-
PDF
- Split View
-
Views
-
Cite
Cite
Aldo Cutaia, Clara Gaetani, Paolo Fonio, Riccardo Faletti, Role of integrated imaging in the diagnosis of an atypical and unresectable cardiac paraganglioma: a case report, European Heart Journal - Case Reports, Volume 7, Issue 8, August 2023, ytad363, https://doi.org/10.1093/ehjcr/ytad363
Close - Share Icon Share
Abstract
Paragangliomas (PGLs) are rare neuroendocrine tumours that originate from extra-adrenal location. Cardiac PGLs can cause severe hypertension, palpitations, and lethal tachyarrhythmias. Diagnosis is based on measurement of plasma or urine metanephrines combined with conventional and nuclear imaging. Effective treatment is represented by surgical resection. We report a case of a 19-year-old patient with recurrent acute pericarditis; integrated imaging detected a large cardiac mass suggestive for PGL.
A 19-year-old male suffered pleuritic chest pain and fever for 4 days; electrocardiogram showed inferior ST elevation and transthoracic echocardiography a 2.2 cm pericardial effusion; these findings led to diagnose acute pericarditis. After a relapse of pericarditis, cardiac magnetic resonance and cardiac computed tomography (CCT) were performed, revealing a cardiac mass with radiological features of PGL. Blood and urine tests detected elevated levels of 3-methoxytyramine and chromogranin A. Gallium-68 positron emission tomography confirmed high metabolic activity of the mass. A negative 123-I-MIBG scintigraphy ruled out the possibility of radiometabolic treatment. A second CCT excluded the chance of surgical resection, due to intra-lesional course of the left anterior descending coronary artery. The young patient was referred to a different centre to achieve reduction of the mass, in order to potentially resect it afterwards.
Cardiac PGLs are rare tumours with significant morbidity related to norepinephrine secretion. In this case, without typical clinical manifestations and with no chance of surgical resection, integrated imaging played a central role in the differential diagnosis between PGL and other cardiac masses, providing both static and dynamic characterization.
Integrated imaging (cardiac computed tomography and cardiac magnetic resonance) is crucial to identify the anatomic relations between cardiac mass, heart chambers, and coronary vessels
Non-invasive diagnosis of PGL should be achieved with nuclear medicine and lab tests.
Introduction
Paragangliomas (PGLs) are rare neuroendocrine tumours that arise from chromaffin cells located outside the adrenal gland. Cardiac paragangliomas represent <1% of all primary cardiac tumors1 that could potentially cause hypertension, excessive sweating, palpitations, and headache.2 Diagnosis is based on measurement of plasma or urine metanephrines and conventional and nuclear imaging.3 Therapeutic management consists mostly of surgical excision and follow-up needs to be extended.4 We report a case of a 19-year-old patient with recurrent acute pericarditis; integrated imaging revealed a large cardiac mass with radiological features suggestive for PGL.
