-
PDF
- Split View
-
Views
-
Cite
Cite
Isabel Mattig, Sima Canaan-Kühl, Christoph Tillmanns, Fabian Knebel, Progression of electrocardiogram changes in an untreated fabry disease: a case report, European Heart Journal - Case Reports, Volume 5, Issue 2, February 2021, ytab045, https://doi.org/10.1093/ehjcr/ytab045
Close - Share Icon Share
Abstract
Fabry disease (FD) is a rare lysosomal storage disorder with multiorgan manifestation and associated with an increased morbidity and mortality. Fabry cardiomyopathy includes left ventricular ‘hypertrophy’ (LVH), cardiac arrhythmias, and heart failure. We report a case of an untreated FD with characteristic findings in electrocardiogram (ECG) over a follow-up period of 10 years.
A 53-year-old man with FD presented to our outpatient department. He suffered from symptomatic ventricular extrasystoles. Echocardiography detected LVH and reduced global longitudinal strain. Twelve years ago, first examination was conducted due to ventricular arrhythmias. Electrocardiogram showed a short PQ minus P-wave (PendQ) interval and negative T-waves. Over time, the number of leads with negative T-waves increased. Moreover, the echocardiography revealed a thickened left ventricular wall. Without any further examinations at that time, the patient was treated for arterial hypertension with proteinuria. Ten years after first symptoms appeared, FD was diagnosed utilizing cardiac magnetic resonance imaging and genetic tests. Hence, enzyme replacement therapy was initiated.
The ECG is a fast diagnostic method and it may — even without additional organ manifestations — provide preliminary suspicion of FD. In particular, as shown in our case, a short PendQ and QT interval indicate FD. Over time, disease progression can be detected through ECG changes. T-waves correlate with an increasing LVH and a reduction in longitudinal function in echocardiographic examinations. Unexplained LVH must be followed by differential diagnosis. In case of confirmed FD, patients should be treated by multidisciplinary teams in experienced centres.
Learning points
Fabry disease has to be considered in the diagnostic workup of any unexplained case of increased left ventricular wall thickness even if no further organs are affected.
Electrocardiogram may provide the first hint. Typical findings include a short PendQ and QT interval, irregular ST segments and T-waves as well as brady- and tachyarrhythmias.
Detection of myocardial replacement fibrosis by cardiac magnetic resonance imaging is another typical feature. The diagnosis is confirmed by biochemical and genetic testing.
Introduction
Fabry disease (FD) is a rare lysosomal storage disorder. It is associated with an increased morbidity and mortality, which results from minimal or absent α-galactosidase A (GLA) activity due to X-linked mutations in the GLA gene.1–3 Symptoms, caused by sphingolipid storage, comprise acroparesthesia, hypohydrosis, angiokeratomas, and involvement of the cardiac, renal, and cerebrovascular system.1,3 Fabry cardiomyopathy is characterized by: (i) symptoms based on small vessel disease and heart failure, (ii) echocardiographic findings like symmetric, non-obstructive left ventricular ‘hypertrophy’ (LVH), diastolic dysfunction, and valve abnormalities, and (iii) brady- and ventricular arrhythmia.3,4 In addition to the aforementioned arrhythmias, typical electrocardiogram (ECG) changes include short PR intervals without pre-excitation, ST segment disturbances, and negative T-waves.5,6 Cardiac magnetic resonance (CMR) imaging often reveals myocardial replacement fibrosis.3 The Fabry Registry of the United States observed reduced life expectancy for women (−4.6 years) and men (−16.5 years), in most cases due to cardiovascular events.7 Enzyme replacement (ERT) and chaperone therapy are available as causal treatments and new approaches are currently under development.3,8 Since best outcomes are obtained with early treatment, immediate diagnosis is essential.
