-
PDF
- Split View
-
Views
-
Cite
Cite
Sémi Souames, Eric Bonnivard, Claude Bazin, Dominique Higuet, High Mutation Rate of TPE Repeats: A Microsatellite in the Putative Transposase of the hobo Element in Drosophila melanogaster, Molecular Biology and Evolution, Volume 20, Issue 11, November 2003, Pages 1826–1832, https://doi.org/10.1093/molbev/msg194
Close - Share Icon Share
Abstract
The hobo transposable element contains a polymorphic microsatellite sequence located in its coding region, the TPE repeats. Previous surveys of natural populations of Drosophila melanogaster have detected at least seven different hobo transposons. These natural populations are geographically structured with regard to TPE polymorphism, and a scenario has been proposed for the invasion process. Natural populations have recently been completely invaded by hobo elements with three TPE repeats. New elements then appeared by mutation, triggering a new stage of invasion by other elements. Since TPE polymorphism appeared over a short period of time, we focused on estimating the mutation rate of these TPE repeats. We used transgenic lines harboring three TPE and/or five TPE hobo elements that had been evolving for at least 16 generations to search for a new TPE repeat polymorphism. We detected three mutants, with four, seven, and eight TPE repeats, respectively. The estimated mutation rate of the TPE repeats is therefore higher than that of neutral microsatellites in D. melanogaster (4.2 × 10−4 versus 6.5 × 10−6). The role of the transposition mechanism and the particular structure of the TPE repeats of the hobo element in this increase in the mutation rate are discussed.
Introduction
The hobo element is a class II transposable element that transposes via a DNA intermediate (for review see Finnegan 1989). It belongs to the hobo-Ac-Tam family, a widely distributed family of transposons (Calvi, Hong, and Gelbart 1991). According to temporal surveys of natural populations of Drosophila melanogaster, it invaded the species during the second half of the twentieth century and is now distributed worldwide (Périquet et al. 1989; Pascual and Périquet 1991; Bonnivard et al. 2000; Bonnivard, Bazin, and Higuet 2002).
The autonomous reference element (HFL1) is 2,959 bp long, with short inverted terminal repeats of 12 bp. It contains two open reading frames (ORFs), ORF0 (96 bp) and ORF1 (1,983 bp). ORF1 encodes the putative hobo transposase and includes the S region containing a tandemly repeated ACTCCAGAA sequence. This motif encodes a threonine-proline-glutamic acid (TPE) motif. The first hobo element to be described was hobo108, which harbors 10 perfect TPE repeats, whereas HFL1 has been described as a three-TPE element. These perfect repeats are flanked by five degenerate ones: three in the 5′ position and two in the 3′ position (Streck, Mac Gaffey, and Beckendorf 1986; Calvi, Hong, and Gelbart 1991).
Bonnivard et al. (2000) and Bonnivard, Bazin, and Higuet (2002) showed that natural populations are highly polymorphic with regard to TPE repeats. Indeed, to date hobo transposons have been detected with two to nine TPE repeats. Moreover, the distribution of this polymorphism in natural populations revealed a geographical structure. Taking into account this geographical pattern that they have detected, the authors propose a two-step invasion scenario. The three-TPE hobo element, which is more frequent in natural populations, has invaded all populations. In a second step, new hobo elements, with different TPE repeats appeared by mutation. Most of these elements are present locally at very low frequencies, apart from the four-TPE and five-TPE hobo elements. The four-TPE element is present in East African and South American populations, whereas the five-TPE element is widely distributed in Europe along a centrifugal frequency gradient that starts in Western Europe. This pattern could suggest that the invasive ability of the hobo elements is correlated with the number of the TPE repeats.
The question we addressed here concerns the high degree of polymorphism of the TPE repeats of the hobo elements in natural populations that suggests that this microsatellite sequence has a high mutation rate (Bonnivard, Bazin, and Higuet 2002). The mean mutation rate of neutral microsatellite loci in D. melanogaster is estimated to be 6.5 × 10−6 (Schug, Mackay, and Aquadro 1997; Schlötterer et al. 1998; Schug et al. 1998), which is low compared with their mutation rate in mammals (e.g., 10−4) (Dallas 1992; Weber and Wong 1993; Ellegren 1995). However, Schlötterer et al. (1998) showed that long microsatellite alleles in D. melanogaster mutate at rates closer to that of mammals (3 × 10−4).
In this paper, we focus on estimating the mutation rate of the S region of the hobo element. Using transgenic lines of D. melanogaster, we show that the S region of the hobo element mutates at a rate 100-times higher than the mean mutation rate of microsatellite loci in the species. However, the rate we estimated remains within the range of mutation rates of long microsatellite alleles in D. melanogaster. This suggests that the transposition mechanism of the hobo element and/or its particular structure could be responsible for this high mutation rate.
