-
PDF
- Split View
-
Views
-
Cite
Cite
Mariacarla Moleti, Maria Di Mauro, Angela Alibrandi, Roberto Vita, Salvatore Benvenga, Francesco Vermiglio, Postpartum Thyroiditis in Women With Euthyroid and Hypothyroid Hashimoto’s Thyroiditis Antedating Pregnancy, The Journal of Clinical Endocrinology & Metabolism, Volume 105, Issue 7, July 2020, Pages e2421–e2428, https://doi.org/10.1210/clinem/dgaa197
Close - Share Icon Share
Abstract
Postpartum thyroiditis (PPT) is defined as the occurrence of de novo autoimmune thyroid disease accompanied by thyroid dysfunction in the first year postpartum. However, hormonal changes resembling the typical pattern of PPT have been reported to occur even in women with pregestational Hashimoto’s thyroiditis (HT) on levothyroxine (LT4).
To evaluate the risk of PPT in women with HT antedating pregnancy.
Retrospective chart review of pregnant women with HT antedating pregnancy seen in a university hospital (2008-2017), who were followed from preconception up to 1 year after delivery.
167 women preconceptionally diagnosed with HT and classified as hypothyroid HT (hypo-HT; n = 98) or euthyroid HT (eu-HT; n = 69), according to their thyroid status at the time of diagnosis.
PPT occurrence and associated clinical characteristics/risk factors.
PPT occurred in 65/167 women, with a rate statistically greater in the eu-HT than in the hypo-HT group (68.1% vs 18.4%; odds ratio [OR] 9.49, 95% confidence interval [CI] 4.62-19.49). Most of the women experiencing PPT in both groups were euthyroid at the time of first-trimester evaluation (39/47 eu-HT [83%] and 16/18 hypo-HT [88.9%]). Multivariate regression analysis showed eu-HT group and first-trimester euthyroidism to be positively associated with PPT occurrence (ORs 10.71 and 3.89, respectively).
PPT may occur in hypo-HT women on LT4 therapy, although significantly less frequently than in eu-HT women. The 4-fold higher risk of PPT in HT women maintaining euthyroidism at first -trimester of gestation suggests that the risk of PPT could be related to the amount of unaffected thyroid tissue.
Women who are thyroid antibody positive in the first trimester of gestation have a 33% to 50% chance of developing postpartum thyroiditis (PPT) in the first year after delivery (1). PPT is deemed to result from the postpartum immunological rebound that follows the partial immunosuppression of pregnancy (2) and is defined as the occurrence of de novo thyroid hormonal abnormalities in the first year after delivery in women who were euthyroid before pregnancy (1). Interestingly, hormonal changes resembling the typical pattern of PPT have been reported in women with pre-existing autoimmune hypothyroidism and adequately replaced on levothyroxine (LT4) (3,4). However, the extent of such event, along with associated distinctive clinical characteristics and/or risk factors, if any, are poorly documented.
To address this issue, we conducted a retrospective analysis of consecutive women diagnosed prior to conception with either euthyroid or hypothyroid Hashimoto’s thyroiditis (HT) who were followed up from preconception to the end of the first year after delivery. The primary objective of our study was to assess the prevalence of PPT in both groups of women. In addition, as a secondary objective, we looked at the impact of PPT on HT women’s thyroid function at 1 year after delivery.
Subjects and Methods
Participants and study design
During a 10-year period (2008-2017), 1378 women were seen in the endocrine outpatient clinic for the diagnosis and treatment of gestational thyroid diseases of the University Hospital of Messina, Italy. On the basis of thyroid-peroxidase autoantibody (TPOAb) positivity and/or ultrasonographic features suggestive of autoimmune thyroiditis (5), 412 women (29.9%) had been diagnosed with HT prior to conception. These 412 women were further classified as hypothyroid-HT (hypo-HT; n = 249) or euthyroid-HT (eu-HT; n = 163), according to whether their serum thyroid-stimulating hormone (TSH) levels at the time of diagnosis were above or within the reference range (0.27-4.2 mU/L) for the nonpregnant population. All women in the hypo-HT group received substitutive LT4 doses to achieve euthyroidism prior to becoming pregnant.
For the purposes of this study, we only included women for whom the following information was available: (i) TSH measurements within 6 months prior to gestation, both during and after pregnancy (at least one determination for each trimester, and at least one determination at 1-2, 3-5, 6-9, and 12 months postpartum); (ii) TPOAb measurement at early pregnancy (≤12 weeks of gestation) and 3 months after delivery; (iii) full clinical data, including age, parity, weight, LT4 dosing prior to and during pregnancy and the postpartum period, and smoking habit; and (iv) details on nutritional iodine status, in terms of iodized salt consumption and/or use of iodine-containing supplements during gestation.
Based on the previously described criteria, 167 women (98 [58.7%] from the Hypo-HT group and 69 [41.3%] from the Eu-HT group) were included in the study.
