-
PDF
- Split View
-
Views
-
Cite
Cite
Veronika M Lieb, Caroline Kleiber, Ehab M R Metwali, Naif M S Kadasa, Omar A Almaghrabi, Christof B Steingass, Reinhold Carle, Fatty acids and triacylglycerols in the seed oils of Saudi Arabian date (Phoenix dactylifera L.) palms, International Journal of Food Science and Technology, Volume 55, Issue 4, April 2020, Pages 1572–1577, https://doi.org/10.1111/ijfs.14383
Close - Share Icon Share
Abstract
Total lipids, fatty acids and triacylglycerols in seeds of the four Saudi Arabian date cultivars ‘Anbra’, ‘Megadwel’, ‘Sacai’ and ‘Sfwai’ were analysed. Total lipid contents ranged between 7.3 and 8.6%. Oleic, lauric and myristic acid represented the most abundant fatty acids in date seed oil. HPLC-DAD-ESI-MSn analysis enabled the identification of 40 triacylglycerols with equivalent carbon number (ECN) 36–54, of which 17 were detected for the first time. Triacylglycerols mainly comprised LaOL, LaLaL, LaML, MML/LaPL and LaOO. ‘Anbra’ oil was characterised by highest proportions of saturated fatty acids and triacylglycerols with ECN 36 and 48–54. Unsaturated fatty acids prevailed in the three remaining varieties. Elevated proportions of triacylglycerols with medium ECNs were found in ‘Megadwel’ and ‘Sfwai’ oils. Aside from highest total lipid contents, ‘Sacai’ seeds predominantly contained triacylglycerols with low ECNs. Multivariate statistical analyses elucidated varietal influences on the fatty acid and triacylglycerol composition of date seed oil.
Introduction
Fruits of the date palm (Phoenix dactylifera L., Arecaceae) are a commercial product of high economic and nutritional relevance in the Middle East. Global date production has constantly expanded to 8.3 million tonnes in 2017. During the past decade, Egypt, Iran, Saudi Arabia and Algeria represented the main date producers. In Saudi Arabia, the fruit provides 6% of daily overall food supply, being as important for the local diet as wheat, vegetables and rice. Dates have a high commercial value as accounting for 22% of the total agricultural gross production in Saudi Arabia in 2013 (FAO, 2019).
Date fruit processing results in vast amounts of unexploited seeds, constituting to 8–19% of total fruit weight (Saafi-Ben Salah et al., 2012). Date pits are still discarded or used as animal feed, despite having promising potential for further applications. For instance, roasted seeds were proposed to be used for the production of a caffeine-free alternative to coffee with comparable flavours (Cohen et al., 2011). As reviewed by Baliga et al. (2011), date seeds exert several pharmacological properties in animal and in vitro studies such as antioxidant, antimutagenic, antiviral, antifungal, anti-inflammatory, antihyperlipidemic, anticancer and immunostimulant activities. Having a fat content of 5–13%, they may further provide a source of edible oils in food applications, cosmetics, pharmaceutics and chemistry (Devshony et al., 1992; Al-Shahib & Marshall, 2003; Besbes et al., 2004b; Saafi-Ben Salah et al., 2012). Devshony et al. (1992) have reported the use of date seed oil for replacing palm oil, coconut oil or tallow in cosmetic formulations. Owing to its ability to protect against UV-A and UV-B radiation, date seed oil may be used as UV protector being comparable to physical sunscreen agents such as titanium dioxide (Besbes et al., 2004b). Moreover, the oil has been shown to prevent oxidative damages in epidermal keratinocytes induced by hydrogen peroxide exposure (Ines et al., 2010). This may be attributed to its high contents of phenolic compounds and tocopherols (Besbes et al., 2004a). Fatty acids in date seed oil have been thoroughly described in literature (Devshony et al., 1992; Al-Shahib & Marshall, 2003; Besbes et al., 2004b; Saafi et al., 2008; Al Juhaimi et al., 2012; Saafi-Ben Salah et al., 2012; Nehdi et al., 2018). Previous studies have indicated that the fatty acid composition in date seeds depends on the fruit variety and cultivation conditions applied (Al-Shahib & Marshall, 2003). Its lipid fraction has been reported to be mainly composed of neutral lipids including acylglycerols, free fatty acids, phospholipids and phytosterols. Within this classification, triacylglycerols (TAGs) represented the major portion (96.9–97.3%) (Besbes et al., 2004c). Even though TAGs have been proposed for oil authentication (Besbes et al., 2004c), their in-depth characterisation in date seed oil derived from defined fruit varieties are lacking to date.
The aim of this study was to determine the varietal influence of date fruits on total lipids, fatty acids and TAGs. For this purpose, fatty acids and TAGs in date seed oils from defined Saudi Arabian fruit varieties were characterised using gas chromatography (GC) and high-performance liquid chromatography (HPLC), respectively. Unsupervised hierarchical cluster analysis (HCA) and principal component analysis (PCA) should be applied for the first time to disclose varietal differences between date seed oils based on their distinct fatty acid and triacylglycerol compositions.
Materials and methods
Chemicals
Acetonitrile for HPLC-MS analysis, n-hexane and isopropanol were purchased from Merck (Darmstadt, Germany). Acetonitrile for HPLC-DAD analysis, ammonium formate, methanol, potassium hydroxide and sodium sulphate were from VWR International (Leuven, Belgium; Fontenay-sous-Bois, France), formic acid from Th. Geyer (Renningen, Germany). Authentic standards of fatty acid methyl esters (FAMEs) (Supelco 37 component FAME mix, cis-11-vaccenic acid methyl ester) were obtained from Sigma-Aldrich Chemie (Steinheim, Germany).
Plant material and sample preparation
Fully ripe P. dactylifera fruits cvs. ‘Anbra’, ‘Megadwel’, ‘Sacai’ and ‘Sfwai’ at the tamr stage were harvested from commercial plantations in Medina (Saudi Arabia) on 07 August 2018 and immediately frozen after harvesting. Seeds were separated manually and milled using an analytical mill (A11 basic; IKA-Werke, Staufen, Germany). Seed dry matter (DM) was determined using a moisture analyser dryer (MB35; Ohaus, Nänikon, Switzerland) operated at 70 °C. Seed lipids were extracted with boiling n-hexane in a Soxhlet apparatus for 4 h. Total lipids in dry matter (%, w/w) were quantitated gravimetrically.