Material and Methods
pP{hobo, white} Construction
The pHfl1 plasmid is a complete three-TPE hobo element cloned in the pBlueScript (Calvi et al. 1991). The pHfl5 is a five-TPE element obtained by substituting an S region with five TPE repeats for the S region of pHfl1 (Béatrice Denis, personal communication). The pHfl plasmids (1 and 5) were digested by two enzymes, KpnI and NaeI, to obtain a restriction fragment that contains the complete hobo element plus 0.5 kb of genomic DNA and a small fragment of pBlueScript. This 3.4-kb fragment was eluted on a 1% agarose gel using the Genelute Kit (Sigma). The KpnI/NaeI fragments containing either the three-TPE hobo element or the five-TPE hobo element were inserted into the KpnI/StuI pUAST plasmid fragment (fig. 1). This plasmid described in Brand and Perrimon (1993) contains the 5′ and the 3′ ends of the P element with the miniwhite reporter gene. The resulting constructs are pP{three-TPE hobo, white+} and pP{five-TPE hobo, white+}.
Microinjections
The yw strain, devoid of P and hobo elements (ME strain), was used as the host strain for the autonomous hobo elements. The constructs pP{three-TPE hobo, white+} and pP{five-TPE hobo, white+} were microinjected separately in dechorionated early embryos. The Δ2.3 helper plasmid (Laski, Rio, and Rubin 1986) is a P transposase source that was coinjected with the constructs to permit the excision of the P{hobo, white+} construct from the pP{hobo, white+} plasmid.
The F0 survival adults were crossed with individuals from the yw strain on standard medium at 25°C. The individual F1 progeny were screened for eye color that ranged from pale yellow to orange. Thus, brothers and sisters with a given eye color were crossed. Four independent transgenic lines with the three-TPE element (lines α, β, χ, and δ) and one line with the five-TPE element (line κ) were obtained.
Establishing the Lines
One hundred lines (hobo T-lines) were established in the laboratory from the four independent transgenic lines bearing the three-TPE element and the transgenic line bearing the five-TPE element. Thirty hobo 3T-lines were obtained from a single pair of heterozygous flies of the initial transgenic lines bearing the three-TPE hobo element. Thirty hobo 5T-lines were obtained from a single pair of heterozygous flies of the initial transgenic line bearing the five-TPE hobo element. Twenty hobo 35T-lines and 20 hobo 53T-lines were obtained by crossing single heterozygous individual from the initial transgenic lines that bore the three-TPE hobo element with single heterozygous individual from the initial transgenic line that bore the five-TPE hobo element. In hobo 35T-lines, the three-TPE element originated on the maternal side, whereas in hobo 53T-lines, it was the five-TPE element that originated on the maternal side. At the first generation, 16 lines (four hobo 3T, four hobo 5T, four hobo 53T and four hobo 35T) were randomly chosen to establish separate lines evolving independently of the hobo T-lines, and called hobo SB-lines. The lines were kept at 25°C without any selection for the eye color phenotype. Every three or four generations, samples of flies were frozen for further analysis after egg laying.
PCR Analysis
PCR analysis with specific primers of the S region (h11−h6, fig. 1) described in Bazin and Higuet (1996) was performed in the two following tests. PCR products were spotted on a 2% agarose gel on a Mupid-21 (Eurogentec), migrated for 3 h on ice at 100 V. The DNA extractions were performed following a modified version of the protocol of Junakovic, Caneva, and Ballario (1984).
Transposition Assay
We used the hobo SB-lines to test the transposition of the hobo elements. As we had initiated the lines from heterozygous individuals, the F1 progeny contained colored-eyes individuals harboring the P{hobo, white+} insert and white-eyes flies that did not harbor this insert. Without transposition events colored-eyes flies are either homozygous or heterozygous for the P{hobo, white+} insert, whereas white-eyes flies are homozygous without this insert. Then, a positive h11−h6 PCR signal in white-eyes flies diagnoses at least one transposition event. These flies were analyzed using a sample of 10 mixed individuals per line (if available) at the first and fourth generations.
Detection of Polymorphism of the TPE Repeats
To determine the detection limits of a new element among others, we used a standard range. N Flies (0 < N < 11) from a transgenic line with one copy of an immobile five-TPE element deleted in 3′ (Béatrice Denis, personal communication) were mixed with N′ flies (N′ = 11−N) from the hobo reference strain 23.5/Cy MRF strain (Yannopoulos et al. 1987). The 23.5/Cy MRF strain is a hobo strain containing only three-TPE hobo elements for which we estimated the number of full-sized copies by scanning densitometry (see below).
The investigation of a new TPE repeat polymorphism was performed at the 16th generation on the hobo T-lines (3T, 5T, 53T, and 35T) and at the 20th generation on hobo SB-lines. The PCR products showing a band corresponding to a putatively new number of TPE repeats were cloned and then sequenced. Once a new hobo element was detected, its frequency was estimated by an individual h11−h6 PCR analysis. Moreover, we analyzed the nearest previous and latest generations in an attempt to investigate the origin and fate of the mutants. DNA extractions were performed on single flies following the protocol of Di Franco et al. (1995).
Southern Blotting and Scanning Densitometry
DNA extractions, according to the Junakovic, Caneva, and Ballario (1984) protocol, were performed on 30 females from the hobo SB-lines. Two micrograms of DNA were digested with the XhoI enzyme at six generations (fifth, seventh, 11th, 14th, 17th, and 20th). The standard Southern blot technique (Sambrook, Fritsch, and Maniatis 1989) was used.
To estimate the number of P{hobo, white+} original inserts, the membranes were hybridized using the 12.8 white gene probe (Bonnivard and Higuet 1999). This probe reveals the endogenous white gene and the miniwhite gene of the P{hobo, white+} insert (fig. 1) that allowed us to count the number of P{hobo, white+} insertion sites in each transgenic line.