Protocol and timing of follow-up
Starting from 2008, we implemented a program of screening of thyroid-dysfunctional women of child-bearing age and pregnant women, aimed at diagnosing and treating at an early stage any maternal thyroid dysfunction potentially harmful to both the mother and the fetus. With specific regard to HT, both hypothyroid women receiving LT4 and euthyroid women are routinely counseled prior to conception regarding the need of thyroid function evaluation if pregnancy is suspected or as soon as it is confirmed. Once pregnant, HT women are tested for thyroid function parameters (TSH and free-thyroxine) at 4- to 6-week intervals, and LT4 titration or prescription are made accordingly, with the goal of maintaining serum TSH levels within the internal trimester-specific upper limit (2.5 mU/L in the first trimester and 3.0 mU/L in the second and third trimester) (6,7). As per protocol, women who are on LT4 during pregnancy are advised to return to their preconception LT4 dosages (if on LT4 prior to conception) or to discontinue LT4 (if euthyroid prior to conception) 1 week after parturition and to have their postpartum thyroid function first tested at 6 weeks after delivery, and at 3, 6, 9, and 12 months thereafter, unless thyroid dysfunction is observed. If thyroid dysfunction is detected, thyroid parameters are monitored monthly until euthyroidism is achieved or permanent thyroid dysfunction is diagnosed. The same follow-up also applies to HT women who are consistently euthyroid during pregnancy.
Definition of PPT and HT progression following PPT
In eu-HT women, PPT was defined by the occurrence of any of these three possibilities within the first 12 months after delivery: (i) transient thyrotoxicosis, followed by transient hypothyroidism (biphasic course); (ii) transient thyrotoxicosis alone; and (iii) transient hypothyroidism alone (8).
In hypo-HT women on LT4 therapy, only the biphasic variant of PPT was taken into account, as the “thyrotoxic only” and the “hypothyroid only” variants might theoretically be due to LT4 over- or underdosing, respectively. Accordingly, in these women PPT was diagnosed only when all of the following changes were observed: (i) marked drop of TSH levels while on LT4 therapy at doses (expressed as μg/kg/day) close to those administered prior to conception, followed by (ii) marked TSH increase and subsequent spontaneous TSH decrease after LT4 withdrawal.
Finally, HT progression following PPT was defined by a persistent need of LT4 replacement at 12 months postpartum (ie, TSH levels above the upper limit of the reference range) in women who were preconceptionally euthyroid or by an increase ≥20% (9) in LT4 replacement doses to achieve preconception TSH levels in women who were preconceptionally hypothyroid.
Biochemical evaluation
Serum concentrations of TSH and TPOAb were measured using the electrochemiluminescence immunoassay system assay (Cobas; Roche Diagnostics, Mannheim, Germany). The detection limits of the assays are 0.005 mU/L for TSH and 5 IU/mL for TPOAb. The manufacturer’s reference ranges for TSH and TPOAb are 0.27 to 4.20 mU/L and 0 to 34 IU/mL, respectively. Precision profiles showed inter- and intra-assay coefficients of variation <5% over the entire measurement range.
The study was approved by the local ethics committee.
Statistical analysis
Numerical data are expressed as mean ± standard deviation, median, and interquartile range, and categorical variables as absolute frequency and percentage.
Since most of the variables were not normally distributed based on the Kolmogorov-Smirnov test, a nonparametric approach was used. In particular, the Mann-Whitney test (comparison between 2 independent samples) and the Wilcoxon test (comparison between 2 dependent samples) were applied.
The chi-square test was used to assess the statistically significant association between categorical variables.
Finally, logistic regression models were used to estimate the dependence of PPT occurrence on various explanatory variables and confounders. Covariates were either continuous/discrete measures (maternal age; years of iodized salt consumption; daily iodine intake by supplements, expressed as μg iodine/day; daily selenium intake by supplements, expressed as μg selenium/day; preconception TSH levels; TPOAb levels at first trimester evaluation) or categorical variables (patient group [eu-HT vs non–eu-HT]; maternal parity [nulliparous vs uni/pluriparous]; history of miscarriages [yes vs no]; smoking habit [yes vs no]; TPOAb positivity at first trimester evaluation [yes vs no]; LT4 therapy during gestation [yes vs no]; maternal euthyroidism at first trimester evaluation, with or without LT4 therapy [yes vs no]). Results were expressed for each covariate as odds ratio (OR), 95% confidence interval (CI), and significance.
Statistical analyses were performed using SPSS Statistics for Windows v20.0 (SPSS, Inc., Chicago, IL, USA). A P-value <0.05 was considered to be statistically significant.
