-
PDF
- Split View
-
Views
-
Cite
Cite
Gian Paolo Rossi, Giulio Ceolotto, Giacomo Rossitto, Giuseppe Maiolino, Maurizio Cesari, Teresa Maria Seccia, Effects of Mineralocorticoid and AT1 Receptor Antagonism on The Aldosterone-Renin Ratio In Primary Aldosteronism—the EMIRA Study, The Journal of Clinical Endocrinology & Metabolism, Volume 105, Issue 6, June 2020, Pages 2060–2067, https://doi.org/10.1210/clinem/dgaa080
Close - Share Icon Share
Abstract
While current guidelines recommend the withdrawal of mineralocorticoid receptor antagonist (MRA) and renin-angiotensin system blockers for the screening and detection of primary aldosteronism (PA), this can worsen hypokalemia and control of high blood pressure (BP) values.
To investigate whether aldosterone/renin ratio (ARR) values were affected by the MRA canrenone and/or by canrenone plus olmesartan treatment in patients with PA.
Within-patient study.
The European Society of Hypertension center of excellence at the University of Padua.
Consecutive patients with an unambiguous diagnosis of PA subtyped by adrenal vein sampling.
Patients were treated for 1 month with canrenone (50–100 mg orally), and for an additional month with canrenone plus olmesartan (10–20 mg orally). Canrenone and olmesartan were up-titrated over the first 2 weeks until BP values and hypokalemia were controlled. Patients with unilateral PA were adrenalectomized; those with bilateral PA were treated medically.
BP, plasma levels of sodium and potassium, renin and aldosterone.
Canrenone neither lowered plasma aldosterone nor increased renin; thus, the high ARR and true positive rate remained unaffected. Addition of the angiotensin type 1 receptor blocker raised renin and slightly lowered aldosterone, which reduced the ARR and increased the false negative rate.
At doses that effectively controlled serum potassium and BP values, canrenone did not preclude an accurate diagnosis in patients with PA. Addition of the angiotensin type 1 receptor blocker olmesartan slightly raised the false negative rate. Hence, MRA did not seem to endanger the accuracy of the diagnosis of PA.
Primary aldosteronism (PA) affects about 6% of the hypertensive patients seen by general practitioners (1, 2), more than 11% of those referred to specialized centers (3), and 17% to 23% of those with drug-resistant hypertension (4). There is compelling evidence that PA patients are at higher risk of atrial fibrillation, left ventricular hypertrophy, stroke, and heart failure (5, 6). Notwithstanding these consequences and its being the most common curable endocrine cause of arterial hypertension, PA is markedly underdiagnosed (1, 7), leaving many patients exposed to hyperaldosteronism over the long term. Besides the fact that the diagnostic work-up of PA is perceived as complex and cumbersome, a major reason for under-detection of PA is the recommendation to withdraw antihypertensive drugs that might affect the plasma levels of renin and aldosterone, the key biomarkers recommended by the guidelines to calculate the aldosterone/renin ratio (ARR).
Mineralocorticoid receptor antagonists (MRAs) are the most effective drugs for controlling hyperaldosteronism, but when patients are being treated with these and other agents, the recognition of PA is seen as challenging. Therefore, withdrawal of MRAs is recommended for 4 to 6 weeks before measuring the ARR. However, this practice, albeit endorsed by the guidelines (8), is not evidence-based and relies on anecdotal experiences and retrospective observational studies (9, 10). Moreover, the few studies conducted so far in patients with drug-resistant hypertension, who are, by definition, on multiple agents, support the possibility of screening for PA patients on MRA (11, 12).
Proving that case detection of PA is feasible without withdrawing or switching treatment is important for at least 3 reasons: (i) PA patients can present with severe drug-resistant arterial hypertension that exposes them to an increased risk of events if treatment is switched or withdrawn; (ii) they are exquisitely responsive to MRA; (iii) hypokalemia can be life-threatening when present and profound, and can blunt aldosterone secretion, thus causing false-negative ARR results. MRAs can effectively control both the hypokalemia and the severe hypertension of patients with a florid PA phenotype, and are often used in combination with blockers of the renin-angiotensin system to minimize the MRA dose and thus their side effects. Therefore, we planned a prospective within-patient study to determine if MRA treatment, either alone or combined with blockade of the angiotensin type 1 receptor (AT1-R), could alter the plasma concentration of aldosterone (PAC), active renin (DRC), and the ARR, thus compromising the identification of PA. To this aim, following the Standards for Reporting Diagnostic accuracy (STARD) guidelines for validating diagnostic tests, which recommend use of a gold standard reference, we used the diagnosis of aldosterone-producing adenoma (APA) (13) as conclusively established by the “five corners” criteria (Online Data Supplement Table 1) (14, 15).
