-
PDF
- Split View
-
Views
-
Cite
Cite
Sandro Ninni, Stanley Nattel, Factor XIa inhibition in atrial fibrillation: insights and knowledge gaps emerging from the PACIFIC-AF trial, Cardiovascular Research, Volume 119, Issue 1, January 2023, Pages e111–e114, https://doi.org/10.1093/cvr/cvac196
Close - Share Icon Share
1. Stroke prevention in atrial fibrillation
Stroke prevention is a cornerstone of the management of patients with atrial fibrillation (AF).1 After a lengthy period during which broad-spectrum and relatively risky vitamin K antagonists were the only option, more safe and specific Factor Xa (FXa) or Factor IIa (FIIa) inhibiting direct oral anticoagulants (DOACs) have become the standard of care.1,2 However, patients taking DOACs still present a residual risk for major bleeding of 1.5–3.6%/year.3 These bleeding events are associated with increased mortality risk, high costs, and compromised adherence to treatment. Therefore, effective new anticoagulant classes with reduced bleeding risk would be of major interest.
2. Rationale for targeting FXI
After endothelial injury, the extrinsic coagulation pathway is triggered by released tissue factor binding to FVII, generating active FVIIa, and leading to FX and FII (thrombin) activation, causing fibrin generation (Figure 1). The intrinsic pathway is classically initiated by FXII activation due to molecular pattern release involving DNA, neutrophil extracellular traps, and exposure to collagen or polyphosphates.4 The intrinsic pathway can also be activated by thrombin generated via the extrinsic pathway and thus plays a significant role in pathologic thrombotic processes. While DOACs target factors figuring in both intrinsic and extrinsic pathways (FXa or FIIa), contributing to bleeding risks, FXIa inhibition specifically targets the intrinsic pathway and in situations where the intrinsic pathway plays a critical role, might maintain efficacy with reduced bleeding risk.
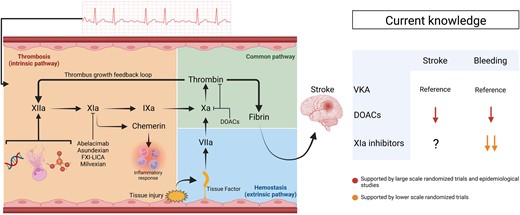
A schematic representation of haemostasis and thrombosis processes in relation to current knowledge regarding anticoagulant drugs in patients with AF. Endothelial injury leads to haemostasis activation involving the extrinsic pathway, following tissue factor release and Factor VII activation. Thrombin and various molecular patterns (DNA, neutrophil extracellular traps, and collagen or polyphosphate groups) lead to the activation of the intrinsic pathway. FXIa is an important component of the intrinsic pathway. FXIa also leads to the generation of chemerin, potentially involved in the inflammatory response. Large-scale studies have demonstrated at least equivalent efficiency for stroke prevention and reduced bleeding risk with DOACs, compared with VKA. Early stage clinical trial studies suggest that FXIa inhibitors have lower bleeding risks than DOACs. The impact of FXIa inhibition on stroke prevention is currently under investigation. DOACs, direct-acting oral anticoagulants; FXI-LICA, Factor XI ligand-conjugated antisense; VKA, vitamin K antagonists.
Previous studies have documented reduced risks of venous thromboembolism (VTE) and stroke in patients with FXI deficiency; while the bleeding risk in haemophilia due to FXI deficiency is relatively mild.5 Based on these observations, efforts have been made to develop FXIa inhibitors with various approaches selectively targeting FXI, such as antisense oligonucleotides (FXI ligand-conjugated antisense), monoclonal antibodies (abelacimab and osocimab), and small molecules (asundexian and milvexian). These molecules were first tested for postoperative VTE prevention, showing similar efficacy to low-molecular-weight heparin with reduced bleeding risk.4
3. The PACIFIC-AF trial
Based on the reasoning presented above, FXIa inhibition was suggested as a potentially interesting strategy for stroke prevention in patients with AF. The PACIFIC-AF trial (official name: Multicentre, Randomized, Active Comparator-controlled, Double-blind, Double-dummy, Parallel Group, Dose-finding Phase 2 Study to Compare the Safety of the Oral FXIa Inhibitor BAY2433334 to Apixaban in Patients With Atrial Fibrillation) was a Phase 2 double-blind, double-dummy, dose-finding randomized controlled trial that compared an oral FXIa inhibitor (asundexian) vs. apixaban in 755 patients with AF at moderate risk for ischaemic stroke (CHA2DS2Vasc ≥ 2 for men and ≥3 for women).6 Patients were randomly assigned to receive asundexian 20 mg/day, asundexian 50 mg/day, or apixaban at guideline-recommended doses for 12 weeks. The primary endpoint encompassed major or clinically relevant non-major bleeding. A double-dummy design with identical-appearing placebos was used to maintain blinding. The mean CHA2DS2VASC score was 3.9 and 14% of patients were also treated with aspirin ≤100 mg per day. Of note, both the 20 and 50 mg dose of asundexian achieved high levels of FXIa inhibition (81 and 92% at trough concentrations, respectively). The occurrence rate of the primary endpoint was significantly reduced in patients treated with asundexian vs. apixaban [ratio of incidence proportions (90% CI): 0.33 (0.09–0.97) for pooled doses of asundexian]. There was no significant difference in bleeding event-rates between the 20 mg and the 50 mg per day groups, consistent with the near-maximal FXIa inhibition with the 20 mg/day dose.
