 2–10 min Temperature: 40° C Other: 150 ml sample was used | Increase in glucose and fructose content after processing | Linhares et al. (2020) |
| Apple juice | Fresh Fuji apples → Washed → Peeled → Chopped into pieces → Pulped in presence of ascorbic acid → Filtered | US type: US probe with 15 mm diameter Frequency: ND Power:525–1,125 W Time interval: 5–12 min Temperature: 30–70° C Other: 200 ml of sample was treated and stored at 4 °C for analysis | Increase in water soluble pectin content | Shen et al. (2021) |
Note. US = ultra-sonication; ND = not defined.
The pectin defragmentation due to cavitation process leads increase in the pectin content and viscosity reduction that was slower than that of untreated juices leading to higher stability of viscous nature in prior (Wang et al., 2019b).
Hence, it can be concluded from above studies that US leads to destruction of cells that leads to the extraction of simple sugars from the intracellular compartment into the fluid matrix and enhances the cell permeability leading to mass transfer; contrary to it due to Maillard reaction which leads to the oxidation and degradation, there can be a reduction in simple sugars. Whereas US leads to breakdown of pectin due to cavitation process that leads to increase in the pectin content and slow reduction in viscosity of juice.
Effect of US on ascorbic acid
Ascorbic acid is also popularly known as Vitamin C and is also a strong antioxidant therefore helping the body fight against free radicals produced during the metabolism of various compounds. It is in charge of several functions, such as iron absorption, management of leukocytes’ absorption of microbes, strengthening and sealing of blood vessels, reduction of cholesterol, and the quickening of wound healing (May & Harrison, 2013). The molecule also plays a key role in regulating the creation of collagen, delaying the ageing process of the skin, and decreasing blood pressure (Crisan et al., 2015). Ascorbic acids exhibit a high degree of sensitivity to light and different processing conditions and their breakdown process occurs via either anaerobic or aerobic routes, additionally fruit juice is exposed to air during ultrasonic processing, and as processing duration increases, oxidation processes intensify, potentially accelerating ascorbic acid breakdown (Wang et al., 2019a, 2019b). Based on the observations in Table 3 juices with high levels of ascorbic acid may be adversely affected by ultrasound treatment as high-intensity conditions promote the production and release of free radicals; depending on processing duration, this might enhance the nutritional degradation. Consequently, an increase in liquid medium temperature may cause ascorbic acid to decompose (Starek et al., 2021). However, some studies (Oladunjoye et al., 2021) showed that cavitation can occasionally assist in removing dissolved oxygen molecules produced due to oxidation process known as degassing which keeps the Vitamin C content unaffected after US processing.
Effect of various us treatments on ascorbic acid, total phenolic content, and carotenoids of fruit juices.
| Type of juice . | Engineering operations . | US processing conditions . | Outcomes . | References . |
|---|---|---|---|---|
| Kiwi juice | Fresh ripened kiwi fruit → Peeled → Crushed → Mixed with water (1:2) | US type: US probe Frequency: 20 kHz Power: 400 W Time interval: 4–16 min Temperature: ND Other: Ice bath was applied to control temperature. 100 ml of sample was refrigerated at 4° C and rest was freeze dried for FTIR analysis | Increased degradation of ascorbic acid as the treatment time increased | Wang et al. (2019a) |
| Tomato juice | Fresh tomatoes → Washed → Dried → Juice extracted via juicer | US type: US probe with 19 mm diameter Frequency: 20 kHz Power: 28–40 W/cm Time interval: 2–10 min Temperature: 37–52 °C Other: Samples were refrigerated at 4 °C | Small reduction in ascorbic acid content | Starek et al. (2021) |
| Strawberry juice | Fresh strawberries → Washed → Sanitised with sodium hypochlorite(100 mg/L) for 15 min → Mixed with water (2:1 of strawberry and water) | US type: US bath Frequency: 40 kHz Power: 110 W Time interval: 5–15 min Temperature: 25–50 °C Other: Sample were cooled and analysed | No difference in ascorbic acid content of treated and control sample | Menelli et al. (2021) |
| Hog plum juice | Matured and ripe Hog plum fruit → Washed with distilled water → Air dried → Peeled → Juice extracted from juicer | US type: US bath Frequency: 40 kHz Power: 400 W Time interval: 5–30 min Temperature: 40–60° C. Other: Treatment carried out in dark to prevent interference due to light. Cooled at room temperature and stored at 4 °C | Increase in ascorbic acid content | Oladunjoye et al. (2021) |