Materials and Methods
Patients
The research protocol was approved by the local institutional review board and all participants gave written informed consent. We prospectively recruited consecutive patients referred to the European Society of Hypertension (ESH) center of excellence of the University of Padua, who at screening were switched to a non-interfering treatment following the Endocrine Society practice guidelines recommendations (8), and who presented a PA phenotype. Hence, after the ARR was shown to be unequivocally elevated twice under treatment with noninterfering agents, all underwent a confirmatory test with captopril challenge, as reported (3). Those wishing to pursue surgical cure were then submitted to adrenal vein sampling (AVS), on average 4 to 8 weeks after the initial screening (8).
Included patients were men and women from 18 to 75 years of age. Exclusion criteria comprised refusal to participate to the study and known history of allergy or intolerance to canrenone, olmesartan, and the contrast medium required for AVS.
Data availability
The datasets generated and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request. The data are protected by privacy safeguards.
Diagnostic criteria
Blood pressure (BP) was measured under attended conditions with a semiautomated device and confirmed by 24-hour BP monitoring (Spacelabs Healthcare, Snoqualmie, WA) in case a white-coat effect was suspected.
The patients underwent a bilaterally simultaneous adrenal vein catheterization under unstimulated conditions (16). Bilateral AVS success was defined as a cortisol-based and an androstenedione-based (17) selectivity index > 2.0. Unilateral PA was diagnosed in patients with lateralization index ≥ 2.0, based on recommendations of experts (18) and results of the largest available AVS registry (19).
The patients who showed unilateral PA at a successful AVS underwent unilateral laparoscopic adrenalectomy, which was followed by clinical and biochemical retesting after 1 month to verify biochemical cure (20). Normalization of plasma aldosterone, renin, and potassium levels after surgery, alongside demonstration on pathology of a CYP11B2-expressing adenoma at immunohistochemistry were required to confirm the diagnosis in all cases (21).
Following an identical protocol, we investigated in parallel PA patients who showed bilateral secretion of aldosterone on AVS, as defined above, indicating bilateral adrenal hyperplasia. These patients were given medical treatment as needed to control their high BP and hypokalemia, if present.
Study Design
The protocol of the study is summarized here as it has been previously published (22), and is reported as online Data Supplement Fig. 1 (14). Briefly, before the initial screening, all patients were instructed to maintain their usual salt intake throughout the screening and study period. They were switched to a non-interfering treatment following the guidelines recommendations (8): angiotensin converting enzyme (ACE) inhibitors, angiotensin type 1 receptor blockers (ARBs), beta-blockers, and diuretics were stopped for 4 weeks and MRAs were withdrawn for 6 weeks. Oral potassium chloride (KCl) supplementation was administered, if necessary, to restore normokalemia using 24-hour urinary potassium excretion as a guide to the number of pills. The patients were switched to receive a long-acting calcium channel blocker, mostly verapamil slow-release (180 mg/day), and/or doxazosin (1–4 mg/day). The plasma concentration values of DRC, PAC, and potassium, and the 24-hour urinary excretion of sodium and potassium were then measured in an ISO 9001-certified laboratory at the initial screening and again on the day of AVS, which was performed on average 4 to 8 weeks after screening, as reported (23). On both occasions a captopril challenge test was performed to confirm the diagnosis.
The MRA canrenone (50 mg/day), chosen because it is the main active metabolite of spironolactone and does not require liver metabolism for activation (24), was then started with up-titration to 100 mg/day during the first 2 weeks if needed to control the hypokalemia and/or BP values, while KCl supplementation was stopped and the background therapy was either kept unchanged or, if necessary, lowered.
