-
PDF
- Split View
-
Views
-
Cite
Cite
Yoshinori Nishijima, Shelby N Hader, Andreas M Beyer, Differential impacts of COVID-19 variants on human microvascular function, Cardiovascular Research, Volume 119, Issue 1, January 2023, Pages e115–e117, https://doi.org/10.1093/cvr/cvad006
Close - Share Icon Share
The purpose of this article is to follow up on our previous report on microvascular endothelial dysfunction post-infection with SARS-CoV-2.1
Since the COVID-19 outbreak caused by SARS-CoV-2 infection, more than 595 million cases were reported worldwide.2 After January 2022, the omicron variant (BA.1, 2, 4, and 5) has been responsible for more than 90% of SARS-CoV-2 infections in the USA.3 Compared with pre-omicron SARS-CoV-2 variants such as delta variant (B.1.617.2), the severity of COVID-19-related hospitalizations among omicron variant SARS-CoV-2 infections is substantially less.4 Previously, we reported that endothelial-dependent dilation in human resistance microvessels was significantly reduced for many months after SARS-CoV-2 infection.1 However, the impact of omicron variant SARS-CoV-2 infection on human arterioles is unknown. In this study, we evaluate the degree of endothelial-dependent function in human arterioles from patients who contracted SARS-CoV-2 in different time periods/variants.
We obtained human tissues as discarded surgical specimens (no consent necessary) as approved by the Institutional Review Board of the Medical College of Wisconsin and in accordance with the Declaration of Helsinki from a total of 47 patients (4 pericardial, 6 visceral, and 37 subcutaneous fat). Negative COVID-19 test results were confirmed in all patients prior to surgery. Three groups were defined as follows: COVID negative [n = 22, 2 males/20 females, 46 ± 3 years, 17 white/5 non-white, body mass index (BMI) 32 ± 1 kg/m2, coronary artery disease (CAD) 0, hypertension 5, cardiac arrythmia 1, ≥ 60 years 4], pre-omicron variants including alpha, beta, and delta (tested positive for COVID-19 between 25 March 2020 to 22 September 2021, n = 12, 2 males and 10 females, 46 ± 4 years, 9 white/3 non-white, BMI 33 ± 2 kg/m2, CAD 1, hypertension 5, hyperlipidaemia 3, >60 years 3, time between positive COVID diagnosis to surgery: average 16 ± 2 months, range 5.8–25 months), and omicron variant (tested positive for COVID-19 between 3 January 2022 to 2 June 2022, n = 13, 4 males/9 females, 44 ± 5 years, 9 white/4 non-white, BMI 31 ± 2 kg/m2, CAD 2, hypertension 3, hyperlipidaemia 4, diabetes mellitus 2, ≥60 years 3, time between COVID diagnosis to surgery: average 3 ± 1 months, range 1–6 months).
Arterioles (average internal diameter, 144 ± 4 µm) were isolated and cannulated onto glass micropipettes under 60 mmHg. Diameter changes to endothelial-dependent vasodilator acetylcholine (ACh) and flow-mediated dilation were measured using videomicrography as previously described and presented as mean ± standard error of the mean.5 Statistical analysis was performed using a two-way analysis of variance, followed by the Student–Newman–Keuls multiple-comparison test.
Vascular responses to endothelial-dependent dilator ACh (panel A) were similar in arterioles from omicron variant subjects compared with COVID negative (at 10−7 M, COVID negative: 59 ± 5%, n = 10 vs. omicron variant: 61 ± 6%, n = 10, P = 0.777), however the dilation was significantly reduced in the pre-omicron variants (at 10−7 M, 31 ± 8%, n = 9, *P < 0.05 vs. omicron). Similar to ACh, flow-mediated dilation (panel B) did not differ in COVID negative and omicron variant (at 100 cmH2O, COVID negative: 90 ± 3%, n = 12 vs. omicron variant: 84 ± 4%, n = 13, P = 0.315) but did with the pre-omicron variants (at 100 cmH2O, 47 ± 3%, n = 12, *P < 0.05 vs. omicron) (Figure 1).
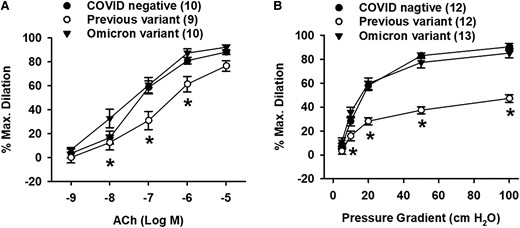
Endothelial-dependent measures of microvascular function was not impaired by SARS-CoV-2 omicron variant infection. (A) ACh-induced dilation was not altered by omicron variant. (B) Maximal flow-mediated dilation was not impaired in omicron variant. *P < 0.05 vs. omicron variant. Ns (isolated vessels/group) indicated in the figure legend.