To estimate the mean copy number of full-sized and deleted hobo elements, the relative amount of DNA between the lanes was first estimated using the signal intensity of the endogenous white gene referring to the yw reference strain. Then, to estimate the number of full-sized hobo elements (2.6-kb XhoI fragments) and deleted elements (<2.6-kb XhoI fragments) by scanning densitometry, the membranes were hybridized with a hobo probe (hobo108) eluted fragment. The mean copy number in a line corresponds to the ratio of the hobo108 signal intensities of the hobo line and that of the CyHBL1 strain (which has one HFL copy per diploid genome [Calvi and Gelbart 1994]) corrected by the ratio of the DNA amount.
Results
Microinjected hobo Elements: Are They Mobile?
Four independent transgenic lines (α, β, χ, and δ) harboring the three-TPE hobo element were obtained. A Southern blot analysis using the 12.8 white gene probe allowed us to estimate that lines α to χ harbor one P{3TPE hobo, white+} insertion site, whereas line δ harbors two insertion sites. We obtained one transgenic line (line κ) harboring one P{five-TPE hobo, white+} insert (fig. 2).
To determine the mobility of hobo elements in hobo T-lines established from the transgenic lines, we tested transposition events in the progeny of the hobo SB-lines. We used an h11−h6 PCR amplification in white flies that should not exhibit an h11−h6 PCR signal in absence of transposition events. This analysis was performed at the first and fourth generations (table 1). The results show that the hobo elements transposed rapidly after the establishment of the lines (i.e., at the first generation) in at least 10 lines, whatever the type of line under test. Moreover, the five-TPE hobo element is active (i.e., transposed) in seven out of 12 lines that initially bore the P{five-TPE hobo, white+} insert, regardless of the genetic context (i.e., the five-TPE element only or both elements in the same line). Two lines (3-D and 35-A) were not tested because of the absence of white-eyes individuals. The fourth generation analysis revealed new transposition events (5-A, 53-A, and 53-B) in lines where no transposition was detected at the first generation. Moreover, in line 3-B at the fourth generation, no PCR signal was detected, whereas it had been at the first generation revealing an intraline heterogeneity. This could suggest a stochastic loss of the element or weak transposition activity of the element. To sum up these results, the hobo elements transposed in all the testable lines at the latest at the fourth generation apart from the 35-B line that never exhibit a positive h11−h6 PCR amplification in this test.
A New TPE Polymorphism
To assess the detection limits of a new element among others, we performed a standard range analysis using the 23.5/Cy MRF strain that bore only three TPE elements and a transgenic line that bore one copy of a five-TPE hobo element. Using scanning densitometry, we have estimated that the 23.5/Cy MRF strain contains approximately 16 copies per diploid genome of the three-TPE hobo element (fig. 3). In a mix of 11 flies, we detected two copies of the five-TPE hobo element in approximately 140 copies of the three-TPE hobo element (i.e., two five-TPE hobo element flies with nine 23.5-strain flies). Thus, each line was studied in a sample of 10 individuals, because in our lines, the copy number remains lower than that of the 23.5 strain (see below).
At the 16th generation, the 87 remaining hobo T-lines out of 100 established lines were analyzed using a sample of 10 flies per line. We detected a new four-TPE hobo element in a hobo 5T-line. At the 20th generation, the 16 hobo SB-lines were analyzed individually using a sample of 10 flies. This analysis revealed two new mutation events. We detected a seven-TPE repeat element in the 5-B line and an eight-TPE repeat element in the 35-C line.
We analyzed further the lines harboring newly appeared hobo elements. We first estimated the intraline frequency of the mutant elements using an individual h11−h6 PCR analysis. At the 16th generation, the four-TPE hobo element was detected in four out of 18 flies analyzed (∼22%). At the 20th generation, the seven-TPE and the eight-TPE hobo elements were, respectively, detected in two and three out of 20 flies analyzed (respectively, 10% and 15%). We therefore investigated the presence of the mutant elements in nearest previous generation for which frozen samples were available. Four samples of 10 mixed flies were analyzed for each line. The four-TPE element was not detected at the 11th generation, suggesting that it must have appeared later. The seven-TPE element was detected at neither the 18th generation nor the 19th generation. The eight-TPE element was detected at the 18th generation in one sample of 10 mixed flies out of four. Lastly, the fate of the mutant elements was investigated in four samples of 10 mixed flies in later generations. The four-TPE and the seven-TPE hobo elements were not detected, respectively, at the 19th and the 29th generations, suggesting that they have disappeared from their respective lines. Nevertheless, when they are not detected, the elements could in fact still have been present, but at a frequency of lower than one individual in 40 (2.5%). The eight-TPE element was detected at the 29th generation in the four samples of 10 mixed flies analyzed. An individual h11−h6 PCR analysis revealed that its frequency increased from 15% at the 20th generation to 40% (eight individuals out of 20 analyzed) at the 29th generation, suggesting a possible invasive ability of this element.