| Mandarin juice | Mandarin fruit harvested → Juice extracted in manual juicer → Filtered to remove peels and seeds → Refrigerated at 4 °C | US type: US probe Frequency: 19 kHz Power: 750 W Time interval: 36 min Temperature: 50° C Other: Water bath was used to maintain temperature | Decrease in ascorbic acid after processing | Cheng et al. (2020) |
| Orange juice | Fresh navel oranges → Washed → Juice extracted from automatic juice extractor | US type: US probe of 13 mm diameter Frequency: 19 kHz Power: ND Time interval: 15–150 s Temperature: 37° C Other: Ice water bath was used to prevent overheating | No difference observed in treated as well as control sample | Gomes et al. (2022) |
| Phenolic compounds | ||||
| Kiwi juice | Fresh ripened kiwi fruit → Peeled → Crushed → Mixed with water (1:2) | US type: US bath Frequency: 20 kHz Power: 400 W Time interval: 4–16 min Temperature: ND Other: Ice bath was applied to control temperature. 100 ml of sample was refrigerated at 4° C and rest was freeze dried for FTIR analysis | Positive effect of US on total phenolic content, flavonoids, gallic acid, and catechin | Wang et al. (2019a) |
| Strawberry juice | Peak ripe staged “Seascape” strawberries were harvested → Smashed into double distilled water (1:2) | US type: US probe Frequency: 20 kHz Power: 400 W Time interval: 4–16 min Temperature: ND Other: Use of pulsed US wave. Ice bath used to control the temperature. 100 ml of juice sample was dried using freeze drier for 48 hr and rest stored at 4 °C | Processing up to 12 min increased total phenols and flavonoid content. Whereas after 16 min of treatment there was a decrease in the content | Wang et al. (2019b) |
| Mandarin juice | Mandarin fruit harvested → Juice extracted in manual juicer → Filtered to remove peels and seeds → Refrigerated at 4 °C. | US type: US probe Frequency: 19 kHz Power: 750 W Time interval: 36 min Temperature: 50° C Other: Water bath was used to maintain temperature | The total phenolic content decreased after treatment | Cheng et al. (2020) |
| Strawberry juice | Fresh strawberries → Washed → Sanitised with sodium hypochlorite(100 mg/L) for 15 min → Mixed with water (2:1 of strawberry and water) | US type: US bath Frequency: 40 kHz Power: 110 W Time interval: 5–15 min Temperature: 25–50° C Other: Samples were cooled and analysed | No effect on phenolic content | Menelli et al. (2021) |
| Sugarcane juice | Sugarcane harvested → Sugarcane culms were grounded in mechanical mill → juice was homogenised → packaged in polypropylene bags (150 ml), and stored and protected from light at −18 °C | US type: US probe with 1.2 cm diameter Frequency: 20 kHz Power: 750 W Time interval: 2–36 min Temperature: 80° C Other: ND | No effect on total phenol content and remained stable | Rodrigues et al. (2021) |
| Cashew apple juice | Fruit harvested → Washed → Fruit separated from the nut → Grounded in a blender → Puree by adding water, sugar and citric acid | US type: S14 US probe Frequency: 24 kHz Power: 85 W Time interval: 15 min Temperature: 60° C Other: Sample stored in sterile glass bottles at 4° C after cooling at room temperature | Phenolic content was retained without any alteration | Deli et al. (2022) |
| Carotenoids | ||||
| Guava juice | Industrial pasteurised guava pulp → diluted in distilled water (15% pulp wt/wt) | US type: US probe with a 1.26 cm2 titanium tip Frequency: 20 kHz Power: 1000 W Time interval: 3–9 min Temperature: 25° C Other: 200 ml of juice sample was treated and then stored at 25° C for 14 days | With increase in treatment time lycopene content decreased gradually | Campoli et al. (2018) |
| Tomato juice | Ripe tomato fruit → Cleaned → Rinsed at 60 °C → Filtered to get rid of peels and seeds | US type: US probe with 6 mm titanium tip Frequency: 25 kHz Power: 200–800 W Time interval: 20 min Temperature: ND Other: 100 g of sample was treated | Higher value for lycopene content was obtained at 400 W | Zhang et al. (2019) |
| Buriti juice | Fresh and mature buriti fruit → Crushed → Mixed with potable water (1:1) | US type: US probe of 25 mm diameter Frequency: 20 kHz Power: 750 W Time interval: 10 min Temperature: ND Other: 100 ml of sample was treated and ice bath was used to control temperature | With increase in energy density the carotenoid content increased | Carvalho et al. (2020) |
| Hog plum juice | Matured and ripe Hog plum fruit → Washed with distilled water → Air dried → Peeled → Juice extracted from juicer | US type: US bath Frequency: 40 kHz Power: 400 W Time interval: 5–30 min Temperature: 40–60° C Other: Treatment carried out in dark to prevent interference due to light. Cooled at room temperature and stored at 4 °C | Carotenoid content increased | Oladunjoye et al. (2021) |