After 1 month, all biochemical measurements were identically repeated in the same laboratory in all patients. We next tested the effect on the same biochemical variables of another month treatment with olmesartan (10 mg/day with up-titration to 20 mg/day, if necessary) on top of canrenone and background therapy (Online Data Supplement Fig. 1) (14). This was because ACE inhibitors or ARBs are often prescribed along with MRAs to minimize their dose-dependent side effects, as erectile dysfunction and gynecomastia are common in men with high doses of these agents.
Biochemical Testing
DRC and PAC were measured using the commercially available chemiluminescent assay LIAISON Direct Renin Kit (DiaSorin, Saluggia, Italy) and LIAISON XL Aldosterone kit (Diasorin), respectively, which we have previously validated in a prospective study (25). Sodium and potassium were measured with standard methods in the same laboratory in serum and in a 24-hour urine collection.
Statistical Analysis
For descriptive purposes data are presented as mean ± SD (or SE as indicated), or median and interquartile range, as appropriate. DRC, PAC, and ARR values showed a skewed distribution and, therefore, were analyzed after achievement of a normal distribution by log transformation. The within-patient comparison of values was undertaken using a repeated measures ANOVA, with Scheffè post hoc test for comparison between different time points. Distribution of categorical variables was investigated by Chi square test. Level of significance was 0.05, and hypothesis tests were 2-sided.
SPSS (IBM SPSS Statistics for Windows, version 25 Bologna, Italy) and GraphPad (vers 0.8.3 for Mac, La Jolla, CA) were used for statistical analysis.
Sample size calculation.
We had estimated beforehand that in this study a sample size of 40 patients provided a 95% power to show a clinically significant 20% change in the ARR at a 5% α value using a two-sided paired t test.
Results
Baseline features
The baseline clinical and biochemical features of the patients were those expected for a cohort of PA patients (Online Data Supplement Table 2)(14). When divided according to unilateral or bilateral PA (and therefore unilateral adrenalectomy or not), the patients showed similar BP values. However, the subgroup who had no lateralized aldosterone excess at AVS showed a milder PA phenotype, featuring lower PAC, and lower ARR values, than the surgically-treated group (Table 1). The adrenalectomized patients harbored a KCNJ5 mutation in 44% and an ATP1A1 mutation in 1 patient, the remaining not showing any known mutation.
| Variable . | Surgically treated (n = 32) . | Medically-treated (n = 10) . | P . |
|---|---|---|---|
| Age (years) | 50 ± 8 | 59 ± 11 | 0.01 |
| Female Sex (n, % F) | 18 (57%) | 5 (50%) | 0.34 |
| BMI (Kg/m2) | 24.5 ± 4.5 | 25.0 ± 5.0 | 0.63 |
| BSA (m2) | 1.83 ± 0.23 | 1.84 ± 0.25 | 0.62 |
| Systolic BP (mmHg) | 155 ± 18 | 149 ± 7 | 0.13 |
| Diastolic BP (mmHg) | 93 ± 13 | 88 ± 11 | 0.26 |
| Serum Creatinine (micromol/L) | 74 ± 16 | 64 ± 5.6 | 0.04 |
| Serum Na + (mmol/L) | 143 ± 2 | 142 ± 2 | 0.34 |
| Serum K + (mmol/L) | 3.3 ± 0.6 | 3.6 ± 0.4 | 0.15 |
| Urinary Na + Excretion (mmol/day) | 152 ± 62 | 161 ± 43 | 0.68 |
| Urinary K + Excretion (mmol/day) | 81 ± 35 | 88 ± 26 | 0.56 |
| DRC (mIU/L) | 2.0 (2.0-2.0) | 2.3 (2.0–3.8) | 0.20 |
| PAC (ng/dL) | 24.6 (16.2–29.9) | 15.5 (13.1–18.8) | 0.01 |