Estimation of the Mutation Rate
To estimate the mutation rate, we used the classical calculation (Schug, Mackay, and Aquadro 1997): μ = number of events/(number of loci × number of generations × number of lines). This calculation method raises the question of the number of loci, which remains a problem in studies dealing with mobile genetic elements. We chose to define the number of loci as the copy number of hobo elements per diploid genome (full-sized and deleted). Due to transposition, the copy number of hobo elements changes over time. To allow for its change, we estimated the mean copy number of hobo elements (mcn) per line. Concerning the mutant detected at the 16th generation, the mcn estimation was performed on four generations (mcnG16). Concerning the two mutants detected at the 20th generation, the mcn estimation was performed on six generations (mcnG20, table 2). The estimation of the mcn per generation and per line was performed by Southern blotting method followed by scanning densitometry on the hobo SB-lines (fig. 3 and table 2). In the calculation described above, the number of loci is defined as the mean mcnG16 for the 87 lines analyzed at the 16th generation and the mean mcnG20 for the 16 lines analyzed at the 20th generation. Then, with three mutation events, the mutation rate can be estimated to be μ = 4.2 × 10−4, 3/([mean mcnG16 × 16 × 87] + [mean mcnG20 × 20 × 16]). To estimate a confidence interval of this mean mutation rate, we defined the maximum mean copy number (mcnmax) and the minimum mean copy number (mcnmin). Using the mcnmax (max mcnG16 = 6.35; max mcnG20 = 10.22) μmin is estimated to be 2.5 × 10−4. Using mcnmin (min mcnG16 = 0.54; min mcnG20 = 0.38) the μmax is estimated to be 3.4 × 10−3. However, the 3-B line that give the minimum mcn have a particularly low number of copies. Hence, this line is related to the 3-A line that shows the same situation. Thus, if we exclude these two lines and use min mcnG16 = 2.30 and min mcnG20 = 2.58, the μmax can be estimated to be 7.4 × 10−4.
Discussion
In this paper, we attempt to estimate the mutation rate of a microsatellite sequence in the coding region of the hobo element: the S region. We used transgenic lines of D. melanogaster that initially bore three-TPE and/or five-TPE repeat elements. We detected three mutation events affecting the TPE repeats. hobo elements with four-TPE and seven-TPE repeats appeared in hobo 5T-lines, and an eight-TPE element appeared in a hobo 35T-line. We estimated the mean mutation rate of the S region to be 4.2 × 10−4 (μmin = 2.5 × 10−4; μmax = 7.4 × 10−4). This estimation took into account the presence of deleted elements that classically do not harbor the TPE repeats in natural populations. Then, these elements are not likely to contribute to the generation of TPE repeats polymorphism. Consequently, the mutation rate is probably underestimated by such a calculation. Nevertheless, our mutation rate is 100-times higher than the estimated mean mutation rate of neutral microsatellites in D. melanogaster (6.5 × 10−6 [Schug, Mackay, and Aquadro 1997; Schlötterer et al. 1998; Schug et al. 1998]). This also is 10-times higher than the mutation rate of the Thre-Gly repeat a region of the period gene of D. melanogaster that could be the target for selection (around 10−5, Rosato et al. 1997). In an attempt to explain the low mutation rate they observed, Schug, Mackay, and Aquadro (1997) suggested that it could be correlated to a low number of replication cycles in the germline of D. melanogaster. However, the mutation rate we observed is within the range of the estimated mutation rate for long-microsatellite alleles (4.5 × 10−4 [Schlötterer et al. 1998; Harr and Schlötterer 2000]). The hobo element is a class II transposable element that transposes after a cut-and-paste mechanism (Finnegan 1989). The element is excised from the donor site at which a DNA repair step is operated, either from the sister chromatid or from the homologous chromosome. This allows the increase in copy number to occur, and each step of DNA repair at the donor site can be considered to constitute an additional replication cycle at the level of the element. Harr and Schlötterer (2000), on the basis of the model proposed by Falush and Iwasa (1999) suggest that sister chromatid exchanges can increase the mutation rate. In our lines, DNA repair should use the sister chromatid as a matrix, insofar as we assume that most of the insertion sites are heterozygous. Moreover, despite the low number of TPE repeats, the S region can be considered to be a long microsatellite, as the motif is longer than classical microsatellites ones. We can therefore suggest that transposition acts as a force that increases the mutation rate by way of gene conversion by sister chromatid exchanges. However, our estimation took into account the result of the transposition process (i.e., the increase in copy number) but did not take into account the transposition rate that cannot be estimated. The sister chromatid exchanges could amplify the effect of the lengths of the TPE repeats on the mutation rate. However, despite the high mutation rate, we cannot test whether the mutational mechanism is DNA polymerase slippage or unequal recombination.
The high mutation rate that we calculated is consistent with the high degree of polymorphism observed in natural populations with regard to the number of TPE repeats. Indeed, since the invasion by the 3TPE hobo element during the 1950s, many new hobo elements have appeared (ranging from two to nine TPE repeats) in natural populations over a short timescale of approximately 600 generations since the 1940s (10 generations per year). Most of them are found at very low frequencies and may represent cryptic polymorphism, but two of these new elements are very frequent: the four-TPE and five-TPE hobo elements (Bonnivard, Bazin, and Higuet 2002). This raises the question of whether they are maintained at high frequencies in natural populations as a result of selective advantage or of genetic drift.
