-
PDF
- Split View
-
Views
-
Cite
Cite
Chao Wu, Xu Yang, Huimin Zhang, Yunhu Song, Cardiac paraganglioma with sulfur subunit B gene mutation: a case report, European Heart Journal - Case Reports, Volume 5, Issue 2, February 2021, ytab025, https://doi.org/10.1093/ehjcr/ytab025
Close - Share Icon Share
Abstract
Pheochromocytoma and paraganglioma is a rare disease with a prevalence of 0.2–0.6% in hypertensive patients from outpatient.
A 21-year-old man complained of blood pressure elevation over one year and persistent hyperhidrosis near 5 years. In local hospital, a mass in the pericardial space with abundant blood flow was observed via echocardiography and confirmed under minimally invasive thoracotomy. With suspicion of malignant cardiac mass, the patient was recommended to transfer for further diagnosis and treatment. Combining evaluation for blood and urinary catecholamine levels, somatostatin receptor imaging, and iodine-131 metaiodobenzylguanidine scintigraphy, he was confirmed with the diagnosis of cardiac paraganglioma with blood supply from the right coronary artery identified via angiography. The cardiac tumour was then surgically resected and confirmed with a pathological diagnosis of paraganglioma. Subsequent genetic test suggested succinate dehydrogenase complex iron sulfur subunit B (SDHB) gene mutation. At 5-month follow-up, the patient was recovered with normal levels of blood catecholamines and catecholamine metabolites.
Cardiac paraganglioma should be considered and evaluated in hypertensive patients with cardiac mass, even in non-typical population. Given a potential risk of developing malignancies, close follow-up is significant in patients with SDHB gene mutations.
Patients with hypertension accompanied by cardiac mass, even if the ‘4P’ symptoms are not typical, cardiac paraganglioma should be considered first and relevant tests should be performed.
Before the surgical resection of cardiac tumour, adequate pre-operative preparations such as high-sodium diet, fluid intake, and medical treatment are used to reduce the risk of severe hypotension after surgery.
Patients with sulfur subunit B gene mutations are at high risk of developing malignancies and follow-up is required to monitoring relapse.
Introduction
Pheochromocytoma and paraganglioma (PPGL) is a rare disease with a prevalence of 0.2–0.6% in hypertensive patients on an outpatient basis.1,2 Typical clinical manifestations of PPGL include ‘4P’ symptoms of headache, face pale, palpitation, and polyidrosis. In addition to localized adrenal pheochromocytomas, ectopic paragangliomas such as urinary bladder paraganglioma3,4 were also reported with non-typical symptoms and indeed prolonged diagnosis. Several genetic mutations such as succinate dehydrogenase complex iron sulfur subunit B (SDHB) mutations also occur in metastatic PPGL patients with increased risk for malignancy. We herein reported a unique case of non-typical paraganglioma in heart identified with SDHB mutation.
Timeline
| Time . | Events . |
|---|---|
| Year −5 | Index presentation |
| Year −1 | Increased blood pressure |
| Month −2 | Reason for initial echocardiography Timing of cardiac computed tomography and positron emission tomography Thoracotomy |