| Mandarin juice | Mandarin fruit harvested → Juice extracted in manual juicer → Filtered to remove peels and seeds → Refrigerated at 4 °C | US type: US probe Frequency: 19 kHz Power: 750 W Time interval: 36 min Temperature: 50° C Other: Water bath was used to maintain temperature | Increment in total carotenoid content | Cheng et al. (2020) |
| Type of juice . | Engineering operations . | US processing conditions . | Outcomes . | References . |
|---|---|---|---|---|
| Kiwi juice | Fresh ripened kiwi fruit → Peeled → Crushed → Mixed with water (1:2) | US type: US probe Frequency: 20 kHz Power: 400 W Time interval: 4–16 min Temperature: ND Other: Ice bath was applied to control temperature. 100 ml of sample was refrigerated at 4° C and rest was freeze dried for FTIR analysis | Increased degradation of ascorbic acid as the treatment time increased | Wang et al. (2019a) |
| Tomato juice | Fresh tomatoes → Washed → Dried → Juice extracted via juicer | US type: US probe with 19 mm diameter Frequency: 20 kHz Power: 28–40 W/cm Time interval: 2–10 min Temperature: 37–52 °C Other: Samples were refrigerated at 4 °C | Small reduction in ascorbic acid content | Starek et al. (2021) |
| Strawberry juice | Fresh strawberries → Washed → Sanitised with sodium hypochlorite(100 mg/L) for 15 min → Mixed with water (2:1 of strawberry and water) | US type: US bath Frequency: 40 kHz Power: 110 W Time interval: 5–15 min Temperature: 25–50 °C Other: Sample were cooled and analysed | No difference in ascorbic acid content of treated and control sample | Menelli et al. (2021) |
| Hog plum juice | Matured and ripe Hog plum fruit → Washed with distilled water → Air dried → Peeled → Juice extracted from juicer | US type: US bath Frequency: 40 kHz Power: 400 W Time interval: 5–30 min Temperature: 40–60° C. Other: Treatment carried out in dark to prevent interference due to light. Cooled at room temperature and stored at 4 °C | Increase in ascorbic acid content | Oladunjoye et al. (2021) |
| Mandarin juice | Mandarin fruit harvested → Juice extracted in manual juicer → Filtered to remove peels and seeds → Refrigerated at 4 °C | US type: US probe Frequency: 19 kHz Power: 750 W Time interval: 36 min Temperature: 50° C Other: Water bath was used to maintain temperature | Decrease in ascorbic acid after processing | Cheng et al. (2020) |
| Orange juice | Fresh navel oranges → Washed → Juice extracted from automatic juice extractor | US type: US probe of 13 mm diameter Frequency: 19 kHz Power: ND Time interval: 15–150 s Temperature: 37° C Other: Ice water bath was used to prevent overheating | No difference observed in treated as well as control sample | Gomes et al. (2022) |
| Phenolic compounds | ||||
| Kiwi juice | Fresh ripened kiwi fruit → Peeled → Crushed → Mixed with water (1:2) | US type: US bath Frequency: 20 kHz Power: 400 W Time interval: 4–16 min Temperature: ND Other: Ice bath was applied to control temperature. 100 ml of sample was refrigerated at 4° C and rest was freeze dried for FTIR analysis | Positive effect of US on total phenolic content, flavonoids, gallic acid, and catechin | Wang et al. (2019a) |
| Strawberry juice | Peak ripe staged “Seascape” strawberries were harvested → Smashed into double distilled water (1:2) | US type: US probe Frequency: 20 kHz Power: 400 W Time interval: 4–16 min Temperature: ND Other: Use of pulsed US wave. Ice bath used to control the temperature. 100 ml of juice sample was dried using freeze drier for 48 hr and rest stored at 4 °C | Processing up to 12 min increased total phenols and flavonoid content. Whereas after 16 min of treatment there was a decrease in the content | Wang et al. (2019b) |
| Mandarin juice | Mandarin fruit harvested → Juice extracted in manual juicer → Filtered to remove peels and seeds → Refrigerated at 4 °C. | US type: US probe Frequency: 19 kHz Power: 750 W Time interval: 36 min Temperature: 50° C Other: Water bath was used to maintain temperature | The total phenolic content decreased after treatment | Cheng et al. (2020) |
| Strawberry juice | Fresh strawberries → Washed → Sanitised with sodium hypochlorite(100 mg/L) for 15 min → Mixed with water (2:1 of strawberry and water) | US type: US bath Frequency: 40 kHz Power: 110 W Time interval: 5–15 min Temperature: 25–50° C Other: Samples were cooled and analysed | No effect on phenolic content | Menelli et al. (2021) |
| Sugarcane juice | Sugarcane harvested → Sugarcane culms were grounded in mechanical mill → juice was homogenised → packaged in polypropylene bags (150 ml), and stored and protected from light at −18 °C | US type: US probe with 1.2 cm diameter Frequency: 20 kHz Power: 750 W Time interval: 2–36 min Temperature: 80° C Other: ND | No effect on total phenol content and remained stable | Rodrigues et al. (2021) |