In our transgenic lines, we checked the fate of the three mutants that we detected. The four-TPE and seven-TPE hobo elements disappeared from their lines, whereas the eight-TPE element is still present at the 29th generation. In the case of the newly appeared eight-TPE element, the increase of its frequency between the 20th and 29th generations (15% to 40%) suggests an invasive ability. The fate of these elements could result either from selective pressures or from genetic drift, as in natural populations. Regarding the fate of the mutants we have detected, it is likely that the mutation rate is underestimated due to putative disappearance of mutants. The studies of microsatellites we referred to mainly focused on neutral microsatellites, whereas the S region of the hobo element is included in the coding region of the putative transposase of the element. This raises the question of the impact of an increase or a decrease in the number of TPE repeats on the activity (transposition or regulation) of the element. Thus, it also raises the question of the selective advantage or disadvantage of the different hobo elements in natural populations and of our mutants. Indeed, changes in the number of TPE repeats could result in a transposase with different properties, and this could include a very active transposase. Such a situation will lead to a higher rate of transposition, which is often associated with more severe deleterious effects that will be counterselected. An impact of this sort on the activity could lead us to underestimate the mutation rate of hobo elements. It is to be noted that no mutation event occurred in hobo 3T-lines (solely three-TPE elements); this could be due to a stochastic effect. However, under the hypothesis that the high mutation rate is related to transposition, the absence of mutant could result from differential ability to transpose of three-TPE and five-TPE hobo elements. This could suggest an impact of the TPE repeats on the ability to transpose, acting either on properties of the transposase or regulation properties of hobo elements. An attempt to assess the impact of the number of TPE repeats on the activity of hobo elements is currently under investigation.
Pierre Capy, Associate Editor
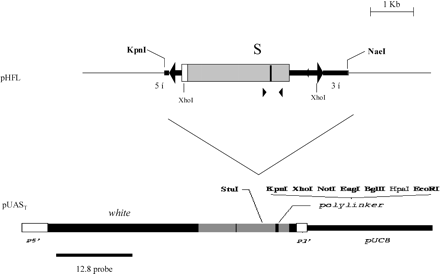
Construction of the pP{three-TPE hobo, white+} and the pP{five-TPE hobo, white+}.The pHfl (hobo element in the pBluescript) is digested by KpnI/NaeI. The 3.4 kb containing the hobo element (2.9 kb) plus 0.5 kb of genomic DNA and pBluescript is inserted in the pUAST plasmid opened by the KpnI/StuI enzymes. The resulting pP{hobo, white+} construct is microinjected in embryos of Drosophila melanogaster.pHfl: white box = ORF0; grey box = ORF1; black box in the ORF1 = S region. ▸ indicates h11 primer. ◂ indicates h6 primer. pUAST: P5′ and P3′ is the ITR 5′ and 3′ plus sequence of the P element. Black box between P5′ and P3′ is the miniwhite gene
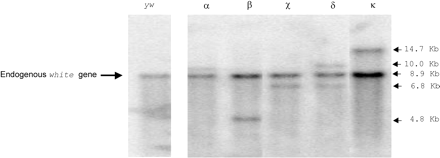
Number of P{hobo, white+} insertion sites in the transgenic lines. Southern blot hybridized using the 12.8 probe that reveals the endogenous white gene and the miniwhite gene of the P{hobo, white+} insert. yw: empty of hobo host strain for the microinjections; lane α to χ: three independent transgenic lines with one insertion site of the P{three-TPE hobo, white+}; lane δ: one transgenic line harboring two insertion sites of the P{three-TPE hobo, white+}; lane κ: one transgenic line harboring one insertion site of the P{five-TPE hobo, white+}

Detection of full-sized and deleted hobo elements at the 11th generation of hobo T-lines. Southern blot first hybridized with a white gene probe (upper image) revealing endogenous white gene and second with hobo108 probe revealing hobo sequences. High-molecular-weight bands (>2.6 kb) correspond to the heterochromatic degenerated hobo sequences (Daniels, Chovnick, and Boussy 1990). The 2.6-kb bands correspond to the internal XhoI fragment of the full-sized hobo element. Low-molecular-weight bands (<2.6 kb) correspond to internally deleted hobo elements. yw is a strain devoid of hobo elements. 23.5/Cy MRF strain is a strain containing full-sized (three-TPE repeats) and deleted hobo elements. CyHBL1 strain is a strain containing one copy of a three-TPE hobo element
Transposition of the hobo Elements in White-Eyes Flies from 16 Lines Detected by h11–h6 PCR Analysis.