Results
The epidemiological and clinical characteristics of women from the hypo-HT and eu-HT groups are shown in Table 1. Prior to conception, almost all hypo-HT women were adequately LT4-replaced, since their serum TSH levels were ≤2.50 mU/L in 95/98 (96.9%) women and >2.51 to 3.50 mU/L in the remaining 3 women. Similarly, among eu-HT women, TSH values were ≤2.50 mU/L in 60/69 women (86.9%) and between 2.51 and 3.50 mU/L in the remaining 9 women (13.1%). Because TSH levels exceeded the upper limit for the gestational period, 43 eu-HT women (62.3%) were started on LT4 therapy during gestation. Of these 43 women, 17 (39.5%), 16 (37.2%), and 10 (23.3%) were started within gestational week 13, 14-19, and after 20, respectively.
| . | Hypo-HT Group (n = 98) . | Eu-HT Group (n = 69) . | P-value . |
|---|---|---|---|
| Age, median (range) | 33 (19–44) | 32.5 (23-43) | ns |
| Parity | ns | ||
| Nulliparous, n (%) | 42 (42.9) | 32 (46.4) | |
| ≥1 pregnancy, n (%) | 56 (57.1) | 37 (53.6) | |
| History of miscarriage, n (%) | 22 (22.4) | 20 (28.9) | ns |
| Smoking habit | ns | ||
| Never, n (%) | 80 (81.6) | 57 (82.6) | |
| Former smoker, n (%) | 15 (15.3) | 11 (15.9) | |
| Current smoker, n (%) | 3 (3.1) | 1 (1.44) | |
| Regular use of iodized salt, n (%) | 57 (58.2) | 35 (50.7) | ns |
| Years of iodized salt use, M ± SD (range) | 8.0 ± 7.8 (1–34) | 6.5 ± 5.3 (1-20) | ns |
| Use of I containing supplements, n (%) | 62 (63.3) | 56 (81.1) | 0.012 |
| I intake by supplements (μg/day), M ± SD (range) | 190.6 ± 33.9 (75–300) | 184.5 ± 26.4 (150-225) | ns |
| Use of Se containing supplements, n (%) | 41 (41.8) | 31 (44.9) | ns |
| Se intake by supplements (μg/day), M ± SD (range) | 40.9 ± 12.6 (30–55) | 38.9 ± 12.2 (30-55) | ns |
| 1st-trim TPOAb positivity, n (%) | 75 (76.5) | 57 (82.6) | ns |
| 1st-trim TPOAb values (IU/mL), M ± SD (range) | 314.1 ± 405 (42.9–2222) | 303.9 ± 446.7 (48.7-2292) | ns |
| Postpartum TPOAb positivity, n (%) | 86 (87.5) | 65 (95.2) | ns |
| Postpartum TPOAB values (IU/mL), M ± SD (range) | 364.7 ± 496.6 (34.6–3054) | 404.4 ± 611.4 (41.1-3138) | ns |
| Preconception TSH (mU/L), M ± SD (range) | 1.05 ± 0.74 (0.10–3.01) | 1.53 ± 0.71 (0.51-3.29) | <0.0001 |
| Timing of preconception TSH sampling (weeks), M ± SD | 15.4 ± 4.9 | 14.5 ± 4.8 | ns |
| Preconception LT4 doses (mcg/kg/d), M ± SD | 1.52 ± 0.44 | NA | — |
| . | Hypo-HT Group (n = 98) . | Eu-HT Group (n = 69) . | P-value . |
|---|---|---|---|
| Age, median (range) | 33 (19–44) | 32.5 (23-43) | ns |
| Parity | ns | ||
| Nulliparous, n (%) | 42 (42.9) | 32 (46.4) | |
| ≥1 pregnancy, n (%) | 56 (57.1) | 37 (53.6) | |
| History of miscarriage, n (%) | 22 (22.4) | 20 (28.9) | ns |
| Smoking habit | ns | ||
| Never, n (%) | 80 (81.6) | 57 (82.6) | |
| Former smoker, n (%) | 15 (15.3) | 11 (15.9) | |
| Current smoker, n (%) | 3 (3.1) | 1 (1.44) | |
| Regular use of iodized salt, n (%) | 57 (58.2) | 35 (50.7) | ns |
| Years of iodized salt use, M ± SD (range) | 8.0 ± 7.8 (1–34) | 6.5 ± 5.3 (1-20) | ns |
| Use of I containing supplements, n (%) | 62 (63.3) | 56 (81.1) | 0.012 |
| I intake by supplements (μg/day), M ± SD (range) | 190.6 ± 33.9 (75–300) | 184.5 ± 26.4 (150-225) | ns |
| Use of Se containing supplements, n (%) | 41 (41.8) | 31 (44.9) | ns |
| Se intake by supplements (μg/day), M ± SD (range) | 40.9 ± 12.6 (30–55) | 38.9 ± 12.2 (30-55) | ns |
| 1st-trim TPOAb positivity, n (%) | 75 (76.5) | 57 (82.6) | ns |
| 1st-trim TPOAb values (IU/mL), M ± SD (range) | 314.1 ± 405 (42.9–2222) | 303.9 ± 446.7 (48.7-2292) | ns |
| Postpartum TPOAb positivity, n (%) | 86 (87.5) | 65 (95.2) | ns |
| Postpartum TPOAB values (IU/mL), M ± SD (range) | 364.7 ± 496.6 (34.6–3054) | 404.4 ± 611.4 (41.1-3138) | ns |
| Preconception TSH (mU/L), M ± SD (range) | 1.05 ± 0.74 (0.10–3.01) | 1.53 ± 0.71 (0.51-3.29) | <0.0001 |
| Timing of preconception TSH sampling (weeks), M ± SD | 15.4 ± 4.9 | 14.5 ± 4.8 | ns |
| Preconception LT4 doses (mcg/kg/d), M ± SD | 1.52 ± 0.44 | NA | — |
Abbreviations: NA, not applicable. ns, not significant; SD, standard deviation; trim, trimester.
