-
PDF
- Split View
-
Views
-
Cite
Cite
Wikler Bernal Torres, Leidy A Giraldo Vinasco, Juan Esteban Gómez, Juan D López Ponce de León, Cardiac paraganglioma: implications and impacts of a rare disease—a case report , European Heart Journal - Case Reports, Volume 8, Issue 3, March 2024, ytae032, https://doi.org/10.1093/ehjcr/ytae032
Close - Share Icon Share
Abstract
Tumour-producing catecholamines arise in the adrenal medulla (pheochromocytomas), as well as in extra-adrenal chromaffin cells (paragangliomas). The origin can be from any location; however, it is very rare in the heart.
A 43-year-old woman with a history of arterial hypertension presented with dyspnoea on moderate exertion, New York Class Association (NYHA) functional classes III and IV, and oedema in the lower extremities. Medical and laboratory evaluation revealed an NT-proBNP of 6046 pg/mL, a left ventricular ejection fraction (LVEF) of 15%, longitudinal strain of −7%, and a mass located on the inner surface of the left atrioventricular groove. Surgical intervention was performed, and the tumour was resected. Pathological report showed an extra-adrenal paraganglioma without neoplastic involvement in the margins of the vena cava. After surgery, the patient showed clinical improvement with NYHA functional class I, LVEF of 56%, and longitudinal strain of −20% on transthoracic echocardiography 4 months after treatment.
Paragangliomas are tumours that are rarely found in the heart, and their diagnosis is difficult. However, early detection and treatment can improve the quality of life of affected patients.
Paragangliomas are rare, with nine cases per million inhabitants, and cardiac involvement occurs in 2% of cases.
The most common manifestations of cardiac paragangliomas are arterial hypertension, palpitations, and the discovery of a cardiac mass on the echocardiogram.
Atrial tachycardia and heart failure can be uncommon clinical manifestations.
Timely detection allows for improved quality of life and can have an impact on cardiac function.
Introduction
Catecholamine-producing tumours, such as pheochromocytomas (adrenal medulla) and paragangliomas (extra-adrenal chromaffin cells), occur in various locations, and cardiac involvement is usually not frequent,1 with a prevalence of 0.1% in hypertensive individuals and 4% in adrenal masses. About 10% of the cases are catecholamine producing.2 The increased epinephrine and norepinephrine levels can lead to symptoms like headaches, palpitations, and excessive sweating, requiring indefinite monitoring.3 This article describes the case of a woman with a cardiac tumour, heart failure (HF), and reduced left ventricular ejection fraction (LVEF).
Summary figure
Case summary
A 43-year-old Hispanic, single woman with a history of arterial hypertension of unspecified duration experiences symptoms for 15 days, including dyspnoea, orthopnoea, New York Class Association (NYHA) functional classes III and IV, and oedema of the lower extremities. She was taking 10 mg of amlodipine orally per day and 25 mg/day of hydrochlorothiazide. She does not use toxic substances and has completed her vaccination against SARS-CoV-2. On physical examination, she weighed 46 kg, had tachycardia (132 b.p.m.), grade I oedema in the lower extremities, and no jugular distension, S3, or cardiac murmur. The electrocardiogram showed atrial tachycardia (Figure 1), and the chest X-ray revealed signs of interstitial oedema. NT-proBNP was 6046 pg/mL (range reference < 125 pg/mL), and the high-sensitivity troponin I was within normal range (13.3 ng/L, range reference 0–20.3 ng/L); electrolytes and haemoglobin were normal, and the transthoracic echocardiography (TTE) showed an LVEF of 19%, longitudinal strain of −7%, and mild mitral insufficiency, without other valvulopathies, with suggestive images of a thrombus inside the left atrium. The right ventricle had a normal-sized cavity with normal wall thickness and normal contractility.