Fatty acid analysis
FAMEs were obtained by base-catalysed transmethylation of fatty acids according to ISO 12966-2 (2011) with some modifications. In brief, 30 µL of the sample was dissolved in 2.5 mL n-hexane and combined with 200 µL methanolic potassium hydroxide (10% w/v). Prior to GC-analysis, the sample was dried over anhydrous sodium sulphate.
Mass spectrometric analysis and quantitation of FAMEs were performed using GC with ion trap mass spectrometry (MS) and flame ionisation detection (FID), respectively, as previously described by Lieb et al. (2019). FAMEs were identified by comparing retention times and mass spectra of the analytes to those of authentic standards. Relative composition of bound fatty acids was expressed as percentage of the total peak area.
Triacylglycerol analysis
Prior to HPLC analysis, samples (50 mg mL−1) were prepared in acetonitrile, isopropanol and n-hexane (2:2:1, v/v/v) and filtered through a 0.2 μm PTFE-filter (Ziemer Chromatographie, Langerwehe, Germany). TAGs were analysed using an Agilent 1100 Series HPLC system equipped with a G1315B diode array detector (DAD) (all from Agilent Technologies, Waldbronn, Germany). Sample tray temperature was set to 30 °C. Injection volume was 10 µL. The column set used consisted of two C18 columns (both Kinetex™, 250 × 4.6 mm i.d., 5 μm particle size; Phenomenex, Aschaffenburg, Germany) connected in series and operated at 20 °C. Acetonitrile (eluent A) and isopropanol (eluent B) represented the mobile phase. The gradient was programmed as follows: from 30 to 70% B in 60 min, from 70 to 30% B in 3 min and isocratic 30% B for 10 min. Total run time was 73 min at a flow rate of 0.9 mL min−1. TAGs were monitored at 210 nm.
TAG identification was performed by coupling the above HPLC system to a 3000 + ion trap mass spectrometer (Bruker, Bremen, Germany) equipped with an electrospray ionisation (ESI) source. ESI-MSn parameters were detailed previously (Lieb et al., 2019).
Relative TAG composition was expressed as percentage of the total peak area. The positional distribution of fatty acids within the TAG molecule was not identified. The equivalent carbon number ECN = CN − 2 × DB was calculated with CN representing the carbon number, that is, the number of acyl carbons of all fatty acid chains, and DB the number of double bonds within the TAG molecule.
Statistics
Three biological replicates (n = 3) of each variety were analysed in analytical duplicates. Univariate statistical analyses were conducted using SAS 3.5 statistical software (SAS Institute, Cary, NC, USA). Shapiro–Wilk's (p ≤ 0.05) and Levene's test (p ≤ 0.01) were applied to test normality and homogeneity of variances, respectively. Significant differences between sample sets with normal distribution and homogeneous variance were determined by analysis of variance (ANOVA) followed by Tukey's multiple-range test (p ≤ 0.05). For data without normal distribution, the non-parametric Kruskal–Wallis test (p ≤ 0.05) was used. HCA and PCA were calculated on the basis of fatty acid and TAG profiles using Solo software version 8.1.1 (Eigenvector Research, Wenatchee, WA, USA).
Results and discussion
Total lipid contents
Total lipid contents in the date seeds assessed ranged between 7.3 and 8.6% (Table 1). Highest values were found in ‘Sacai’ seeds (8.6 ± 0.0%), followed by ‘Sfwai’ (7.8 ± 0.1%), ‘Megadwel’ (7.6 ± 0.2%) and ‘Anbra’ (7.3 ± 0.1%). Total lipid contents determined herein were in good agreement with those reported in the literature (5.4–12.7%) (Al-Shahib & Marshall, 2003; Besbes et al., 2004b; Saafi-Ben Salah et al., 2012).
Total lipid contents (% of DM) and fatty acids (%) in seeds of four Saudi Arabian P. dactylifera varieties
| . | Anbra . | Megadwel . | Sacai . | Sfwai . |
|---|---|---|---|---|
| Total lipids | 7.3 ± 0.1c | 7.6 ± 0.2b | 8.6 ± 0.0a | 7.8 ± 0.1b |
| Fatty acids | ||||
| C12:0 (La) | 22.1 ± 0.2a | 21.3 ± 0.2b | 20.3 ± 0.1c | 20.6 ± 0.5c |
| C14:0 (M) | 12.3 ± 0.1b | 11.2 ± 0.0d | 12.8 ± 0.1a | 11.4 ± 0.2c |
| C16:0 (P) | 10.2 ± 0.1b | 9.3 ± 0.0c | 10.1 ± 0.1b | 10.6 ± 0.0a |
| C18:0 (S) | 4.0 ± 0.0a | 3.3 ± 0.0b | 2.9 ± 0.0d | 3.1 ± 0.0c |
| C18:1n9 (O) | 40.3 ± 0.4b | 43.1 ± 0.2a | 42.4 ± 0.3a | 42.8 ± 0.3a |
| C18:2n6 (L) | 7.8 ± 0.1b | 9.0 ± 0.0a | 9.0 ± 0.5a | 8.5 ± 0.4a |
| Othersa | 3.3 ± 0.6a | 2.7 ± 0.1a | 2.5 ± 0.1a | 2.9 ± 0.0a |
| SFA | 51.0 ± 0.3a | 47.0 ± 0.3b | 47.8 ± 0.2b | 47.7 ± 0.6b |
| MUFA | 41.1 ± 0.2c | 43.9 ± 0.2a | 43.2 ± 0.3b | 43.7 ± 0.3ab |
| PUFA | 7.8 ± 0.1b | 9.1 ± 0.0a | 9.0 ± 0.5a | 8.6 ± 0.4a |
| . | Anbra . | Megadwel . | Sacai . | Sfwai . |
|---|---|---|---|---|
| Total lipids | 7.3 ± 0.1c | 7.6 ± 0.2b | 8.6 ± 0.0a | 7.8 ± 0.1b |
| Fatty acids | ||||
| C12:0 (La) | 22.1 ± 0.2a | 21.3 ± 0.2b | 20.3 ± 0.1c | 20.6 ± 0.5c |
| C14:0 (M) | 12.3 ± 0.1b | 11.2 ± 0.0d | 12.8 ± 0.1a | 11.4 ± 0.2c |
| C16:0 (P) | 10.2 ± 0.1b | 9.3 ± 0.0c | 10.1 ± 0.1b | 10.6 ± 0.0a |
| C18:0 (S) | 4.0 ± 0.0a | 3.3 ± 0.0b | 2.9 ± 0.0d | 3.1 ± 0.0c |
| C18:1n9 (O) | 40.3 ± 0.4b | 43.1 ± 0.2a | 42.4 ± 0.3a | 42.8 ± 0.3a |
| C18:2n6 (L) | 7.8 ± 0.1b | 9.0 ± 0.0a | 9.0 ± 0.5a | 8.5 ± 0.4a |
| Othersa | 3.3 ± 0.6a | 2.7 ± 0.1a | 2.5 ± 0.1a | 2.9 ± 0.0a |
| SFA | 51.0 ± 0.3a | 47.0 ± 0.3b | 47.8 ± 0.2b | 47.7 ± 0.6b |
| MUFA | 41.1 ± 0.2c | 43.9 ± 0.2a | 43.2 ± 0.3b | 43.7 ± 0.3ab |
| PUFA | 7.8 ± 0.1b | 9.1 ± 0.0a | 9.0 ± 0.5a | 8.6 ± 0.4a |
Means ± standard deviations (n = 3). Different letters within a row indicate significant differences of means (p ≤ 0.05).