| Day −23 | Timing of blood tests and genetic test |
| Day −15 | Timing of iodine-131 metaiodobenzylguanidine scintigraphy |
| Day −10 | Timing of coronary angiography |
| Day 0 | Surgery |
| Day +1 | Timing of pathology |
| Month +5 | Follow-up |
| Time . | Events . |
|---|---|
| Year −5 | Index presentation |
| Year −1 | Increased blood pressure |
| Month −2 | Reason for initial echocardiography Timing of cardiac computed tomography and positron emission tomography Thoracotomy |
| Day −23 | Timing of blood tests and genetic test |
| Day −15 | Timing of iodine-131 metaiodobenzylguanidine scintigraphy |
| Day −10 | Timing of coronary angiography |
| Day 0 | Surgery |
| Day +1 | Timing of pathology |
| Month +5 | Follow-up |
| Time . | Events . |
|---|---|
| Year −5 | Index presentation |
| Year −1 | Increased blood pressure |
| Month −2 | Reason for initial echocardiography Timing of cardiac computed tomography and positron emission tomography Thoracotomy |
| Day −23 | Timing of blood tests and genetic test |
| Day −15 | Timing of iodine-131 metaiodobenzylguanidine scintigraphy |
| Day −10 | Timing of coronary angiography |
| Day 0 | Surgery |
| Day +1 | Timing of pathology |
| Month +5 | Follow-up |
| Time . | Events . |
|---|---|
| Year −5 | Index presentation |
| Year −1 | Increased blood pressure |
| Month −2 | Reason for initial echocardiography Timing of cardiac computed tomography and positron emission tomography Thoracotomy |
| Day −23 | Timing of blood tests and genetic test |
| Day −15 | Timing of iodine-131 metaiodobenzylguanidine scintigraphy |
| Day −10 | Timing of coronary angiography |
| Day 0 | Surgery |
| Day +1 | Timing of pathology |
| Month +5 | Follow-up |
Case presentation
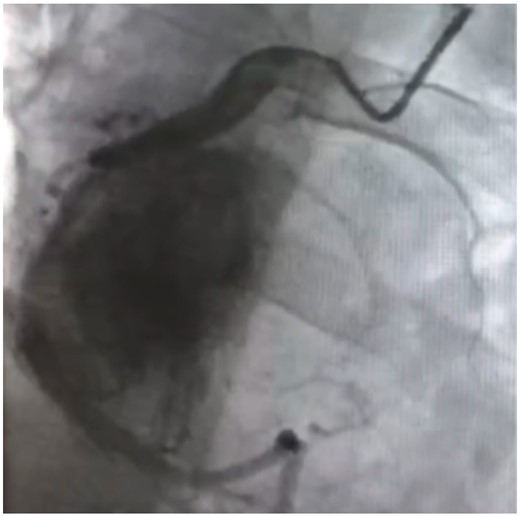
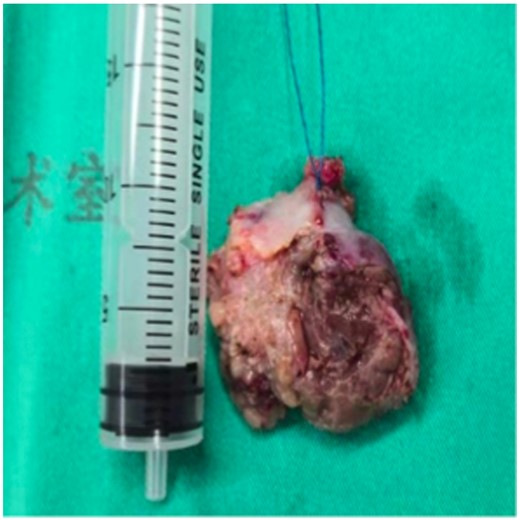
A 21-year-old male patient was admitted to the local hospital with a blood pressure gradually increasing from 134/92 mmHg to 162/112 mmHg over a year, without any medications. Symptoms such as perspiration, headache, and face pale were negative, except for persistent hyperhidrosis occurring near 5 years. Notably, a mass was observed in the right heart (possibly in the pericardial space) via echocardiography, with an anteroposterior diameter of the left atrium (LA) of 33 mm, a left ventricular end-diastolic anteroposterior diameter (LV) of 52 mm, and a left ventricular ejection fraction (LVEF) of 65%. None abnormality was detected via abdominal ultrasound and double lower limb arteriovenous ultrasound. Cardiac computed tomography (CT) revealed an abnormal density shadow with rich blood supply in the right atrioventricular sulcus, and a right coronary aneurysm was suspected. He was given betaxolol 25 mg b.i.d. (from 4 to 2 weeks prior to surgery), nifedipine sustained-release tablets 30 mg qd (from 4 to 3 weeks prior to surgery), and cefuroxime 0.25 g b.i.d. (for prevention of incision infection). Then, a minimally invasive thoracotomy was performed in the local hospital, for resecting the cardiac mass. However, positron emission tomography (PET) CT presented an abnormal glucose concentration in the mass, indicating a malignant lesion. Given the malignant