| Linea . | Generation 1 . | Type of Element . | Generation 4 . | Type of Element . |
|---|---|---|---|---|
| 3-A | − | − | ||
| 3-B | + | Three TPE | − | |
| 3-C | + | Three TPE | + | Three TPE |
| 3-D | nw | − | ||
| 5-A | − | + | Five TPE | |
| 5-B | + | Five TPE | + | Five TPE |
| 5-C | + | Five TPE | + | Five TPE |
| 5-D | + | Five TPE | + | Five TPE |
| 53-A | − | + | Three TPE | |
| 53-B | + | Five TPE | + | Three and five TPE |
| 53-C | + | Three and five TPE | + | Three and five TPE |
| 53-D | + | Three and five TPE | + | Three and five TPE |
| 35-A | nw | nw | ||
| 35-B | − | − | ||
| 35-C | + | Three TPE | + | Three TPE |
| 35-D | + | Five TPE | nte |
| Linea . | Generation 1 . | Type of Element . | Generation 4 . | Type of Element . |
|---|---|---|---|---|
| 3-A | − | − | ||
| 3-B | + | Three TPE | − | |
| 3-C | + | Three TPE | + | Three TPE |
| 3-D | nw | − | ||
| 5-A | − | + | Five TPE | |
| 5-B | + | Five TPE | + | Five TPE |
| 5-C | + | Five TPE | + | Five TPE |
| 5-D | + | Five TPE | + | Five TPE |
| 53-A | − | + | Three TPE | |
| 53-B | + | Five TPE | + | Three and five TPE |
| 53-C | + | Three and five TPE | + | Three and five TPE |
| 53-D | + | Three and five TPE | + | Three and five TPE |
| 35-A | nw | nw | ||
| 35-B | − | − | ||
| 35-C | + | Three TPE | + | Three TPE |
| 35-D | + | Five TPE | nte |
Note.—Minus sign (–) indicates no h11-h6 PCR signal; plus sign (+) indicates positive h11−h6 PCR signal; nw indicates no white-eyes flies in the line; nt indicates nontested line.
aSamples of 10 flies except 3-A (two), 3-B (five), 3-C (three), and 53-B (three) at generation 1.
Transposition of the hobo Elements in White-Eyes Flies from 16 Lines Detected by h11–h6 PCR Analysis.
| Linea . | Generation 1 . | Type of Element . | Generation 4 . | Type of Element . |
|---|---|---|---|---|
| 3-A | − | − | ||
| 3-B | + | Three TPE | − | |
| 3-C | + | Three TPE | + | Three TPE |
| 3-D | nw | − | ||
| 5-A | − | + | Five TPE | |
| 5-B | + | Five TPE | + | Five TPE |
| 5-C | + | Five TPE | + | Five TPE |
| 5-D | + | Five TPE | + | Five TPE |
| 53-A | − | + | Three TPE | |
| 53-B | + | Five TPE | + | Three and five TPE |
| 53-C | + | Three and five TPE | + | Three and five TPE |
| 53-D | + | Three and five TPE | + | Three and five TPE |
| 35-A | nw | nw | ||
| 35-B | − | − | ||
| 35-C | + | Three TPE | + | Three TPE |
| 35-D | + | Five TPE | nte |
| Linea . | Generation 1 . | Type of Element . | Generation 4 . | Type of Element . |
|---|---|---|---|---|
| 3-A | − | − | ||
| 3-B | + | Three TPE | − | |
| 3-C | + | Three TPE | + | Three TPE |
| 3-D | nw | − | ||
| 5-A | − | + | Five TPE | |
| 5-B | + | Five TPE | + | Five TPE |
| 5-C | + | Five TPE | + | Five TPE |
| 5-D | + | Five TPE | + | Five TPE |
| 53-A | − | + | Three TPE | |
| 53-B | + | Five TPE | + | Three and five TPE |
| 53-C | + | Three and five TPE | + | Three and five TPE |
| 53-D | + | Three and five TPE | + | Three and five TPE |
| 35-A | nw | nw | ||
| 35-B | − | − | ||
| 35-C | + | Three TPE | + | Three TPE |
| 35-D | + | Five TPE | nte |
Note.—Minus sign (–) indicates no h11-h6 PCR signal; plus sign (+) indicates positive h11−h6 PCR signal; nw indicates no white-eyes flies in the line; nt indicates nontested line.
aSamples of 10 flies except 3-A (two), 3-B (five), 3-C (three), and 53-B (three) at generation 1.
Estimation per Line of the Mean Copy Number of hobo Elements per Diploid Genome by Scanning Densitometry.
