-
PDF
- Split View
-
Views
-
Cite
Cite
Michelle Feijen, Meindert Palmen, Hildo J Lamb, Eleonora P M Corssmit, Maria Louisa Antoni, A case report of an intracardiac paraganglioma attached to the left main coronary artery in a patient with a succinate dehydrogenase complex subunit D mutation, European Heart Journal - Case Reports, Volume 7, Issue 10, October 2023, ytad418, https://doi.org/10.1093/ehjcr/ytad418
Close - Share Icon Share
Abstract
Cardiac paragangliomas are extremely rare neuroendocrine tumours derived from neural crest cells that represent <2% of all paragangliomas. Approximately 35–40% of all paragangliomas are associated with inherited syndromes such as mutation in the succinate dehydrogenase (SDH) enzyme.
A 44-year-old male with an SDH complex subunit D (SDHD) mutation was diagnosed with an intracardiac paraganglioma attached to the left main coronary artery. Multimodality imaging, including gallium dotatate positron emission tomography computed tomography, cardiac magnetic resonance imaging, and coronary computed tomography angiography (CCTA) confirmed the suspected intracardiac paraganglioma. During follow-up with a CCTA, the mass showed growth, and surgical removal was recommended to anticipate on the risk of compression of the left main coronary artery. Prior to surgery, coronary angiography was performed, which showed no coronary calcifications. The highly vascularized paraganglioma was visible near the left main and proximal left anterior descending artery. The intracardiac paraganglioma was successfully removed through a median sternotomy with cardiopulmonary bypass, without any complications. The post-operative course was uneventful, and histological examination confirmed the diagnosis of a paraganglioma.
Intracardiac paragangliomas in the vicinity of the left main coronary artery are rare, and surgical removal may be challenging. Therefore, screening and the use of multiple imaging modalities in patients with SDHD mutations prior to surgery is of major importance.
The yearly estimated incidence paragangliomas is 2–10 cases per 1 000 000. Approximately 25% of patients have a succinate dehydrogenase complex mutation.
Screening for intracardiac paragangliomas in patients with succinate dehydrogenase complex subunit D mutation is important, since these tumours remain asymptomatic until they compress a cardiac structure such as a coronary artery.
A ‘wait and scan’ policy to determine tumour growth is essential for adequate timing of surgical intervention.
To identify surgical challenges and surgical risks, multimodality imaging is imperative.
Introduction
Pheochromocytomas and paragangliomas (together called PPGL) are rare neuroendocrine tumours derived from neural crest cells.1 During embryonic development, these cells migrate alongside major vessels, cranial nerves, autonomic nerves, and ganglia. In adulthood, paragangliomas can arise in chromaffin cells of the adrenal medulla (known as pheochromocytoma), or in paraganglionic tissue of sympathetic paravertebral thoracic, abdominal, or pelvic ganglia. Generally, pheochromocytomas and sympathetic paragangliomas produce catecholamines. The optimal biochemical test to diagnose pheochromocytoma is measuring the plasma-free metanephrines.2 On the other hand, paraganglioma originating in parasympathetic paraganglionic tissue in the head and neck region (HNPGLs), in the carotid body, along the vagal nerve and in the jugular foramen, typically do not produce catecholamines.1,3,4 However, some patients with HNPGLs have biochemically active tumours, often characterized by the excretion of 3-methoxythyramine (3-MT), the 3-0-methylated metabolite of dopamine.2
The clinical presentation of PPGL with catecholamine secretion manifests in symptoms such as hypertension, sweating, episodic headache, and palpitations. Conversely, patients with non-secreting tumours can remain asymptomatic or present with symptoms related to mass effect. Since ∼35–40% of all paragangliomas are associated with inherited syndromes, genetic counselling and screening is recommended for all patients with PPGL, as well as their first-degree relatives. Of interest, germline mutations in the succinate dehydrogenase complex (SDHx) genes are responsible for ∼25% of PPGL.5 The reported incidence of paragangliomas has been reported to be 2–10 cases per million per year, with a prevalence rate ranging from 1:2500 to 1:6500.1,3 Notably, only 2% of all PPGLs are cardiac.
In this case report, we discuss the rare case of a 44-year-old male with a succinate dehydrogenase complex subunit D (SDHD) mutation predisposing him to familiar paragangliomas. Specifically, he developed an intracardiac paraganglioma attached to the left main coronary artery.
