-
PDF
- Split View
-
Views
-
Cite
Cite
Fariba Heidarizadeh, Maryam Kolahi, Marziye Akhond, Enhancing the qualitative and nutritional properties of strawberry juice through chitosan treatment, International Journal of Food Science and Technology, Volume 58, Issue 10, October 2023, Pages 5214–5226, https://doi.org/10.1111/ijfs.16623
Close - Share Icon Share
Abstract
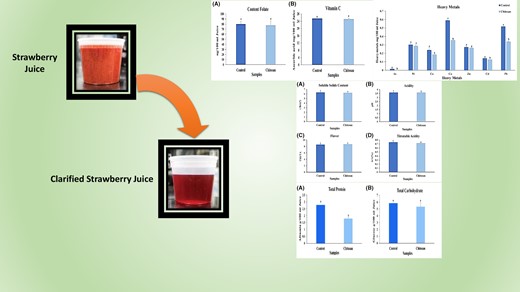
Strawberry (Fragaria x ananassa Duch.) belongs to the Rosaceae family. The fruit is very beneficial, with a delicious taste, attractive colour, and high vitamin and mineral content. The effect of chitosan on juice's transparency and phytochemical properties from the Camarosa cultivar was evaluated. Using chitosan as a clarifying agent for strawberry juice significantly reduced turbidity, phenolic content, flavonoids, protein and antioxidant properties. In contrast, the quantity of soluble solids, titratable acidity, pH, folate, vitamin C, anthocyanin and carbohydrates showed no significant changes. Changes were also observed in the colour-related parameters of the juice. In order to assess potential contamination in strawberry fruit, the levels of heavy metals and pesticide residues were analysed separately using ICP and GC–MS techniques. There was a significant reduction in the levels of Cu, As, Co, Pb and Cd in strawberry juice after treatment with chitosan. Reducing heavy metals in fruit juice using chitosan helps improve the fruit juice's safety, nutritional properties and health benefits. It can be posited that chitosan affords the production of a more nutritious and healthier fruit juice as compared to natural strawberry fruit. Using chitosan as a clarifying agent produces a juice with extraordinary clarity, improves the safety, health benefits and nutritional value, and produces a consumer-friendly product.
Introduction
Strawberries (Fragaria × ananassa Duch.) have become an increasingly popular fruit due to their colour, texture and taste, leading to a rising demand in the market (Rapuru et al., 2022); beyond their delicious flavour, strawberries are associated with several health benefits. These include reducing and even preventing heart disease due to their antioxidants and polyphenols, which protect heart health and prevent various types of cancer (Davis et al., 2007). Furthermore, their high vitamin C content helps keep skin and hair healthy, while the fibre aids in weight loss and digestion. Additionally, consuming strawberries can lower total cholesterol, LDL and other markers of atherosclerosis (Aloo et al., 2023). Despite the numerous health benefits of strawberries, they must be handled and stored carefully due to their susceptibility to injury. Therefore, converting strawberries into juice can help reduce food waste.
Juices are an important part of modern diets in many countries, and they are one of the best drinks that, due to having salts and vitamins, while quenching thirst, provide a significant part of the body's need for vitamins. Since juices are expected to be transparent products, the production process of these drinks requires a clarification step that allows the removal of suspended solids of different origins (Abd El-Hack et al., 2020). The purpose of clarifying fresh juices is to reduce suspended solids such as skin, and fruit flesh particles, remove smaller sized substances such as unwanted yeasts and bacteria, and protein and pectic substances of plant cells that cause viscosity and turbidity of juice (Castro Marin et al., 2021). Food industry specialists are looking for natural compounds to increase the shelf life of drinks, develop active packaging, improve clarification methods and produce new functional drinks (Abd El-Hack et al., 2020). The conventional methods used for clarifying strawberry juice, such as filtration, centrifugation, fining agents and enzyme treatment, have certain drawbacks compared to chitosan. Here are some drawbacks of these traditional methods: Filtration is commonly employed for juice clarification. However, it can be time-consuming and require multiple filtration steps to achieve sufficient clarity (Lu et al., 2021). Additionally, the fine filtration process can result in the loss of certain desirable compounds, including flavour and aroma components, which may affect the overall quality of the juice. Centrifugation is another widely used method for juice clarification. While it can effectively remove larger particles and sediment, it may not efficiently remove smaller colloidal particles. Moreover, centrifugation requires specialised equipment and may involve high energy consumption, making it less cost-effective for large-scale production. Fining agents, such as gelatine, bentonite and activated carbon, are often used to clarify fruit juices (Ghosh et al., 2018). These agents work by adsorbing and removing impurities from the juice. However, using chemical fining agents raises concerns about their potential impact on the nutritional and sensory properties of the juice. Some fining agents may leave residues or introduce foreign flavours, affecting the overall taste and quality of the clarified juice. Enzymatic treatment is a popular method for juice clarification due to its numerous advantages over traditional mechanical processes (Sharma et al., 2017). The enzymes used in juice clarification typically include cellulases, pectinases and amylases, each targeting specific components in the juice. While enzymatic treatment for juice clarification is well-accepted and advantageous, it has challenges and limitations. It is time-consuming, sensitive to temperature and pH, expensive or less accessible, flavour changing and possible enzyme deactivation.
In comparison, chitosan offers several advantages as a clarification method for strawberry juice. Chitosan exhibits excellent clarifying properties and can efficiently remove suspended particles, turbidity and impurities from the juice. Its positively charged amino groups enable strong electrostatic interactions with negatively charged contaminants, improving clarification. Chitosan is derived from chitin, a natural biopolymer found in crustacean shells. It is considered a safe and environmentally friendly substance. Chitosan does not have harmful residues in the clarified juice, ensuring its safety for consumption. Unlike some conventional methods, chitosan clarification has minimal impact on the sensory characteristics of the juice. It removes unwanted particles while preserving the strawberry's natural flavour, aroma and nutritional components. Chitosan is a sustainable alternative to synthetic chemical fining agents. It is biocompatible and non-toxic, making it a more environmentally friendly option for juice clarification. Considering these drawbacks of conventional methods and the advantages offered by chitosan, our study explores the potential of chitosan as a superior alternative for enhancing the quality and clarity of strawberry juice.