| . | Hypo-HT Group (n = 98) . | Eu-HT Group (n = 69) . | P-value . |
|---|---|---|---|
| Age, median (range) | 33 (19–44) | 32.5 (23-43) | ns |
| Parity | ns | ||
| Nulliparous, n (%) | 42 (42.9) | 32 (46.4) | |
| ≥1 pregnancy, n (%) | 56 (57.1) | 37 (53.6) | |
| History of miscarriage, n (%) | 22 (22.4) | 20 (28.9) | ns |
| Smoking habit | ns | ||
| Never, n (%) | 80 (81.6) | 57 (82.6) | |
| Former smoker, n (%) | 15 (15.3) | 11 (15.9) | |
| Current smoker, n (%) | 3 (3.1) | 1 (1.44) | |
| Regular use of iodized salt, n (%) | 57 (58.2) | 35 (50.7) | ns |
| Years of iodized salt use, M ± SD (range) | 8.0 ± 7.8 (1–34) | 6.5 ± 5.3 (1-20) | ns |
| Use of I containing supplements, n (%) | 62 (63.3) | 56 (81.1) | 0.012 |
| I intake by supplements (μg/day), M ± SD (range) | 190.6 ± 33.9 (75–300) | 184.5 ± 26.4 (150-225) | ns |
| Use of Se containing supplements, n (%) | 41 (41.8) | 31 (44.9) | ns |
| Se intake by supplements (μg/day), M ± SD (range) | 40.9 ± 12.6 (30–55) | 38.9 ± 12.2 (30-55) | ns |
| 1st-trim TPOAb positivity, n (%) | 75 (76.5) | 57 (82.6) | ns |
| 1st-trim TPOAb values (IU/mL), M ± SD (range) | 314.1 ± 405 (42.9–2222) | 303.9 ± 446.7 (48.7-2292) | ns |
| Postpartum TPOAb positivity, n (%) | 86 (87.5) | 65 (95.2) | ns |
| Postpartum TPOAB values (IU/mL), M ± SD (range) | 364.7 ± 496.6 (34.6–3054) | 404.4 ± 611.4 (41.1-3138) | ns |
| Preconception TSH (mU/L), M ± SD (range) | 1.05 ± 0.74 (0.10–3.01) | 1.53 ± 0.71 (0.51-3.29) | <0.0001 |
| Timing of preconception TSH sampling (weeks), M ± SD | 15.4 ± 4.9 | 14.5 ± 4.8 | ns |
| Preconception LT4 doses (mcg/kg/d), M ± SD | 1.52 ± 0.44 | NA | — |
| . | Hypo-HT Group (n = 98) . | Eu-HT Group (n = 69) . | P-value . |
|---|---|---|---|
| Age, median (range) | 33 (19–44) | 32.5 (23-43) | ns |
| Parity | ns | ||
| Nulliparous, n (%) | 42 (42.9) | 32 (46.4) | |
| ≥1 pregnancy, n (%) | 56 (57.1) | 37 (53.6) | |
| History of miscarriage, n (%) | 22 (22.4) | 20 (28.9) | ns |
| Smoking habit | ns | ||
| Never, n (%) | 80 (81.6) | 57 (82.6) | |
| Former smoker, n (%) | 15 (15.3) | 11 (15.9) | |
| Current smoker, n (%) | 3 (3.1) | 1 (1.44) | |
| Regular use of iodized salt, n (%) | 57 (58.2) | 35 (50.7) | ns |
| Years of iodized salt use, M ± SD (range) | 8.0 ± 7.8 (1–34) | 6.5 ± 5.3 (1-20) | ns |
| Use of I containing supplements, n (%) | 62 (63.3) | 56 (81.1) | 0.012 |
| I intake by supplements (μg/day), M ± SD (range) | 190.6 ± 33.9 (75–300) | 184.5 ± 26.4 (150-225) | ns |
| Use of Se containing supplements, n (%) | 41 (41.8) | 31 (44.9) | ns |
| Se intake by supplements (μg/day), M ± SD (range) | 40.9 ± 12.6 (30–55) | 38.9 ± 12.2 (30-55) | ns |
| 1st-trim TPOAb positivity, n (%) | 75 (76.5) | 57 (82.6) | ns |
| 1st-trim TPOAb values (IU/mL), M ± SD (range) | 314.1 ± 405 (42.9–2222) | 303.9 ± 446.7 (48.7-2292) | ns |
| Postpartum TPOAb positivity, n (%) | 86 (87.5) | 65 (95.2) | ns |
| Postpartum TPOAB values (IU/mL), M ± SD (range) | 364.7 ± 496.6 (34.6–3054) | 404.4 ± 611.4 (41.1-3138) | ns |
| Preconception TSH (mU/L), M ± SD (range) | 1.05 ± 0.74 (0.10–3.01) | 1.53 ± 0.71 (0.51-3.29) | <0.0001 |
| Timing of preconception TSH sampling (weeks), M ± SD | 15.4 ± 4.9 | 14.5 ± 4.8 | ns |
| Preconception LT4 doses (mcg/kg/d), M ± SD | 1.52 ± 0.44 | NA | — |
Abbreviations: NA, not applicable. ns, not significant; SD, standard deviation; trim, trimester.