Our previous data collected before the summer of 2021 (pre-omicron periods) suggested that microvascular endothelial function is impaired for months in humans post-SARS-CoV-2 infection and this impairment may contribute to post-acute sequelae of SARS-CoV-2 infection. In contrast, this study indicates that microvascular endothelial function is not altered by SARS-CoV-2 omicron variant infection. Similarly, Skow et al.6 reported that macro- and microvascular function, arterial stiffness, and indices of cardiac autonomic function did not differ from COVID-19 negative subjects compared with subjects who contracted the omicron variant of COVID-19 in young adults. This evidence suggests that infection of the omicron variant has less impact on microvascular endothelial function and may be directly related to less severe disease progress, and as such, cause less impact on future adverse cardiovascular events.
There are several potential limitations to our study. Pre-omicron/omicron variants were determined by the date of positive COVID test (before September 2021 vs. after January 2022), not by specific polymerase chain reaction genotyping for each variant. As all tissues were de-identified, we could not obtain information about the severity of COVID-19-related symptoms or different treatment regimens. Vaccine status was not available for all subjects, however, among COVID positive patients (both pre-omicron and omicron variants), 4 patients were fully vaccinated and boosted, 3 patients were fully vaccinated, and 2 patients were not vaccinated.
In conclusion, we observed no impact on microvascular endothelial function in patients who contracted the omicron variant of COVID-19.
Funding
This work was supported by the National Institutes of Health R01HL133029 to A.M.B. and the American Heart Assosiation AHA963959 to A.M.B.
Data Availability
The data underlying this article will be shared on reasonable request to the corresponding author.
CRediT Taxonomy Information
Shelby N. Hader, Yoshinori Nishijima, PhD, D.V.M., Andreas M. Beyer, PhD.
References
Authors
 Biography: Yoshinori Nishijima graduated in veterinary medicine at Rakuno Gakuen University in Hokkaido, Japan, and received his master’s and PhD degrees from The Ohio State University in Columbus, Ohio, under the supervision of Dr Robert Hamlin. He continued his work as a postdoctoral fellow at the laboratories of Drs. Jay Zweier, Sandor Györke, and Cynthia Carnes and received the American Heart Association Postdoctoral Fellowship Award based on Calcium regulation during heart failure. In 2011, he joined the vascular biology research group of Drs. David Zhang, David Gutterman, and Andreas Beyer as a postdoctoral researcher/staff scientist at the Medical College of Wisconsin. His research interests include understanding the vascular regulation by ion channels such as transient receptor potential channels and K+ channels. Currently, he is working on the role of toll-like proteins and mitochondrial DNA in regulating microvascular dysfunction in long COVID syndrome.
Biography: Yoshinori Nishijima graduated in veterinary medicine at Rakuno Gakuen University in Hokkaido, Japan, and received his master’s and PhD degrees from The Ohio State University in Columbus, Ohio, under the supervision of Dr Robert Hamlin. He continued his work as a postdoctoral fellow at the laboratories of Drs. Jay Zweier, Sandor Györke, and Cynthia Carnes and received the American Heart Association Postdoctoral Fellowship Award based on Calcium regulation during heart failure. In 2011, he joined the vascular biology research group of Drs. David Zhang, David Gutterman, and Andreas Beyer as a postdoctoral researcher/staff scientist at the Medical College of Wisconsin. His research interests include understanding the vascular regulation by ion channels such as transient receptor potential channels and K+ channels. Currently, he is working on the role of toll-like proteins and mitochondrial DNA in regulating microvascular dysfunction in long COVID syndrome.
 Biography: Shelby N. Hader is a Research Technologist III in the Beyer/Gutterman Lab. She is also a graduate student pursuing her Master’s in Medical Physiology. Prior to starting at the Medical College of Wisconsin, she received her BA from Lawrence University. Her primary goal is to analyse the vascular reactivity of human coronary arterioles and adipose microvessels within healthy and diseased patients. Some of her projects include the cardiotoxicity of chemotherapy upon the microvasculature along with measuring the differences between fission and fusion of mitochondria in human arterioles. Additionally, Shelby provides research support for multiple projects in the lab via imaging, dissection of discarded tissue, and rat/mice colony maintenance.
