-
PDF
- Split View
-
Views
-
Cite
Cite
Stephany H Donze, Layla Damen, Eva F Mahabier, Anita C S Hokken-Koelega, Improved Mental and Motor Development During 3 Years of GH Treatment in Very Young Children With Prader-Willi Syndrome, The Journal of Clinical Endocrinology & Metabolism, Volume 103, Issue 10, October 2018, Pages 3714–3719, https://doi.org/10.1210/jc.2018-00687
Close - Share Icon Share
Abstract
Infants and toddlers with Prader-Willi syndrome (PWS) have mental and motor developmental delay. Short-term data suggest a positive effect of GH on mental and motor development in infants and children with PWS. There are, however, no longer-term results about the effects of GH treatment on mental and motor development.
To investigate the longer-term effects of GH on psychomotor development in infants and toddlers with PWS and the effect of age at start of GH treatment on psychomotor development.
Prospective cohort study during 3 years of GH treatment.
The PWS Reference Center in the Netherlands.
All children were treated with GH 1 mg/m2/d (≈0.035 mg/kg/d).
Mental and motor developmental age assessed with Bayleys Scales of Infant Development II and expressed as percentage of the expected development (100%).
During 3 years of GH, mean (SEM) mental development increased from 58.1% (2.8) at baseline to 79.6% (3.7), and motor development increased from 41.9% (2.9) to 78.2% (3.9; both P < 0.01). A lower baseline psychomotor development and a younger age at start of GH treatment were associated with a higher increase in mental and motor development (P < 0.01).
Mental and motor development increased significantly during 3 years of GH treatment, reducing the gap between infants with PWS and healthy peers. A younger age at start of GH treatment leads to greater improvement in psychomotor development.
Prader-Willi syndrome (PWS) is a neurodevelopmental disorder resulting from the absence of locus q11-q13 (PWS region) on the paternally derived chromosome 15, caused by a paternal deletion, maternal uniparental disomy (mUPD), imprinting center defect, or paternal chromosomal translocation. Early features of the syndrome include muscular hypotonia, feeding difficulties with poor weight gain, and mental and motor developmental delay. When children get older, they develop short stature, hyperphagia with an increased risk of obesity, neurobehavioral problems, and mild to moderate cognitive impairment with an average IQ between 60 and 70 (1–4).
Long-term continuous GH treatment in children with PWS improves body composition, linear growth, physical strength, and adaptive functioning (5, 6). Short-term data suggest a positive effect of GH on mental and motor development in infants and children with PWS (7–10).
In 29 infants with PWS aged 6 months to 3 years, we found a significant improvement in mental and motor development after 1 year of GH treatment compared with randomized control subjects. Mental and motor development declined in infants who were not treated with GH (7). To our knowledge, there are currently no reports on the longer-term effects of GH on mental and motor development in infants with PWS. In this prospective cohort study, we investigated the effects of 3 years of GH treatment on psychomotor development in a large group of infants with PWS who started GH treatment at a very young age. We hypothesized that psychomotor development would not only improve during the first year of GH treatment but would continue to improve during the second and third years, thereby reducing the disparity compared with infants without PWS.
Because there could be an age-dependent effect of GH on psychomotor development, we also investigated if starting GH at a younger age would correlate with greater mental and motor development during 3 years of GH. We hypothesized that starting GH treatment at a younger age would result in a greater improvement in psychomotor development.
Patients and Methods
Patients
The study group consisted of 63 infants and toddlers with PWS. All study subjects fulfilled the following inclusion criteria: (1) genetically confirmed diagnosis of PWS, (2) naive to GH treatment at time of enrollment, (3) uncomplicated pregnancy and delivery, (4) maximum age of 3 years at first evaluation, and (5) at least 2 Bayley Scales of Infant Development II (BSID-II) tests during 3 years of GH treatment. Six of 63 infants participated in our previous study investigating psychomotor development in infants and toddlers with PWS during 1 year of GH treatment compared with randomized control subjects (7). The study protocol was approved by the Medical Ethics Committee of the Erasmus Medical Centre (Rotterdam, Netherlands) and by the collaborating centers. Written informed consent was obtained from the parents.
Design
All participants were followed at the Dutch PWS Reference Centre. To investigate the effect of GH on mental and motor development, BSID-II scores were analyzed from the start of GH treatment. Children were treated with Genotropin (Pfizer Inc., New York, NY) administered subcutaneously once daily at bedtime (1 mg/m2/d; ≈0.035 mg/kg/d) for at least 3 consecutive years. The GH dose was regularly adjusted based on calculated body surface area and serum IGF-I levels. IGF-I levels were expressed as SD scores, adjusted for age and sex (11). GH dose was lowered if serum IGF-I was >3 SD score.