DM, dry matter; MUFA, monounsaturated fatty acids; PUFA, polyunsaturated fatty acids; SFA, saturated fatty acids.
Including the minor fatty acids C8:0, C10:0, C15:0, C16:1n7, C17:0, C18:1n7, C18:3n3, C20:0, C20:1n9, C21:0, C22:0, C23:0, C24:0.
Total lipid contents (% of DM) and fatty acids (%) in seeds of four Saudi Arabian P. dactylifera varieties
| . | Anbra . | Megadwel . | Sacai . | Sfwai . |
|---|---|---|---|---|
| Total lipids | 7.3 ± 0.1c | 7.6 ± 0.2b | 8.6 ± 0.0a | 7.8 ± 0.1b |
| Fatty acids | ||||
| C12:0 (La) | 22.1 ± 0.2a | 21.3 ± 0.2b | 20.3 ± 0.1c | 20.6 ± 0.5c |
| C14:0 (M) | 12.3 ± 0.1b | 11.2 ± 0.0d | 12.8 ± 0.1a | 11.4 ± 0.2c |
| C16:0 (P) | 10.2 ± 0.1b | 9.3 ± 0.0c | 10.1 ± 0.1b | 10.6 ± 0.0a |
| C18:0 (S) | 4.0 ± 0.0a | 3.3 ± 0.0b | 2.9 ± 0.0d | 3.1 ± 0.0c |
| C18:1n9 (O) | 40.3 ± 0.4b | 43.1 ± 0.2a | 42.4 ± 0.3a | 42.8 ± 0.3a |
| C18:2n6 (L) | 7.8 ± 0.1b | 9.0 ± 0.0a | 9.0 ± 0.5a | 8.5 ± 0.4a |
| Othersa | 3.3 ± 0.6a | 2.7 ± 0.1a | 2.5 ± 0.1a | 2.9 ± 0.0a |
| SFA | 51.0 ± 0.3a | 47.0 ± 0.3b | 47.8 ± 0.2b | 47.7 ± 0.6b |
| MUFA | 41.1 ± 0.2c | 43.9 ± 0.2a | 43.2 ± 0.3b | 43.7 ± 0.3ab |
| PUFA | 7.8 ± 0.1b | 9.1 ± 0.0a | 9.0 ± 0.5a | 8.6 ± 0.4a |
| . | Anbra . | Megadwel . | Sacai . | Sfwai . |
|---|---|---|---|---|
| Total lipids | 7.3 ± 0.1c | 7.6 ± 0.2b | 8.6 ± 0.0a | 7.8 ± 0.1b |
| Fatty acids | ||||
| C12:0 (La) | 22.1 ± 0.2a | 21.3 ± 0.2b | 20.3 ± 0.1c | 20.6 ± 0.5c |
| C14:0 (M) | 12.3 ± 0.1b | 11.2 ± 0.0d | 12.8 ± 0.1a | 11.4 ± 0.2c |
| C16:0 (P) | 10.2 ± 0.1b | 9.3 ± 0.0c | 10.1 ± 0.1b | 10.6 ± 0.0a |
| C18:0 (S) | 4.0 ± 0.0a | 3.3 ± 0.0b | 2.9 ± 0.0d | 3.1 ± 0.0c |
| C18:1n9 (O) | 40.3 ± 0.4b | 43.1 ± 0.2a | 42.4 ± 0.3a | 42.8 ± 0.3a |
| C18:2n6 (L) | 7.8 ± 0.1b | 9.0 ± 0.0a | 9.0 ± 0.5a | 8.5 ± 0.4a |
| Othersa | 3.3 ± 0.6a | 2.7 ± 0.1a | 2.5 ± 0.1a | 2.9 ± 0.0a |
| SFA | 51.0 ± 0.3a | 47.0 ± 0.3b | 47.8 ± 0.2b | 47.7 ± 0.6b |
| MUFA | 41.1 ± 0.2c | 43.9 ± 0.2a | 43.2 ± 0.3b | 43.7 ± 0.3ab |
| PUFA | 7.8 ± 0.1b | 9.1 ± 0.0a | 9.0 ± 0.5a | 8.6 ± 0.4a |
Means ± standard deviations (n = 3). Different letters within a row indicate significant differences of means (p ≤ 0.05).
DM, dry matter; MUFA, monounsaturated fatty acids; PUFA, polyunsaturated fatty acids; SFA, saturated fatty acids.
Including the minor fatty acids C8:0, C10:0, C15:0, C16:1n7, C17:0, C18:1n7, C18:3n3, C20:0, C20:1n9, C21:0, C22:0, C23:0, C24:0.
Fatty acids
Gas chromatographic analyses enabled the identification of 19 fatty acids in Saudi Arabian date seed oils (Table 1). C12:0, C14:0, C16:0, C18:0, C18:1n9 and C18:2n6 were the prevalent fatty acids, contributing to 96.7–97.5% of total bound fatty acids. Minor constituents each representing < 1% were C8:0, C10:0, C15:0, C16:1n7, C17:0, C18:1n7, C18:3n3, C20:0, C20:1n9, C21:0, C22:0, C23:0 and C24:0. Al Juhaimi et al. (2012) and Nehdi et al. (2018) have additionally identified trace amounts of C6:0, C13:0, C16:1n9, C17:1n7, C20:1n7, C26:0 and 9,10-epoxystearic acid in Saudi-Arabian date seed oils. All aforementioned minor constituents were not found in our study.