This study aimed to evaluate the effect of chitosan treatment on the quality of strawberry juice containing features like physicochemical (pH, acidity, total soluble solids), total polyphenol, total antioxidant capacity, nutritional, pesticide residue, and colour parameters (L*, a*, b*) and heavy metal content.
Materials and methods
This study utilised chemicals and solvents from Merck and Sigma-Aldrich without extra purification. Various apparatus were used in this research, including UV–Vis spectrophotometer (Model 6705, Jenway, UK) and Optima 8300 ICP-AES (PerkinElmer, USA). Additionally, a pH meter (Az 86 502, Taiwan), Turb55 turbidity meter (WTW GmbH, Germany), magnetic stirring machine (LABINCO L-81, Amsterdam) together with a digital refractometer (PAL-α ATAGO CO. LTD., Tokyo, Japan) were also employed, together. A miniature high-sensitivity spectrometer with optic fibre cable using an LED lamp (model HSMSV900 from HSM 201-202-203 series) was used to assess the colour of strawberry juices.
Preparation of strawberry juice
Strawberries of the Camarosa cultivar were purchased from the market. They were then washed with distilled water and dried in the open air. After that, they were crushed in a mortar and filtrated through a cheesecloth. The extracted juice was stored at 4 °C until further use.
Preparation of water-soluble chitosan
The high molecular weight chitosan solution was mixed with 0.5% acetic acid (v/v) and then stirred at 60 °C for 1 h to prepare a 1% (w/v) chitosan solution.
Clarification of strawberry juice
To 45 mL of fruit juice, 5 mL of 1% chitosan solution was added. The control tube had water instead of a chitosan solution. The tubes were incubated at room temperature for 1 h and centrifuged at 10 000 r.p.m. for 10 min to obtain clear fruit juice.
Turbidity measurement
The turbidity of strawberry juice was determined in terms of NTU using a turbidimeter (German WTW model turb555).
The pH and titratable acidity
Titratable acidity (T.A.) was determined by titrating 1 mL of strawberry juice diluted in 5 mL of deionised water against NaOH (0.1 N) solution to an end point of 8.2, and the results were expressed as % citric acid (Mandha et al., 2023).
Total soluble solids (TSS) assay
A few drops of the strawberry juice were put on the sensitive plate of the refractometer, and total soluble solids were read and recorded in terms of Brix (Rehman et al., 2022).
Flavour test
The ratio of flavour was calculated by dividing the TSS index by the T.A. index (Usanmaz et al., 2022).
Total phenolic compound assay
The concentration of total phenolic compounds in strawberry juice was obtained based on the Folin–Ciocalteu assay. The strawberry juice (0.2 mL) was transferred to the Falcon tube, then 1.5 mL of 10% Folin reagent and 1.5 mL of 5% sodium carbonate were added to the extract, and the resulting mixture was covered with foil and stored in the dark environment for 60 min. The mixtures were left at room temperature for 15 min, and the total phenol content was determined by colourimetric method at 765 nm. Total phenol content was calculated based on the calibration curve and expressed as Gallic acid equivalent (mg GAE g−1) (Munteanu & Apetrei, 2021).
Flavonoid content assay
Total flavonoid content was determined utilising the aluminium chloride colourimetric assay (Shraim et al., 2021). 1 mL of the extract prepared was transferred to the Falcon tube, 1 mL of 96% ethanol was added and then 0.1 mL of 10% aluminium chloride was added. After 6 min of adding aluminium chloride, 0.1 mL of 1 M sodium acetate trihydrate was added, and 2.8 mL of distilled water was added to the resulting solution. The absorbance of the samples was read at 433 nm using a spectrophotometer after 30 min in the dark environment, and the total flavonoid compounds were expressed as mg of quercetin per 100 g of fresh weight. A calibration curve was plotted using quercetin as standard. The total flavonoid content in strawberry juice was expressed in mg of quercetin per 100 mL (Munteanu & Apetrei, 2021).
Total anthocyanin content assay
0.2 g of fruit tissue was ground well with 5 mL of methanol: hydrochloric acid (1:99 volume by volume), then added 5 mL of the methanolic acid solution, and the sample was transferred to a test tube and kept in the dark environment of the refrigerator for 24 h. Then it was centrifuged for 5 min at 10 000 r.p.m., and the absorbance was read at a wavelength of 500 nm. The amount of anthocyanin in terms of pelargonidin-3-glycoside, the dominant anthocyanin of strawberries, was calculated (Cruz et al., 2022).
Antioxidant activity by diphenyl-1-picryl-hydroxyl diphenyl-1-picryl-hydroxyl (DPPH) assay
The antioxidant activity of the samples was determined by the method of Brand-Williams, based on the free radical scavenging properties of DPPH. The prepared extract (0.1 mL) was mixed with 3.9 mL DPPH solution (0.002% in methanol). The absorbance of the samples was determined at 515 nm. The percentage inhibition of DPPH was calculated using eqn 1 (Munteanu & Apetrei, 2021).
Total protein assay
Modifying Lowry's method with bovine serum albumin (BSA) as the standard determined the total protein concentration. A standard curve was prepared, and the absorbance of the samples was measured at 660 nm utilising a UV–visible spectrophotometer. The reagents needed to measure cytoplasmic proteins were prepared with the following protocols. Folin reagent: 5 mL of Folin 2 N was diluted to 50 mL with distilled water.
Reagent A: To prepare this reagent, 2 g of NaOH was dissolved in distilled water and diluted to 100 mL with distilled water. Then 10 g of sodium carbonate was added to it and shaken until it was completely dissolved. (Note: this solution can only be stored for 24–48 h). Reagent B: 1 g of aqueous copper sulphate was dissolved in distilled water and brought to a volume of 1 mL. Then, one drop of sulphuric acid was added per 100 mL solution to clarify it. Reagent C: 4.91 g of sodium potassium tartrate (KNaC4H4O6·4H2O) was dissolved in distilled water and diluted to 100 mL. Reagent ABC: 15 mL of reagent A, 0.75 mL of reagent B and 0.75 mL of reagent C were poured into a 50 mL Erlenmeyer flask and mixed thoroughly.