We report a case of a patient with FD who presented with ventricular arrhythmias and characteristic ECG findings. Both aspects appeared approximately 10 years before FD was diagnosed. To our knowledge, this is the first report since 1999, presenting retrospectively ECG changes in an untreated FD.9
Timeline
| 2008 | First cardiovascular evaluation due to symptomatic ventricular arrhythmia: electrocardiogram (ECG) showed a short PendQ interval and negative T-waves, echocardiography revealed a slightly thickened left ventricular wall. A cardiac magnetic resonance (CMR) has been recommended, but no further examinations for LVH were conducted. |
| 2012 | Follow-up with ECG. |
| 2015 | Repeated cardiovascular examination with ECG, ergometry, and echocardiography. Detection of arterial hypertension and proteinuria. Proteinuria was interpreted as a result of arterial hypertension. |
| June–July 2018 | Follow-up examination at a new cardiology department. CMR revealed T1 mapping with typical findings for FD. Referral to the Charité Fabry Centrum. |
| September 2018 | Biochemical and genetic diagnosis of FD (mutation: c.902G>A, p.(Arg301Gln) hemizygote, exon 6). |
| November 2018 | Multidisciplinary evaluation of multiorgan involvement and start of ERT (Agalsidase Beta). |
| 2019 | CMR showed increased T1 relaxation time and reduced left ventricular mass (-14 g). |
| 2020 | New cardiology outpatient department within Charité Fabry Centrum: Systematic retrospective evaluation of ECG findings. |
| 2008 | First cardiovascular evaluation due to symptomatic ventricular arrhythmia: electrocardiogram (ECG) showed a short PendQ interval and negative T-waves, echocardiography revealed a slightly thickened left ventricular wall. A cardiac magnetic resonance (CMR) has been recommended, but no further examinations for LVH were conducted. |
| 2012 | Follow-up with ECG. |
| 2015 | Repeated cardiovascular examination with ECG, ergometry, and echocardiography. Detection of arterial hypertension and proteinuria. Proteinuria was interpreted as a result of arterial hypertension. |
| June–July 2018 | Follow-up examination at a new cardiology department. CMR revealed T1 mapping with typical findings for FD. Referral to the Charité Fabry Centrum. |
| September 2018 | Biochemical and genetic diagnosis of FD (mutation: c.902G>A, p.(Arg301Gln) hemizygote, exon 6). |
| November 2018 | Multidisciplinary evaluation of multiorgan involvement and start of ERT (Agalsidase Beta). |
| 2019 | CMR showed increased T1 relaxation time and reduced left ventricular mass (-14 g). |
| 2020 | New cardiology outpatient department within Charité Fabry Centrum: Systematic retrospective evaluation of ECG findings. |
| 2008 | First cardiovascular evaluation due to symptomatic ventricular arrhythmia: electrocardiogram (ECG) showed a short PendQ interval and negative T-waves, echocardiography revealed a slightly thickened left ventricular wall. A cardiac magnetic resonance (CMR) has been recommended, but no further examinations for LVH were conducted. |
| 2012 | Follow-up with ECG. |
| 2015 | Repeated cardiovascular examination with ECG, ergometry, and echocardiography. Detection of arterial hypertension and proteinuria. Proteinuria was interpreted as a result of arterial hypertension. |
| June–July 2018 | Follow-up examination at a new cardiology department. CMR revealed T1 mapping with typical findings for FD. Referral to the Charité Fabry Centrum. |