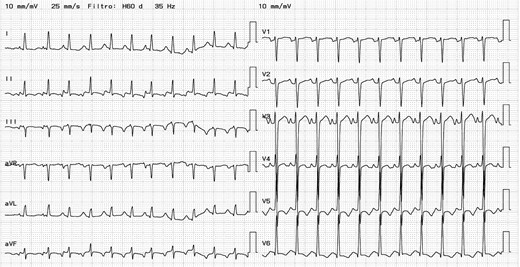
Electrocardiogram. Heart rate 145 b.p.m. QRS duration 90 ms. RP>PR. Atrial tachycardia.
Subsequently, we performed a transoesophageal echocardiogram to characterize the lesion better. It revealed an LVEF of 15% and a probable mass located in the right atrium, without clarifying whether it was extra- or intracardiac (Figure 2; Supplementary material online, Videos S1 and S2). The cardiac magnetic resonance imaging (CMRI) reported changes consistent with dilated cardiomyopathy with an LVEF of 21%, showing diffuse interstitial fibrosis. There was a mass centred on the inner surface of the left atrioventricular groove, with a possible myxoma in the coronary sinus (heterogeneous delayed enhancement; Figure 3). We initiated furosemide (10 mg intravenous every 8 h) to treat the congestive state. The cardiac medical team decided to perform a surgical intervention on the lesion, and the patient was informed and aware of the risks of the proposed surgical procedure. Before the surgery, the team performed a computed tomography (CT) scan of the heart. In the venous phase, it revealed a solid mass located on the inferior aspect of the left atrioventricular groove, with a high degree of vascularity through the right coronary artery, suggesting the presence of a paraganglioma (Figure 4).
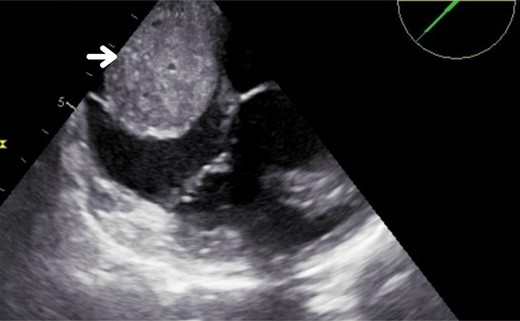
Transoesophageal echocardiogram. The arrow indicates the 50 × 40 mm image in the right atrium.
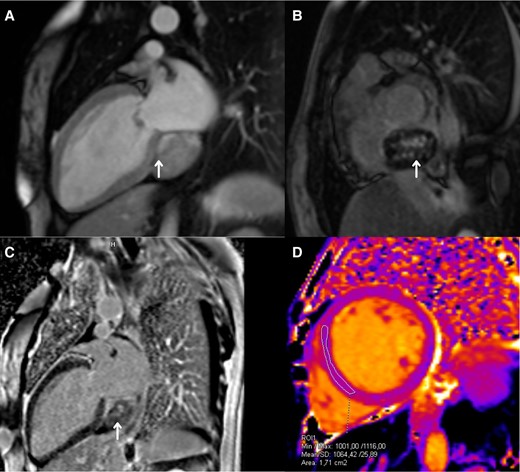
Cardiac magnetic resonance imaging. (A) Two-chamber long-axis view. T1 contrast enhanced with early post-gadolinium enhancement (arrow). (B) Short-axis view. T1 contrast enhanced, a late enhancement over the atrioventricular groove, with areas of intense enhancement in the central portion of the lesion (arrow). (C) Two-chamber long-axis view. T1 contrast enhanced, a late enhancement over the atrioventricular groove, with areas of intense enhancement in the central portion of the lesion (arrow). (D) Native (simple) T1 map in the mid-ventricular short-axis view, showing mild alterations.