Biography: Shelby N. Hader is a Research Technologist III in the Beyer/Gutterman Lab. She is also a graduate student pursuing her Master’s in Medical Physiology. Prior to starting at the Medical College of Wisconsin, she received her BA from Lawrence University. Her primary goal is to analyse the vascular reactivity of human coronary arterioles and adipose microvessels within healthy and diseased patients. Some of her projects include the cardiotoxicity of chemotherapy upon the microvasculature along with measuring the differences between fission and fusion of mitochondria in human arterioles. Additionally, Shelby provides research support for multiple projects in the lab via imaging, dissection of discarded tissue, and rat/mice colony maintenance.
 Biography: Andreas M. Beyer, PhD, Associate Professor, Medicine, Physiology (Research Title or General Area: Microvascular (dys)function in cardiovascular health and disease—focus on mitochondria), received a Diploma of Biochemical Engineering at the University of Applied Science in Giessen-Friedberg, Germany, before moving to the University of Iowa to obtain a PhD in Genetics under the mentorship of Curt D. Sigmund. After completing his graduate research, he pursued postdocs in Cardiovascular Physiology under the guidance of Kamal Rahmouni/Val Sheffield (University of Iowa) and Julian Lombard (Medical College of Wisconsin). In 2011, he joined the rank of faculty in the Department of Medicine and Physiology at the Medical College of Wisconsin. His lab has been externally independently funded since 2012.Research program Beyer lab: As part of an integrated group of investigators, the Beyer lab is a leader among those studying the human microcirculation. Our ground-breaking findings led to the re-evaluation of how microvascular tone is regulated in patients and as such we are well-positioned and have all the necessary expertise to further define physiological and molecular mechanisms microvascular function contribute to the development and progression of cardiovascular disease. My research focuses on translational aspects of vascular pathophysiology with an emphasis on microvascular endothelial (dys)function. The mechanisms that cause microvascular dysfunction remain ill-defined and represent a critical gap in knowledge. We have made fundamental contributions to understanding signalling events regulating human microvascular function by establishing that the mechanisms of flow-mediated dilation (FMD) change over the lifespan and shift with the onset of CAD. In healthy patients, FMD is regulated by the vasoprotective dilator nitric oxide.These findings informed a new line of investigation focused on understanding how external stress, including unrelated pathologies, damages the microcirculation and increases the risk of major adverse cardiovascular events long term. Website: https://www.mcw.edu/departments/cardiovascular-center-heart/members/faculty-and-labs/andreas-beyer-lab
Biography: Andreas M. Beyer, PhD, Associate Professor, Medicine, Physiology (Research Title or General Area: Microvascular (dys)function in cardiovascular health and disease—focus on mitochondria), received a Diploma of Biochemical Engineering at the University of Applied Science in Giessen-Friedberg, Germany, before moving to the University of Iowa to obtain a PhD in Genetics under the mentorship of Curt D. Sigmund. After completing his graduate research, he pursued postdocs in Cardiovascular Physiology under the guidance of Kamal Rahmouni/Val Sheffield (University of Iowa) and Julian Lombard (Medical College of Wisconsin). In 2011, he joined the rank of faculty in the Department of Medicine and Physiology at the Medical College of Wisconsin. His lab has been externally independently funded since 2012.Research program Beyer lab: As part of an integrated group of investigators, the Beyer lab is a leader among those studying the human microcirculation. Our ground-breaking findings led to the re-evaluation of how microvascular tone is regulated in patients and as such we are well-positioned and have all the necessary expertise to further define physiological and molecular mechanisms microvascular function contribute to the development and progression of cardiovascular disease. My research focuses on translational aspects of vascular pathophysiology with an emphasis on microvascular endothelial (dys)function. The mechanisms that cause microvascular dysfunction remain ill-defined and represent a critical gap in knowledge. We have made fundamental contributions to understanding signalling events regulating human microvascular function by establishing that the mechanisms of flow-mediated dilation (FMD) change over the lifespan and shift with the onset of CAD. In healthy patients, FMD is regulated by the vasoprotective dilator nitric oxide.These findings informed a new line of investigation focused on understanding how external stress, including unrelated pathologies, damages the microcirculation and increases the risk of major adverse cardiovascular events long term. Website: https://www.mcw.edu/departments/cardiovascular-center-heart/members/faculty-and-labs/andreas-beyer-lab
Author notes
Conflict of interest: None declared.