| ARR (ng/mIU) | 95.8 (67.0–136.7) | 63.6 (34.5–94.2) | 0.04 |
| Variable . | Surgically treated (n = 32) . | Medically-treated (n = 10) . | P . |
|---|---|---|---|
| Age (years) | 50 ± 8 | 59 ± 11 | 0.01 |
| Female Sex (n, % F) | 18 (57%) | 5 (50%) | 0.34 |
| BMI (Kg/m2) | 24.5 ± 4.5 | 25.0 ± 5.0 | 0.63 |
| BSA (m2) | 1.83 ± 0.23 | 1.84 ± 0.25 | 0.62 |
| Systolic BP (mmHg) | 155 ± 18 | 149 ± 7 | 0.13 |
| Diastolic BP (mmHg) | 93 ± 13 | 88 ± 11 | 0.26 |
| Serum Creatinine (micromol/L) | 74 ± 16 | 64 ± 5.6 | 0.04 |
| Serum Na + (mmol/L) | 143 ± 2 | 142 ± 2 | 0.34 |
| Serum K + (mmol/L) | 3.3 ± 0.6 | 3.6 ± 0.4 | 0.15 |
| Urinary Na + Excretion (mmol/day) | 152 ± 62 | 161 ± 43 | 0.68 |
| Urinary K + Excretion (mmol/day) | 81 ± 35 | 88 ± 26 | 0.56 |
| DRC (mIU/L) | 2.0 (2.0-2.0) | 2.3 (2.0–3.8) | 0.20 |
| PAC (ng/dL) | 24.6 (16.2–29.9) | 15.5 (13.1–18.8) | 0.01 |
| ARR (ng/mIU) | 95.8 (67.0–136.7) | 63.6 (34.5–94.2) | 0.04 |
For ARR calculation, the minimum value of DRC was set at 2.0 mIU/L. Normal values on a 100–200 mmol Na+/day: DRC > 2.8 mIU/L, PAC < 12 ng/dL, ARR < 20.6 (ng/mIU). Data are presented as mean ± SD or median and interquartile range, as appropriate.
Abbreviations: ARR, aldosterone/renin ratio; BP, blood pressure; BMI, body mass Index; BSA, body surface area; DRC, direct active renin concentration; PAC, plasma aldosterone concentration.
| Variable . | Surgically treated (n = 32) . | Medically-treated (n = 10) . | P . |
|---|---|---|---|
| Age (years) | 50 ± 8 | 59 ± 11 | 0.01 |
| Female Sex (n, % F) | 18 (57%) | 5 (50%) | 0.34 |
| BMI (Kg/m2) | 24.5 ± 4.5 | 25.0 ± 5.0 | 0.63 |
| BSA (m2) | 1.83 ± 0.23 | 1.84 ± 0.25 | 0.62 |
| Systolic BP (mmHg) | 155 ± 18 | 149 ± 7 | 0.13 |
| Diastolic BP (mmHg) | 93 ± 13 | 88 ± 11 | 0.26 |
| Serum Creatinine (micromol/L) | 74 ± 16 | 64 ± 5.6 | 0.04 |
| Serum Na + (mmol/L) | 143 ± 2 | 142 ± 2 | 0.34 |
| Serum K + (mmol/L) | 3.3 ± 0.6 | 3.6 ± 0.4 | 0.15 |
| Urinary Na + Excretion (mmol/day) | 152 ± 62 | 161 ± 43 | 0.68 |
| Urinary K + Excretion (mmol/day) | 81 ± 35 | 88 ± 26 | 0.56 |
| DRC (mIU/L) | 2.0 (2.0-2.0) | 2.3 (2.0–3.8) | 0.20 |
| PAC (ng/dL) | 24.6 (16.2–29.9) | 15.5 (13.1–18.8) | 0.01 |
| ARR (ng/mIU) | 95.8 (67.0–136.7) | 63.6 (34.5–94.2) | 0.04 |
| Variable . | Surgically treated (n = 32) . | Medically-treated (n = 10) . | P . |
|---|---|---|---|
| Age (years) | 50 ± 8 | 59 ± 11 | 0.01 |
| Female Sex (n, % F) | 18 (57%) | 5 (50%) | 0.34 |
| BMI (Kg/m2) | 24.5 ± 4.5 | 25.0 ± 5.0 | 0.63 |
| BSA (m2) | 1.83 ± 0.23 | 1.84 ± 0.25 | 0.62 |
| Systolic BP (mmHg) | 155 ± 18 | 149 ± 7 | 0.13 |
| Diastolic BP (mmHg) | 93 ± 13 | 88 ± 11 | 0.26 |
| Serum Creatinine (micromol/L) | 74 ± 16 | 64 ± 5.6 | 0.04 |
| Serum Na + (mmol/L) | 143 ± 2 | 142 ± 2 | 0.34 |
| Serum K + (mmol/L) | 3.3 ± 0.6 | 3.6 ± 0.4 | 0.15 |
| Urinary Na + Excretion (mmol/day) | 152 ± 62 | 161 ± 43 | 0.68 |
| Urinary K + Excretion (mmol/day) | 81 ± 35 | 88 ± 26 | 0.56 |
| DRC (mIU/L) | 2.0 (2.0-2.0) | 2.3 (2.0–3.8) | 0.20 |
| PAC (ng/dL) | 24.6 (16.2–29.9) | 15.5 (13.1–18.8) | 0.01 |
| ARR (ng/mIU) | 95.8 (67.0–136.7) | 63.6 (34.5–94.2) | 0.04 |
For ARR calculation, the minimum value of DRC was set at 2.0 mIU/L. Normal values on a 100–200 mmol Na+/day: DRC > 2.8 mIU/L, PAC < 12 ng/dL, ARR < 20.6 (ng/mIU). Data are presented as mean ± SD or median and interquartile range, as appropriate.