4. PACIFIC-AF trial in perspective
The PACIFIC-AF trial was the first study to investigate FXIa inhibition for stroke prevention in patients with AF. The bleeding-rate results are in accordance with previous Phase 2 studies of asundexian in VTE prevention.4 Furthermore, the PACIFIC-AF trial included patients representative of most studies investigating anticoagulation strategies in AF. Of note, no conclusions can be drawn regarding efficacy, as the study was not powered to test for changes in stroke risk.
5. Relevance for basic cardiovascular investigators
If FXIa inhibition proves to be safer and as effective as existing DOACs for AF-related thromboembolism, they will represent a significant advance in stroke-prevention therapy for AF. This development underlines the importance of stroke as the most important clinical complication of AF and the limitations to our understanding of the mechanistic determinants of AF-related stroke. For basic investigators, this work highlights a significant lacune in our experimental repertoire: the lack of clinically relevant experimental models for AF-related thromboembolism. Limitations to such animal models include species-dependent differences in coagulation systems compared with humans and a lack of AF-related atrial thrombus and/or thromboembolism in large-animal models.
A variety of mechanistic contributors to AF-related thromboembolism have been suggested, including mechanical stasis, particularly in the left-atrial appendage, changes in blood coagulability, endothelial damage, atrial inflammation, and atrial fibrosis.7,8 The relative contribution of these mechanisms is difficult to assess in the absence of solid, clinically relevant animal models of AF-related thromboembolism. FXIa cleaves the adipokine prochemerin to a more active proinflammatory and adipogenic form, creating a link between FXIa activity and inflammation.9 Proteomic analyses of plasma samples from patients presenting VTE point to links between FXIa activity and immune pathways related to thrombosis, inflammation, extracellular matrix interaction, lipid metabolism, and apoptosis.10 Thus, pleiotropic actions of FXIa might contribute to stroke prevention, an interesting possibility that has also been raised for FXa. Clearly, further work is needed to better understand the mechanisms underlying AF-related stroke risk and identify the potential value of various mechanisms as stroke-prevention targets.
6. Conclusions
Stroke is the most important clinical complication of AF and improved prevention remains a highly relevant clinical goal. The PACIFIC-AF study supports the potential value of FXIa inhibition for effective stroke prevention with reduced bleeding risk. The basic mechanisms linking AF to stroke risk remain unclear and better experimental models for AF-related thromboembolism are needed to advance this area.
Funding
The study was funded by Fédération Française de Cardiologie, the Groupement de Coopération Sanitaire Interrégional des Centres Hospitaliers Universitaires Amiens Caen Lille Rouen (G4), Canadian Institutes of Health Research (1484010), and Heart and Stroke Foundation of Canada (22-0031958).
Authors
 Biography: Dr Sandro Ninni, graduated in medicine at the University of Lille in 2016 and obtained a PhD degree based on an academic thesis on the determinant and impact of inflammatory response following cardiac surgery under the supervision of Prof. David Montaigne, at the University of Lille. He completed training in Cardiac electrophysiology at Lille University hospital, combining it with translational research at the UMR1011 ‘nuclear receptor, metabolic and cardiovascular diseases’ (Prof. Bart Staels). He joined the Heart and Lung Institute at the Lille University Hospital as Associate Professor in Cardiology, particularly in cardiac electrophysiology and developed ‘cardiovascular translational research’ projects focused on cardiac arrhythmia and its association with inflammatory response and metabolic disorders. He joined the Montreal Heart Institute (Prof. Stanley Nattel) for a Postdoctoral fellowship in 2022. His main lines of research centre around pathophysiological mechanisms involved arrhythmias with a special focus placed on inflammation and metabolic disorders. He is an elected member of the scientific committee of the Société Française de Cardiologie: Groupe de Réflexion sur la Recherche Cardiovasculaire (GRRC).