Occurrence of PPT
Starting from 1 week after delivery, all eu-HT women who had been started on LT4 stopped this therapy, while all hypo-HT women returned to their preconception LT4 dosing.
Overall, PPT occurred in 65/167 women (38.9%), but with a rate statistically greater in the eu-HT group (47/69; 68.1%) than in the hypo-HT group (18/98; 18.4%) (χ 2= 42.1, P < 0.0001, OR 9.49, 95% CI 4.62-19.49). However, the 2 groups did not differ in the timing of PPT occurrence (eu-HT 12.1 ± 1.5 vs hypo-HT 11.3 ± 1.1 weeks, P = 0.53).
As previously stated, in the hypo-HT group, only the classical variant of PPT was considered. Fig. 1 depicts TSH variations, along with changes in LT4 therapy, at 5 points in time after delivery (T1 = 6.7 ± 0.9 weeks; T2 = 13.6 ± 1.2 weeks; T3 = 19.1 ± 1.3 weeks; T4 = 23.6 ± 1.6 weeks; T5 = 51.3 ± 1.4 weeks) in hypo-HT women diagnosed with PPT.
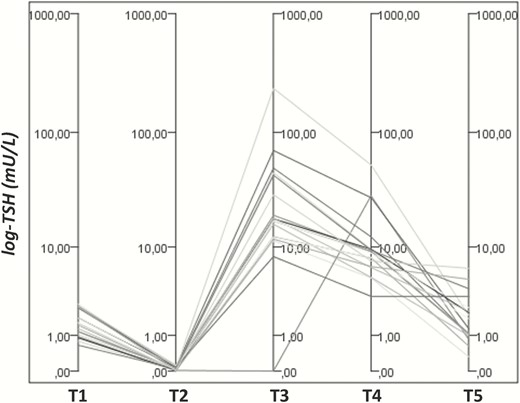
TSH variations on/off LT4 therapy in hypo-HT women diagnosed with PPT. A significant reduction in TSH levels was observed between T1 and T2 (1.06 ± 0.72 mU/L vs 0.07 ± 0.10, P-value = 0.00042), despite similar LT4 dosages at these points in time (1.51 ± 0.39 mcg/kg/die vs 1.57 ± 0.44, P-value = 0.062). At T3, all the women had discontinued LT4 therapy, and their TSH values markedly increased up to 33.2 ± 56.6 (range 0.03-234.9 mU/L) to spontaneously decrease to 9.73 ± 12.07 mU/L at T4. Following this point, LT4 therapy was restored in all hypo-HT women, with the exception of 1 woman who showed a later PPT onset (TSH suppression up to 24 weeks postpartum followed by the previously described pattern of TSH changes between 28 and 33 weeks after delivery). The 5 time points after delivery (T1 through T5) are as follows: T1 = 6.7 ± 0.9 weeks; T2 = 13.6 ± 1.2 weeks; T3 = 19.1 ± 1.3 weeks; T4 = 23.6 ± 1.6 weeks; and T5 = 51.3 ± 1.4 weeks.
In the eu-HT group, biphasic PPT was diagnosed in 35/47 women (74.5%), and the “only thyrotoxic” or the “only hypothyroid” variants in 7 (14.9%) and 5 women (10.6%), respectively.
There was no difference in the frequency of PPT between women found to be TPOAb positive and TPOAb negative at early gestation, either in the whole series, (53/132 [40.1%] vs 12/35 [34.3%], χ 2 = 0.4, P = 0.53) or in the 2 groups (hypo-HT group: PPT TPOAb positive 15/75 [20%] vs PPT TPOAb negative 3/23 [13%]; χ 2 = 0.2, P = 0.65; eu-HT group: PPT TPOAb positive 38/57 [66.7%] vs PPT TPOAb negative 9/12 [75%], χ 2 = 0.05, P = 0.82).