The oils assessed herein were mainly composed of saturated (47.0–51.0%) and monounsaturated fatty acids (41.1–43.9%). C18:1n9 (40.3–43.1%) was the predominant fatty acid in all samples, followed by C12:0 (20.3–22.1%) and C14:0 (11.2–12.8%).
Nehdi et al. (2018) have described similar fatty acid profiles in six Saudi Arabian oil samples. In contrast, our seed oils derived from various varieties evidently differed regarding their fatty acid composition. ‘Anbra’ seed oil was characterised by significantly higher proportions of C12:0 (22.1 ± 0.2%) and C18:0 (4.0 ± 0.0%) as well as lower proportions of C18:1n9 (40.3 ± 0.4%) and C18:2n6 (7.8 ± 0.1%). As a result, this sample showed elevated proportions of saturated (51.0 ± 0.3%) and lowest proportions of unsaturated fatty acids (41.1 ± 0.2% MUFA, 7.8 ± 0.1% PUFA) compared to the remaining date seed oils (47.0–47.8% SFA, 43.2–43.9% MUFA, 8.6–9.1% PUFA). In contrast, ‘Megadwel’, ‘Sacai’ and ‘Sfwai’ oils displayed significantly higher proportions of unsaturated fatty acids, in particular, of the diunsaturated C18:2n6. Date seed oils containing high proportions of unsaturated fatty acids in combination with low concentrations of phenolic compounds are prone to oxidation (Besbes et al., 2005).
The fatty acid compositions determined herein were comparable to those reported in various Saudi-Arabian date seed oils (Al-Shahib & Marshall, 2003; Al Juhaimi et al., 2012; Nehdi et al., 2018). However, they differed from those reported for fruits from other provenances. For instance, seed oils of Tunisian fruits have been reported to show a broader variation in their fatty acid profiles (Besbes et al., 2004b; Saafi et al., 2008; Saafi-Ben Salah et al., 2012). In addition, date seeds originating from the United Arab Emirates and Israel had higher proportions of unsaturated and lower proportions of saturated fatty acids compared to our Saudi Arabian oils (Devshony et al., 1992; Habib et al., 2013).
Triacylglycerols
TAG compositions in P. dactylifera seed oils are compiled in Table 2. A total of 40 TAGs with ECN ranging between 36 and 54 was identified using MSn, of which 17 TAGs have been unprecedented to date, that is, LaLaLa, MMM, LaLaS, LaMS, MMP, LaPP, SLL, MMS, MPP, LaPS, PPP, LaSS, MPS, PPS, MSS, PSS and SSS. To the best of our knowledge, such a comprehensive qualitative and quantitative profiling of TAGs in seed oil from defined P. dactylifera varieties was conducted for the first time. Holčapek et al. (2005) have previously reported 66 TAGs in date seed oil derived from fruits of an unknown variety and origin. Compared to our results, they were able to identify a larger number of TAGs by considering the minor fatty acids C8:0, C10:0, C17:0, C20:0, C20:1n9, C22:0, C23:0 and C24:0.
Triacylglycerols in seed oils of four Saudi Arabian P. dactylifera varieties
| TAGsa . | ECN . | Anbra . | Megadwel . | Sacai . | Sfwai . |
|---|---|---|---|---|---|
| LaLaL | 38 | 7.8 ± 0.1bc | 7.5 ± 0.1c | 8.0 ± 0.2b | 8.4 ± 0.0a |
| LaLL | 40 | 2.7 ± 0.1d | 3.6 ± 0.0a | 3.1 ± 0.1c | 3.4 ± 0.0b |
| LaML | 40 | 7.8 ± 0.0b | 6.8 ± 0.1d | 8.2 ± 0.1a | 7.3 ± 0.1c |
| LaLaO | 40 | 4.4 ± 0.0b | 4.1 ± 0.1c | 4.1 ± 0.0c | 4.6 ± 0.0a |
| MLL | 42 | 1.3 ± 0.0c | 1.5 ± 0.0b | 1.6 ± 0.0a | 1.3 ± 0.0c |
| LaOL | 42 | 9.9 ± 0.0c | 11.4 ± 0.1a | 10.8 ± 0.1b | 10.9 ± 0.1b |
| MML/LaPL | 42 | 7.4 ± 0.0b | 6.8 ± 0.1c | 7.6 ± 0.1a | 7.4 ± 0.1b |
| LaMO | 42 | 4.8 ± 0.1a | 4.1 ± 0.0c | 4.8 ± 0.1ab | 4.6 ± 0.1b |
| OLL | 44 | 1.2 ± 0.1b | 2.1 ± 0.0a | 1.3 ± 0.0b | 1.2 ± 0.0b |
| PLL | 44 | 1.2 ± 0.0c | 1.5 ± 0.0a | 1.3 ± 0.0b | 1.4 ± 0.0a |
| MOL | 44 | 5.2 ± 0.1c | 5.5 ± 0.0b | 6.0 ± 0.0a | 5.2 ± 0.0c |
| LaOO | 44 | 6.1 ± 0.1c | 6.8 ± 0.1ab | 6.5 ± 0.1b | 7.0 ± 0.1a |