Anthropometry
All children were examined every 3 months. Standing height was measured using a Harpenden Stadiometer and supine height with a Harpenden Infantometer (Holtain Ltd., Crosswell, UK). Weight was measured on a calibrated electric scale (Servo Balance KA-20-150S; Servo Berkel Prior, Katwijk, Netherlands). Head circumference was measured with a measuring tape.
Height, weight, body mass index, and head circumference were expressed as SD scores using Growth Analyzer 4.0 (available at www.growthanalyser.org), adjusting for sex and age according to Dutch reference values (12, 13).
Psychomotor development
Mental and motor development were measured annually using BSID-II. BSID-II is an individually administered and standardized instrument for developmental diagnostics that can be used to evaluate the mental and motor development of young children with a “developmental age” between 0 and 3.5 years. BSID-II yields two scores: mental developmental age (in months) and motor developmental age (in months). The mental scale consists of items in relation to visual and auditory information processing, language development, memory, eye-hand coordination, imitation, and problem solving. The motor scale assesses fine and gross motor skills. All tests were performed by one experienced psychologist (E.M.) in our PWS Reference Centre (Erasmus University Medical Centre, Rotterdam, Netherlands), and the results were compared with reference data derived from healthy infants and toddlers of comparable age (14, 15). Mental and motor development were expressed as percentage of the expected mental and motor development for their age (%ed) and calculated as follows: (developmental age/chronological age)100.
Body composition
Body composition was annually assessed using dual-energy X-ray absorptiometry (Lunar Prodigy; GE Health Care, Chalfont St Giles, UK). Total fat mass (FM; kg) and lean body mass (LBM; kg) were measured. LBM was calculated as fat-free mass minus bone-mineral content. All scans were made on the same machine, and quality assurance was performed daily. The intra-assay coefficient of variation was 0.41% to 0.88% for fat tissue and 1.57% to 4.49% for LBM (16). Because reference values for dual-energy X-ray absorptiometry in this age group are not yet available, FM and LBM were expressed as percentage of total body weight (FM% and LBM%).
Statistical analysis
Statistical analyses were performed with SPSS 24.0 (SPSS Inc., Chicago, IL). Data were expressed as mean (SD). In case of a non-Gaussian distribution, data were expressed as median [interquartile range (IQR)]. Test results of psychomotor development were expressed as developmental age divided by chronological age multiplied by 100, reflecting the percentage of the expected development (%ed) for that age. To compare psychomotor development with normal development (100%ed), a one-sided t test was used. Sex and genotypic differences in baseline psychomotor development were calculated by independent samples t tests. Correlations between baseline psychomotor development and age, head circumference, and LBM% were calculated by Pearson correlation analysis in case of a Gaussian distribution and by Spearman correlation analysis in case of a non-Gaussian distribution.
Mental and motor development during 3 years of GH were analyzed using linear mixed model analysis with years of GH use (0 = baseline, 1 = 1 year of GH treatment, 2 = 2 years of GH treatment, and 3 = 3 years of GH treatment) as a categorical independent variable and a first-order autoregressive covariance matrix. The effects of genotype, age at start of GH, and change in head circumference and body composition on psychomotor development during GH treatment were determined by using these variables as factors (in case of nominal or ordinal variables) or covariates (in case of scale variables) in the model. Results were adjusted for age and baseline psychomotor development. The effect of age at start of GH on psychomotor development was only corrected for baseline psychomotor development. A P value <0.05 was considered statistically significant.
Results
Baseline characteristics
Sixty-three infants and toddlers with PWS (35 boys, 28 girls) participated in the current evaluation of psychomotor development during 3 years of GH treatment. Baseline clinical characteristics are shown in Table 1. Thirty-one children had a deletion (49.2%), 31 children had mUPD (49.2%), and one child a paternal translocation (1.6%). Birth weight SD score, height SD score, weight for age and height SD score, and head circumference for age SD score were all significantly <0 SD score. GH treatment was started at a median (IQR) age of 1.0 year (0.7 to 1.6).
| . | PWS (n = 63) . |
|---|---|
| Boys/girls, n | 35/28 |
| Genetic subtype: deletion/mUPD/translocation | 31/31/1 |
| Gestational age, wk | 39.8 (38.0 to 41.2) |
| Birth weight (SD score) | −1.1a (−1.8 to −0.6) |
| Age at start GH treatment, y | 1.0 (0.7 to 1.6) |
| Height for age (SD score) | −1.7a (−2.6 to −1.0) |
| Weight for age (SD score) | −1.8a (−2.5 to −0.7) |
| Weight for height (SD score) | −0.6a (−1.4 to 0.5) |
| FM% | 28.6 (26.2 to 34.6) |
| Head circumference for age (SD score) | −1.3a (−1.7 to −0.5) |
| . | PWS (n = 63) . |
|---|---|
| Boys/girls, n | 35/28 |
| Genetic subtype: deletion/mUPD/translocation | 31/31/1 |
| Gestational age, wk | 39.8 (38.0 to 41.2) |
| Birth weight (SD score) | −1.1a (−1.8 to −0.6) |
| Age at start GH treatment, y | 1.0 (0.7 to 1.6) |
| Height for age (SD score) | −1.7a (−2.6 to −1.0) |
| Weight for age (SD score) | −1.8a (−2.5 to −0.7) |
| Weight for height (SD score) | −0.6a (−1.4 to 0.5) |
| FM% | 28.6 (26.2 to 34.6) |
| Head circumference for age (SD score) | −1.3a (−1.7 to −0.5) |
Data expressed as median (IQR).