| September 2018 | Biochemical and genetic diagnosis of FD (mutation: c.902G>A, p.(Arg301Gln) hemizygote, exon 6). |
| November 2018 | Multidisciplinary evaluation of multiorgan involvement and start of ERT (Agalsidase Beta). |
| 2019 | CMR showed increased T1 relaxation time and reduced left ventricular mass (-14 g). |
| 2020 | New cardiology outpatient department within Charité Fabry Centrum: Systematic retrospective evaluation of ECG findings. |
| 2008 | First cardiovascular evaluation due to symptomatic ventricular arrhythmia: electrocardiogram (ECG) showed a short PendQ interval and negative T-waves, echocardiography revealed a slightly thickened left ventricular wall. A cardiac magnetic resonance (CMR) has been recommended, but no further examinations for LVH were conducted. |
| 2012 | Follow-up with ECG. |
| 2015 | Repeated cardiovascular examination with ECG, ergometry, and echocardiography. Detection of arterial hypertension and proteinuria. Proteinuria was interpreted as a result of arterial hypertension. |
| June–July 2018 | Follow-up examination at a new cardiology department. CMR revealed T1 mapping with typical findings for FD. Referral to the Charité Fabry Centrum. |
| September 2018 | Biochemical and genetic diagnosis of FD (mutation: c.902G>A, p.(Arg301Gln) hemizygote, exon 6). |
| November 2018 | Multidisciplinary evaluation of multiorgan involvement and start of ERT (Agalsidase Beta). |
| 2019 | CMR showed increased T1 relaxation time and reduced left ventricular mass (-14 g). |
| 2020 | New cardiology outpatient department within Charité Fabry Centrum: Systematic retrospective evaluation of ECG findings. |
Case presentation
A 53-year-old Caucasian man with FD presented to our cardiology department for regular follow-up. He reported symptomatic ventricular extrasystoles with palpitations for over ten years while he has been in good physical shape. Moreover, he complained of pain and cramps in the lower limbs. In the past, he had two operations (retractile testicles, operation of his knee), but denied further comorbidities. The family history for FD was negative. The physical examination revealed an arrhythmic pulse and a mild atrial hypertension. The ECG showed a shortened PendQ interval, i. e. PQ interval minus P-wave, of 40 ms and negative T-waves (Figure 1). The echocardiographic examination presented LVH about 15 mm and a mildly reduced global longitudinal strain (Figure 2, Video 1). The left ventricular ejection fraction was in a normal range and no valvular damage was observed. N-terminal prohormone of brain natriuretic peptide (NT-proBNP) was mildly elevated (160 ng/L, normal range <121 ng/L).
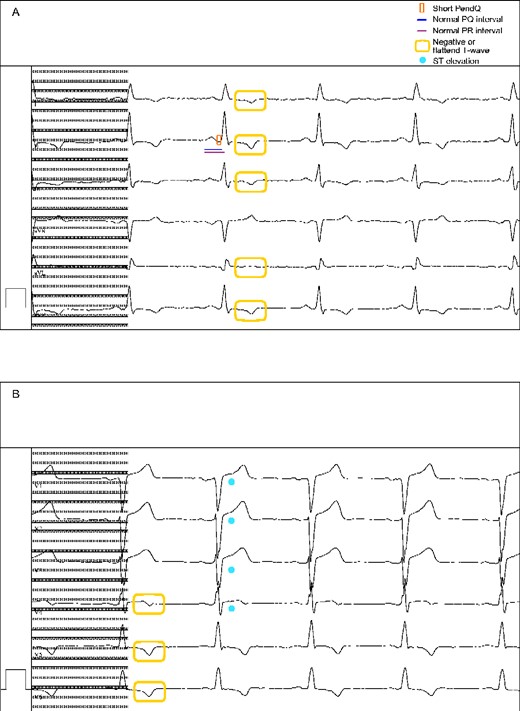
Electrocardiogram (2020) presenting (A) a short PendQ interval (red box), a normal PQ (blue line) and PR interval (purple line), (A, B) negative or flattened T waves (yellow box) as well as (B) ST elevations (light blue point). 50 mm/s, 10 mm/mV.
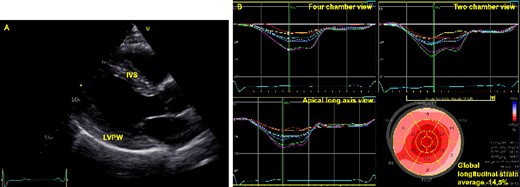
(A) Echocardiographic imaging of the left ventricle in parasternal long axis view showing thickened left ventricular wall. IVS, interventricular septum (15 mm); LVPW, left ventricular posterior wall (15 mm). (B) Left ventricular global longitudinal strain visualized with automated function imaging. Notice the reduced global longitudinal strain and the impaired contractility posterior and lateral.