potential, life-threatening location, and abundant blood supply, need for heart transplantation was thus suspected. He was then transferred to our hospital for further diagnosis and treatment. Upon physical examination, he presented with a blood pressure fluctuating within 130–140/80–90 mmHg, body mass index (BMI) of 20.34 kg/m2, and heart rate of 100–110 beats per minute. The blood pressure of lower extremity exceeds the upper extremity. All pulses were apparent without vascular murmurs. After admission, the levels of blood norepinephrine, dopamine, methoxy norepinephrine, and urinary norepinephrine were evaluated (Table 1). The cardiac troponin I was 0.000 ng/mL, myoglobin was 9.560 ng/mL, and creatine kinase MB was 0.595 ng/mL. Echocardiography presented LA 33 mm, LV 53 mm, LVEF 65%, and a solid mass in the pericardial space (39 mm × 35 mm × 39 mm). Computed tomography scanning of double kidney, adrenal gland, and renal artery was normal. Somatostatin receptor imaging revealed abnormalities in the right atrioventricular sulcus, indicating a cardiac paraganglioma (Figure 1), in concordant with iodine-131 metaiodobenzylguanidine scintigraphy (Figure 2). Coronary angiography revealed a blood supply of the mass from the right coronary artery (Figure 3, Supplementary material online, Video S1). During the hospitalization (3 weeks prior to surgery), the patient complained of severe substernal pain related to breath, which aggravated in the supine position, slightly relieved after sitting up, and relieved after injecting morphine 3 mg. Oral nifedipine sustained-release tablets were withdrawn and symptom was recovered with terazosin 2 mg once per day. His blood pressure was 110–140/80–90 mmHg and the heart rate was 100–110 beats per minute. For surgery preparation, a high-sodium diet was started 2 weeks before the operation, terazosin was gradually added from 2 mg b.i.d. (from 2 to 1 week prior to surgery) to 2 mg t.i.d. (from 1 week prior to surgery), and betaxolol was added to 25 mg t.i.d. (from 2 weeks prior to surgery). His blood pressure was then controlled to 100–121/56–77 mmHg and the heart rate was 80–100 beats per minute. Hydroxyethyl starch 500 mL qd and 0.9% saline 500 mL qd were also administered intravenously for 3 days before the operation. Then the patient was evaluated with recovered blood volume, and surgical resection of cardiac tumour was performed to completely remove the dark red, ductile tumour (3 cm × 4 cm) (Figure 4). The pathological diagnosis was confirmed as cardiac paraganglioma. Immunohistochemical results presented CgA (+), S100 (+), NSE (+), CK (−), EMA (−), CD34 (−), calretinin (+), and CD68 (−). The genetic test showed SDHB gene mutation, nucleotide mutation c.725G>A, and amino acid mutation p. Arg242His. At the 5-month follow-up, his blood pressure was recovered to 110/70 mmHg under atenolol tablets 6.25 mg t.i.d., and the heart rate was 80 beats per minute. The assay of blood catecholamines and catecholamine metabolites was negative. The informed written consent was obtained from the patient.
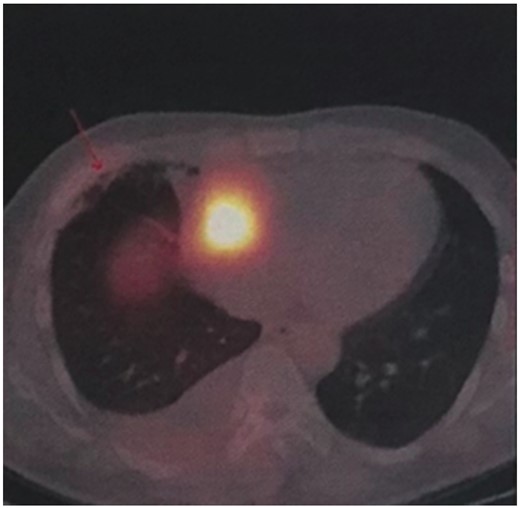
Somatostatin receptor imaging reveals abnormalities in the right atrioventricular sulcus.