| LaSL/MPL | 44 | 4.4 ± 0.1a | 3.6 ± 0.1c | 3.9 ± 0.1b | 3.7 ± 0.1bc |
| MMO/LaPO | 44 | 5.0 ± 0.0a | 4.1 ± 0.0b | 4.8 ± 0.1b | 5.0 ± 0.0a |
| OOL | 46 | 3.0 ± 0.1c | 4.7 ± 0.0a | 3.3 ± 0.1b | 2.9 ± 0.1c |
| POL | 46 | 4.0 ± 0.0c | 4.6 ± 0.0a | 4.3 ± 0.0b | 4.3 ± 0.0b |
| MOO | 46 | 3.3 ± 0.1b | 3.1 ± 0.0c | 3.5 ± 0.0a | 3.2 ± 0.0bc |
| PPL/MSL | 46 | 2.2 ± 0.0a | 1.7 ± 0.0c | 1.9 ± 0.0b | 1.9 ± 0.1b |
| LaSO/MPO | 46 | 2.8 ± 0.0a | 2.1 ± 0.0c | 2.4 ± 0.0b | 2.4 ± 0.0b |
| OOO | 48 | 2.3 ± 0.1c | 3.0 ± 0.1a | 2.3 ± 0.0c | 2.4 ± 0.0b |
| SOL | 48 | 1.5 ± 0.0a | 1.6 ± 0.0a | 1.2 ± 0.0b | 1.3 ± 0.0b |
| POO | 48 | 2.4 ± 0.0b | 2.4 ± 0.0b | 2.5 ± 0.0b | 2.6 ± 0.0a |
| PSL | 48 | 1.1 ± 0.0a | 0.8 ± 0.0b | 0.7 ± 0.0c | 0.8 ± 0.0b |
| PPO/MSO | 48 | 1.3 ± 0.0a | 0.9 ± 0.1d | 1.1 ± 0.0c | 1.2 ± 0.0b |
| SOO | 50 | 1.0 ± 0.0a | 1.0 ± 0.1ab | 0.8 ± 0.0c | 0.9 ± 0.0bc |
| Othersb | 5.7 ± 0.6a | 4.7 ± 0.4ab | 4.1 ± 0.3b | 4.5 ± 0.2b | |
| Trisaturated TAGs | 3.2 ± 0.4a | 2.7 ± 0.5a | 2.3 ± 0.2a | 2.6 ± 0.1a | |
| Disaturated TAGs | 50.9 ± 0.3a | 43.6 ± 0.4c | 48.5 ± 0.1b | 48.7 ± 0.1b | |
| Diunsaturated TAGs | 39.2 ± 0.3c | 43.5 ± 0.0a | 42.0 ± 0.1b | 41.9 ± 0.1b | |
| Triunsaturated TAGs | 6.7 ± 0.4b | 10.2 ± 0.0a | 7.1 ± 0.1b | 6.8 ± 0.1b | |
| TAGsa . | ECN . | Anbra . | Megadwel . | Sacai . | Sfwai . |
|---|---|---|---|---|---|
| LaLaL | 38 | 7.8 ± 0.1bc | 7.5 ± 0.1c | 8.0 ± 0.2b | 8.4 ± 0.0a |
| LaLL | 40 | 2.7 ± 0.1d | 3.6 ± 0.0a | 3.1 ± 0.1c | 3.4 ± 0.0b |
| LaML | 40 | 7.8 ± 0.0b | 6.8 ± 0.1d | 8.2 ± 0.1a | 7.3 ± 0.1c |
| LaLaO | 40 | 4.4 ± 0.0b | 4.1 ± 0.1c | 4.1 ± 0.0c | 4.6 ± 0.0a |
| MLL | 42 | 1.3 ± 0.0c | 1.5 ± 0.0b | 1.6 ± 0.0a | 1.3 ± 0.0c |
| LaOL | 42 | 9.9 ± 0.0c | 11.4 ± 0.1a | 10.8 ± 0.1b | 10.9 ± 0.1b |
| MML/LaPL | 42 | 7.4 ± 0.0b | 6.8 ± 0.1c | 7.6 ± 0.1a | 7.4 ± 0.1b |
| LaMO | 42 | 4.8 ± 0.1a | 4.1 ± 0.0c | 4.8 ± 0.1ab | 4.6 ± 0.1b |
| OLL | 44 | 1.2 ± 0.1b | 2.1 ± 0.0a | 1.3 ± 0.0b | 1.2 ± 0.0b |
| PLL | 44 | 1.2 ± 0.0c | 1.5 ± 0.0a | 1.3 ± 0.0b | 1.4 ± 0.0a |
| MOL | 44 | 5.2 ± 0.1c | 5.5 ± 0.0b | 6.0 ± 0.0a | 5.2 ± 0.0c |
| LaOO | 44 | 6.1 ± 0.1c | 6.8 ± 0.1ab | 6.5 ± 0.1b | 7.0 ± 0.1a |
| LaSL/MPL | 44 | 4.4 ± 0.1a | 3.6 ± 0.1c | 3.9 ± 0.1b | 3.7 ± 0.1bc |
| MMO/LaPO | 44 | 5.0 ± 0.0a | 4.1 ± 0.0b | 4.8 ± 0.1b | 5.0 ± 0.0a |
| OOL | 46 | 3.0 ± 0.1c | 4.7 ± 0.0a | 3.3 ± 0.1b | 2.9 ± 0.1c |
| POL | 46 | 4.0 ± 0.0c | 4.6 ± 0.0a | 4.3 ± 0.0b | 4.3 ± 0.0b |
| MOO | 46 | 3.3 ± 0.1b | 3.1 ± 0.0c | 3.5 ± 0.0a | 3.2 ± 0.0bc |
| PPL/MSL | 46 | 2.2 ± 0.0a | 1.7 ± 0.0c | 1.9 ± 0.0b | 1.9 ± 0.1b |
| LaSO/MPO | 46 | 2.8 ± 0.0a | 2.1 ± 0.0c | 2.4 ± 0.0b | 2.4 ± 0.0b |
| OOO | 48 | 2.3 ± 0.1c | 3.0 ± 0.1a | 2.3 ± 0.0c | 2.4 ± 0.0b |
| SOL | 48 | 1.5 ± 0.0a | 1.6 ± 0.0a | 1.2 ± 0.0b | 1.3 ± 0.0b |
| POO | 48 | 2.4 ± 0.0b | 2.4 ± 0.0b | 2.5 ± 0.0b | 2.6 ± 0.0a |
| PSL | 48 | 1.1 ± 0.0a | 0.8 ± 0.0b | 0.7 ± 0.0c | 0.8 ± 0.0b |
| PPO/MSO | 48 | 1.3 ± 0.0a | 0.9 ± 0.1d | 1.1 ± 0.0c | 1.2 ± 0.0b |
| SOO | 50 | 1.0 ± 0.0a | 1.0 ± 0.1ab | 0.8 ± 0.0c | 0.9 ± 0.0bc |
| Othersb | 5.7 ± 0.6a | 4.7 ± 0.4ab | 4.1 ± 0.3b | 4.5 ± 0.2b | |
| Trisaturated TAGs | 3.2 ± 0.4a | 2.7 ± 0.5a | 2.3 ± 0.2a | 2.6 ± 0.1a | |
| Disaturated TAGs | 50.9 ± 0.3a | 43.6 ± 0.4c | 48.5 ± 0.1b | 48.7 ± 0.1b | |
| Diunsaturated TAGs | 39.2 ± 0.3c | 43.5 ± 0.0a | 42.0 ± 0.1b | 41.9 ± 0.1b | |
| Triunsaturated TAGs | 6.7 ± 0.4b | 10.2 ± 0.0a | 7.1 ± 0.1b | 6.8 ± 0.1b | |
Means ± standard deviations (n = 3). Different letters within a row indicate significant differences of means (p ≤ 0.05). TAGs, triacylglycerols; ECN, equivalent carbon number.
aAbbreviations, see Table 1.
bIncluding the minor TAGs LaLaLa, LaLaM, LaLaP/LaMM, LLL, MMM/LaLaS/LaMP, LaMS/MMP/LaPP, SLL, MMS/MPP/LaPS, PPP/LaSS/MPS, SSL, PSO, PPS/MSS, SSO, PSS, SSS.