Summary figure
| May 2021 | First hospitalization for acute pericarditis. Patient discharged asymptomatic after medical therapy. |
| December 2021 | Recurrence of symptoms and pericardial effusion, restart of medical therapy. |
| 15 March 2022 | Cardiac magnetic resonance: mass with the involvement of cardiac structures but no endocavitary growth, pericardial effusion. |
| 23 March 2022 | Cardiac computed tomography (CCT): slightly intra-lesion course of the proximal tract of left anterior descending. The mass showed also rich vascularization. |
| Late March 2022 | Lab tests: 3-methossy-thiramine resulted a high increase. |
| April 2022 | Intermittent fever, dry cough, and thoracic pain. Pericardial effusion. |
| Late April 2022 | Positron emission tomography with 68Ga: intense uptake of the mass (standardized uptake value or SUV max 33.7). |
| 18 July 2022 | Follow-up CCT: increase of the mass. |
| May 2021 | First hospitalization for acute pericarditis. Patient discharged asymptomatic after medical therapy. |
| December 2021 | Recurrence of symptoms and pericardial effusion, restart of medical therapy. |
| 15 March 2022 | Cardiac magnetic resonance: mass with the involvement of cardiac structures but no endocavitary growth, pericardial effusion. |
| 23 March 2022 | Cardiac computed tomography (CCT): slightly intra-lesion course of the proximal tract of left anterior descending. The mass showed also rich vascularization. |
| Late March 2022 | Lab tests: 3-methossy-thiramine resulted a high increase. |
| April 2022 | Intermittent fever, dry cough, and thoracic pain. Pericardial effusion. |
| Late April 2022 | Positron emission tomography with 68Ga: intense uptake of the mass (standardized uptake value or SUV max 33.7). |
| 18 July 2022 | Follow-up CCT: increase of the mass. |
| May 2021 | First hospitalization for acute pericarditis. Patient discharged asymptomatic after medical therapy. |
| December 2021 | Recurrence of symptoms and pericardial effusion, restart of medical therapy. |
| 15 March 2022 | Cardiac magnetic resonance: mass with the involvement of cardiac structures but no endocavitary growth, pericardial effusion. |
| 23 March 2022 | Cardiac computed tomography (CCT): slightly intra-lesion course of the proximal tract of left anterior descending. The mass showed also rich vascularization. |
| Late March 2022 | Lab tests: 3-methossy-thiramine resulted a high increase. |
| April 2022 | Intermittent fever, dry cough, and thoracic pain. Pericardial effusion. |
| Late April 2022 | Positron emission tomography with 68Ga: intense uptake of the mass (standardized uptake value or SUV max 33.7). |
| 18 July 2022 | Follow-up CCT: increase of the mass. |
| May 2021 | First hospitalization for acute pericarditis. Patient discharged asymptomatic after medical therapy. |
| December 2021 | Recurrence of symptoms and pericardial effusion, restart of medical therapy. |
| 15 March 2022 | Cardiac magnetic resonance: mass with the involvement of cardiac structures but no endocavitary growth, pericardial effusion. |
| 23 March 2022 | Cardiac computed tomography (CCT): slightly intra-lesion course of the proximal tract of left anterior descending. The mass showed also rich vascularization. |
| Late March 2022 | Lab tests: 3-methossy-thiramine resulted a high increase. |
| April 2022 | Intermittent fever, dry cough, and thoracic pain. Pericardial effusion. |
| Late April 2022 | Positron emission tomography with 68Ga: intense uptake of the mass (standardized uptake value or SUV max 33.7). |
| 18 July 2022 | Follow-up CCT: increase of the mass. |
Case presentation
A previously healthy 19-year-old male was admitted to hospital for acute chest pain and fever. He underwent chest X-ray (which detected enlargement of cardiac shadow), electrocardiogram (aVF and III ST elevation) and transthoracic echocardiography (2.2 cm pericardial effusion). Since the clinical presentation was consistent with acute pericarditis, he was treated with ibuprofen (600 mg) and colchicine (1 mg) and was discharged in good condition after one week; colchicine was maintained as therapy (0.5 mg). At 1 month follow-up, pericardial effusion persisted at echocardiography (0.7 cm) and so did asthenia. After seven months, another episode of important asthenia associated with pericardial effusion occurred, so a cardiac magnetic resonance (CMR) was requested. The CMR detected the presence of a large mass (54 × 42 mm) in contiguity with the epicardial profile of the basal and middle anterior wall of the left ventricle. The mass was hypointense with a peripheral hyperintense rim in T2-weighted sequences and hyperintense in T1-weighted sequences. It also showed rich contrast enhancement after Gadolinium injection except for a central core (Figure 1). At the diffusion-weighted whole-body imaging with background body signal suppression (DWIBS) sequences, the mass did not show a signal increase. Inversion recovery turbo field echo sequences to evaluate late gadolinium enhancement were not performed. These findings strongly suggested the presence of a primary cardiac neoplasm. A cardiac computed tomography (CCT) was performed, confirming hypervascularization of the mass, with a central hypodense scar, located superiorly to the antero-septal wall of the left ventricle. It also confirmed the intra-lesional course of the left anterior descending (LAD) artery (Figure 2). Cardiac structures did not show signs of infiltration. The patient was referred to cardiac surgery consultation where three different metanephrines were tested: 3-methoxytyramine resulted increased both in blood and in urine samples [6058 μg/L—n.v. < 550 μg/L]. Moreover, a marker of neuroendocrine tumours (chromogranin A) was tested resulting a high increase [627 ng/mL—n.v. < 100 ng/mL]. These findings suggested the diagnosis of cardiac PGL.