| Cashew apple juice | Fruit harvested → Washed → Fruit separated from the nut → Grounded in a blender → Puree by adding water, sugar and citric acid | US type: S14 US probe Frequency: 24 kHz Power: 85 W Time interval: 15 min Temperature: 60° C Other: Sample stored in sterile glass bottles at 4° C after cooling at room temperature | Phenolic content was retained without any alteration | Deli et al. (2022) |
| Carotenoids | ||||
| Guava juice | Industrial pasteurised guava pulp → diluted in distilled water (15% pulp wt/wt) | US type: US probe with a 1.26 cm2 titanium tip Frequency: 20 kHz Power: 1000 W Time interval: 3–9 min Temperature: 25° C Other: 200 ml of juice sample was treated and then stored at 25° C for 14 days | With increase in treatment time lycopene content decreased gradually | Campoli et al. (2018) |
| Tomato juice | Ripe tomato fruit → Cleaned → Rinsed at 60 °C → Filtered to get rid of peels and seeds | US type: US probe with 6 mm titanium tip Frequency: 25 kHz Power: 200–800 W Time interval: 20 min Temperature: ND Other: 100 g of sample was treated | Higher value for lycopene content was obtained at 400 W | Zhang et al. (2019) |
| Buriti juice | Fresh and mature buriti fruit → Crushed → Mixed with potable water (1:1) | US type: US probe of 25 mm diameter Frequency: 20 kHz Power: 750 W Time interval: 10 min Temperature: ND Other: 100 ml of sample was treated and ice bath was used to control temperature | With increase in energy density the carotenoid content increased | Carvalho et al. (2020) |
| Hog plum juice | Matured and ripe Hog plum fruit → Washed with distilled water → Air dried → Peeled → Juice extracted from juicer | US type: US bath Frequency: 40 kHz Power: 400 W Time interval: 5–30 min Temperature: 40–60° C Other: Treatment carried out in dark to prevent interference due to light. Cooled at room temperature and stored at 4 °C | Carotenoid content increased | Oladunjoye et al. (2021) |
| Mandarin juice | Mandarin fruit harvested → Juice extracted in manual juicer → Filtered to remove peels and seeds → Refrigerated at 4 °C | US type: US probe Frequency: 19 kHz Power: 750 W Time interval: 36 min Temperature: 50° C Other: Water bath was used to maintain temperature | Increment in total carotenoid content | Cheng et al. (2020) |
Note. US = ultra-sonication; ND = not defined.
Effect of various us treatments on ascorbic acid, total phenolic content, and carotenoids of fruit juices.
| Type of juice . | Engineering operations . | US processing conditions . | Outcomes . | References . |
|---|---|---|---|---|
| Kiwi juice | Fresh ripened kiwi fruit → Peeled → Crushed → Mixed with water (1:2) | US type: US probe Frequency: 20 kHz Power: 400 W Time interval: 4–16 min Temperature: ND Other: Ice bath was applied to control temperature. 100 ml of sample was refrigerated at 4° C and rest was freeze dried for FTIR analysis | Increased degradation of ascorbic acid as the treatment time increased | Wang et al. (2019a) |
| Tomato juice | Fresh tomatoes → Washed → Dried → Juice extracted via juicer | US type: US probe with 19 mm diameter Frequency: 20 kHz Power: 28–40 W/cm Time interval: 2–10 min Temperature: 37–52 °C Other: Samples were refrigerated at 4 °C | Small reduction in ascorbic acid content | Starek et al. (2021) |
| Strawberry juice | Fresh strawberries → Washed → Sanitised with sodium hypochlorite(100 mg/L) for 15 min → Mixed with water (2:1 of strawberry and water) | US type: US bath Frequency: 40 kHz Power: 110 W Time interval: 5–15 min Temperature: 25–50 °C Other: Sample were cooled and analysed | No difference in ascorbic acid content of treated and control sample | Menelli et al. (2021) |
| Hog plum juice | Matured and ripe Hog plum fruit → Washed with distilled water → Air dried → Peeled → Juice extracted from juicer | US type: US bath Frequency: 40 kHz Power: 400 W Time interval: 5–30 min Temperature: 40–60° C. Other: Treatment carried out in dark to prevent interference due to light. Cooled at room temperature and stored at 4 °C | Increase in ascorbic acid content | Oladunjoye et al. (2021) |
| Mandarin juice | Mandarin fruit harvested → Juice extracted in manual juicer → Filtered to remove peels and seeds → Refrigerated at 4 °C | US type: US probe Frequency: 19 kHz Power: 750 W Time interval: 36 min Temperature: 50° C Other: Water bath was used to maintain temperature | Decrease in ascorbic acid after processing | Cheng et al. (2020) |
| Orange juice | Fresh navel oranges → Washed → Juice extracted from automatic juice extractor | US type: US probe of 13 mm diameter Frequency: 19 kHz Power: ND Time interval: 15–150 s Temperature: 37° C Other: Ice water bath was used to prevent overheating | No difference observed in treated as well as control sample | Gomes et al. (2022) |