Triacylglycerols in seed oils of four Saudi Arabian P. dactylifera varieties
| TAGsa . | ECN . | Anbra . | Megadwel . | Sacai . | Sfwai . |
|---|---|---|---|---|---|
| LaLaL | 38 | 7.8 ± 0.1bc | 7.5 ± 0.1c | 8.0 ± 0.2b | 8.4 ± 0.0a |
| LaLL | 40 | 2.7 ± 0.1d | 3.6 ± 0.0a | 3.1 ± 0.1c | 3.4 ± 0.0b |
| LaML | 40 | 7.8 ± 0.0b | 6.8 ± 0.1d | 8.2 ± 0.1a | 7.3 ± 0.1c |
| LaLaO | 40 | 4.4 ± 0.0b | 4.1 ± 0.1c | 4.1 ± 0.0c | 4.6 ± 0.0a |
| MLL | 42 | 1.3 ± 0.0c | 1.5 ± 0.0b | 1.6 ± 0.0a | 1.3 ± 0.0c |
| LaOL | 42 | 9.9 ± 0.0c | 11.4 ± 0.1a | 10.8 ± 0.1b | 10.9 ± 0.1b |
| MML/LaPL | 42 | 7.4 ± 0.0b | 6.8 ± 0.1c | 7.6 ± 0.1a | 7.4 ± 0.1b |
| LaMO | 42 | 4.8 ± 0.1a | 4.1 ± 0.0c | 4.8 ± 0.1ab | 4.6 ± 0.1b |
| OLL | 44 | 1.2 ± 0.1b | 2.1 ± 0.0a | 1.3 ± 0.0b | 1.2 ± 0.0b |
| PLL | 44 | 1.2 ± 0.0c | 1.5 ± 0.0a | 1.3 ± 0.0b | 1.4 ± 0.0a |
| MOL | 44 | 5.2 ± 0.1c | 5.5 ± 0.0b | 6.0 ± 0.0a | 5.2 ± 0.0c |
| LaOO | 44 | 6.1 ± 0.1c | 6.8 ± 0.1ab | 6.5 ± 0.1b | 7.0 ± 0.1a |
| LaSL/MPL | 44 | 4.4 ± 0.1a | 3.6 ± 0.1c | 3.9 ± 0.1b | 3.7 ± 0.1bc |
| MMO/LaPO | 44 | 5.0 ± 0.0a | 4.1 ± 0.0b | 4.8 ± 0.1b | 5.0 ± 0.0a |
| OOL | 46 | 3.0 ± 0.1c | 4.7 ± 0.0a | 3.3 ± 0.1b | 2.9 ± 0.1c |
| POL | 46 | 4.0 ± 0.0c | 4.6 ± 0.0a | 4.3 ± 0.0b | 4.3 ± 0.0b |
| MOO | 46 | 3.3 ± 0.1b | 3.1 ± 0.0c | 3.5 ± 0.0a | 3.2 ± 0.0bc |
| PPL/MSL | 46 | 2.2 ± 0.0a | 1.7 ± 0.0c | 1.9 ± 0.0b | 1.9 ± 0.1b |
| LaSO/MPO | 46 | 2.8 ± 0.0a | 2.1 ± 0.0c | 2.4 ± 0.0b | 2.4 ± 0.0b |
| OOO | 48 | 2.3 ± 0.1c | 3.0 ± 0.1a | 2.3 ± 0.0c | 2.4 ± 0.0b |
| SOL | 48 | 1.5 ± 0.0a | 1.6 ± 0.0a | 1.2 ± 0.0b | 1.3 ± 0.0b |
| POO | 48 | 2.4 ± 0.0b | 2.4 ± 0.0b | 2.5 ± 0.0b | 2.6 ± 0.0a |
| PSL | 48 | 1.1 ± 0.0a | 0.8 ± 0.0b | 0.7 ± 0.0c | 0.8 ± 0.0b |
| PPO/MSO | 48 | 1.3 ± 0.0a | 0.9 ± 0.1d | 1.1 ± 0.0c | 1.2 ± 0.0b |
| SOO | 50 | 1.0 ± 0.0a | 1.0 ± 0.1ab | 0.8 ± 0.0c | 0.9 ± 0.0bc |
| Othersb | 5.7 ± 0.6a | 4.7 ± 0.4ab | 4.1 ± 0.3b | 4.5 ± 0.2b | |
| Trisaturated TAGs | 3.2 ± 0.4a | 2.7 ± 0.5a | 2.3 ± 0.2a | 2.6 ± 0.1a | |
| Disaturated TAGs | 50.9 ± 0.3a | 43.6 ± 0.4c | 48.5 ± 0.1b | 48.7 ± 0.1b | |
| Diunsaturated TAGs | 39.2 ± 0.3c | 43.5 ± 0.0a | 42.0 ± 0.1b | 41.9 ± 0.1b | |
| Triunsaturated TAGs | 6.7 ± 0.4b | 10.2 ± 0.0a | 7.1 ± 0.1b | 6.8 ± 0.1b | |
| TAGsa . | ECN . | Anbra . | Megadwel . | Sacai . | Sfwai . |
|---|---|---|---|---|---|
| LaLaL | 38 | 7.8 ± 0.1bc | 7.5 ± 0.1c | 8.0 ± 0.2b | 8.4 ± 0.0a |
| LaLL | 40 | 2.7 ± 0.1d | 3.6 ± 0.0a | 3.1 ± 0.1c | 3.4 ± 0.0b |
| LaML | 40 | 7.8 ± 0.0b | 6.8 ± 0.1d | 8.2 ± 0.1a | 7.3 ± 0.1c |
| LaLaO | 40 | 4.4 ± 0.0b | 4.1 ± 0.1c | 4.1 ± 0.0c | 4.6 ± 0.0a |
| MLL | 42 | 1.3 ± 0.0c | 1.5 ± 0.0b | 1.6 ± 0.0a | 1.3 ± 0.0c |
| LaOL | 42 | 9.9 ± 0.0c | 11.4 ± 0.1a | 10.8 ± 0.1b | 10.9 ± 0.1b |
| MML/LaPL | 42 | 7.4 ± 0.0b | 6.8 ± 0.1c | 7.6 ± 0.1a | 7.4 ± 0.1b |
| LaMO | 42 | 4.8 ± 0.1a | 4.1 ± 0.0c | 4.8 ± 0.1ab | 4.6 ± 0.1b |
| OLL | 44 | 1.2 ± 0.1b | 2.1 ± 0.0a | 1.3 ± 0.0b | 1.2 ± 0.0b |