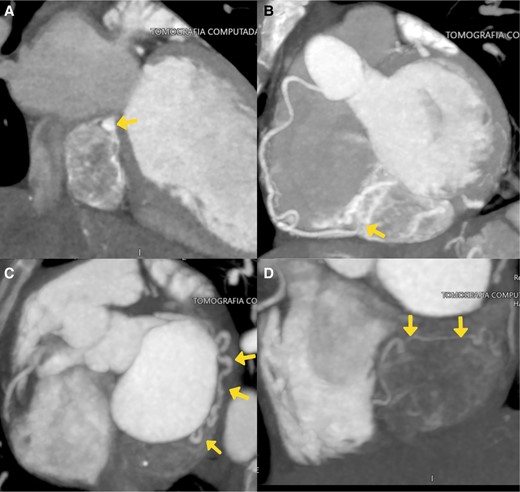
Axial computed tomography of the heart. (A) Two-chamber long-axis view in venous phase. The coronary sinus (arrow) is compressed cephalically by the mass located in the atrioventricular groove, below the inferior wall of the left atrium. Progressive enhancement with intratumoural vessels indicative of its high vascularity. (B) Maximum intensity projection of the contrast. Intratumoural vascularization from the right coronary artery (arrow). (C and D) Maximum intensity projection of the contrast, where the main nutrient vessel originating from the circumflex artery is delineated (arrows), along with another vessel originating from the posterolateral branch of the right coronary artery. There is a prominent arterial vessel located within the lesion (D, arrows).
Additional studies, such as abdominal and thoracic tomography, ruled out the presence of metastasis. The coronary arteriography ruled out coronary artery disease, and it identified a fistula between the circumflex artery and the posterior lateral branch of the right coronary artery, which supplies blood to the tumour in the atrioventricular groove. During the surgery, we spotted a highly vascularized, semisolid mass measuring 5 × 6 cm. It was in the posterior region of the heart, in contact with the inferior vena cava, the atrioventricular groove, and the coronary sinus. We performed a reconstruction of the interatrial septum, atrioseptoplasty with pericardial patch, reconstruction of the inferior vena cava, and coronary sinus with pericardial patch. While we were manipulating the mass, the patient experienced a hypertensive crisis; therefore, we administered alpha- and beta-blockers. Serum and urinary metanephrine levels were within normal ranges. The pathological report confirmed the diagnosis of an extra-adrenal paraganglioma (Figure 5).
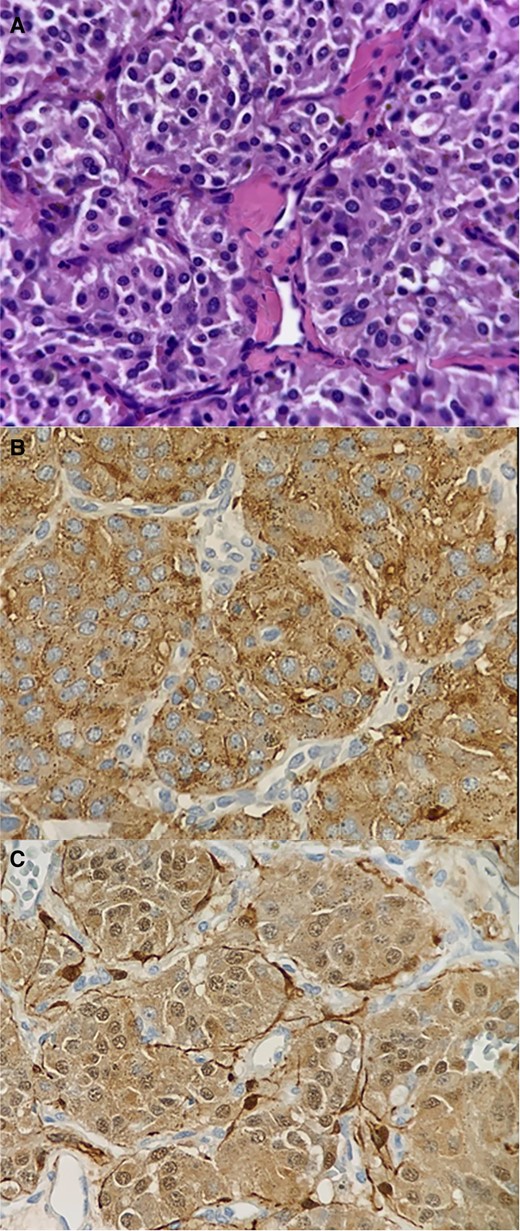
Histology (A) and immunohistochemistry (B and C). (A) Neoplastic lesion composed of intermediate-sized oval or ovoid cells with nuclei showing fine granular chromatin, exhibiting mild cytological atypia, and possessing pink cytoplasm. These cells form an ‘organoid’ pattern within thin fibrous septa (zellballen pattern). Dilated small blood vessels are observed. No areas of necrosis are present. (B and C) The neoplastic cells show positivity for CD56, synaptophysin, chromogranin, and S-100, while being negative for CKAE1-AE3, PAX-8, and PAGF. The vascular marker CD31 highlights the presence of small capillary vessels. The proliferation index KI-67 is <2%. The histological and immunophenotypic profile is consistent with a non-malignant neuroendocrine neoplasia, resembling a paraganglioma.