| . | hobo 3T-Lines . | hobo 5T-Lines . | hobo 53T-Lines . | hobo 35T-Lines . | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Generation . | 3-A . | 3-B . | 3-C . | 3-D . | 5-A . | 5-B . | 5-C . | 5-D . | 53-A . | 53-B . | 53-C . | 53-D . | 35-A . | 35-B . | 35-C . | 35-D . |
| 5 | 0.89 | 0.63 | 2.17 | 3.21 | 1.34 | 4.01 | 1.91 | 2.25 | 2.71 | 2.27 | 2.96 | 3.25 | 3.46 | 2.16 | 3.79 | 3.08 |
| 7 | 0.56 | 0.58 | 3.23 | 5.30 | 2.33 | 7.40 | 3.31 | 4.10 | 2.52 | 5.24 | 2.48 | 3.82 | 3.60 | 1.94 | 3.73 | 2.75 |
| 11 | — | — | 3.22 | — | — | 4.46 | — | — | — | 6.10 | 2.64 | — | — | 1.21 | 2.87 | 3.82 |
| 14 | 0.72 | 0.41 | 4.51 | 4.13 | 3.25 | 9.52 | 8.47 | 5.51 | 4.46 | 4.98 | 6.98 | 3.15 | 6.60 | 3.91 | 8.02 | 4.87 |
| mcnG16/line | 0.72 | \(\underline{0.54}\) | 3.28 | 4.21 | 2.30 | 6.35 | 4.56 | 3.95 | 3.23 | 4.65 | 3.76 | 3.41 | 4.55 | 2.30 | 4.60 | 3.63 |
| Mean mcnG16 | 3.5 | |||||||||||||||
| 17 | 2.91 | 0.01 | 5.39 | 0.03 | 5.19 | 4.44 | 4.73 | 3.79 | 2.41 | 3.49 | 2.86 | 2.54 | 9.49 | 1.65 | 2.01 | 2.52 |
| 20 | 2.81 | 0.29 | 5.14 | 5.63 | 7.16 | 3.76 | 32.66 | 7.57 | 8.79 | 5.09 | 6.62 | 5.88 | 14.30 | 4.59 | 15.23 | 5.13 |
| mcnG20/line | 1.58 | \(\underline{0.38}\) | 3.94 | 3.66 | 3.85 | 5.60 | 10.22 | 4.64 | 4.18 | 4.53 | 4.09 | 3.73 | 7.49 | 2.58 | 3.70 | 5.94 |
| Mean mcnG20 | 4.4 | |||||||||||||||
| . | hobo 3T-Lines . | hobo 5T-Lines . | hobo 53T-Lines . | hobo 35T-Lines . | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Generation . | 3-A . | 3-B . | 3-C . | 3-D . | 5-A . | 5-B . | 5-C . | 5-D . | 53-A . | 53-B . | 53-C . | 53-D . | 35-A . | 35-B . | 35-C . | 35-D . |
| 5 | 0.89 | 0.63 | 2.17 | 3.21 | 1.34 | 4.01 | 1.91 | 2.25 | 2.71 | 2.27 | 2.96 | 3.25 | 3.46 | 2.16 | 3.79 | 3.08 |
| 7 | 0.56 | 0.58 | 3.23 | 5.30 | 2.33 | 7.40 | 3.31 | 4.10 | 2.52 | 5.24 | 2.48 | 3.82 | 3.60 | 1.94 | 3.73 | 2.75 |
| 11 | — | — | 3.22 | — | — | 4.46 | — | — | — | 6.10 | 2.64 | — | — | 1.21 | 2.87 | 3.82 |
| 14 | 0.72 | 0.41 | 4.51 | 4.13 | 3.25 | 9.52 | 8.47 | 5.51 | 4.46 | 4.98 | 6.98 | 3.15 | 6.60 | 3.91 | 8.02 | 4.87 |
| mcnG16/line | 0.72 | \(\underline{0.54}\) | 3.28 | 4.21 | 2.30 | 6.35 | 4.56 | 3.95 | 3.23 | 4.65 | 3.76 | 3.41 | 4.55 | 2.30 | 4.60 | 3.63 |
| Mean mcnG16 | 3.5 | |||||||||||||||
| 17 | 2.91 | 0.01 | 5.39 | 0.03 | 5.19 | 4.44 | 4.73 | 3.79 | 2.41 | 3.49 | 2.86 | 2.54 | 9.49 | 1.65 | 2.01 | 2.52 |
| 20 | 2.81 | 0.29 | 5.14 | 5.63 | 7.16 | 3.76 | 32.66 | 7.57 | 8.79 | 5.09 | 6.62 | 5.88 | 14.30 | 4.59 | 15.23 | 5.13 |
| mcnG20/line | 1.58 | \(\underline{0.38}\) | 3.94 | 3.66 | 3.85 | 5.60 | 10.22 | 4.64 | 4.18 | 4.53 | 4.09 | 3.73 | 7.49 | 2.58 | 3.70 | 5.94 |
| Mean mcnG20 | 4.4 | |||||||||||||||
Note.—Underlined indicates the minimum mcnG16 and minimum mcnG20. Bold indicates the maximum mcnG16 and maximum mcnG20.
Estimation per Line of the Mean Copy Number of hobo Elements per Diploid Genome by Scanning Densitometry.
| . | hobo 3T-Lines . | hobo 5T-Lines . | hobo 53T-Lines . | hobo 35T-Lines . | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Generation . | 3-A . | 3-B . | 3-C . | 3-D . | 5-A . | 5-B . | 5-C . | 5-D . | 53-A . | 53-B . | 53-C . | 53-D . | 35-A . | 35-B . | 35-C . | 35-D . |
| 5 | 0.89 | 0.63 | 2.17 | 3.21 | 1.34 | 4.01 | 1.91 | 2.25 | 2.71 | 2.27 | 2.96 | 3.25 | 3.46 | 2.16 | 3.79 | 3.08 |
| 7 | 0.56 | 0.58 | 3.23 | 5.30 | 2.33 | 7.40 | 3.31 | 4.10 | 2.52 | 5.24 | 2.48 | 3.82 | 3.60 | 1.94 | 3.73 | 2.75 |
| 11 | — | — | 3.22 | — | — | 4.46 | — | — | — | 6.10 | 2.64 | — | — | 1.21 | 2.87 | 3.82 |