The juice samples were diluted 1:19 with phosphate buffer, and 0.5 mL was taken from them and transferred to the test tube. Then 2.5 mL of ABC reagent was added and kept at room temperature for 15 min. 1.5 mL of diluted Folin reagent was added to each tube, and after 45 min, the absorption of the samples was read at 660 nm. Finally, the protein content was reported based on mg of albumin in 100 mL strawberry juice (Niemi et al., 2023).
Vitamin C content assay
Hernandez's method was used to measure vitamin C content. Metaphosphoric acid solution (5%) was added to strawberry juice, and titration was carried out with dichlorophenol indophenol (DCIP 0.01%) until the solution changed colour from colourless to pink. The vitamin C content was reported based on mg of ascorbic acid in 100 mL of fruit juice (Honfo et al., 2022).
Folate measurement
Folate measurements of strawberry juice were carried out on the sample containing chitosan and control samples. Folate content was determined using Folate AccuBind ELISA test system kits (Monobind Inc., Lake Forest, CA 92630, USA) (Ahmed et al., 2022).
Heavy metals analysis in strawberry juice
Heavy metals were determined by the Dániel method (Dániel et al., 1997; Anastácio et al., 2018). Three milliltres of juice was transferred to a 100 mL beaker, and about 10 mL of 65% nitric acid was added. It was kept under the hood for 24 h, so the plant sample was well-digested in acid and turned brown. The resulting solution was exposed to heat using a heater at a temperature of 90 °C to remove acid vapours; after cooling the solution, 1 mL of hydrogen peroxide (30%) was added to it, and heating was continued until all acid vapours were removed. Then, the digested samples were diluted with 25 mL of deionised water in a volumetric flask. The concentration of heavy metals (As, Cu, Co, Pb, Ni, Zn and Cd) was measured by the inductively coupled plasma atomic emission spectrometry (ICP-OES) analysis (ICP) method (Motalab et al., 2022).
Colour analysis
To measure the colour of strawberry juices, a miniature emission spectrometer was used in part related to the colour colouring index. This device has standards for measuring CIE values CIE1931, CIE1960 and CIE1976 colour measurement standards. To qua a*, b* and L* characteristics were used to quantify the colour values related to X, Y and Z characteristics. Colour parameters: L* represents brightness (0 = black, 100 = white), a* represents green, and red (a*, green, +a*, red), and b* represents blue, and yellow (−b*, blue, +b*, yellow). The relationship between characteristics a*, b* and L* with X, Y and Z is in the form of Relations eqns 2–4 (Chen et al., 2019):
Pesticide residue in strawberry juice
The residual amount of pesticides in the fruit was measured by gas chromatography-mass spectrometry with detector model C5975 and autosampler model 7693 with helium capsule and capillary column (Bebek Markovinović et al., 2022).
Statistical analysis
Data analyses were performed using the SPSS20.0 software package (SPSS Inc., Chicago, IL, USA). All experimental data were presented as the mean ± S.D. In order to ensure greater statistical accuracy, every test was conducted three times and the means were compared using the independent t-test and the Duncan test. The level of significance was set at P < 0.05 for all tests.
Result and discussion
Effect of clarifying chitosan on quality features of strawberry juice
Turbidity
The results of the present study indicated that the turbidity of the control sample was 702.11 ± 1.76, whereas the sample containing chitosan had significantly reduced turbidity to 12.29 ± 1.46 (t = 520.06) (P < 0.05) (Fig. 1A). This decrease was in agreement with previous studies, which reported that adding chitosan to pomegranate juice and various juices, respectively, decreased the turbidity of the juices (Lachowicz et al., 2018).
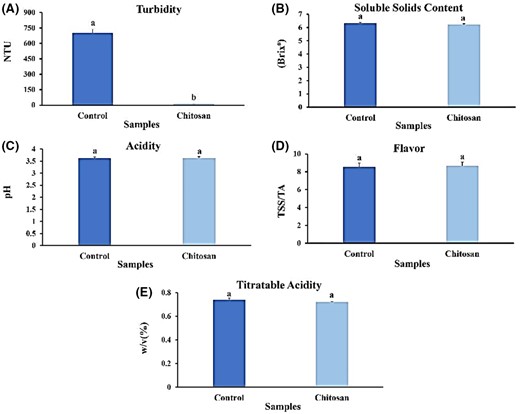
Effect of clarifying chitosan on qualifying features of strawberry juice. (A) Turbidity; (B) TSS; (C) pH; (D) taste index; (E) TA. Bars represent the means of triplicate ± SE. Values with different letters are statistically significantly different at P < 0.05.
The effect of several clarifying agents on beriberi juice reduced the turbidity of the juice (Chen et al., 2019). In another research on chitosan in the clarification of apple, grape, lemon and orange juices, the results showed that after adding the chitosan solution, the turbidity of the juice was observed. The distinct impacts of clarifying agents and their reaction time on checkerberry juice demonstrate that the diverse polyphenolic compounds in the juice significantly decrease the level of turbidity. According to the correlation coefficient results, polyphenolic compounds, especially polymeric procyanidins, have an essential effect on the turbidity of cherry juice (Lachowicz et al., 2018). Using non-starch polysaccharides such as chitosan can inhibit the formation of protein-phenol masses and turbidity of juices. This matter is accomplished by establishing non-specific hydrogen bonds and ionic interactions that prevent the formation of insoluble protein-phenol masses, thus decreasing the overall haze in the juice. In turn, forming a water-soluble tannin–polysaccharide protein complex produces clearer juice (Lachowicz et al., 2018). Incorporating chitosan into pomegranate juice showed a lower decrease in the overall anthocyanin content compared to the protein-based clarifier. This inference was based on the findings of the experiment, which revealed that the protein-based clarifiers had a stronger ability to bind and decrease the anthocyanin content (Erkan-Koç et al., 2015).
Total soluble solids
The findings indicated that there was no notable difference in total soluble solids between the control sample, with a reading of 6.33 °Brix, and the chitosan-included sample, measuring at 6.23 °Brix (t = 1) (P < 0.05) (Fig. 1B). However, the introduction of chitosan to apple juice exhibited a higher quantity of soluble solids compared to the control sample (Ghorbel-Bellaaj et al., 2012; Rajaselvi et al., 2023). Another study observed a significant disparity in total soluble solids when chitosan was added to orange juice (Martín-Diana et al., 2009). Still, the amount of dissolved solids in fruit juice remained relatively constant post-chitosan treatment. This feature appears to correlate with the sugar content in the juice as, typically, the concentration of sugar remains steady (Chatterjee et al., 2004; Chen et al., 2023).