FD was suspected in 2018 after performing a CMR. The CMR presented LVH [left ventricular mass (LVM) 146 g] and short T1 relaxation time, which is indicative of sphingolipid storage in an early FD. Moreover, it showed prolonged T1 time with an intramural hyperenhancement in the inferolateral wall in late enhancement sequences as a sign for fibrosis (Figure 3, Video 2). Biochemical testing revealed a reduced GLA activity [<2.8 µmol/L/h (limit of quantification), normal range ≥15.3 µmol/L/h] and an increased concentration of lyso-globotriaosylceramide (lyso-Gb3, 20.0 ng/mL, normal range ≤1.8 ng/mL). The diagnosis was confirmed by a hemizygous pathogenetic mutation in exon 6 of the GLA gene [c.902G>A, p.(Arg301Gln)]. Afterwards, the patient was admitted to the Department of Nephrology to evaluate organ involvement in a multidisciplinary approach. Arterial hypertension was treated with an angiotensin receptor blocker. Kidney biopsy was performed due to unclear aetiology of proteinuria in the presence of arterial hypertension. It showed ‘zebra bodies’ and vacuoles in podocytes as a renal involvement of FD. Neurological examination detected bilateral carpal tunnel syndrome and cerebrovascular magnetic resonance showed microbleeds and white matter lesions. According to the detected mutation, an ERT with Agalsidase Beta was started in 2018. The patient presented regularly for follow-up examinations in our Fabry Centrum (CeRKiD). In 2019, CMR detected increased T1 relaxation time (1079 ms in 2019 vs. 1060 ms in 2018) and reduced LVM (−14 g) and, in 2020, lyso-Gb3 concentration decreased to a level of 9 ng/mL.
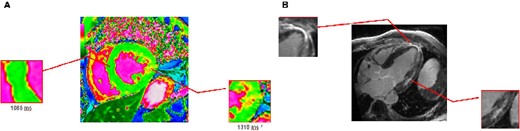
Cardiovascular magnetic resonance T1 mapping (3 T MR) visualized short T1 time (1085 ms), indicative of sphingolipid storage (A). The same image displays areas of prolonged T1 time (1310 ms, A), which correlates with late gadolinium enhancement as a surrogate for fibrosis in Fabry disease (B).
In retrospective analysis, ECG characteristics of FD have already been present in 2008 and progressed over time (Figure 4). In particular, the first ECG (2008) showed a sinus rhythm with a PendQ interval of 40 ms, a PQ interval of 160 ms, a QT interval of 360 ms, and negative T-waves in II, III, aVF, V5, and V6. ST elevations were present in V1-V4 with a maximum of 0.2 mV in V2 and V3. Seven years later, the ECG showed additional negative T-waves in I and V4. The duration of the PQ interval remained stable over time while PendQ decreased. The ECG findings correlated well with echocardiographic measurements. While the diameter of the interventricular septum was constant with 15-16mm, the posterior wall exhibited an increasing thickness from 11 mm (2008) to 14 mm (2018, before ERT) and up to 15 mm (2020, two years after ERT was started). In addition, in 2020, lateral and posterior longitudinal left ventricular function was reduced.
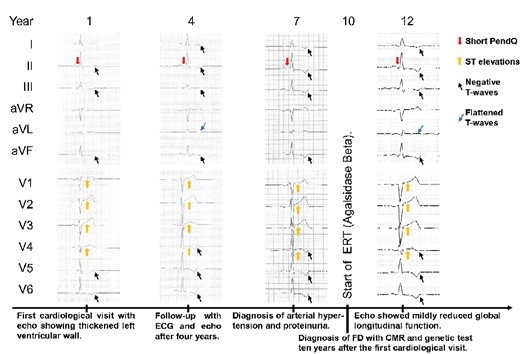
Timeline with typical ECG findings for FD, especially the short PendQ interval, i.e. PQ minus P-wave, and changes of the ST segments and T-waves (2008–2020). 50 mm/s, 10 mm/mV. ECG, electrocardiogram; FD, Fabry disease; CMR, cardiac magnetic resonance imaging; ERT, enzyme replacement therapy.