Abbreviations: ARR, aldosterone/renin ratio; BP, blood pressure; BMI, body mass Index; BSA, body surface area; DRC, direct active renin concentration; PAC, plasma aldosterone concentration.
Effect of MRA and ARB in the surgically treated patients
In the surgically-cured APA patients, the BP, serum potassium, PAC, DRC, and ARR values, measured twice at baseline (B1 and B2), after 1 month of MRA add-on treatment, and again after another month of combined MRA and ARB therapy are presented in Figs. 1 to 3. All values were similar at B1 and B2. After MRA treatment, BP values fell and serum potassium levels increased (Fig. 1A and Fig. 2A), while the PAC values remained elevated and not significantly different from baseline values (Fig. 3 panel A), although in 3 patients they fell below 12 ng/dL. Of note, DRC remained suppressed and the ARR continued to be elevated in all but 1 case, who had an ARR of 17.2 ng/mIU.
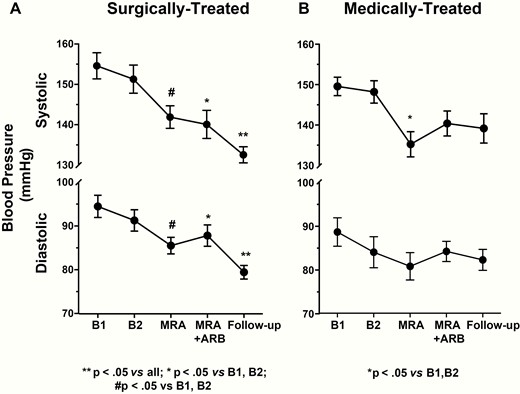
Systolic and diastolic blood pressure values in surgically and medically treated PA patients. A significant decrease in blood pressure was observed in both groups after 1 month of treatment with the mineralocorticoid receptor antagonist (MRA) canrenone; a further significant decrease was found only in adrenalectomized patients. Mean ± SD.
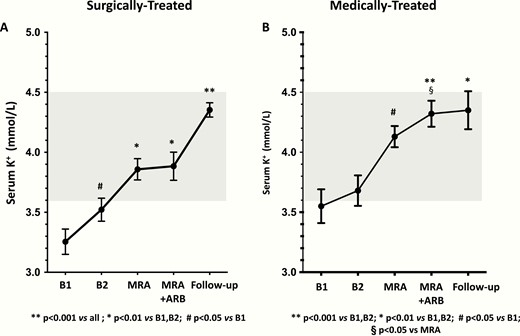
Serum potassium (K+) in surgically and medically treated APA patients. After 1 month of treatment with the MRA, a significant increase was observed in both groups; a further significant increase was found in adrenalectomized patients. The shaded area denotes the normal range. Mean ± SD.