| Phenolic compounds | ||||
| Kiwi juice | Fresh ripened kiwi fruit → Peeled → Crushed → Mixed with water (1:2) | US type: US bath Frequency: 20 kHz Power: 400 W Time interval: 4–16 min Temperature: ND Other: Ice bath was applied to control temperature. 100 ml of sample was refrigerated at 4° C and rest was freeze dried for FTIR analysis | Positive effect of US on total phenolic content, flavonoids, gallic acid, and catechin | Wang et al. (2019a) |
| Strawberry juice | Peak ripe staged “Seascape” strawberries were harvested → Smashed into double distilled water (1:2) | US type: US probe Frequency: 20 kHz Power: 400 W Time interval: 4–16 min Temperature: ND Other: Use of pulsed US wave. Ice bath used to control the temperature. 100 ml of juice sample was dried using freeze drier for 48 hr and rest stored at 4 °C | Processing up to 12 min increased total phenols and flavonoid content. Whereas after 16 min of treatment there was a decrease in the content | Wang et al. (2019b) |
| Mandarin juice | Mandarin fruit harvested → Juice extracted in manual juicer → Filtered to remove peels and seeds → Refrigerated at 4 °C. | US type: US probe Frequency: 19 kHz Power: 750 W Time interval: 36 min Temperature: 50° C Other: Water bath was used to maintain temperature | The total phenolic content decreased after treatment | Cheng et al. (2020) |
| Strawberry juice | Fresh strawberries → Washed → Sanitised with sodium hypochlorite(100 mg/L) for 15 min → Mixed with water (2:1 of strawberry and water) | US type: US bath Frequency: 40 kHz Power: 110 W Time interval: 5–15 min Temperature: 25–50° C Other: Samples were cooled and analysed | No effect on phenolic content | Menelli et al. (2021) |
| Sugarcane juice | Sugarcane harvested → Sugarcane culms were grounded in mechanical mill → juice was homogenised → packaged in polypropylene bags (150 ml), and stored and protected from light at −18 °C | US type: US probe with 1.2 cm diameter Frequency: 20 kHz Power: 750 W Time interval: 2–36 min Temperature: 80° C Other: ND | No effect on total phenol content and remained stable | Rodrigues et al. (2021) |
| Cashew apple juice | Fruit harvested → Washed → Fruit separated from the nut → Grounded in a blender → Puree by adding water, sugar and citric acid | US type: S14 US probe Frequency: 24 kHz Power: 85 W Time interval: 15 min Temperature: 60° C Other: Sample stored in sterile glass bottles at 4° C after cooling at room temperature | Phenolic content was retained without any alteration | Deli et al. (2022) |
| Carotenoids | ||||
| Guava juice | Industrial pasteurised guava pulp → diluted in distilled water (15% pulp wt/wt) | US type: US probe with a 1.26 cm2 titanium tip Frequency: 20 kHz Power: 1000 W Time interval: 3–9 min Temperature: 25° C Other: 200 ml of juice sample was treated and then stored at 25° C for 14 days | With increase in treatment time lycopene content decreased gradually | Campoli et al. (2018) |
| Tomato juice | Ripe tomato fruit → Cleaned → Rinsed at 60 °C → Filtered to get rid of peels and seeds | US type: US probe with 6 mm titanium tip Frequency: 25 kHz Power: 200–800 W Time interval: 20 min Temperature: ND Other: 100 g of sample was treated | Higher value for lycopene content was obtained at 400 W | Zhang et al. (2019) |
| Buriti juice | Fresh and mature buriti fruit → Crushed → Mixed with potable water (1:1) | US type: US probe of 25 mm diameter Frequency: 20 kHz Power: 750 W Time interval: 10 min Temperature: ND Other: 100 ml of sample was treated and ice bath was used to control temperature | With increase in energy density the carotenoid content increased | Carvalho et al. (2020) |
| Hog plum juice | Matured and ripe Hog plum fruit → Washed with distilled water → Air dried → Peeled → Juice extracted from juicer | US type: US bath Frequency: 40 kHz Power: 400 W Time interval: 5–30 min Temperature: 40–60° C Other: Treatment carried out in dark to prevent interference due to light. Cooled at room temperature and stored at 4 °C | Carotenoid content increased | Oladunjoye et al. (2021) |
| Mandarin juice | Mandarin fruit harvested → Juice extracted in manual juicer → Filtered to remove peels and seeds → Refrigerated at 4 °C | US type: US probe Frequency: 19 kHz Power: 750 W Time interval: 36 min Temperature: 50° C Other: Water bath was used to maintain temperature | Increment in total carotenoid content | Cheng et al. (2020) |
| Type of juice . | Engineering operations . | US processing conditions . | Outcomes . | References . |
|---|---|---|---|---|
| Kiwi juice | Fresh ripened kiwi fruit → Peeled → Crushed → Mixed with water (1:2) | US type: US probe Frequency: 20 kHz Power: 400 W Time interval: 4–16 min Temperature: ND Other: Ice bath was applied to control temperature. 100 ml of sample was refrigerated at 4° C and rest was freeze dried for FTIR analysis | Increased degradation of ascorbic acid as the treatment time increased | Wang et al. (2019a) |