Interestingly, we found that in both groups most of the women who developed PPT were euthyroid at the time of first trimester evaluation (hypo-HT: 16/18 [88.9%]; eu-HT: 39/47 [83%]). In particular, the rate of PPT in hypo-HT women who maintained euthyroidism at early gestation with their preconception LT4 dosage (16/68; 23.5%) was 3.5-fold greater compared to that found in women who were not euthyroid at the same point in time (2/30; 6.7%), although this difference was only borderline significant. By contrast, the same comparison in the eu-HT group showed that the rate of PPT was significantly higher among women who were euthyroid at early gestation (39/52; 75%) compared to that found among women who experienced early thyroid insufficiency (8/17; 47.1%) (Fig. 2). In addition, in the whole cohort, women who developed PPT increased/started LT4 therapy later in gestation than women who did not experience PPT (mean gestational week of first LT4 increase/prescription: PPT women 17.4 ± 5.7 [median: 18] vs non-PPT women 13.7 ± 5.8 [median: 12], P 0.002).
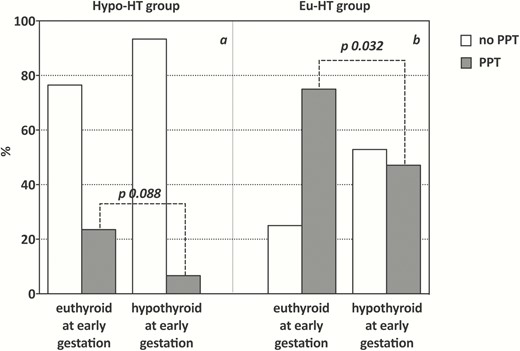
Prevalence of PPT in hypo-HT (A) and eu-HT (B) women according to their thyroid status at early gestation. In both groups, the rate of PPT among women who were euthyroid at the time of first trimester evaluation was higher than that observed among women found to be hypothyroid at the same point in time, this difference being statistically significant in eu-HT (χ 2 = 4.61, P-value = 0.032) and only borderline significant in hypo-HT group (Yates corrected χ 2 = 2.9, P-value = 0.088).
Outcome of PPT at the 1-Year Follow-Up and Predictivity of PPT Occurrence
The risk of thyroid function deterioration at 12 months postpartum was higher in hypo-HT than in eu-HT women (54/98 [55.1%] vs 23/69 [33.3%], χ 2 = 7.72, P = 0.0054, OR 0.41, 95% CI 0.21-0.77).
At the end of the follow up period (12 months postpartum), the risk of thyroid function worsening (defined by a need of daily LT4 dosage >20% of the preconception dose to achieve baseline TSH levels) was insignificantly greater in hypo-HT women who experienced PPT compared to those from the same group who did not [12/18 (66.6%) vs 42/80 (52.5%), χ 2 = 1.19, P = 0.27, OR 1.81, 95% CI 0.62-5.29). By contrast, at 12 months after delivery 20/47 (42.6%) eu-HT women experiencing PPT and only 3/22 (13.6%) eu-HT women without PPT were found to have TSH levels above the upper limit of the reference range for the nonpregnant population, the 2 rates being statistically different (χ 2 = 5.64, P = 0.018, OR 4.69, 95% CI 1.21-18.06).
To assess the possible dependency of PPT on explanatory variables and confounders, univariate logistic regression models were estimated (Table 2). These analyses showed a significant positive association of eu-HT group (OR = 9.49, 95% CI 4.62-19.49), daily iodine intake by supplements (OR = 1.04, 95% CI 1.00-1.01), euthyroidism at first trimester of gestation (with or without LT4 therapy) (OR = 3.13, 95% CI 1.43-6.87), and LT4 therapy during gestation (OR = 0.18, 95% CI 0.07-0.45) with PPT occurrence. The significant associations of eu-HT group and euthyroidism at first trimester of gestation were confirmed by multivariate analysis (OR = 10.71, 95% CI 4.29-26.70, P < 0.0001 and OR = 3.89, 95% CI 1.43-10.61, P 0.008, respectively).