Summary figure
| November 2009 | Genetic counselling revealed a succinate dehydrogenase complex subunit D mutation (c.416T>C p.leu139pro) No catecholamine excess |
| April 2021 | Screening with thoracic and abdominal magnetic resonance imaging (MRI) revealed a nodule of 9 mm in diameter in the left lateral mediastinum |
| June 2021 | Gallium dotatate positron emission tomography–computed tomography (68Ga-octreotide) showed an active mass caudally from the the pulmonary trunk, 23 × 11 mm, suggestive of a paraganglioma |
| January 2022 | Transthoracic echocardiography: normal right and left ventricular function, no valve abnormalities, no signs of external compression Cardiac MRI: intracardiac lesion just below the left main coronary artery and left anterior descending (LAD) of 16 × 16 × 12 mm, no fat, no late gadolinium enhancement. These results suggested a cardiac paraganglioma ‘wait and scan’ strategy |
| February 2022 | Coronary computed tomography angiography: suspected paraganglioma ventrocaudal from the left main coronary artery and proximal LAD (18 × 14 × 14 mm) |
| September 2022 | Coronary computed tomography angiography: growth of the mass ventrocaudal from the left main coronary artery and proximal LAD (20 × 17 × 12 mm) |
| October 2022 | Coronary angiography: no signs of coronary artery disease, suspected paraganglioma visible around the left main coronary artery and LAD |
| January 2023 | Uncomplicated resection of the paraganglioma. Histological confirmation of the paraganglioma |
| November 2009 | Genetic counselling revealed a succinate dehydrogenase complex subunit D mutation (c.416T>C p.leu139pro) No catecholamine excess |
| April 2021 | Screening with thoracic and abdominal magnetic resonance imaging (MRI) revealed a nodule of 9 mm in diameter in the left lateral mediastinum |
| June 2021 | Gallium dotatate positron emission tomography–computed tomography (68Ga-octreotide) showed an active mass caudally from the the pulmonary trunk, 23 × 11 mm, suggestive of a paraganglioma |
| January 2022 | Transthoracic echocardiography: normal right and left ventricular function, no valve abnormalities, no signs of external compression Cardiac MRI: intracardiac lesion just below the left main coronary artery and left anterior descending (LAD) of 16 × 16 × 12 mm, no fat, no late gadolinium enhancement. These results suggested a cardiac paraganglioma ‘wait and scan’ strategy |
| February 2022 | Coronary computed tomography angiography: suspected paraganglioma ventrocaudal from the left main coronary artery and proximal LAD (18 × 14 × 14 mm) |
| September 2022 | Coronary computed tomography angiography: growth of the mass ventrocaudal from the left main coronary artery and proximal LAD (20 × 17 × 12 mm) |
| October 2022 | Coronary angiography: no signs of coronary artery disease, suspected paraganglioma visible around the left main coronary artery and LAD |
| January 2023 | Uncomplicated resection of the paraganglioma. Histological confirmation of the paraganglioma |
| November 2009 | Genetic counselling revealed a succinate dehydrogenase complex subunit D mutation (c.416T>C p.leu139pro) No catecholamine excess |
| April 2021 | Screening with thoracic and abdominal magnetic resonance imaging (MRI) revealed a nodule of 9 mm in diameter in the left lateral mediastinum |
| June 2021 | Gallium dotatate positron emission tomography–computed tomography (68Ga-octreotide) showed an active mass caudally from the the pulmonary trunk, 23 × 11 mm, suggestive of a paraganglioma |
| January 2022 | Transthoracic echocardiography: normal right and left ventricular function, no valve abnormalities, no signs of external compression Cardiac MRI: intracardiac lesion just below the left main coronary artery and left anterior descending (LAD) of 16 × 16 × 12 mm, no fat, no late gadolinium enhancement. These results suggested a cardiac paraganglioma ‘wait and scan’ strategy |
| February 2022 | Coronary computed tomography angiography: suspected paraganglioma ventrocaudal from the left main coronary artery and proximal LAD (18 × 14 × 14 mm) |
| September 2022 | Coronary computed tomography angiography: growth of the mass ventrocaudal from the left main coronary artery and proximal LAD (20 × 17 × 12 mm) |
| October 2022 | Coronary angiography: no signs of coronary artery disease, suspected paraganglioma visible around the left main coronary artery and LAD |
| January 2023 | Uncomplicated resection of the paraganglioma. Histological confirmation of the paraganglioma |