| PLL | 44 | 1.2 ± 0.0c | 1.5 ± 0.0a | 1.3 ± 0.0b | 1.4 ± 0.0a |
| MOL | 44 | 5.2 ± 0.1c | 5.5 ± 0.0b | 6.0 ± 0.0a | 5.2 ± 0.0c |
| LaOO | 44 | 6.1 ± 0.1c | 6.8 ± 0.1ab | 6.5 ± 0.1b | 7.0 ± 0.1a |
| LaSL/MPL | 44 | 4.4 ± 0.1a | 3.6 ± 0.1c | 3.9 ± 0.1b | 3.7 ± 0.1bc |
| MMO/LaPO | 44 | 5.0 ± 0.0a | 4.1 ± 0.0b | 4.8 ± 0.1b | 5.0 ± 0.0a |
| OOL | 46 | 3.0 ± 0.1c | 4.7 ± 0.0a | 3.3 ± 0.1b | 2.9 ± 0.1c |
| POL | 46 | 4.0 ± 0.0c | 4.6 ± 0.0a | 4.3 ± 0.0b | 4.3 ± 0.0b |
| MOO | 46 | 3.3 ± 0.1b | 3.1 ± 0.0c | 3.5 ± 0.0a | 3.2 ± 0.0bc |
| PPL/MSL | 46 | 2.2 ± 0.0a | 1.7 ± 0.0c | 1.9 ± 0.0b | 1.9 ± 0.1b |
| LaSO/MPO | 46 | 2.8 ± 0.0a | 2.1 ± 0.0c | 2.4 ± 0.0b | 2.4 ± 0.0b |
| OOO | 48 | 2.3 ± 0.1c | 3.0 ± 0.1a | 2.3 ± 0.0c | 2.4 ± 0.0b |
| SOL | 48 | 1.5 ± 0.0a | 1.6 ± 0.0a | 1.2 ± 0.0b | 1.3 ± 0.0b |
| POO | 48 | 2.4 ± 0.0b | 2.4 ± 0.0b | 2.5 ± 0.0b | 2.6 ± 0.0a |
| PSL | 48 | 1.1 ± 0.0a | 0.8 ± 0.0b | 0.7 ± 0.0c | 0.8 ± 0.0b |
| PPO/MSO | 48 | 1.3 ± 0.0a | 0.9 ± 0.1d | 1.1 ± 0.0c | 1.2 ± 0.0b |
| SOO | 50 | 1.0 ± 0.0a | 1.0 ± 0.1ab | 0.8 ± 0.0c | 0.9 ± 0.0bc |
| Othersb | 5.7 ± 0.6a | 4.7 ± 0.4ab | 4.1 ± 0.3b | 4.5 ± 0.2b | |
| Trisaturated TAGs | 3.2 ± 0.4a | 2.7 ± 0.5a | 2.3 ± 0.2a | 2.6 ± 0.1a | |
| Disaturated TAGs | 50.9 ± 0.3a | 43.6 ± 0.4c | 48.5 ± 0.1b | 48.7 ± 0.1b | |
| Diunsaturated TAGs | 39.2 ± 0.3c | 43.5 ± 0.0a | 42.0 ± 0.1b | 41.9 ± 0.1b | |
| Triunsaturated TAGs | 6.7 ± 0.4b | 10.2 ± 0.0a | 7.1 ± 0.1b | 6.8 ± 0.1b | |
Means ± standard deviations (n = 3). Different letters within a row indicate significant differences of means (p ≤ 0.05). TAGs, triacylglycerols; ECN, equivalent carbon number.
aAbbreviations, see Table 1.
bIncluding the minor TAGs LaLaLa, LaLaM, LaLaP/LaMM, LLL, MMM/LaLaS/LaMP, LaMS/MMP/LaPP, SLL, MMS/MPP/LaPS, PPP/LaSS/MPS, SSL, PSO, PPS/MSS, SSO, PSS, SSS.
Disaturated TAGs prevailed in the date seed oils assessed (43.6–50.9%), followed by diunsaturated (39.2–43.5%), triunsaturated (6.7–10.2%) and trisaturated TAGs (2.3–3.2%). As depicted in Fig. 1a, major TAGs were those with ECN 42 (24.0–25.4%) and 44 (23.4–24.1%). LaOL (9.9–11.4%), LaLaL (7.5–8.4%), LaML (6.8–8.2%), MML/LaPL (6.8–7.6%) and LaOO (6.1–7.0%) represented the most abundant TAGs. In contrast, LaLaO, LaOO and LaMO have been identified as the most important TAGs in date seed oil of an unknown variety (Holčapek et al., 2005). Besbes et al. (2004c) have also quantitated TAGs in date seed oils of two defined fruit varieties from Tunisia using gas chromatography. In their study, TAGs were classified according to their CN ignoring the presence of unsaturated fatty acids. In accordance with their results, TAGs with CN 48, that is, LaLL, LaOL, LaOO, LaSL/MPL, LaSO/MPO and PPP/LaSS/MPS, prevailed in our seed oils (Fig. 1b). Besbes et al. (2004c) have reported a profile characterised by the abundance of TAGs with medium molecular weight (26.46–27.24%), whereas we found merely low proportions of TAGs with CN 36–42 (13.1–14.3%).