Moreover, the impact of various clarifying agents on berry juice was investigated, revealing a decrease in soluble solids content post-clarification. With sugars forming the majority of soluble solids in fruit juices, our research demonstrates that chitosan does not substantially alter the sugar content. This conclusion is derived from the collective data from our trials, reinforcing the concept that sugars are a critical component of soluble solids in fruit juices (Anastácio et al., 2018).
pH
The current research found that the amount of acidity in the sample containing chitosan and the control sample was the same, and there was no significant difference (t = 0.136; P < 0.05) (Fig. 1C). This result is consistent with previous studies, which showed that several clarifying compounds had no significant effect on the pH of bayberry juice (Chen et al., 2019). Similarly, researchers found that chitosan had no significant impact on the pH of pomegranate juice when used as a clarifying agent (Erkan-Koç et al., 2015).
Flavour
This study found that chitosan has a limited impact on the taste of the sample (Fig. 1D). These results are confirmed by the research on the change in flavour due to the change in the activity of glycolytic and alcoholic fermentation enzymes, such as alcohol hydrogenase (ADH) and pyruvate decarboxylase (PDC) (Chen et al., 2023). Therefore, the incorporation of chitosan in food products can improve their taste. Further research is needed to evaluate the effects of chitosan on taste.
Titratable acidity
The titratable acidity of the chitosan-containing juice samples was not significantly different from the control samples. Figurich is consistent with previous studies on various fruits (Chen et al., 2023). Chitosan did not significantly affect the titratable acidity and soluble solids in apple, grape, lemon, pomegranate and orange juices (Erkan-Koç et al., 2015; Dereli et al., 2023); On the other hand, the titratable acidity decreased following clarification with several clarifying compounds in berry juice (Chen et al., 2019). This discrepancy may be due to the different types of clarifying agents used in the studies. Nevertheless, using chitosan as a clarifying agent minimises the titratable acidity of various fruit juices (Erkan-Koç et al., 2015).
Effect of clarifying chitosan on phytochemical features of strawberry juice
Phenolic content
Adding chitosan slightly reduces the total phenolic content, which showed the control sample had higher total phenolic content (499.13 ± 9.57 mg gallic acid per100 mL of fruit juice) than the sample with chitosan (377.98 ± 12.25 mg gallic acid per 100 mL of fruit juice) (t = 13.492) (P < 0.05) (Fig. 2A).
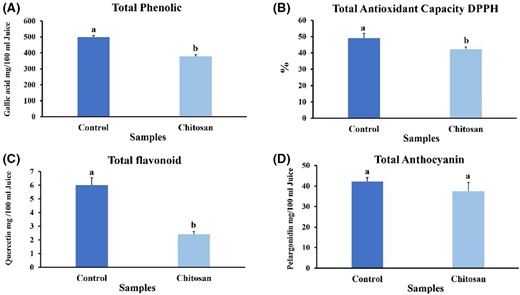
Effect of clarifying chitosan on phytochemical features of strawberry juice. (A) Phenolic content, (B) antioxidant capacity (C) flavonoids content, (D) anthocyanin content. Bars represent the means of triplicate ± SE. Values with different letters are statistically significantly different at P < 0.05.
This study was consistent with previous results that different clarifiers, including chitosan, reduced phenolic content to a limited extent (Dereli et al., 2023). Furthermore, the effect of several clarifying agents on beriberi juice showed that adding chitosan reduced the phenolic content of beriberi juice by 26% (Chen et al., 2019). They studied the effects of different concentrations of fining agents on Czech berry juice and their reaction times. The utilisation of various fining agents, taking into consideration their type and concentration, exerts a notable influence on the content of polyphenolic compounds. (Lachowicz et al., 2018). A study of pomegranate juice clarification without the use of clarifiers and in the presence of polysaccharide clarifiers showed that the reduction of phenolic compounds during clarification resulted from their natural oxidation. However, polysaccharide-based clarifiers have not been found to affect the amount of phenolic compounds significantly. According to our results, the partial reduction of phenolic compounds was probably due to the polycationic properties of chitosan, and this partial reduction of phenolic compounds did not affect the nutritional value of the juice (Dereli et al., 2023).
Flavonoids content
The result of flavonoid content in strawberry juice showed that flavonoid compounds have decreased in chitosan-containing significantly (t = 10.505, P < 0.05) compared to the control sample (59.66%) (Fig. 2C). It seems the optimal interaction between flavonoids (quercetin, quercetin, artemethin, etc.) and chitosan is through van der Waals and hydrogen bonding between the polar groups of the flavonoid and chitosan. Furthermore, chitosan has a strong affinity for negatively charged phenolic compounds such as flavan-3-ols (Erkan-Koç et al., 2015; Dereli et al., 2023). Therefore, the decrease in flavonoid content could be attributed to flavanol-3-ol, the major flavonoid in strawberry juice. This decrease was further evidenced by the red/green factor (a*), which decreased from 79.60 ± 1.7 in control samples to 67.23 ± 0.3 in chitosan samples.
Anthocyanin content
The data on anthocyanins indicated that there was no statistically significant difference between the control and the chitosan-infused samples (t = 1.790; P < 0.05) (Fig. 2D). Using chitosan led to only a slight decrease in anthocyanin levels in bayberry juice. It was noted that clarifying agents based on polysaccharides retain more antioxidant compounds than other types of clarifiers (Chen et al., 2019). Erkan-Koç et al. (2015) investigated the effects of natural sedimentation and clarification agents—both protein-based (such as albumin, casein and gelatine) and polysaccharide-based (like chitosan and xanthan gum)—on total phenolics, hydrolysable tannins, anthocyanins and the antioxidant activity of pomegranate juice. They discovered that protein-based agents resulted in more significant reductions in total phenolics (7.2%–17.2%), hydrolysable tannins (16.7%–59.5%) and anthocyanins (11.7%–23.7%; P < 0.05) compared to natural sedimentation. In contrast, the effects of polysaccharide-based agents were akin to those of natural sedimentation (P > 0.05). This suggests that protein-based clarifying agents are more prone to decrease anthocyanin content than polysaccharide-based ones (Erkan-Koç et al., 2015; Dereli et al., 2023).