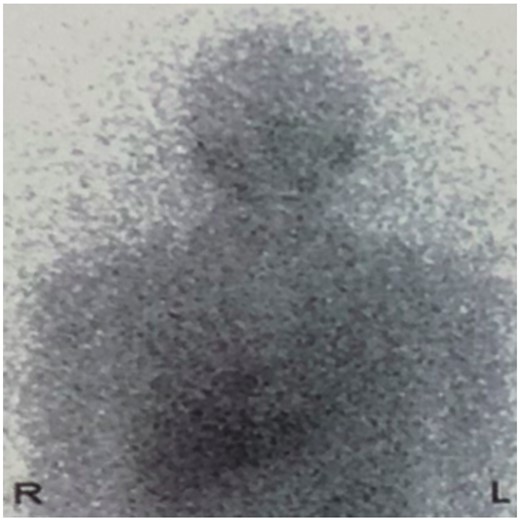
Iodine-131 metaiodobenzylguanidine shows radiotracer accumulation in the right upper chest.
| . | Before operation . | After operation . | Normal range . |
|---|---|---|---|
| WBC (109/L) | 9.33 | — | — |
| Haemoglobin (g/L) | 151 | — | — |
| PLT (109/L) | 423 | — | — |
| K+ (mmol/L) | 3.70 | — | — |
| Scr (µmol/L) | 74 | — | — |
| LDL-C (mmol/L) | 2.86 | — | — |
| cTnI (ng/mL) | 0.00 | — | — |
| Blood norepinephrine | 6.635 | <0.078 | <0.548 ng/mL |
| Blood dopamine | 0.331 | <0.020 | <0.08 ng/mL |
| Blood methoxy norepinephrine | 0.913 | 0.127 | 0.01–0.168 ng/mL |
| Urinary norepinephrine | 2857 | 250 | 28–615 µg/24 h |
| . | Before operation . | After operation . | Normal range . |
|---|---|---|---|
| WBC (109/L) | 9.33 | — | — |
| Haemoglobin (g/L) | 151 | — | — |
| PLT (109/L) | 423 | — | — |
| K+ (mmol/L) | 3.70 | — | — |
| Scr (µmol/L) | 74 | — | — |
| LDL-C (mmol/L) | 2.86 | — | — |
| cTnI (ng/mL) | 0.00 | — | — |
| Blood norepinephrine | 6.635 | <0.078 | <0.548 ng/mL |
| Blood dopamine | 0.331 | <0.020 | <0.08 ng/mL |
| Blood methoxy norepinephrine | 0.913 | 0.127 | 0.01–0.168 ng/mL |
| Urinary norepinephrine | 2857 | 250 | 28–615 µg/24 h |
cTNI, cardiac troponin I; LDL-C, low-density lipoprotein cholesterol; PLT, blood platelet; Scr, serum creatinine; WBC, white blood cell.
| . | Before operation . | After operation . | Normal range . |
|---|---|---|---|
| WBC (109/L) | 9.33 | — | — |
| Haemoglobin (g/L) | 151 | — | — |
| PLT (109/L) | 423 | — | — |
| K+ (mmol/L) | 3.70 | — | — |
| Scr (µmol/L) | 74 | — | — |
| LDL-C (mmol/L) | 2.86 | — | — |
| cTnI (ng/mL) | 0.00 | — | — |
| Blood norepinephrine | 6.635 | <0.078 | <0.548 ng/mL |
| Blood dopamine | 0.331 | <0.020 | <0.08 ng/mL |
| Blood methoxy norepinephrine | 0.913 | 0.127 | 0.01–0.168 ng/mL |
| Urinary norepinephrine | 2857 | 250 | 28–615 µg/24 h |
| . | Before operation . | After operation . | Normal range . |
|---|---|---|---|
| WBC (109/L) | 9.33 | — | — |
| Haemoglobin (g/L) | 151 | — | — |
| PLT (109/L) | 423 | — | — |
| K+ (mmol/L) | 3.70 | — | — |
| Scr (µmol/L) | 74 | — | — |
| LDL-C (mmol/L) | 2.86 | — | — |
| cTnI (ng/mL) | 0.00 | — | — |
| Blood norepinephrine | 6.635 | <0.078 | <0.548 ng/mL |
| Blood dopamine | 0.331 | <0.020 | <0.08 ng/mL |
| Blood methoxy norepinephrine | 0.913 | 0.127 | 0.01–0.168 ng/mL |
| Urinary norepinephrine | 2857 | 250 | 28–615 µg/24 h |
cTNI, cardiac troponin I; LDL-C, low-density lipoprotein cholesterol; PLT, blood platelet; Scr, serum creatinine; WBC, white blood cell.