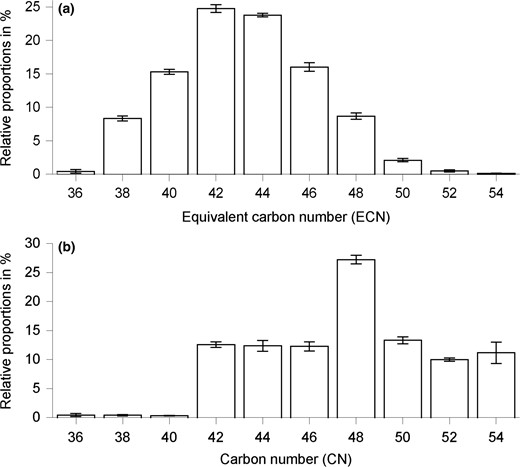
Distribution of TAGs in P. dactylifera seed oils classified according to the ECNs (a) and CNs (b).
Comparing all varieties analysed herein, ‘Anbra’ seed oil had significantly higher proportions of disaturated TAGs (50.9 ± 0.3%), being predominantly composed of TAGs with ECN 36 and 48–54 such as SOL, PSL, PPO/MSO and SOO. In comparison to the other samples, ‘Megadwel’ exhibited significantly highest proportions of unsaturated TAGs (43.5 ± 0.0% di- and 10.2 ± 0.0% triunsaturated TAGs). ‘Sacai’ mainly contained TAGs with the low ECNs 40 to 44. ‘Megadwel’ and ‘Sfwai’ stood out for their high proportions of TAGs with medium ECN (46–48).
Nehdi et al. (2010) have thoroughly analysed TAGs in Canary island date palm (Phoenix canariensis Chabaud) seeds, identifying LaMP (15.31%), LaOO/PLL/MPL (12.86%) and LaPO (11.33%) as major TAGs in the seed oil of ripe samples. Despite their genetic relationship, the aforementioned TAGs amounted to a total of merely 17.0% in our P. dactylifera seed oils.
Multivariate statistics
Unsupervised HCA and PCA were used for the first time to classify Saudi Arabian date seed oils according to their fatty acid and triacylglycerol compositions (Fig. 2). The first principal components (PCs) of the model explained 74.5% of the total variance among the data set with contributions of 50.3% by PC 1 and 24.3% by PC 2, respectively. ‘Megadwel’ was clearly separated from the remaining varieties. As revealed by the PCA loadings plot (Fig. 2b’), particularly TAGs with medium ECNs ranging between 40 and 48 contributed to the aforementioned clustering. In contrast, higher proportions of saturated fatty acids and triacylglycerols promoted the segregation of ‘Anbra’ oils in a second cluster. Owing to their similar lipid profile, ‘Sacai’ and ‘Sfwai’ fruits formed a heterogeneous cluster being only separated by PC 2.
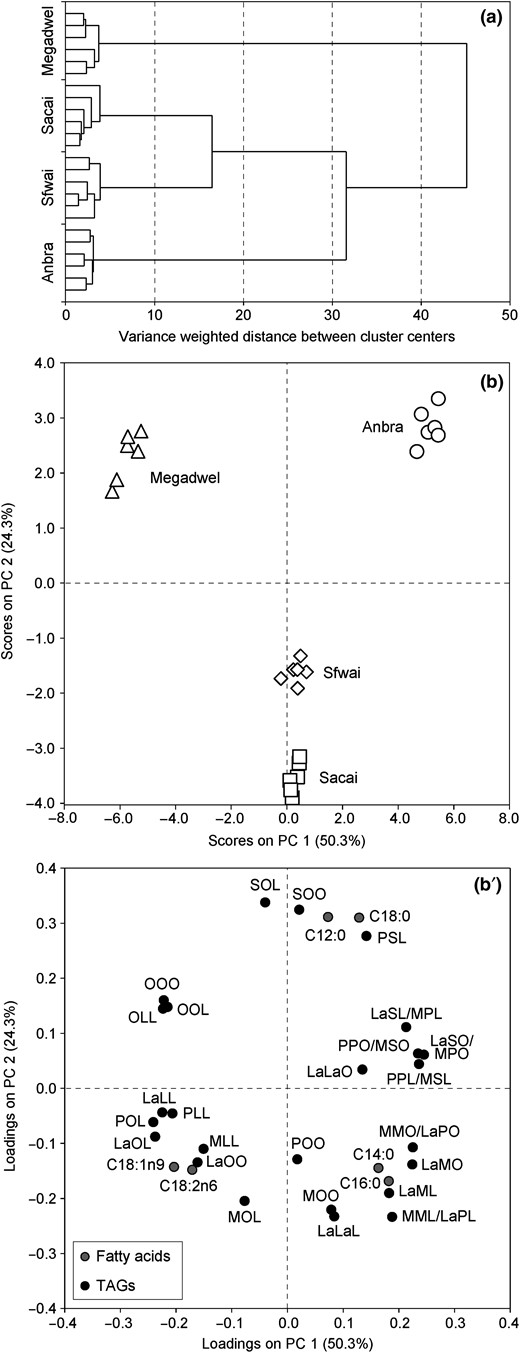
HCA dendrogram (a), PCA scores (b) and loadings plots (b’) based on fatty acids and triacylglycerols in P. dactylifera seed oils.
Conclusions
GC-FID, GC-MS and HPLC-DAD-ESI-MSn analyses provided comprehensive insights into the lipid composition of date seed oils from four Saudi Arabian fruit varieties. Such a detailed profiling of triacylglycerols in date seed oils derived from defined cultivars was reported for the first time, presenting an oil with TAGs ranging between ECN 36 and 54. In addition, the impact of the fruit variety on total lipids, fatty acids and triacylglycerols in date seeds has so far not been investigated. Multivariate statistical tools applied enabled the varietal differentiation based on fatty acid and triacylglycerol compositions.
Instead of being discarded, hitherto unexploited date seeds may be used for recovering oleic-lauric oils. P. dactylifera seed oil may find a wide range of applications in the food and cosmetic industry. For instance, it may provide a liquid source in oil blends for targeted fat formulations or substitute palm oil and olein fractions derived thereof in diverse commercial products.
Acknowledgments
This work was funded by University of Jeddah, Deanship of Scientific Research, Jeddah, under grant No. (UJ-11-18-ICP). The authors, therefore, acknowledge all the technical and financial supports.
Conflict of interest
The authors declare no conflict of interest in this work.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethical guidelines
Ethics approval was not required for this research.