Studies have shown that polysaccharide-based agents like chitosan cannot clot with phenolic compounds, instead forming water-soluble complexes with phenol. The reduction in anthocyanin content is likely due to oxidative degradation (Lachowicz et al., 2018).
Antioxidant capacity
The findings from the analysis of strawberry juice's antioxidant capacity revealed a significant decrease when chitosan was present in the sample compared to the control sample (13.86%) (t = 3.734; P < 0.05) (Fig. 2B).
When examining the impact of various clarifying agents on berry juice, it was observed that the antioxidant capacity was notably reduced in samples containing chitosan. However, in the case of pomegranate juice, the addition of chitosan did not affect its antioxidant capacity. Furthermore, investigating the influence of different concentrations of smoothing agents and their reaction times on chokeberry juice, it was found that the usage of varying dosages of the smoothing agent had a significant impact on the antioxidant activity. The decrease in phenolic and flavonoid compounds in the juice led to a decline in its antioxidant capacity.
Additionally, a positive and significant correlation was observed between antioxidant activity and the content of phenol and flavonoid compounds. This suggests that phenolic compounds play a crucial role in the observed reduction (Chen et al., 2019).
Effect of clarifying chitosan on nutritional features of strawberry juice
Protein content
The analysis comparing the average total protein content in strawberry juice yielded significant findings, indicating a notable 35.65% decrease in the sample supplemented with chitosan compared to the control sample (t = 9324; P < 0.05) (Fig. 3A). This outcome aligns with a previous study conducted by Chen et al. (2019), where they observed an 88% reduction in protein content in berries with chitosan supplementation. The decline in protein levels can be attributed to the interaction between polyphenols and proteins, a common factor contributing to beverage clouding. Moreover, several studies have demonstrated that the addition of chitosan in apple, grape, lemon and orange juices can significantly reduce protein content (Chatterjee et al., 2004; Chen et al., 2023). Additionally, the investigation highlighted a strong correlation between the turbidity of fruit juices treated with polysaccharide-based clarifying agents and their protein content. These findings suggest that chitosan, as a clarifying agent, has the potential to decrease protein content and enhance the clarity of fruit juices. This research underscores the impact of chitosan on protein levels and its potential role in improving the visual appearance of fruit juices (Chen et al., 2019).
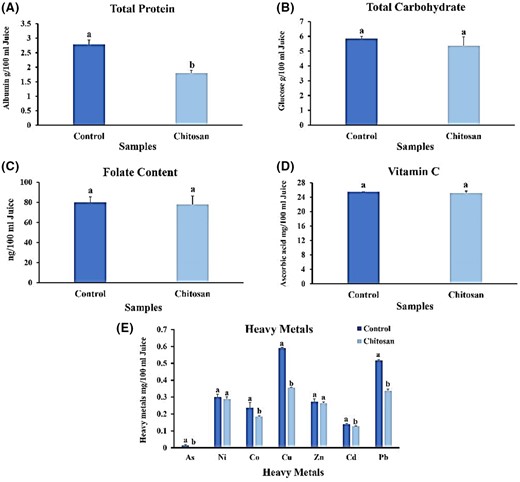
Effect of clarifying chitosan on nutritional features of strawberry juice. (A) Protein content; (B) total soluble carbohydrate content; (C) folate content; (D) vitamin C content; and (E) heavy-metal content. Bars represent the means of triplicate ± SE. Values with different letters are statistically significantly different at P < 0.05.
Total soluble carbohydrate content
The analysis conducted on total soluble carbohydrate content in the study indicated no significant difference between the control samples and those supplemented with chitosan (t = 1.272; P < 0.05) (Fig. 3B). Including chitosan as a clarifying agent across various juices resulted in a slight decrease in total soluble carbohydrate content, suggesting a minimal impact on the nutritional value. These findings imply that the presence of chitosan does not significantly alter the overall content of total soluble carbohydrates in the studied juices. Consequently, the nutritional value associated with carbohydrates remains relatively unaffected when adding chitosan (Rajaselvi et al., 2023).
Folate measurement
The folate content in the control sample was measured to be 80 ± 8.9, while the sample supplemented with chitosan showed a slightly lower value of 77.8 ± 7.5. However, statistical analysis revealed no significant difference between the two samples (P < 0.05) (Fig. 3C). These results indicate that the addition of chitosan does not have a substantial impact on folate levels in the studied samples. Although a slight numerical difference was observed, it did not reach statistical significance. Therefore, the study does not provide strong evidence to support the claim that chitosan significantly affects folate levels. While this study did not find a significant effect on folate levels, it is important to consider other potential benefits and applications of chitosan. Future research and studies can further explore its potential use as a dietary supplement and its implications for public health.
Vitamin C content
The results of the comparison of vitamin C content in strawberry juice showed that the sample containing chitosan had no significant difference compared with the control sample (t = 1272; P < 0.05) (Fig. 3D). Martin Diana et al. studied the effect of chitosan on orange juice; the results showed that chitosan did not have a significant effect on vitamin C content during the first day of storage. Presumably, increasing the storage time and using chitosan at high concentrations resulted in a decrease in vitamin C content; a decrease in vitamin C content was observed as the storage time was increased and chitosan was used at higher concentrations (Martín-Diana et al., 2009). The results of research on the effects of chitosan on several different fruit juices showed that the vitamin C content in the juice was not significantly reduced due to the addition of chitosan, and the nutritional value of the juice was maintained in terms of vitamin C content (Ghorbel-Bellaaj et al., 2012; Rajaselvi et al., 2023). Thus, it can be concluded that chitosan does not significantly affect vitamin C content.
Effect of clarifying chitosan on the amount of heavy metal in strawberry juice
Investigation of heavy metals in the strawberry juice control sample showed that the highest heavy metal content was associated with copper, lead, nickel, zinc, cobalt and cadmium, and the lowest concentration was associated with arsenic. Adding chitosan to strawberry juice significantly reduced copper, arsenic, cobalt, lead and cadmium content. These changes could be due to chelation, electrostatic interactions or ion exchange properties of chitosan (Vakili et al., 2019). On the other hand, no significant difference in zinc and nickel concentrations was observed compared with the control sample (t = 0.827; P < 0.05) (Fig. 3E).