| Tomato juice | Fresh tomatoes → Washed → Dried → Juice extracted via juicer | US type: US probe with 19 mm diameter Frequency: 20 kHz Power: 28–40 W/cm Time interval: 2–10 min Temperature: 37–52 °C Other: Samples were refrigerated at 4 °C | Small reduction in ascorbic acid content | Starek et al. (2021) |
| Strawberry juice | Fresh strawberries → Washed → Sanitised with sodium hypochlorite(100 mg/L) for 15 min → Mixed with water (2:1 of strawberry and water) | US type: US bath Frequency: 40 kHz Power: 110 W Time interval: 5–15 min Temperature: 25–50 °C Other: Sample were cooled and analysed | No difference in ascorbic acid content of treated and control sample | Menelli et al. (2021) |
| Hog plum juice | Matured and ripe Hog plum fruit → Washed with distilled water → Air dried → Peeled → Juice extracted from juicer | US type: US bath Frequency: 40 kHz Power: 400 W Time interval: 5–30 min Temperature: 40–60° C. Other: Treatment carried out in dark to prevent interference due to light. Cooled at room temperature and stored at 4 °C | Increase in ascorbic acid content | Oladunjoye et al. (2021) |
| Mandarin juice | Mandarin fruit harvested → Juice extracted in manual juicer → Filtered to remove peels and seeds → Refrigerated at 4 °C | US type: US probe Frequency: 19 kHz Power: 750 W Time interval: 36 min Temperature: 50° C Other: Water bath was used to maintain temperature | Decrease in ascorbic acid after processing | Cheng et al. (2020) |
| Orange juice | Fresh navel oranges → Washed → Juice extracted from automatic juice extractor | US type: US probe of 13 mm diameter Frequency: 19 kHz Power: ND Time interval: 15–150 s Temperature: 37° C Other: Ice water bath was used to prevent overheating | No difference observed in treated as well as control sample | Gomes et al. (2022) |
| Phenolic compounds | ||||
| Kiwi juice | Fresh ripened kiwi fruit → Peeled → Crushed → Mixed with water (1:2) | US type: US bath Frequency: 20 kHz Power: 400 W Time interval: 4–16 min Temperature: ND Other: Ice bath was applied to control temperature. 100 ml of sample was refrigerated at 4° C and rest was freeze dried for FTIR analysis | Positive effect of US on total phenolic content, flavonoids, gallic acid, and catechin | Wang et al. (2019a) |
| Strawberry juice | Peak ripe staged “Seascape” strawberries were harvested → Smashed into double distilled water (1:2) | US type: US probe Frequency: 20 kHz Power: 400 W Time interval: 4–16 min Temperature: ND Other: Use of pulsed US wave. Ice bath used to control the temperature. 100 ml of juice sample was dried using freeze drier for 48 hr and rest stored at 4 °C | Processing up to 12 min increased total phenols and flavonoid content. Whereas after 16 min of treatment there was a decrease in the content | Wang et al. (2019b) |
| Mandarin juice | Mandarin fruit harvested → Juice extracted in manual juicer → Filtered to remove peels and seeds → Refrigerated at 4 °C. | US type: US probe Frequency: 19 kHz Power: 750 W Time interval: 36 min Temperature: 50° C Other: Water bath was used to maintain temperature | The total phenolic content decreased after treatment | Cheng et al. (2020) |
| Strawberry juice | Fresh strawberries → Washed → Sanitised with sodium hypochlorite(100 mg/L) for 15 min → Mixed with water (2:1 of strawberry and water) | US type: US bath Frequency: 40 kHz Power: 110 W Time interval: 5–15 min Temperature: 25–50° C Other: Samples were cooled and analysed | No effect on phenolic content | Menelli et al. (2021) |
| Sugarcane juice | Sugarcane harvested → Sugarcane culms were grounded in mechanical mill → juice was homogenised → packaged in polypropylene bags (150 ml), and stored and protected from light at −18 °C | US type: US probe with 1.2 cm diameter Frequency: 20 kHz Power: 750 W Time interval: 2–36 min Temperature: 80° C Other: ND | No effect on total phenol content and remained stable | Rodrigues et al. (2021) |
| Cashew apple juice | Fruit harvested → Washed → Fruit separated from the nut → Grounded in a blender → Puree by adding water, sugar and citric acid | US type: S14 US probe Frequency: 24 kHz Power: 85 W Time interval: 15 min Temperature: 60° C Other: Sample stored in sterile glass bottles at 4° C after cooling at room temperature | Phenolic content was retained without any alteration | Deli et al. (2022) |
| Carotenoids | ||||
| Guava juice | Industrial pasteurised guava pulp → diluted in distilled water (15% pulp wt/wt) | US type: US probe with a 1.26 cm2 titanium tip Frequency: 20 kHz Power: 1000 W Time interval: 3–9 min Temperature: 25° C Other: 200 ml of juice sample was treated and then stored at 25° C for 14 days | With increase in treatment time lycopene content decreased gradually | Campoli et al. (2018) |