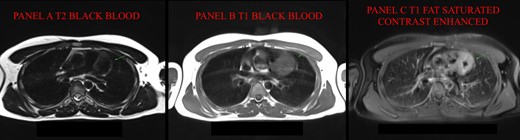
Cardiac magnetic resonance (MR). (A) In T2 black blood (BB), mass is hypointense with hyperintense rim; (B) in T1 turbo spin echo (TSE) BB, mass is hyperintense. (C) T1-TSE fat saturated with contrast (FS C+) shows a better view of the hypointense core without contrast enhancement. MR, magnetic resonance; BB, black blood; TSE, turbo spin echo.
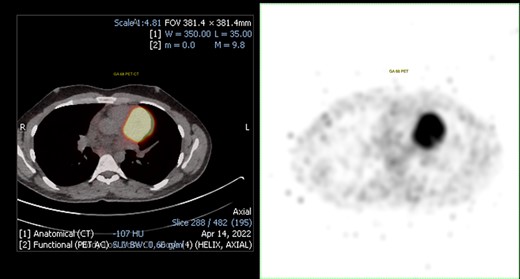
68Ga positron emission tomography (PET). On the left, PET-CT fusion imaging that shows the presence of mediastinal uptake in correspondence of the mass (SUV max 33.7). On the right, non-fusion PET image. PET, positron emission tomography
The patient was later admitted to hospital for recurrence of acute pericarditis with high fever, cough, and thoracic pain, with normal I-troponin levels (3 ng/L) and increased C-reactive protein (196 mg/L).
The patient underwent a positron emission tomography (PET) with 68Ga to assess the expression of somatostatin receptors. The mass showed intense uptake (SUV max 33.7), supporting the diagnostic hypothesis of PGL (Figure 3). A 123-iodine metaiodobenzylguanidine scintigraphy was performed to assess the possibility of radio-metabolic treatment, resulting negative. Cardiac computed tomography was repeated, showing a dimensional increase of the mass (56 × 49 mm compared to previous CCT 49 × 47 mm).
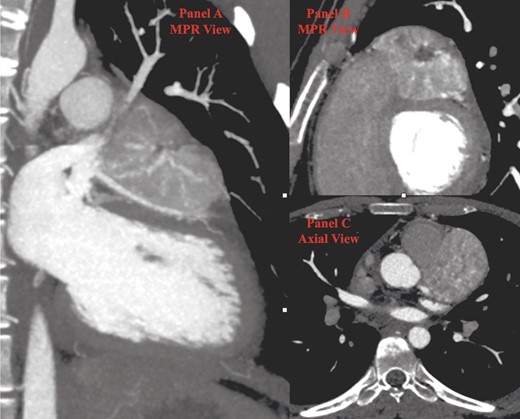
Cardiac computed tomography (CT). (A) In the first multiplanar reconstruction (MPR) view, the course of left anterior descending (LAD) artery is indissociable from the inferior surface of the paraganglioma (PGL). (B) The second MPR view shows the typical aspect of PGL with a central hypodense scar surrounded by an inhomogeneous enhancing tissue. (C) Axial view. CT, computed tomography; LAD, left anterior descending; PGL, paraganglioma.
At present time, resection of the mass is not achievable, given its proximity to LAD. The patient was referred to a different centre to try an experimental treatment to reduce the mass and to allow subsequent surgery. Further information is not currently available.