The results show the commercial potential of chitosan in the adsorption of heavy metals in aqueous solution. The results indicated that copper had the highest absorption capacity with increasing chitosan concentration, followed by lead, cadmium and zinc, and with increasing chitosan, heavy metal absorption also increased significantly (Zhang et al., 2021). Chitosan has been used as a binding agent to reduce metal ions (mercury, iron, nickel, lead, copper and zinc) in wastewater and has reduced heavy metal concentrations in wastewater. In agreement with previous results, strawberry juice revealed that maximum copper and lead absorption could be achieved (Gamage & Shahidi, 2007; El-Naggar et al., 2022). The parameters of chitosan structure, amount of adsorbent, identification and initial concentration of heavy metals, treatment time, solution pH and temperature significantly affect the absorption properties of chitosan. Various processes, such as adsorption, ion exchange and chelation, are involved in the formation of complexes between chitosan and metal ions. It has been observed that ion exchange is the primary process for calcium, while adsorption with some chelation processes is more important for other metals, such as Cu and Pb.
Furthermore, the increase in adsorption capacity due to the binding of some amine groups further confirmed their participation in the complex formation. Chelate formation requires the participation of two or more groups of the same molecule to form a complex. Thus, ionic metals seek to bind to two or more amine groups present on a single chitosan molecule. The nitrogenous amine group of chitosan is the major coordination site for metal ions. This problem is because the electron pair on nitrogen can form recessive bonds with metals like zinc and copper. Although other nitrogen-containing polymers can absorb metal ions, chitosan is superior in this respect as it can absorb more metal ions due to the presence of a nitrogen group. The involvement of ligands other than the amine group in the complex formation cannot be ignored since the concentration of the metal ions to be adsorbed in some cases is greater than the number of amine groups in the sample. In general, the uptake of ionic metals by chitosan is very pH sensitive; increasing the pH from 4 to 7 increased the uptake of metal ions by chitosan. This thread suggests that more amine groups will be at higher pH values. The decrease in metal ion adsorption at acidic pH values can be attributed to the metal ion coordination with the nitrogen electron pair having to compete with H3O+ at lower pH (Zhang et al., 2021).
Effect of clarifying chitosan on colour parameters in strawberry juice
The results of comparing the mean colour parameters in strawberry juice showed that the parameters b* and L* in the control sample and the sample containing chitosan were not significantly different, but the parameter a* (15.54%) showed a significant decrease (P < 0.05) (Fig. 4).
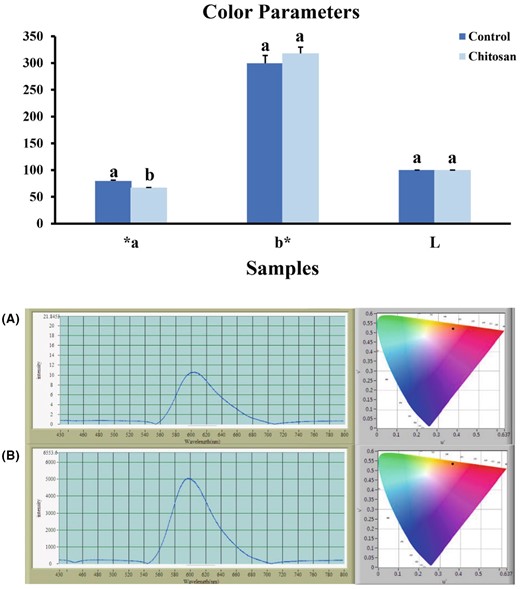
Effect of chitosan on colour parameters in strawberry. Bars represent the means of triplicate ± SE. Values with different letters are statistically significantly different at P < 0.05. (A) control sample; (B) clarified strawberry juice.
The red appearance of strawberries or their products plays an essential role in their acceptability and helps improve the market value of the fruit. This parameter affects the first human encounter with the product and the decision to buy the product (Lachowicz et al., 2018). The effect of chitosan on the colour parameter of strawberries showed that the values of L*, a* and b* in the control sample were significantly lower than in the clarified sample (Wu et al., 2022) (Fig. 4).
Residual pesticides
The remaining fifty-seven pesticide residues in strawberry juice were estimated. The results, based on the permissible limit set by the National Standard Organization and the Iranian Plant Pest Disposal Organization, indicated that the tested plant showed no contamination with pesticide residues. The greenhouse cultivation conditions of strawberries and the possibility of pollution control have led to the non-detection of pesticides in the samples (Bebek Markovinović et al., 2022; Table 1).