Echocardiographic imaging of the left ventricle (apical four-, three-, and two-chamber view) and left ventricular global longitudinal strain visualized with automated function imaging.
Cardiac magnetic resonance imaging, SSFP Cine sequence, four chamber view with symmetric left ventricular hypertrophy.
Discussion
Our case report shows that even simple diagnostic methods like ECGs can provide suspicion of FD and correlate with disease progression. In contrast to the previous case series, no shortened PQ interval was found with respect to our patient.6,10
Namdar et al. developed an ECG formula to detect FD in patients with LVH (PendQ <40ms and QT duration <440 ms), which showed a sensitivity of 100% and specificity of 99%.10 Ten years prior to FD was diagnosed (2008), the ECG of our patient presented a PendQ duration of 40 ms and a QT duration of 360 ms. In retrospect, the ECG provided preliminary evidence for FD, which could be confirmed by CMR, genetic test, and kidney biopsy afterwards.
Conduction abnormalities in FD occur in progressive cardiomyopathy.11 Echocardiographic surrogate markers for arrhythmias include an increased LVM, a reduced left ventricular function, and a left atrial reservoir dysfunction.11
The National Society of Genetic Counselors of the USA recommends a diagnostic algorithm in case of suspected FD. This includes genetic tests for women (always) and for men (if GAL enzyme activity of leukocytes is reduced).12 Since FD is an X-linked mutation, heterozygotes women show variable levels of GAL enzyme activity and, therefore, a measurement is not required.12 After confirmation of FD, a multidisciplinary diagnostic approach should comprise laboratory measurements, ECG, echocardiography and/or CMR, imaging of the brain, pulmonary function test, hearing, ophthalmologic and psychiatric examination, and organ biopsies in unclear cases.12 Patients should be treated with ERT or chaperone therapy in specialized centres.8,12 Furthermore, measurements of lyso-Gb3, a metabolite of globotriaosylceramide (Gb3) and thus a biomarker for FD, may support diagnosing FD.13 Depending on the level of Gb3, conclusions may be drawn regarding the patient’s response to therapy.13
Previous studies detected a recovery of ECG changes under ERT.14,15 Prinz et al.14 reported a regression of ST-segment disturbances and an improvement of left ventricular function. Moreover, ERT leads to a shortening in QRS duration due to reduced LVM.15 Consequently, it may be assumed that ECG changes (most likely associated with progressive glycolipidglobotriaosylceramide deposition) are declining under ERT.6 In our case, a regression of ECG changes has not yet been detected allegedly due to a rather short treatment period.
Conclusion
In conclusion, typical ECG changes can provide preliminary suspicion of FD. Moreover, differential diagnosis of unclear LVH in adult patients should include storage disorders like FD. If a respective diagnosis has been confirmed, patients should be treated by multidisciplinary teams in experienced centres including regular follow-up examinations.
Lead author biography
Isabel Mattig, M.D., is 26 years old. In 2018, she completed medical studies at the Charité, Universitätsmedizin Berlin, Germany. Since 2019, she is following a residency in cardiology and internal medicine at the Department for Cardiology and Angiology at the Charité, Universitätsmedizin Berlin, Germany.
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Slide sets: A fully edited slide set detailing these cases and suitable for local presentation is available online as Supplementary data.
Consent: Consent for publication has been obtained, in line with the COPE best practice guidelines, and the patient, who is being reported on, is aware of the possible consequences of that reporting.
Conflict of interest: S.C.K. received honoraria and travel grants from Sanofi-Genzyme, Takeda-Shire, Amicus, and Greenovation. FK received honoraria from Sanofi-Genzyme, Takeda-Shire. The other authors have no conflicts of interest to declare.
Funding: We acknowledge support from the German Research Foundation (DFG) and the Open Access Publication Fund of Charité – Universitätsmedizin Berlin.
References
Author notes
Mattig Isabel and Canaan-Kühl Sima contributed equally to this work.





Comments