The patient was transferred to the intensive care unit for postoperative management. Subsequently, we initiated treatment with metoprolol succinate 25 mg per day and angiotensin receptor–neprilysin inhibitor (ARNI) 24/26 mg twice per day. However, due to the hypotension that persisted despite the suspension of ARNI, we decided not to continue with this medication. Based on the above, we decided to initiate management with ivabradine 5 mg twice a day to control heart rate better. In addition, we initiated treatment with furosemide 40 mg per day, spironolactone 25 mg per day, and empagliflozin 10 mg per day. Ten days later, we conducted a TTE, which revealed an LVEF of 35%. The patient was discharged with the previously described treatment. After 4 months, the patient is in NYHA functional class I. Additionally, the follow-up TTE reports an LVEF of 56% and a longitudinal strain of −20% (see Supplementary material online, Videos S1 and S2). We did not discontinue the medication during the follow-up period.
Discussion
Paragangliomas are uncommon tumours that originate from chromaffin cells of the neural crest.4 Their incidence is estimated to be between 1.5 and 9 cases per million people, and ∼2% of these cases involve cardiac paragangliomas.4 The main location is in the posterior mediastinum, adjacent to the left atrium.5,6 They affect adults between the ages of 30 and 60, and they are most common in men.5 In this case, both the gender and the location differ, as the tumour was found on the inferior aspect of the left atrioventricular groove, compressing the coronary sinus.
Most paragangliomas are asymptomatic until the tumour reaches a size significant enough to cause obstructive effects. However, hormonally active tumours show symptoms such as high blood pressure, tachycardia, and anxiety.7 The observed atrial tachycardia could be a manifestation of cardiac tumours; tumour invasion can disrupt the conduction system and account for such an alteration. Nonetheless, arrhythmias are not specific to cardiac paragangliomas, and this manifestation is not described in the literature.6
In this case, it manifested as HF with reduced LVEF and left cavity dilation. Typically, we perform a TTE as part of the diagnostic workup for HF, but in this case, the tumour was incidentally detected. In addition, LVEF assessment and global longitudinal strain are important to identify significant risks and changes during treatment.8
The pathophysiology of HF in the context of paragangliomas could manifest in various ways. When dilated cardiomyopathy is induced due to exposure to catecholamines, clinical symptoms like congestive HF, pulmonary oedema, or cardiogenic shock may often appear. However, when paragangliomas are functionally inactive or produce low levels of catecholamines, symptoms may show due to the compression of blood vessels and flow obstruction. The obstruction of the venous sinus can elevate transcapillary pressure, subsequently increasing perfusion pressure in the myocardium, even when having healthy coronary arteries. This obstruction can lead to vessel congestion, affecting coronary blood flow and resulting in cardiac dysfunction and the progression of HF. This is similar to what we observed in this case, where the mass compression on the venous sinus influenced venous drainage, contributing as both a mechanical and haemodynamic factor to the symptoms and the development of HF. It is important to note that these manifestations can be transient if detected promptly.7,8