| Entries . | Pesticides . | Test result . |
|---|---|---|
| (mg kg−1) . | ||
| 1 | Thiometon | N.D |
| 2 | Beta-HCH | N.D |
| 3 | Atrazine | N.D |
| 4 | Diazinon | N.D |
| 5 | Pirimicarb | N.D |
| 6 | Alachlor | N.D |
| 7 | Metalaxyl | N.D |
| 8 | Alderin | N.D |
| 9 | Pirimiphos-Methyl | N.D |
| 10 | Malathion | N.D |
| 11 | Fenthion | N.D |
| 12 | Triadimefon | N.D |
| 13 | Bromophos-methyl | N.D |
| 14 | Penconazole | N.D |
| 15 | Fipronil | N.D |
| 16 | 2,4′-DDE | N.D |
| 17 | 4,4-DDE | N.D |
| 18 | 2,4′-DDD | N.D |
| 19 | 4,4-DDD | N.D |
| 20 | 4,4′-DDT | N.D |
| 21 | Endrin | N.D |
| 22 | Oxadiazon | N.D |
| 23 | Dieldrin | N.D |
| 24 | Buprofezin | N.D |
| 25 | Endosulfan alpha | N.D |
| 26 | Endosulfan-beta | |
| 27 | Endosulfan sulphate | |
| 28 | Iprodione | N.D |
| 29 | Ethion | N.D |
| 30 | Propiconazole 1 | N.D |
| 31 | Propiconazole 2 | |
| 32 | Tebuconazole | N.D |
| 33 | Bromopropylate | N.D |
| 34 | Methoxychlor | N.D |
| 35 | Fenpropathrin | N.D |
| 36 | Bitertanol | N.D |
| 37 | Bromophos-ethyl | N.D |
| 38 | Buprofezin | N.D |
| 39 | Edifenohos | N.D |
| 40 | Butachlor | N.D |
| 41 | Fenitrothion | N.D |
| 42 | Imazalil | N.D |
| 43 | Phosalone | N.D |
| 44 | Pyridaben | N.D |
| 45 | Chlordane | N.D |
| 46 | Dialifos | N.D |
| 47 | Iprobenfos | N.D |
| 48 | Metribuzin | N.D |
| 49 | Phosphamidon | N.D |
| 50 | Chlorthion | N.D |
| 51 | Flamprop-m-isopropyl | N.D |
| 52 | Hexaconazole | N.D |
| 53 | Permethrin l | N.D |
| 54 | Permethrin 2 | |
| 55 | Cypermethrin 1 | N.D |
| 56 | Cypermethrin 2 | N.D |
| 57 | Deltamethrin | N.D |
| Entries . | Pesticides . | Test result . |
|---|---|---|
| (mg kg−1) . | ||
| 1 | Thiometon | N.D |
| 2 | Beta-HCH | N.D |
| 3 | Atrazine | N.D |
| 4 | Diazinon | N.D |
| 5 | Pirimicarb | N.D |
| 6 | Alachlor | N.D |
| 7 | Metalaxyl | N.D |
| 8 | Alderin | N.D |
| 9 | Pirimiphos-Methyl | N.D |
| 10 | Malathion | N.D |
| 11 | Fenthion | N.D |
| 12 | Triadimefon | N.D |
| 13 | Bromophos-methyl | N.D |
| 14 | Penconazole | N.D |
| 15 | Fipronil | N.D |
| 16 | 2,4′-DDE | N.D |
| 17 | 4,4-DDE | N.D |
| 18 | 2,4′-DDD | N.D |
| 19 | 4,4-DDD | N.D |
| 20 | 4,4′-DDT | N.D |
| 21 | Endrin | N.D |
| 22 | Oxadiazon | N.D |
| 23 | Dieldrin | N.D |
| 24 | Buprofezin | N.D |
| 25 | Endosulfan alpha | N.D |
| 26 | Endosulfan-beta | |
| 27 | Endosulfan sulphate | |
| 28 | Iprodione | N.D |
| 29 | Ethion | N.D |
| 30 | Propiconazole 1 | N.D |
| 31 | Propiconazole 2 | |
| 32 | Tebuconazole | N.D |
| 33 | Bromopropylate | N.D |
| 34 | Methoxychlor | N.D |
| 35 | Fenpropathrin | N.D |
| 36 | Bitertanol | N.D |
| 37 | Bromophos-ethyl | N.D |
| 38 | Buprofezin | N.D |
| 39 | Edifenohos | N.D |
| 40 | Butachlor | N.D |
| 41 | Fenitrothion | N.D |
| 42 | Imazalil | N.D |
| 43 | Phosalone | N.D |
| 44 | Pyridaben | N.D |
| 45 | Chlordane | N.D |
| 46 | Dialifos | N.D |
| 47 | Iprobenfos | N.D |
| 48 | Metribuzin | N.D |
| 49 | Phosphamidon | N.D |
| 50 | Chlorthion | N.D |
| 51 | Flamprop-m-isopropyl | N.D |
| 52 | Hexaconazole | N.D |
| 53 | Permethrin l | N.D |
| 54 | Permethrin 2 | |
| 55 | Cypermethrin 1 | N.D |
| 56 | Cypermethrin 2 | N.D |
| 57 | Deltamethrin | N.D |
| Entries . | Pesticides . | Test result . |
|---|---|---|
| (mg kg−1) . | ||
| 1 | Thiometon | N.D |
| 2 | Beta-HCH | N.D |
| 3 | Atrazine | N.D |
| 4 | Diazinon | N.D |
| 5 | Pirimicarb | N.D |
| 6 | Alachlor | N.D |
| 7 | Metalaxyl | N.D |
| 8 | Alderin | N.D |
| 9 | Pirimiphos-Methyl | N.D |
| 10 | Malathion | N.D |
| 11 | Fenthion | N.D |
| 12 | Triadimefon | N.D |
| 13 | Bromophos-methyl | N.D |
| 14 | Penconazole | N.D |
| 15 | Fipronil | N.D |
| 16 | 2,4′-DDE | N.D |
| 17 | 4,4-DDE | N.D |
| 18 | 2,4′-DDD | N.D |
| 19 | 4,4-DDD | N.D |
| 20 | 4,4′-DDT | N.D |
| 21 | Endrin | N.D |
| 22 | Oxadiazon | N.D |
| 23 | Dieldrin | N.D |
| 24 | Buprofezin | N.D |
| 25 | Endosulfan alpha | N.D |
| 26 | Endosulfan-beta | |
| 27 | Endosulfan sulphate | |
| 28 | Iprodione | N.D |
| 29 | Ethion | N.D |
| 30 | Propiconazole 1 | N.D |
| 31 | Propiconazole 2 | |
| 32 | Tebuconazole | N.D |
| 33 | Bromopropylate | N.D |
| 34 | Methoxychlor | N.D |
| 35 | Fenpropathrin | N.D |
| 36 | Bitertanol | N.D |
| 37 | Bromophos-ethyl | N.D |
| 38 | Buprofezin | N.D |
| 39 | Edifenohos | N.D |
| 40 | Butachlor | N.D |
| 41 | Fenitrothion | N.D |
| 42 | Imazalil | N.D |
| 43 | Phosalone | N.D |
| 44 | Pyridaben | N.D |
| 45 | Chlordane | N.D |
| 46 | Dialifos | N.D |
| 47 | Iprobenfos | N.D |
| 48 | Metribuzin | N.D |
| 49 | Phosphamidon | N.D |
| 50 | Chlorthion | N.D |
| 51 | Flamprop-m-isopropyl | N.D |
| 52 | Hexaconazole | N.D |
| 53 | Permethrin l | N.D |
| 54 | Permethrin 2 | |
| 55 | Cypermethrin 1 | N.D |
| 56 | Cypermethrin 2 | N.D |
| 57 | Deltamethrin | N.D |
| Entries . | Pesticides . | Test result . |
|---|---|---|
| (mg kg−1) . | ||
| 1 | Thiometon | N.D |
| 2 | Beta-HCH | N.D |
| 3 | Atrazine | N.D |
| 4 | Diazinon | N.D |
| 5 | Pirimicarb | N.D |
| 6 | Alachlor | N.D |
| 7 | Metalaxyl | N.D |
| 8 | Alderin | N.D |
| 9 | Pirimiphos-Methyl | N.D |
| 10 | Malathion | N.D |
| 11 | Fenthion | N.D |
| 12 | Triadimefon | N.D |
| 13 | Bromophos-methyl | N.D |
| 14 | Penconazole | N.D |
| 15 | Fipronil | N.D |
| 16 | 2,4′-DDE | N.D |
| 17 | 4,4-DDE | N.D |
| 18 | 2,4′-DDD | N.D |
| 19 | 4,4-DDD | N.D |
| 20 | 4,4′-DDT | N.D |
| 21 | Endrin | N.D |
| 22 | Oxadiazon | N.D |
| 23 | Dieldrin | N.D |
| 24 | Buprofezin | N.D |
| 25 | Endosulfan alpha | N.D |
| 26 | Endosulfan-beta | |
| 27 | Endosulfan sulphate | |
| 28 | Iprodione | N.D |
| 29 | Ethion | N.D |
| 30 | Propiconazole 1 | N.D |
| 31 | Propiconazole 2 | |