| November 2009 | Genetic counselling revealed a succinate dehydrogenase complex subunit D mutation (c.416T>C p.leu139pro) No catecholamine excess |
| April 2021 | Screening with thoracic and abdominal magnetic resonance imaging (MRI) revealed a nodule of 9 mm in diameter in the left lateral mediastinum |
| June 2021 | Gallium dotatate positron emission tomography–computed tomography (68Ga-octreotide) showed an active mass caudally from the the pulmonary trunk, 23 × 11 mm, suggestive of a paraganglioma |
| January 2022 | Transthoracic echocardiography: normal right and left ventricular function, no valve abnormalities, no signs of external compression Cardiac MRI: intracardiac lesion just below the left main coronary artery and left anterior descending (LAD) of 16 × 16 × 12 mm, no fat, no late gadolinium enhancement. These results suggested a cardiac paraganglioma ‘wait and scan’ strategy |
| February 2022 | Coronary computed tomography angiography: suspected paraganglioma ventrocaudal from the left main coronary artery and proximal LAD (18 × 14 × 14 mm) |
| September 2022 | Coronary computed tomography angiography: growth of the mass ventrocaudal from the left main coronary artery and proximal LAD (20 × 17 × 12 mm) |
| October 2022 | Coronary angiography: no signs of coronary artery disease, suspected paraganglioma visible around the left main coronary artery and LAD |
| January 2023 | Uncomplicated resection of the paraganglioma. Histological confirmation of the paraganglioma |
Case presentation
A now 44-year-old male was diagnosed with an SDHD mutation in 2009. He was genetically screened because of first-degree relatives diagnosed with paragangliomas based on an SDHD mutation, according to the international consensus on initial screening and follow-up of asymptomatic SDHx mutation carriers.5 At that time, he did not experience any symptoms, he had no relevant medical history, and did not take any medication. A left-sided carotid body tumour was surgically removed at age 31; furthermore, a right-sided vagal body and carotid body tumour were followed up with a ‘wait and scan’ strategy. In 2021, plasma screening revealed normal normetanephrine and metanephrine; however, the plasma 3-MT was increased four times the upper reference limit, suggesting a biochemically active paraganglioma. Thoracic and abdominal magnetic resonance imaging (MRI) identified an extracardiac (high mediastinal) and an intracardiac tumour. Additional 68Ga-octreotide positron emission tomography–computed tomography (PET–CT) was performed, which showed strongly increased gallium dotatate uptake between the carotid and the subclavian artery (high mediastinal PPGL) and caudal from the pulmonary truncus and confirmed the suspected intracardiac paraganglioma (Figure 1A and B).
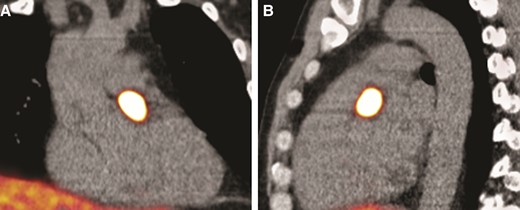
(A) 68Ga-octreotide positron emission tomography–computed tomography coronal plane demonstrates an active mass in the heart suspected of a cardiac paraganglioma, size 23 × 11 mm. (B) 68Ga-octreotide positron emission tomography–computed tomography in the sagittal plane shows the active mass.
The patient was referred to the cardiac outpatient clinic for further analysis. He had no chest pain (at exercise), shortness of breath, or palpitations. A physical examination revealed normal cardiac and pulmonary sounds and the absence of peripheral oedema. The electrocardiogram showed a normal sinus rhythm, and transthoracic echocardiography showed normal biventricular function without valve abnormalities. However, the tumour was not visualized, therefore, and for tumour characterization, a subsequent cardiac MRI was scheduled. The lesion had an intermediate signal on T1-weighted images (Figure 2A and C–E). The tumour showed high signal on T2-weighted acquisition with fat suppression (Figure 2B) and no signal on mDixon fat reconstruction images (Figure 2F–H), indicating the absence of fatty content in the lesion. Perfusion images showed contrast late in the arterial phase (Figure 2I), and the lesion showed synchronous motion with the coronary arteries and aorta in the cine images (Figure 2J). Furthermore, there was no late gadolinium enhancement in the lesion, indicating a lack of fibrotic components (Figure 2K–M). The lesion was highly vascularized and located just below the distal left main coronary artery (Summary figure). Cardiac MRI supported the most likely diagnosis of a cardiac paraganglioma.