It is important to measure metanephrine levels for diagnostic guidance, which have a sensitivity and specificity of 99 and 89% (plasma) and 97 and 93% (urine).6,7 In this case, the results came back normal. However, this does not exclude a secretory tumour, as there can be minimal releases of catecholamines that are difficult to detect and require measurement during episodes. Computed tomography and CMRI are valuable tools for identifying the location and relationship with adjacent structures4 and for surgical planning.6 In this case, we observed a mass that initially suggested a myxoma in the coronary sinus. When analysing the results from the echocardiogram, CMRI, and CT, the most common feature we observed was the localization in the atrioventricular groove. Nevertheless, it could be found in the left or right atrium.5 It is noteworthy to highlight the findings in T1, which revealed delayed enhancement in the atrioventricular groove and areas of intense enhancement in the central part of the tumour lesion (Figure 3). Furthermore, we observed a marked vascularization in the tomography (Figure 4). The vascularization and blood supply to the tumour originated from the coronary arteries, which we confirmed through the coronary arteriography. All these characteristics strongly indicate a cardiac paraganglioma diagnosis, and we believe that the use of multimodality imaging played a key role in improving the diagnostic accuracy prior to surgery. The definitive confirmation requires histopathological examination with staining for chromogranin, synaptophysin, and S-100.1
Regarding medical management, there is no specific approach, and it involves controlling blood pressure through α- and β-adrenergic blockade, along with the administration of diuretics in cases of volume overload.7
Regarding surgical management, the high vascularity makes biopsy challenging due to the risk of bleeding. The presence of the fistula from the circumflex and right coronary arteries that supplied the tumour ruled out alternatives other than surgery. The definitive treatment is the complete resection of the tumour aiming to reduce the risk of local invasion and metastasis, which has been described in up to 10% of cases.9 In some situations, cardiac transplantation may be necessary, especially for difficult resect paragangliomas, particularly when having myocardial invasion.6 The recurrence rate can be as high as 25% in long-term follow-up, and currently, there are no specific signs of recurrence described nor a structured follow-up plan. However, we recommend performing regular interval imaging to assess the tumour’s evolution.9
In this case, the predominant symptoms are associated with arrhythmia, HF, and the incidental finding of a cardiac tumour. Following the management, the patient experienced recovery; therefore, it is of vital importance a complete surgical eradication. At the 4-month follow-up, the patient had a NYHA functional class I and an LVEF of 56%. Currently, there is no specific evidence regarding LVEF post-improvement treatment. However, based on the guidelines,10 we recommend maintaining the treatment. Therefore, we are continuing with the proposed plan.
Cardiac paragangliomas are rare and difficult to diagnose; however, early detection and appropriate treatment can improve symptoms and quality of life.
Lead author biography

Wikler Bernal Torres is currently a cardiology fellow at Universidad ICESI and Fundación Valle del Lili in Cali. He is currently working in the Department of Cardiology.
Supplementary material
Supplementary material is available at European Heart Journal – Case Reports online.
Acknowledgements
Fundación Valle del Lili, Universidad ICESI, Cali-Colombia, Mauricio M. González, Ana María Arrunategui, Paula A. Vesga Reyes, Eduardo A. Cadavid Alvear, and Álvaro D. Peña González.
Consent: The authors confirm that written consent for submission and publication of this case report, including image(s) and associated text, has been obtained from the patient in line with COPE guidance.
Funding: None declared.
Data availability
The data from this case study are available on request from the corresponding author.
References
Author notes
Mauricio M. González, Ana María Arrunategui, Carlos Vesga Reyes, Paula A. Vesga Reyes, Eduardo A. Cadavid Alvear, and Álvaro D. Peña González contributed equally to this case report.
Conflict of interest: None declared.





Comments