| 14 | 0.72 | 0.41 | 4.51 | 4.13 | 3.25 | 9.52 | 8.47 | 5.51 | 4.46 | 4.98 | 6.98 | 3.15 | 6.60 | 3.91 | 8.02 | 4.87 |
| mcnG16/line | 0.72 | \(\underline{0.54}\) | 3.28 | 4.21 | 2.30 | 6.35 | 4.56 | 3.95 | 3.23 | 4.65 | 3.76 | 3.41 | 4.55 | 2.30 | 4.60 | 3.63 |
| Mean mcnG16 | 3.5 | |||||||||||||||
| 17 | 2.91 | 0.01 | 5.39 | 0.03 | 5.19 | 4.44 | 4.73 | 3.79 | 2.41 | 3.49 | 2.86 | 2.54 | 9.49 | 1.65 | 2.01 | 2.52 |
| 20 | 2.81 | 0.29 | 5.14 | 5.63 | 7.16 | 3.76 | 32.66 | 7.57 | 8.79 | 5.09 | 6.62 | 5.88 | 14.30 | 4.59 | 15.23 | 5.13 |
| mcnG20/line | 1.58 | \(\underline{0.38}\) | 3.94 | 3.66 | 3.85 | 5.60 | 10.22 | 4.64 | 4.18 | 4.53 | 4.09 | 3.73 | 7.49 | 2.58 | 3.70 | 5.94 |
| Mean mcnG20 | 4.4 | |||||||||||||||
| . | hobo 3T-Lines . | hobo 5T-Lines . | hobo 53T-Lines . | hobo 35T-Lines . | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Generation . | 3-A . | 3-B . | 3-C . | 3-D . | 5-A . | 5-B . | 5-C . | 5-D . | 53-A . | 53-B . | 53-C . | 53-D . | 35-A . | 35-B . | 35-C . | 35-D . |
| 5 | 0.89 | 0.63 | 2.17 | 3.21 | 1.34 | 4.01 | 1.91 | 2.25 | 2.71 | 2.27 | 2.96 | 3.25 | 3.46 | 2.16 | 3.79 | 3.08 |
| 7 | 0.56 | 0.58 | 3.23 | 5.30 | 2.33 | 7.40 | 3.31 | 4.10 | 2.52 | 5.24 | 2.48 | 3.82 | 3.60 | 1.94 | 3.73 | 2.75 |
| 11 | — | — | 3.22 | — | — | 4.46 | — | — | — | 6.10 | 2.64 | — | — | 1.21 | 2.87 | 3.82 |
| 14 | 0.72 | 0.41 | 4.51 | 4.13 | 3.25 | 9.52 | 8.47 | 5.51 | 4.46 | 4.98 | 6.98 | 3.15 | 6.60 | 3.91 | 8.02 | 4.87 |
| mcnG16/line | 0.72 | \(\underline{0.54}\) | 3.28 | 4.21 | 2.30 | 6.35 | 4.56 | 3.95 | 3.23 | 4.65 | 3.76 | 3.41 | 4.55 | 2.30 | 4.60 | 3.63 |
| Mean mcnG16 | 3.5 | |||||||||||||||
| 17 | 2.91 | 0.01 | 5.39 | 0.03 | 5.19 | 4.44 | 4.73 | 3.79 | 2.41 | 3.49 | 2.86 | 2.54 | 9.49 | 1.65 | 2.01 | 2.52 |
| 20 | 2.81 | 0.29 | 5.14 | 5.63 | 7.16 | 3.76 | 32.66 | 7.57 | 8.79 | 5.09 | 6.62 | 5.88 | 14.30 | 4.59 | 15.23 | 5.13 |
| mcnG20/line | 1.58 | \(\underline{0.38}\) | 3.94 | 3.66 | 3.85 | 5.60 | 10.22 | 4.64 | 4.18 | 4.53 | 4.09 | 3.73 | 7.49 | 2.58 | 3.70 | 5.94 |
| Mean mcnG20 | 4.4 | |||||||||||||||
Note.—Underlined indicates the minimum mcnG16 and minimum mcnG20. Bold indicates the maximum mcnG16 and maximum mcnG20.
We would like to thank Marcela Antivilo, Céline Gomez, and Chantal Labellie for their helpful technical assistance, Monika Ghosh for language revision, and Béatrice Denis for gracefully providing us plasmids and strains. This work was supported by GDR 2157-CNRS “Evolution des éléments transposables: du génome aux populations.”
Literature Cited
Bazin, C., and D. Higuet.
Bonnivard, E., C. Bazin, B. Denis, and D. Higuet.
Bonnivard, E., C. Bazin, and D. Higuet.
Bonnivard, E., and D. Higuet.
Brand, A., and N. Perrimon.
Calvi, B. R., and W. M. Gelbart.
Calvi, B. R., T. J. Hong, and W. M. Gelbart.
Dallas, J. F.
Daniels, S. B., A. Chovnick, and I. A. Boussy.
Di Franco, C., A. Terrinoni, D. Galuppi, and N. Junakovic.
Falush, D., and Y. Iwasa.
Finnegan, D. J.
Harr, B., and C. Schlötterer.
Junakovic, N., R. Caneva, and P. Ballario.
Laski, F. A., D. C. Rio, and G. M. Rubin.
Pascual, L., and G. Périquet.
Périquet, G., M. H. Hamelin, Y. Bigot, and A. Lepissier.
Rosato, E., A. A. Peixoto, R Costa, and C. P. Kyriacou.
Sambrook, J., E. F. Fritsch, and T. Maniatis.
Schlötterer, C., R. Ritter, B. Harr, and G. Brem.
Schug, M. D., C. M. Hutter, M. A. Noor, and C. F. Aquadro.
Schug, M. D., T. F. Mackay, and C. F. Aquadro.
Streck, R. D., J. E. MacGaffey, and S. K. Beckendorf.
Weber, J. L., and C. Wong.