Discussion
Among the typical ‘4P’ symptoms of PPGLs, only hyperhidrosis occurred in this case, with a hypermetabolic state of decreased BMI. Patients with hypertension accompanied by cardiac mass, even if atypical, should be considered with a potential of paraganglioma and indicate relevant neuroendocrine tests. In addition, SDHB gene mutation was identified. Previous studies have established 14 different genes susceptible in PPGLs, with SDHB mutations mainly in extra-adrenal tumours.5 Of note, 30% of patients with metastatic PPGL appear with pathogenic SDHB mutations.6 Malignant tumours, identified as the presence of metastases in non-chromaffin tissue, are with a prevalence of 10–17% in overall PPGLs.7 Remarkably, Pasini and Stratakis8 documented a prevalence of 36% of SDHB mutations in malignant PPGLs. A high risk of developing malignancy is thus implied in PPGL patients with SDHB gene mutations.5 Indeed, patients with metastatic disease should be tested for potential SDHB gene mutations.
In the local hospital, due to the limited understandings for this disease, the scarcity, and life-threatening location for the mass with abundant blood supply observed during the operation, the clinicians did not take a biopsy or remove the tumour, but efficiently transferred the patient into our hospital for future diagnosis and treatment. Also, given the malignant potential of tumour, the local clinician suspected a potential for tumour tissue invasion on cardiac walls and then promoted a potential solution for cardiac transplantation. Yet, it is lacking of sufficient evidence and not regularly recommended. In this case, chest pain occurred and a plausible explanation may be that a large amount of catecholamine was released and then caused coronary spasm and myocardial ischaemia. In addition, small focal necrosis might occur in the cardiac paraganglioma, which could cause chemical stimulation. Notably, majority of PPGLs secrete excessive amounts of catecholamines. If left untreated or misdiagnosed, the morbidity and mortality of PPGL patients due to cardiovascular diseases can be extremely high.9,10 Moreover, paraganglioma might gradually grow and invade adjacent tissues and organs, which requires for timely surgical resection. Pre-operative medical treatment should be performed 7–14 days before surgery to normalize blood pressure and heart rate. Coadministration of β-adrenergic receptor blockers is indicated for pre-operative heart rate control after administration of α-adrenergic receptor blockers. Previous studies recommended a target blood pressure of less than 130/80 mmHg while seated, and a target heart rate of 60–70 beats per minute.11 However, these targets should be adjusted according to age and accompanying diseases.11 After β-adrenergic receptor blockade starting a few days, a high-sodium diet and fluid intake therapy should be started to reverse catecholamine-induced blood volume contraction preoperatively and thus to reduce severe hypotension risk after tumour removal.12 We also suggest to monitor blood pressure and heart rate accompanied with adjustment of associated therapy in post-operative period. Also, blood or urinary catecholamines and their metabolites are recommended to be regularly followed up after operation for possible recurrent or metastatic diseases.
Lead author biography
Huimin Zhang, MD, PhD, Professor, Chief Physician, Master Tutor. Assistant Director of the Hypertension Center of Fuwai Hospital. Vice Chairman of the Hypertension Integrated Care Organization of National Center for Cardiovascular Diseases. Director of the China Hypertension Alliance. Member of the Hypertension Committee of the Chinese Medical Doctor Association. She is good at diagnosis and treatment of various secondary hypertension, refractory hypertension and peripheral vascular diseases. She is invited to write the chapter of arteritis in the book ‘The Heart in Rheumatologic, Inflammatory and Autoimmune Diseases: Pathophysiology, Clinical Aspects and Therapeutic Approaches’ by Elsevier Press.
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Acknowledgements
We appreciate the multiple contributions from Jun Cai, Haiying Wu, Wenjun Ma, Xianliang Zhou, Lei Song, Yan Zhao, Jingjing Xu, Jin Bian, Luyun Fan from Departement of Hypertension, Yuan Li, Haining Sun, Tao Yang from Department of Cardiovascular Surgery, Fuwai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The authors confirm that written consent for submission and publication of this case report including images and associated text has been obtained from the patient in line with COPE guidance.
Conflict of interest: None declared.
Funding: None declared.
References
Author notes
Wu Chao and Yang Xu contriubted equally to this case report.





Comments