Biography: Dr Sandro Ninni, graduated in medicine at the University of Lille in 2016 and obtained a PhD degree based on an academic thesis on the determinant and impact of inflammatory response following cardiac surgery under the supervision of Prof. David Montaigne, at the University of Lille. He completed training in Cardiac electrophysiology at Lille University hospital, combining it with translational research at the UMR1011 ‘nuclear receptor, metabolic and cardiovascular diseases’ (Prof. Bart Staels). He joined the Heart and Lung Institute at the Lille University Hospital as Associate Professor in Cardiology, particularly in cardiac electrophysiology and developed ‘cardiovascular translational research’ projects focused on cardiac arrhythmia and its association with inflammatory response and metabolic disorders. He joined the Montreal Heart Institute (Prof. Stanley Nattel) for a Postdoctoral fellowship in 2022. His main lines of research centre around pathophysiological mechanisms involved arrhythmias with a special focus placed on inflammation and metabolic disorders. He is an elected member of the scientific committee of the Société Française de Cardiologie: Groupe de Réflexion sur la Recherche Cardiovasculaire (GRRC).
 Biography: Dr Nattel received his MD from McGill University in 1974, undertook Internal Medicine and Clinical Pharmacology training at McGill from 1974 to 1978, then obtained clinical and basic research training at Indiana University and University of Pennsylvania (1978–1981) before returning to McGill in 1981. In 1987, he transferred to the University of Montreal and Montreal Heart Institute, where he directed the Research Centre between 1990 and 2004. He is currently Paul-David Chair in Cardiovascular Electrophysiology at the University of Montreal and Director of the Electrophysiology Research Programme at the Montreal Heart Institute. He is Editor in Chief of the Canadian Journal of Cardiology, Associate Editor of Cardiovascular Research, Rhythm Disorders Section Editor in JACC, and is on the editorial board of many other journals including Cardiovascular Research, Circulation Research, Circulation Arrhythmia and Electrophysiology, Drugs, JACC Clinical Electrophysiology, Journal of Molecular and Cellular Cardiology, Journal of Cardiovascular Electrophysiology, Journal of Cardiovascular Pharmacology, and Nature Reviews in Cardiology. His research focuses on clinically relevant mechanisms of cardiac bioelectricity, particularly atrial fibrillation, proarrhythmia, cardiac remodelling, ion channel molecular physiology, and mechanisms of drug action. His lab uses a wide range of molecular, cellular, whole-animal, and mathematical modeling methods to study clinically relevant basic mechanisms and identify novel therapeutic targets. He has supervised over 170 research trainees, published over 725 papers in peer-review journals, has an h index of 149 and is a Fellow of the Royal Society of Canada (Academy of Science), the Royal College of Physicians of Canada, the American College of Cardiology and the Heart Rhythm Society.
Biography: Dr Nattel received his MD from McGill University in 1974, undertook Internal Medicine and Clinical Pharmacology training at McGill from 1974 to 1978, then obtained clinical and basic research training at Indiana University and University of Pennsylvania (1978–1981) before returning to McGill in 1981. In 1987, he transferred to the University of Montreal and Montreal Heart Institute, where he directed the Research Centre between 1990 and 2004. He is currently Paul-David Chair in Cardiovascular Electrophysiology at the University of Montreal and Director of the Electrophysiology Research Programme at the Montreal Heart Institute. He is Editor in Chief of the Canadian Journal of Cardiology, Associate Editor of Cardiovascular Research, Rhythm Disorders Section Editor in JACC, and is on the editorial board of many other journals including Cardiovascular Research, Circulation Research, Circulation Arrhythmia and Electrophysiology, Drugs, JACC Clinical Electrophysiology, Journal of Molecular and Cellular Cardiology, Journal of Cardiovascular Electrophysiology, Journal of Cardiovascular Pharmacology, and Nature Reviews in Cardiology. His research focuses on clinically relevant mechanisms of cardiac bioelectricity, particularly atrial fibrillation, proarrhythmia, cardiac remodelling, ion channel molecular physiology, and mechanisms of drug action. His lab uses a wide range of molecular, cellular, whole-animal, and mathematical modeling methods to study clinically relevant basic mechanisms and identify novel therapeutic targets. He has supervised over 170 research trainees, published over 725 papers in peer-review journals, has an h index of 149 and is a Fellow of the Royal Society of Canada (Academy of Science), the Royal College of Physicians of Canada, the American College of Cardiology and the Heart Rhythm Society.
References
Author notes
Conflict of interest: Sa.Ni. is a speaker for BMS/Pfizer and Bayer. St.Na. is a consultant for LQT Therapeutics and Glasco Smith Klein.