Discussion
In our case report, the first clinical presentation was consistent with a diagnosis of idiopathic acute pericarditis, initially referred to Post-Acute COVID-19 syndrome.5 Because of a subsequent relapse, a CMR was performed, revealing a mass superiorly to the left ventricle characterized by MR signal compatible with PGL, as found by other authors.6,7 On DWIBS sequences, the mass lacked the signal increase3 usually seen in PGLs. Laboratory findings and high PET uptake confirmed the diagnosis of cardiac PGL. Cardiac computed tomography guaranteed a better anatomic detail and showed that the mass was in contiguity with LAD (Figure 4), indissociable from the proximal tract, thus making the patient ineligible for biopsy or surgery. The combination of CCT and CMR assured an optimal diagnostic work-up, combining tissue characterization from CMR with coronary and general anatomy details from CCT.8 Differential diagnosis included angioma (which has slower and more homogeneous enhancement), myxofibrosarcoma (often already metastatic), and angiosarcoma (occurs mainly in the right atrium or pericardium).9 Given the suggestive anamnesis, the highly specific CMR ‘salt and pepper’ appearance [(enhancing parenchyma of the mass (salt) and flow voids of the vessels (pepper)] (Figure 5) and the high blood values of metanephrines and chromogranin A, the presumptive diagnosis appeared extremely solid. Considering that pericardial effusion associated with PGL is a very uncommon finding described only in a few case reports in literature,10 we could hypothesize a ‘compression effect’ of the mass causing pericardial effusion and a key role of unknown microenvironmental factors related to the spread of an acute inflammatory event.
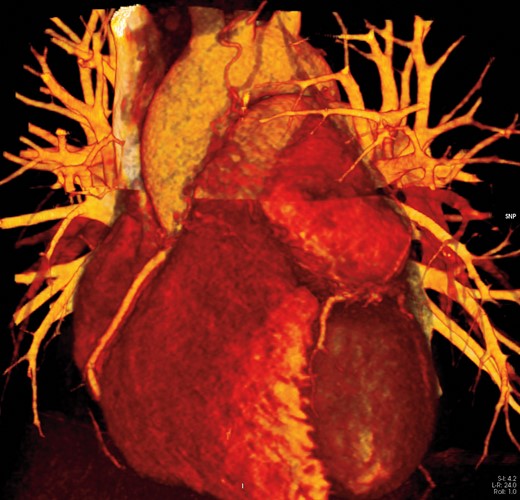
3D image of the mass and its relationship with left anterior descending (LAD). LAD, left anterior descending.
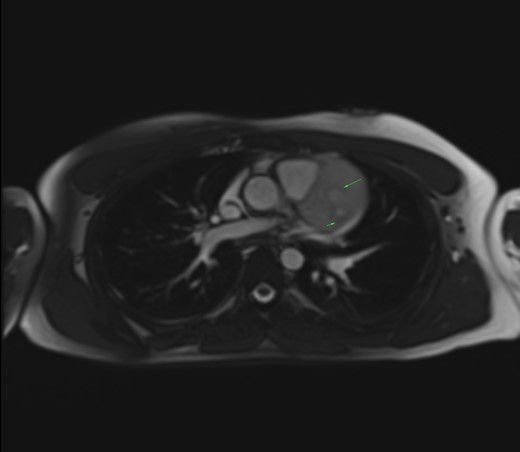
Salt and pepper appearance on SSFP (steady state free precession) cine sequences. Long arrow: enhancing parenchyma of the mass (salt). Short arrow: signal flow voids of the vessels (pepper).
Conclusions
Cardiac imaging has a crucial role in the diagnosis of cardiac PGL, especially if it presents with atypical clinical features and when surgical resection and biopsy are at very high risk. In conclusion, CCT and CMR, combined with laboratory tests and nuclear medicine imaging, play a fundamental role in distinguishing PGL from other cardiac masses.
Lead author biography
 Radiology resident at second year of training in Molinette Hospital, Turin.
Radiology resident at second year of training in Molinette Hospital, Turin.
Consent: The patient consent for submission and publication was obtained from the patient in line with COPE guidelines.
Funding: None.
Data availability
The data underlying this article are available in the article and in its online supplementary material.
References
Author notes
Conflict of interest: None declared.




Comments