| Tomato juice | Ripe tomato fruit → Cleaned → Rinsed at 60 °C → Filtered to get rid of peels and seeds | US type: US probe with 6 mm titanium tip Frequency: 25 kHz Power: 200–800 W Time interval: 20 min Temperature: ND Other: 100 g of sample was treated | Higher value for lycopene content was obtained at 400 W | Zhang et al. (2019) |
| Buriti juice | Fresh and mature buriti fruit → Crushed → Mixed with potable water (1:1) | US type: US probe of 25 mm diameter Frequency: 20 kHz Power: 750 W Time interval: 10 min Temperature: ND Other: 100 ml of sample was treated and ice bath was used to control temperature | With increase in energy density the carotenoid content increased | Carvalho et al. (2020) |
| Hog plum juice | Matured and ripe Hog plum fruit → Washed with distilled water → Air dried → Peeled → Juice extracted from juicer | US type: US bath Frequency: 40 kHz Power: 400 W Time interval: 5–30 min Temperature: 40–60° C Other: Treatment carried out in dark to prevent interference due to light. Cooled at room temperature and stored at 4 °C | Carotenoid content increased | Oladunjoye et al. (2021) |
| Mandarin juice | Mandarin fruit harvested → Juice extracted in manual juicer → Filtered to remove peels and seeds → Refrigerated at 4 °C | US type: US probe Frequency: 19 kHz Power: 750 W Time interval: 36 min Temperature: 50° C Other: Water bath was used to maintain temperature | Increment in total carotenoid content | Cheng et al. (2020) |
Note. US = ultra-sonication; ND = not defined.
Several observations about the impact of US on ascorbic acid content of juices can be found in the literature. Some studies have reported an increase in the Vitamin C content (Oladunjoye et al., 2021; Wang et al., 2019a), others a decrease (Cheng et al., 2020; Starek et al., 2021) whereas some reported no change at all (Gomes et al., 2022; Menelli et al., 2021). This can be due to the different treatments applied as higher frequency, power, and temperature reduce ascorbic acid levels, while lower frequency, power, and temperature can increase it with moderate conditions show no change.
Effect of US on phenolic compounds
The reducing qualities of phenolic compounds as hydrogen- or electron-donating agents predict their potential for action as free-radical scavengers (antioxidants) additionally, they have the ability to chelate metals, especially iron and copper, which inhibit the formation of free radicals catalysed by metals (Vuolo et al., 2019). According to the Table 3 following US application, there is an increase in phenolic compounds. Although, these bioactive compounds are not synthesised during food processing; rather, the cavitation phenomenon—which disrupts juice cells and releases intracellular components—is responsible for this increase (Lepaus et al., 2023). The outcomes of US-treated sample of kiwi juice demonstrated that the total phenolic content was considerably enhanced by ultrasonic treatment. The total phenolic content of the US-treated samples was the highest and was twice as high as that of the control (Wang et al., 2019a). The total phenolic content increased with the US treatment, although it reduced to some extent after some time; however, the values were significantly higher than the other treatments such as pasteurisation (Cheng et al., 2020). Samples with higher concentrations of phenols exhibit greater antioxidant capacity as sonication significantly improves juice’s overall antioxidant activity by enhancing the phenolic (Shahid et al., 2021).
The extractability of these compounds increases with the treatment due to the cavitation process. Therefore, it is essential to preserve these compounds due to their high scavenging capacity for free oxygen species (Shaik & Chakraborty, 2023).
Effect of US on carotenoids
Carotenoids are found in a variety of photosynthetic organisms, including algae, plants, bacteria, fungi, and some species of animals. Carotenoids, such as lutein, zeaxanthin, lycopene, and β-carotene, are the main antioxidants found in fruits and act as scavengers of reactive oxygen species, additionally reducing the risk of prostate, colon, and lung cancers (Kapoor et al., 2022). The main dietary source of carotenoids in human diet is coloured fruits and vegetables such as mango, persimmon, peach, papaya fruits, apricots, peaches, citrus fruits, tomatoes, green leafy vegetables, pumpkin, red paprika, carrots, capsicum pods, etc. (Saini et al., 2022). Carotenoids in animals have numerous functions, including being precursors of Vitamin A, antioxidants, photo protectors, immune system boosters, and reproductive factors (Maoka, 2020).