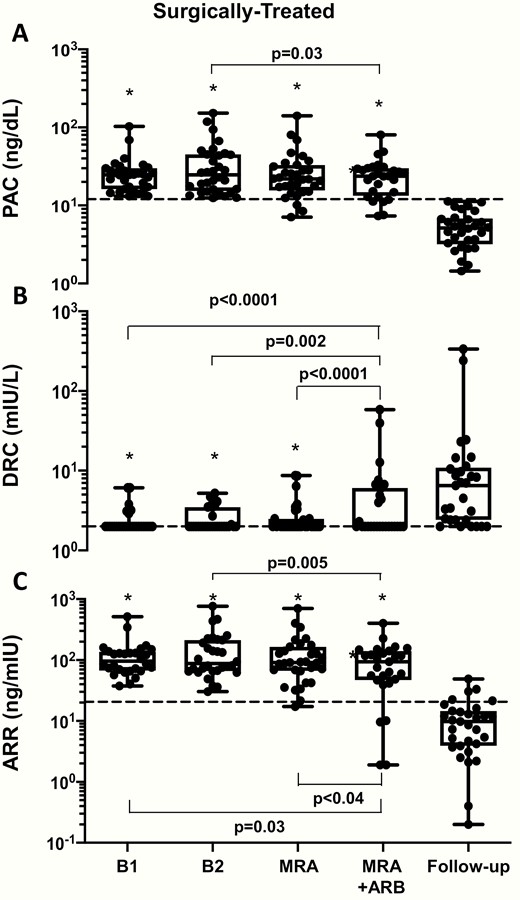
The box and whisker plots show the plasma aldosterone concentration (PAC), direct renin concentration (DRC), and aldosterone/renin ratio (ARR) in surgically treated APA patients measured twice at baseline (B1 and B2). After 1 month of MRA treatment neither PAC nor DRC (and therefore, the ARR) changed significantly. Dotted line indicates 15 ng/dL for PAC, 2 mIU/L for DRC and 20.6 for ARR. Median (interquartile range). * p<.001 vs Follow-up.
The rate of true positive results, that is, PA patients with ARR above our threshold for PA identification (20.6 ng/mIU) was 97% (P = 0.99) (Online Data Supplement Table 3) (14). Of note, these changes occurred without changes in 24-hour urinary potassium and sodium excretion (Online Data Supplement Fig. 2) (14).
Olmesartan did not change PAC, but 5 patients turned negative, in that they showed values below 15 ng/dL. Moreover, olmesartan raised DRC in 9% of the 32 patients (P < 0.01). Hence, the ARR fell and the rate of false negative patients increased to 12%, although not significantly (Online Data Supplement Table 3) (14).
Effects of MRA and ARB in the medically-treated patients
In the patients who were medically treated because of bilateral PA, the MRA lowered BP and increased serum potassium (Fig. 1B and Fig. 2B) in a similar fashion as in the surgically treated patients. The MRA did not change the PAC, DRC, or the ARR values from baseline (Fig. 4), thus leaving the rate of true positive results unchanged (Online Data Supplement Table 3) (14).
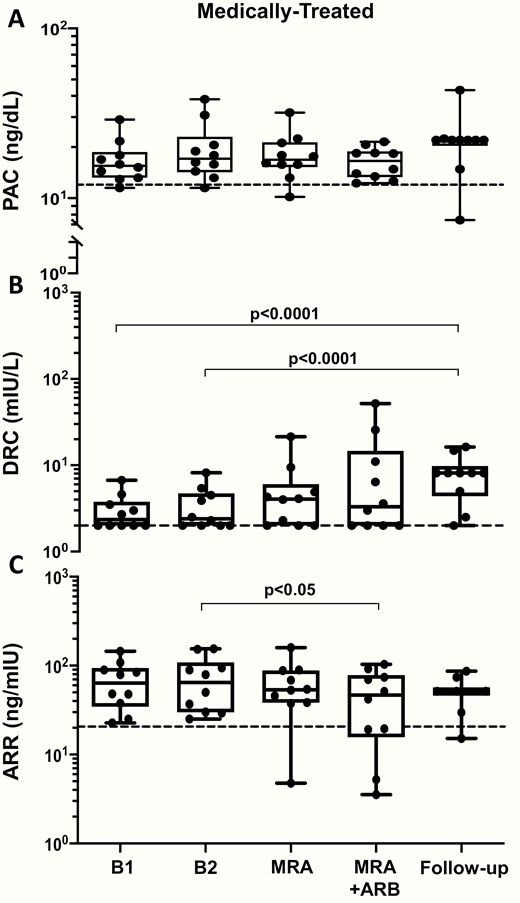
The box and whisker plots show the plasma aldosterone concentration (PAC), direct renin concentration (DRC), and aldosterone/renin ratio (ARR) in medically treated APA patients measured twice at baseline (B1 and B2). After 1 month of MRA treatment no significant change was found. Dotted line indicates 15 ng/dL for PAC, 2 mIU/L for DRC, and 20.6 for ARR. Median (interquartile range).