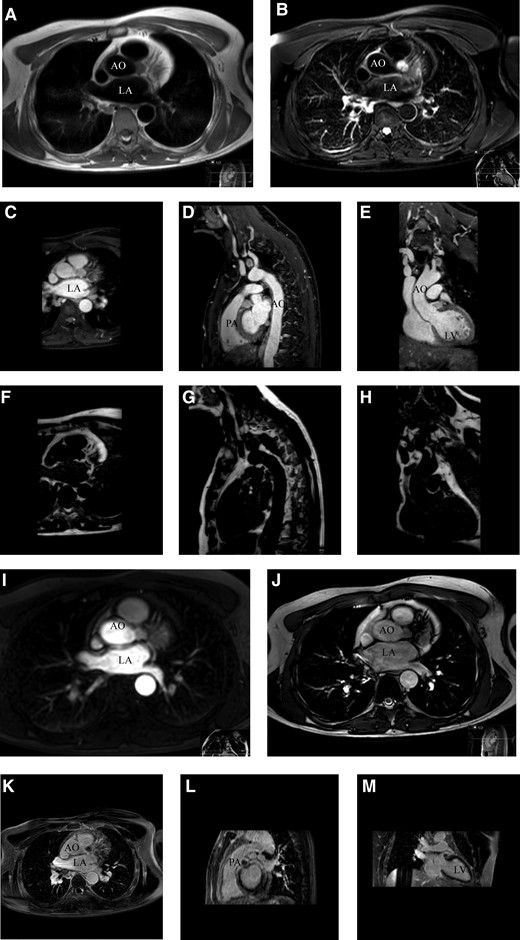
(A) Axial plane black blood turbo spin echo T1-weighted cardiac magnetic resonance imaging, tumour shows an intermediated signal. (B) Axial plane, black blood turbo spin echo T2-weighted spectral attenuated inversion recovery that suppresses fat, tumour shows a high signal, indicating a lack of fat content. (C–E) Axial, sagittal, and coronal plane of mDixon water reconstruction T1-weighted three-dimensional non-contrast-enhanced magnetic resonance angiography with respiratory navigator gating, showing an intermediate tumour signal. (F–H) Axial, sagittal, and coronal plane of mDixon fat reconstruction T1-weighted three-dimensional non-contrast-enhanced magnetic resonance angiography with respiratory navigation gating, indicating the absence of fatty content in the tumour. (I) Axial plane of the perfusion image in which contrast appears in the tumour in the late arterial phase. (J) Axial plane of the cine TRA bTEE and (K–M) axial, sagittal, and coronal plane show the absence of late gadolinium enhancement. The white arrow points to the tumour in all panels. Ao, aorta; bTEE, balanced turbo field echo; LA, left atrium; LV, left ventricle; PA, pulmonary artery; TRA, transverse.
A coronary computed tomography angiography (CCTA) was scheduled to visualize the exact anatomical location and the relation with surrounding structures. Coronary computed tomography angiography demonstrated the paraganglioma with a dimension of 18 × 14 × 14 mm caudal from the left main ostium, transitioning in the proximal left anterior descending (LAD) (Figure 3A–C). The case was presented in a multidisciplinary heart team and discussed with a cardiologist, cardiothoracic surgeon, vascular surgeon, and the endocrinologist. Coronary computed tomography angiography showed no signs of compression of the left main or LAD. The multidisciplinary team decided to remain conservative, since paragangliomas are typically slow-growing and rarely malignant. The preferred and only available curative treatment is en bloc resection, which, in this case, includes cardiac surgery (with cardiopulmonary bypass and the risk of coronary artery bypass grafting). Therefore, the patient was followed up with a ‘wait and scan’ policy. A follow-up CCTA scan was scheduled 6 months later and revealed growth (20 × 17 × 14 mm) (Figure 3C–E).