| Covariates . | OR . | 95% CI . | P-value . |
|---|---|---|---|
| Eu-HT group | 9.49 | 4.62–19.49 | <0.0001 |
| Maternal age (years) | 0.95 | 0.89–1.01 | 0.147 |
| Parity (0 vs ≥ 1) | 0.94 | 0.63–1.41 | 0.767 |
| History of miscarriage (yes vs no) | 1.06 | 0.62–1.81 | 0.835 |
| Smoking habit (yes vs no) | 0.86 | 0.43–1.72 | 0.670 |
| Years of iodized salt consumption | 0.98 | 0.93–1.03 | 0.402 |
| Iodine intake by supplements (μg/day) | 1.004 | 1.00–1.01 | 0.028 |
| Selenium intake by supplements (μg/day) | 0.99 | 0.962–1.038 | 0.968 |
| Preconception TSH (mU/L) | 1.17 | 0.76–1.79 | 0.486 |
| First-trimester euthyroidism | 3.13 | 1.43–6.87 | 0.004 |
| LT4 during pregnancy | 0.18 | 0.07–0.45 | <0.0001 |
| First-trimester TPOAb positivity (yes vs no) | 1.23 | 0.56–2.71 | 0.606 |
| First-trimester TPOAb values (IU/mL) | 1.000 | 0.99–1.00 | 0.533 |
| Covariates . | OR . | 95% CI . | P-value . |
|---|---|---|---|
| Eu-HT group | 9.49 | 4.62–19.49 | <0.0001 |
| Maternal age (years) | 0.95 | 0.89–1.01 | 0.147 |
| Parity (0 vs ≥ 1) | 0.94 | 0.63–1.41 | 0.767 |
| History of miscarriage (yes vs no) | 1.06 | 0.62–1.81 | 0.835 |
| Smoking habit (yes vs no) | 0.86 | 0.43–1.72 | 0.670 |
| Years of iodized salt consumption | 0.98 | 0.93–1.03 | 0.402 |
| Iodine intake by supplements (μg/day) | 1.004 | 1.00–1.01 | 0.028 |
| Selenium intake by supplements (μg/day) | 0.99 | 0.962–1.038 | 0.968 |
| Preconception TSH (mU/L) | 1.17 | 0.76–1.79 | 0.486 |
| First-trimester euthyroidism | 3.13 | 1.43–6.87 | 0.004 |
| LT4 during pregnancy | 0.18 | 0.07–0.45 | <0.0001 |
| First-trimester TPOAb positivity (yes vs no) | 1.23 | 0.56–2.71 | 0.606 |
| First-trimester TPOAb values (IU/mL) | 1.000 | 0.99–1.00 | 0.533 |
Statistically significant P-values are in bold.
| Covariates . | OR . | 95% CI . | P-value . |
|---|---|---|---|
| Eu-HT group | 9.49 | 4.62–19.49 | <0.0001 |
| Maternal age (years) | 0.95 | 0.89–1.01 | 0.147 |
| Parity (0 vs ≥ 1) | 0.94 | 0.63–1.41 | 0.767 |
| History of miscarriage (yes vs no) | 1.06 | 0.62–1.81 | 0.835 |
| Smoking habit (yes vs no) | 0.86 | 0.43–1.72 | 0.670 |
| Years of iodized salt consumption | 0.98 | 0.93–1.03 | 0.402 |
| Iodine intake by supplements (μg/day) | 1.004 | 1.00–1.01 | 0.028 |
| Selenium intake by supplements (μg/day) | 0.99 | 0.962–1.038 | 0.968 |
| Preconception TSH (mU/L) | 1.17 | 0.76–1.79 | 0.486 |
| First-trimester euthyroidism | 3.13 | 1.43–6.87 | 0.004 |
| LT4 during pregnancy | 0.18 | 0.07–0.45 | <0.0001 |
| First-trimester TPOAb positivity (yes vs no) | 1.23 | 0.56–2.71 | 0.606 |
| First-trimester TPOAb values (IU/mL) | 1.000 | 0.99–1.00 | 0.533 |
| Covariates . | OR . | 95% CI . | P-value . |
|---|---|---|---|
| Eu-HT group | 9.49 | 4.62–19.49 | <0.0001 |
| Maternal age (years) | 0.95 | 0.89–1.01 | 0.147 |
| Parity (0 vs ≥ 1) | 0.94 | 0.63–1.41 | 0.767 |
| History of miscarriage (yes vs no) | 1.06 | 0.62–1.81 | 0.835 |
| Smoking habit (yes vs no) | 0.86 | 0.43–1.72 | 0.670 |
| Years of iodized salt consumption | 0.98 | 0.93–1.03 | 0.402 |
| Iodine intake by supplements (μg/day) | 1.004 | 1.00–1.01 | 0.028 |
| Selenium intake by supplements (μg/day) | 0.99 | 0.962–1.038 | 0.968 |
| Preconception TSH (mU/L) | 1.17 | 0.76–1.79 | 0.486 |
| First-trimester euthyroidism | 3.13 | 1.43–6.87 | 0.004 |
| LT4 during pregnancy | 0.18 | 0.07–0.45 | <0.0001 |
| First-trimester TPOAb positivity (yes vs no) | 1.23 | 0.56–2.71 | 0.606 |
| First-trimester TPOAb values (IU/mL) | 1.000 | 0.99–1.00 | 0.533 |
Statistically significant P-values are in bold.
Discussion
Per definition, PPT is the occurrence of de novo autoimmune thyroid disease accompanied by thyroid dysfunction (excluding Graves’ disease) in the first year after delivery (1). However, since the most important risk factor for PPT is the evidence of TPOAb positivity during the first trimester of pregnancy, it has been suggested that PPT should be better considered as an exacerbation of a pre-existing autoimmune thyroiditis (2,10). This being the case, PPT can occur also in women with a known previous diagnosis of autoimmune primary hypothyroidism, provided they have enough viable residual thyroid tissue where thyroiditis can take place (10,11). This possibility was first suggested by Caixàs et al in 1999 (3), who reported that 12 of 18 women with autoimmune hypothyroidism antedating pregnancy and treated with LT4 displayed changes in thyroid hormone levels and in LT4 requirements over the first year after delivery that were suggestive of PPT. Similarly, in 2015 Sergi et al found that two-thirds of women with treated hypothyroidism antedating pregnancy had fluctuations in thyroid function consistent with PPT (4).