The addition of the ARB did not further decrease the BP values, but significantly increased the serum potassium compared with the values seen after MRA treatment alone (Fig. 2B). Olmesartan did not affect PAC, but raised DRC in all except 4 patients. As a result of these changes, there was a trend (chi square P = 0.8) toward lower ARR values, resulting in 4 patients with false negative results (i.e., a 40% rate) (Online Data Supplement Table 3) (14). As in the surgically treated patients, the 24-hour urinary potassium and sodium excretion did not change significantly (Online Data Supplement Fig. 2) (14).
Effect of adrenalectomy
After surgery, 50% of the patients were cured from hypertension, as they needed no antihypertensive medications (Fig. 1A). The BP values dropped in a highly significant manner (on average by 22 mm Hg and 13 mm Hg for systolic and diastolic, respectively), notwithstanding the tapering of antihypertensive medications. It is worth noting that serum potassium levels rose to normal values in all patients (Fig. 2A), despite no detectable changes in 24-hour urinary sodium and potassium excretion (Online Data Supplement Fig. 2) (14).
The PAC values fell into the normal range in a highly significant manner (Fig. 3A), which, along with normalization of serum potassium, demonstrated biochemical cure in all patients (Fig. 2). DRC increased significantly; however, notwithstanding resolution of hypertension it remained ≤ 2.0 mIU/L in 6 (19%) of the patients (Fig. 3B), in 3 of whom this persistent renin suppression could be attributed to concomitant treatment with bisoprolol (26). As a result of these changes, the ARR did not fall below 20.6 ng/mIU in 15% of the patients.
Effect of medical treatment
At follow-up, in medically-treated patients the PAC values remained elevated in all but one case; the DRC values were no longer suppressed except in one case receiving bisoprolol (Fig. 4). Likewise none of these patients were cured of hypertension; on average they required 2.7 antihypertensive drugs to control their high BP (Fig. 1B).
The changes of serum potassium and sodium, as well as the 24-hour urinary potassium and sodium excretion values, did not show differences between groups allocated to either treatment (Online Data Supplement Fig. 2) (14).
Discussion
PA is the most common and probably the least investigated endocrine cause of hypertension because of the hurdles encountered in detection (1). A major factor among these impediments is the belief that treatment with antihypertensive agents must be withdrawn for a long period of time (up to 6 weeks for MRAs) in order to obtain an unbiased assessment of the ARR. There is a diffuse concern that such a long withdrawal of antihypertensive agents, and particularly of MRAs, which are effective in lowering BP and controlling hypokalemia, can expose patients to the risks of uncontrolled hypertension and/or hypokalemia-induced arrhythmias. Fragmentary evidence against this view has, however, been provided by observational studies (27–29), but not in prospective studies.
Canrenone is the main active metabolite of the prototypical MRA, spironolactone, but unlike the latter, does not require hepatic activation, thus minimizing potential confounders due interindividual differences of liver function (24).
In this study we prospectively recruited a selected cohort of PA patients wishing to achieve cure and therefore undergoing adrenal vein sampling (AVS). We found that the administration of canrenone neither lowered plasma aldosterone nor did it raise active renin. Thus, it did not factitiously lower the ARR and did not raise the false negative rate (Figs. 3 and 4) (Online Data Supplement Table 3) (14). Albeit seen also in the patients where unilateral PA could not be identified, these results were more evident in the patients with a florid PA phenotype due to unilateral PA that was surgically cured after adrenalectomy. In the latter group, the diagnosis of APA was conclusively determined following the STARD recommendations (13), and by applying the strictest criteria available to date, including immunochemical demonstration of CYP11B2-expressing cells in the adenoma found in the excised adrenal gland (Online Data Supplement Table 1) (14, 15, 21). Being powered to demonstrate a clinically significant 20% change in the ARR, (16) this study showed that prescription of an MRA did not seem to preclude the diagnosis of PA.