| 32 | Tebuconazole | N.D |
| 33 | Bromopropylate | N.D |
| 34 | Methoxychlor | N.D |
| 35 | Fenpropathrin | N.D |
| 36 | Bitertanol | N.D |
| 37 | Bromophos-ethyl | N.D |
| 38 | Buprofezin | N.D |
| 39 | Edifenohos | N.D |
| 40 | Butachlor | N.D |
| 41 | Fenitrothion | N.D |
| 42 | Imazalil | N.D |
| 43 | Phosalone | N.D |
| 44 | Pyridaben | N.D |
| 45 | Chlordane | N.D |
| 46 | Dialifos | N.D |
| 47 | Iprobenfos | N.D |
| 48 | Metribuzin | N.D |
| 49 | Phosphamidon | N.D |
| 50 | Chlorthion | N.D |
| 51 | Flamprop-m-isopropyl | N.D |
| 52 | Hexaconazole | N.D |
| 53 | Permethrin l | N.D |
| 54 | Permethrin 2 | |
| 55 | Cypermethrin 1 | N.D |
| 56 | Cypermethrin 2 | N.D |
| 57 | Deltamethrin | N.D |
Conclusion
Using chitosan at a concentration of 1 g L−1 proved optimal for achieving enhanced clarity in strawberry juice. This clarification resulted in a significant reduction in turbidity by 98%. Notably, the nutritional composition of the strawberry juice remained largely unaffected, as indicated by the maintenance of soluble solids, pH, organic acid compounds, vitamin C, folate, anthocyanin, protein and carbohydrate content. While a slight decrease was observed in specific parameters, such as phenol content and antioxidant properties, it is noteworthy that using chitosan at the desired concentration did not significantly reduce the quantity of valuable phytochemical compounds in the juice. The remarkable improvement in juice clarity also contributed to increased customer acceptability. The enhancement of colour in strawberry juice, an essential quality parameter, emerged as one of the notable advantages of utilising chitosan as a clarifying agent. Furthermore, incorporating chitosan played a pivotal role in reducing heavy metal levels in fruit juice, enhancing its nutritional properties and safety. This research's findings emphasise that adding chitosan to strawberry juice achieves extraordinary clarity and improves its overall safety and nutritional value, resulting in a consumer-friendly product. Therefore, chitosan is highly recommended as a practical and effective method for preparing strawberry juice. The outcomes of this study offer valuable scientific knowledge, supporting the innovation and development of new functional juices while garnering consumer support. These findings contribute to the establishment of a robust scientific knowledge base in the field, driving advancements and ensuring the production of high-quality functional beverages.
Acknowledgments
The authors gratefully acknowledge the financial support (grant no.1400) of this research by the Shahid Chamran University of Ahvaz.
Author contributions
Fariba Heidarizadeh: Investigation (equal); methodology (equal); project administration (equal); writing – original draft (equal); writing – review and editing (equal). Maryam Kolahi: Investigation (equal); methodology (equal); project administration (equal); writing – original draft (equal); writing – review and editing (equal). Marziye Akhond: Investigation (equal); methodology (equal).
Funding information
This work was supported by the Shahid Chamran University of Ahvaz.
Conflict of interest
The authors declare that there is no conflict of interest.
Ethical approval
This material is the author's original work, which is yet to be previously published elsewhere. All authors have been personally and actively involved in substantial work leading to the paper and will take public responsibility for its content.
Consent to participate
Informed consent was obtained from all participants included in the study.
Consent to publish
The participant has consented to submit the case report to the journal.
Peer review
The peer review history for this article is available at https://www.webofscience.com/api/gateway/wos/peer-review/10.1111/ijfs.16623.
Data availability statement
All data generated or analysed during this study are included in this published article.
References
This article investigates the presence and levels of trace metals, which are of concern due to their potential toxicological effects. This research provides valuable insights into the quality and safety of fruit juices, contributing to consumer health and regulatory considerations regarding trace metal contamination.
The authors investigate the effectiveness of chitosan, a natural biopolymer, in removing impurities and enhancing the clarity of fruit juice. This research contributes to developing sustainable and eco-friendly methods for fruit juice clarification, highlighting the potential application of chitosan in the food processing industry.
This article describes a study investigating the clarification of pomegranate and strawberry juices using different clarification agents. The authors assess the impact of these agents on turbidity, anthocyanins, colour, phenolics and antioxidant activity of the juices. This research provides valuable insights into optimizing clarification processes for pomegranate and strawberry juices, helping to enhance their quality and maintain their beneficial compounds.
This article provides a comprehensive review of the research progress in utilising chitosan and its derivatives to absorb and remove heavy metals. The authors discuss the effectiveness of chitosan-based materials in the remediation of heavy metal-contaminated environments, highlighting the adsorption mechanisms and factors influencing their performance. This review serves as a valuable resource for understanding the potential applications of chitosan and its derivatives as eco-friendly and efficient adsorbents for heavy-metal removal.