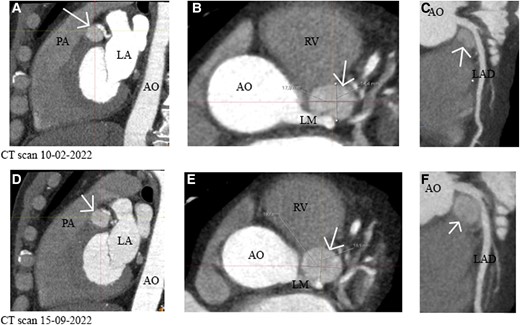
(A–C) (Sagittal, axial, and curved planar reformatted plane) Computed tomography of 10 February 2022 showed an intracardiac lesion caudal from the left main that transitions into the left anterior descending of 18 × 14 × 14 mm without encasement of the left main or left anterior descending. (D–F) (Sagittal, axial, and curved planar reformatted plane) A repeated computed tomography scan shows growth of the suspected paraganglioma. Size 20 × 17 × 14 mm. The white arrow points to the tumour. AO, aorta; LA, left atrium; LAD, left anterior descending; LM, left main; RV, right ventricle; PA, pulmonary artery.
Surgical removal was now recommended to anticipate on risk of compression of the left main coronary artery. Prior to surgery, coronary angiogram showed no coronary calcifications, while the highly vascularized paraganglioma was visible near the left main artery and proximal LAD (Figure 4). Additional preoperative blood tests did not show (nor-)methanephrine excess, and therefore, the patient was not pretreated with alpha-blockers. Furthermore, the vascular surgeon was consulted for the high mediastinal PPGL, but it was decided to remain conservative (absence of growth).
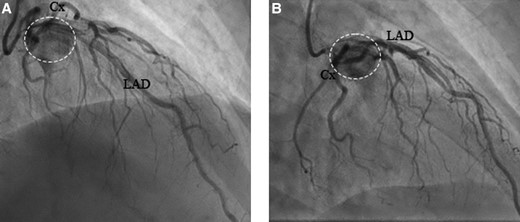
(A) −35/29 RSO and (B) −35/2 RAO, coronary angiography showing the inflow of contrast into the tumour. Cx, circumflex artery; LAD, left anterior descending artery; RAO, right anterior oblique; RSO, superior angulation. The dashed circle points at the tumour.
The paraganglioma was removed through median sternotomy. After the initiation of cardiopulmonary bypass, aortic cross-clamping was applied, and the aorta was circularly transected at the level of the sino-tubular junction. The tumour was carefully dissected from its localization at the medial/pulmonary side of the left main coronary artery and could be removed below the main pulmonary artery without the need for transection of the main pulmonary artery (Figure 5A and B). The source of the blood supply appeared to originate from the left main coronary artery, and after removal of the tumour, a small defect in the left main coronary artery was repaired using a 7-0 polypropylene suture (Figure 5C). Post-operative echocardiogram showed no regional wall motion abnormalities, and electrocardiogram demonstrated no signs of cardiac ischaemia, with post-operative enzyme release being within the normal range. The post-operative course was uneventful, and the patient was discharged at the fourth post-operative period.
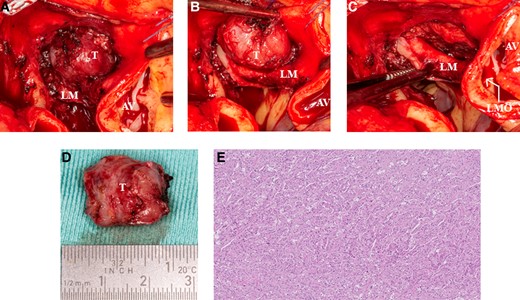
(A) Intraoperative image of the cardiac mass. (B) Intraoperative view of the close relation with the left main artery and the left anterior descending artery. (C) Image of the excised cardiac paraganglioma. (D) Image of the small defect of the left main artery meaning that the left anterior descending artery was the feeding artery. (E) Histopathology of the mass shows a Zellballen pattern. Haematoxylin–eosin stain ×5 original magnification. AV, aortic valve; LF, left main; LMO, left main ostium; T, tumour.
Complete removal of the tumour was confirmed with histological examination. The dimension of the tumour was 20 × 18 × 11 mm (Figure 5D). An examination showed tumour cells in a typical ‘zellballen’ pattern, and the diagnosis of a paraganglioma was confirmed (Figure 5E).
Discussion
Paragangliomas are rare neuroendocrine tumours, with <2% located within the thorax, making cardiac paragangliomas extremely rare.6 Most of these are located in the posterior mediastinum or in the left atrium in the atrio-ventricular groove and around the root of great vessels, and they may restrict the blood flow to the coronary arteries.6–8 Furthermore, <1% of all cardiac tumours are paragangliomas.6 This report describes the rare case of an intracardiac paraganglioma that was attached to the left main artery transitioning into the proximal LAD.