Of note, at a dose that further raised serum potassium but provided no more BP lowering, the addition of the ARB raised renin in some PA patients in both the surgically and in the medically treated cohorts (Figs. 3 and 4), an effect that could be valuable in PA patients with persistent hypokalemia despite MRA treatment and/or in male patients in whom estrogen-like side effects impede the up-titration of the MRA.
In the majority of the patients, both surgically and medically treated, the ARB raised DRC and also slightly lowered PAC, and therefore factitiously decreased the ARR, suggesting that the diagnosis of PA could have been missed if the PA patients were screened while on ARBs. This seemed to be the case particularly when the ARR was only borderline elevated in the less florid patients, as in our subgroup of medically treated PA patients (Table 1). The adrenals from these patients could not be obtained for ethical reasons; thus it remains uncertain if some of them, who remained true positive with the ARB, also harbored an APA that escaped diagnosis, or if they had bilateral adrenal hyperplasia with scattered aldosterone-producing cell clusters (7).
On the whole, we believe that these findings can be important for multiple reasons. First, they provide evidence that concomitant MRA treatment did not preclude the correct diagnosis in patients with a surgically curable form of PA, who may present with stage 3 or resistant hypertension and/or prominent hypokalemia, both of which need correction during the screening phase. There is consensus that the MRAs are the most efficacious drugs in these patients and thus they are recommended by the ESH/European Society of Cardiology guidelines for the treatment of resistant hypertension (4). Moreover, the patients with stage 3 or resistant hypertension need to be identified in a timely manner not only because they are at the highest risk, but also because they can be cured with adrenalectomy, both biochemically and clinically. In patients with PA, both unilateral and bilateral, canrenone effectively corrected the hypokalemia and lowered BP, even though it was administered at a relatively low dose. As these hemodynamic and biochemical derangements increase the risk of surgery in these patients, the possibility of correction, as documented in this study, is very appealing.
It might be argued that the lack of a control group of normotensive or essential hypertensive patients is a limitation of this study. However, our predefined aim was not to validate the use of MRA and/or MRA plus ARB for the screening of PA from the hypertensive population at large, but instead to prove the concept that these treatments do not prevent identification of patients who had a conclusive diagnosis of PA (22). Moreover, whether different MRAs, such as apararenone, esaxerenone, and finerenone (30–31), administered for longer periods of time can furnish the same results remains to be ascertained through specific research. However, as canrenone is the main active metabolite of spironolactone, the results obtained with this MRA, which does not require activation in the liver, likely mimic those obtainable with the parent drug (24).
Recent observational retrospective studies showed that the persistence of suppressed renin levels in medically treated PA patients can denote a higher risk of incident atrial fibrillation and renal damage, and might be an index of inadequate mineralocorticoid receptor blockade (6, 32). In our subtyped PA patients DRC remained suppressed notwithstanding achievement of normotension and normokalemia, which indicated adequate mineralocorticoid receptor blockade. However, it raised after addition of olmesartan, suggesting that use of plasma renin as a guide for effective control of the hyperaldosteronism could be inaccurate in PA patients receiving ARBs and likely also ACE inhibitors.
In summary, at this stage of research the following conclusion seems reasonable: MRA treatment with canrenone at doses that control hypertension and hypokalemia does not preclude diagnosis in patients with PA. Based on these results, at our institution we now allow use of MRA during the detection of PA and even at subtyping by means of AVS if renin is not elevated (18). This allowed a remarkable simplification of the workup and a considerable advancement toward widening the detection of the most common curable form of arterial hypertension in the population of hypertensive patients.
Abbreviations
- ACE
angiotensin converting enzyme
- APA
aldosterone-producing adenoma
- ARR
aldosterone/renin ratio
- AT1-R
angiotensin type 1 receptor
- AVS
adrenal vein sampling
- BP
blood pressure
- DRC
direct renin concentration (active renin)
- ESH
European Society of Hypertension
- MRA
mineralocorticoid receptor antagonist
- PA
primary aldosteronism
- PAC
plasma concentration of aldosterone
Acknowledgments
G.P.R. had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Financial Support: Italian Ministry of Health (RF2011-02352318) and University of Padova (DOR1625891/16; DOR1670784/16; BIRD163255/16).
Additional Information
Disclosures. The authors have nothing to disclose.
Data Availability: The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References