Some of the studies in Table 3 showed increase in the total lycopene content. The cavitation action during thermo-sonication damages the cell wall and releases the chemicals, which is why the carotenoid content increased (Manzoor et al., 2021). However, a rise in carotenoid content during the sonication process is also linked to disruptions in cell structure and the deactivation of enzymes during cavitation, as in the case of lipoxygenase (Cheng et al., 2020). Increased power during US treatment may encourage the release of lycopene crystals from the lycopene-protein complex and their dispersion into the serum (Zhang et al., 2019). Also it was observed that US reduced the lycopene content gradually with increase in time in guava juice (Table 3). According to Campoli et al. (2018), although it is being more accessible than the control samples, the chemicals were liberated from the matrix of food and got exposed to higher deterioration due to cavitation.
However, a variety of factors, such as the kind of processing (power, frequency, temperature, and time), as well as the characteristics of the sample (composition, volume, consistency/texture, and presence of lipids or fibres) affect the carotenoid content and its bio accessibility (Campoli et al., 2018; Suo et al., 2022).
Therefore US can be used to extract the carotenoid from the inner cell matrix due to cavitation phenomenon making it more accessible for the benefit of the consumers (Cheng et al., 2020).
Effect of US on anti-oxidant activity
Fruit juices are abundant in naturally occurring bioactive substances, such as vitamins, anthocyanin, and phenolic compounds, which have antioxidant properties and support the health. Vitamin C and phenolic compounds are the strong antioxidants that help scavenging the free radicals and protect the body from oxidative stress (Roobab et al., 2023).
According to Araujo et al. (2021), there is a positive relation between increase in these bioactive compounds and antioxidant activity after implication of ultrasound treatment. US leads to release of bonded antioxidants such as phenols and ascorbic acid therefore increasing the overall antioxidant activity in the fruit juices leading to inhibition of development of numerous diseases such as heart diseases, cancers arteriosclerosis, and improve the health (Aslam et al., 2023; Shahid et al., 2021). Similarly, Hasheminya & Dehghannya (2022) observed increase in antioxidant properties of black carrot juice after implication of ultrasound treatment. Ascorbic acid concentrations can rise as a result of cavitation during the processing, which is linked to an increase in antioxidant activity.
According to Wang et al. (2019a, 2019b) and Carvalho et al. (2020) observed that increasing US energy density led to a significant increase in antioxidant activity as well as colour parameters (brightness, redness, and yellowness). However, higher ultrasonic intensity has been linked to compound degradation since higher intensity produces more hydroxyl radicals, which in turn cause bioactive chemicals to break down chemically. Araujo et al. (2021) claim that the process of producing these radicals is what encourages phenolic compounds to undergo hydroxylation. Furthermore, this compound may deteriorate due to high pressures and temperatures caused by the bursting of bubbles.
In order to preserve nutritional content, more research is necessary to better understand and identify the parameters (time, power, frequency, and amplitude) that should be used for juice processing through US application.
Conclusion
Fruit juices are extremely vulnerable to the harsh processing conditions which can alter their nutritional value, freshness, flavour, and colour. Although thermal treatments enable microbiological decontamination, they degrade the nutritional value, flavour, and colour of fruit juices due to high temperatures; need a greater space, and have a high energy consumption and wastage. This has increased demand of non-thermal technologies like US, which uses mechanical waves leading to cavitation that enhance microbial destruction and improve the permeability of proteins, carbohydrates, lipids, enzymes, and other bioactive compounds preserving juice quality. Although there are benefits of US, still there are barriers to its industrialisation due to reluctance to use sound energy for chemical reactions and a lack of appropriate equipment for scaling up the process. Further investigations are required to understand how US affects structure, nutrients, and bioactive compounds in fruit juices; particularly carbohydrates as well as the impact on climacteric and non-climacteric fruit juices such as coconut, olives, acai, avocado that are also a rich source of fatty acids and proteins.
Data availability
The corresponding author will provide the created datasets during the current investigation, upon reasonable request.
Author contributions
Neha Pathania (Investigation, Formal analysis, Visualisation, Writing—original draft) and Praveen Kumar Dubey (Project administration, Conceptualisation, Investigation, Visualisation, Supervision, Writing—review & editing)
Funding
None declared.
Conflicts of interest
The authors declare no conflict of interest.
Acknowledgements
The authors express gratitude to the Department of Food Technology and Nutrition, School of Agriculture, Lovely Professional University, Punjab, for providing the necessary facilities to conduct this review.