Historically, paragangliomas were thought to be sporadic tumours; however, in the last two decades, it was found that 35–40% are associated with inherited syndromes.1,4 Mutations in one of the four subunits of the SDH (SDHA, -B, -C, or -D) enzyme account for 25% of these familiar paragangliomas.4 Of interest, SDHD mutations, like in our case, are transmitted autosomal-dominant mutations with maternal genomic imprinting. Patients are, therefore, affected only if the inherited defect allele is paternal.8 Because of an autosomal-dominant inheritance pattern, genetic counselling is recommended for all first-degree relatives.5
Patients with an SDHD mutation usually have non-secreting paragangliomas in the head and neck region.8 Nevertheless, these tumours can be widely distributed from the skull to the pelvic floor.5 Patients with non-secreting paragangliomas remain asymptomatic or start showing pain or symptoms related to mass effect at a late stadium. Therefore, these tumours are difficult to diagnose. In case of cardiac paragangliomas, symptoms may arise as the tumour starts to compress the coronary arteries or cardiac tissue. Severe symptoms such as chest pain or shortness of breath can appear. Another critical issue is that these tumours may restrict the coronary artery blood flow.
Current follow-up practice is not guided by robust evidence and differs widely among clinics.5 In 2021, Amar et al. recommended screening adults with a paternal SDHD mutation initially with blood pressure measurement, blood testing (plasma-free metanephrines and 3-MT screening for patients with SDHx mutations), head and neck MRI, abdominal and pelvic MRI, and PET–CT. After a negative screening, yearly screening with blood pressure measurements and blood testing is advised.5 Furthermore, screening with head and neck MRI, and thoracic, abdominal, and pelvic MRI are advised every 2–3 years. Our case highlights the importance of screening in patients with an SDHD mutation, since the cardiac paraganglioma was detected before significant coronary compression and/or symptoms were present.
En bloc surgical resection is the preferred and only curative treatment for patients with paragangliomas. However, resection may be challenging, because anatomical localization and paragangliomas are highly vascularized and lack encapsulation.9 Intracardiac paragangliomas have another challenge since they may restrict the coronary artery blood flow. Moreover, during surgical removal, the risk of bleeding or damaging the coronary arteries is present.6 Thus, adequate surgical planning requires multiple imaging modalities to exactly locate the tumour to analyse its blood supply and to define its intricate relationship with surrounding vital structures. Therefore, in the present case, additional transthoracic echocardiogram, cardiac MRI, CCTA, and coronary angiography were performed.
Conclusion
Intracardiac paragangliomas in the vicinity of the left main coronary artery are rare, and surgical removal may be challenging. Therefore, screening and the use of multiple imaging modalities in patients with SDHD mutations prior to surgery is of major importance.
Lead author biography
 Michelle Feijen is a PhD candidate and a cardiology resident at the Department of Cardiology at the Leiden University Medical Center, The Netherlands. She obtained her Medical degree in 2018 at the Vrije Universiteit Amsterdam and worked as a cardiology resident at the Northwest Clinics in Alkmaar. Her PhD thesis focuses on optimization of heart failure patients with a device.
Michelle Feijen is a PhD candidate and a cardiology resident at the Department of Cardiology at the Leiden University Medical Center, The Netherlands. She obtained her Medical degree in 2018 at the Vrije Universiteit Amsterdam and worked as a cardiology resident at the Northwest Clinics in Alkmaar. Her PhD thesis focuses on optimization of heart failure patients with a device.
Acknowledgements
The authors would like to thank Prof. Dr J. Wouter Jukema and Prof. Dr Jerry Braun for their scientific input, providing their time and expertise making this case report possible. Furthermore, the authors would like to thank Gerrit Kracht for his assistance with the surgical images.
Consent: All procedures performed involving the human participant were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The patient provided written informed consent for publication of this case report, including images and associated text in line with COPE guidance.
Funding: This work was supported by the general funds of the Department of Cardiology of the Leiden University Medical Center, Leiden, The Netherlands. The funders were not involved in study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication. The authors confirm that they have no competing interest.
Data availability
The data underlying this article are available in this article and in its online supplementary material.
References
Author notes
Conflict of interest: None declared.




Comments