To evaluate the frequency of PPT occurring in women with already-known autoimmune thyroiditis, we carried out a retrospective analysis in a large cohort of well-documented pregnant women who were diagnosed with HT prior to conception and who were either replaced with LT4 because hypothyroid at diagnosis (hypo-HT group) or not receiving LT4 because consistently euthyroid before gestation (eu-HT group). In the whole cohort, PPT occurred in almost 40% of HT women, although the risk of PPT was roughly 10-fold higher in eu-HT women than in those with established hypothyroidism prior to conception and adequately LT4 replaced. Among the latter women, the prevalence of PPT was 18%, which is probably an underestimated rate, as we only considered women with the classical variant of PPT, in whom this diagnosis was unquestionable. In fact, we decided not to take into account those hypo-HT women showing biochemical patterns suggestive of the “only hypothyroid” or the “only thyrotoxic” variants of PPT while on LT4 therapy, as we could not definitely rule out that the observed changes would result from suboptimal or excessive replacement LT4 doses, respectively. By contrast, among eu-HT women, all the variants of PPT were diagnosed, the biphasic one accounting for almost three-fourths of all the observed cases. This frequency is definitely higher than that reported in the literature, which ranges between 22% (12) and 50% (13). One possible explanation for this finding may be that, according to our protocol, women found to have abnormal TSH over the postpartum period are routinely scheduled to have their thyroid function tested monthly. This approach may have enabled us to promptly detect any subtle and transient increase in thyroid hormone levels due to the leakage of thyroid hormones from destroyed thyrocytes, and the subsequent progression toward hypothyroidism. Equally related to the close postpartum monitoring might also be the higher than expected prevalence of PPT, which in our cohort was diagnosed in two-thirds of eu-HT women. However, this rate virtually coincides with the 69.6% of PPT among TPOAb-positive women as very recently reported by one of us in a contemporary series of pregnant women from the same area of the women included in the present study (14) and is very close to the rate of 60% found in TPOAb-positive women from a mild to moderate iodine deficient area and receiving iodine supplements during pregnancy (13).
In our view, the most intriguing finding in this study is the identification of maternal thyroid status at early pregnancy as predictive for PPT occurrence, with euthyroidism at early gestation being associated in our HT series to a 4-fold higher risk of developing PPT. Indeed, we found that almost 90% of the hypo-HT women and 83% of the eu-HT women who went on to develop PPT had TSH levels within the upper limit of the trimester-specific reference range (2.5 mU/L) at the time of the first gestational evaluation and did not require any LT4 increase or prescription at this point in gestation. This finding is congruent with the presence of enough intact, functional thyrocytes to respond to the chorionic gonadotrophin stimulation at early pregnancy and for thyroiditis to take place in the postpartum.
As a secondary objective of our study, we looked at the impact of PPT on women’s thyroid function at 1 year following delivery. In our series, thyroid function deterioration occurred in both hypo-HT and eu-HT women, although to a greater extent in the former. However, among the eu-HT women, PPT occurrence was associated with a more than 4-fold increased risk of TSH elevation above the upper limit of the reference range for the nonpregnant population at 12-month evaluation. Whether this finding is consistent with permanent hypothyroidism or it represents the tail end of a still ongoing disease that will spontaneously resolve later cannot be said on the basis of our results due to the relatively short follow-up period. Overall, 42% of eu-HT women experiencing PPT failed to regain euthyroidism at 12-months postpartum, a rate that is appreciably higher than the 10% to 20% usually reported (11), but similar to those found in 3 Italian studies (Stagnaro-Green et al (15): 92/169 [54.4%]; Filippi et al (16): 28/57 [49.1%]; Benvenga et al (14): 34/63 [54.0%]).
In conclusion, this study shows that PPT may also occur in hypo-HT women already on LT4 therapy prior to gestation, although to a definitely lower rate than in eu-HT women. On the basis of our results, we speculate that the risk of PPT occurrence in HT women is related—at least partly—to the amount of unaffected parenchymal thyroid tissue exposed to the postpartum autoimmune attack. This is supported by the evidence that women who maintained euthyroidism at early pregnancy (with or without LT4) had a 4-fold higher risk of developing PPT compared to those whose thyroid was unable to cope with the increased functional demands at the same stage of pregnancy. To the best of our knowledge, this information has never been provided before and is worth being prospectively explored, as it may represent a novel and early marker to identify HT women who carry a higher risk for PPT.
Additional Information
Disclosure Summary: All the authors declare that no competing financial interests exist.
Data Availability: The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References