P < 0.01, compared with 0 SD score.
| . | PWS (n = 63) . |
|---|---|
| Boys/girls, n | 35/28 |
| Genetic subtype: deletion/mUPD/translocation | 31/31/1 |
| Gestational age, wk | 39.8 (38.0 to 41.2) |
| Birth weight (SD score) | −1.1a (−1.8 to −0.6) |
| Age at start GH treatment, y | 1.0 (0.7 to 1.6) |
| Height for age (SD score) | −1.7a (−2.6 to −1.0) |
| Weight for age (SD score) | −1.8a (−2.5 to −0.7) |
| Weight for height (SD score) | −0.6a (−1.4 to 0.5) |
| FM% | 28.6 (26.2 to 34.6) |
| Head circumference for age (SD score) | −1.3a (−1.7 to −0.5) |
| . | PWS (n = 63) . |
|---|---|
| Boys/girls, n | 35/28 |
| Genetic subtype: deletion/mUPD/translocation | 31/31/1 |
| Gestational age, wk | 39.8 (38.0 to 41.2) |
| Birth weight (SD score) | −1.1a (−1.8 to −0.6) |
| Age at start GH treatment, y | 1.0 (0.7 to 1.6) |
| Height for age (SD score) | −1.7a (−2.6 to −1.0) |
| Weight for age (SD score) | −1.8a (−2.5 to −0.7) |
| Weight for height (SD score) | −0.6a (−1.4 to 0.5) |
| FM% | 28.6 (26.2 to 34.6) |
| Head circumference for age (SD score) | −1.3a (−1.7 to −0.5) |
Data expressed as median (IQR).
P < 0.01, compared with 0 SD score.
Baseline percentage of expected mental and motor development (%) are shown in Fig. 1. Mean (SEM) baseline mental and motor development were 58.1% (16.1) and 41.9% (13.9), respectively, both being significantly lower than in healthy reference subjects (P < 0.001). There was a large variation in psychomotor development, with mental development ranging from 24% to 91% of healthy peers and motor development ranging from 15% to 78%. Baseline motor development was significantly lower than mental development (P < 0.001). Mental and motor development were positively correlated (r = 0.69, P < 0.001), meaning that infants with a higher baseline motor developmental score had a higher baseline mental developmental score and vice versa.
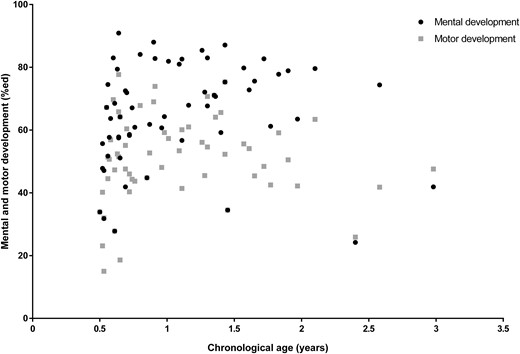
Mental and motor development before start of GH in the total group compared with healthy reference subjects (100%). Black dots represent mental development; gray squares represent motor development. Mental development was less affected than motor development (P < 0.001). A younger age was significantly associated with lower baseline mental development but not motor development (P = 0.01 and P = 0.29, respectively).
Baseline psychomotor development was not different between boys and girls or between infants with a deletion or an mUPD (all P > 0.07). We also found no significant association between head circumference and baseline mental and motor development (P = 0.40 and P = 0.08, respectively). A younger age was significantly associated with lower baseline mental development but not with motor development (P = 0.01 and P = 0.29, respectively). Baseline height SD score was weakly associated with baseline motor development (r = 0.26, P = 0.047), but baseline LBM% was not significantly associated with baseline motor development (P = 0.47).
Psychomotor development during 3 years of GH treatment
During the first year of GH, mean (SEM) mental development increased from 58.1% (2.8) to 65.7% (1.6), and motor development increased from 41.9% (2.9) to 54.8% (1.7) (both P < 0.01). After the first year of GH, mean (SEM) mental and motor development increased further to 79.6 (3.7) and 78.2% (3.9), respectively, after 3 years of GH (both P < 0.01; Fig. 2), further reducing the disparity between infants with PWS and healthy peers of the same age. In spite of this improvement, average mental and motor development after 3 years of GH was still significantly lower compared with healthy reference subjects (both P < 0.001). The change in mental development during 3 years of GH was significantly associated with the change in motor development (r = 0.71, P < 0.001).
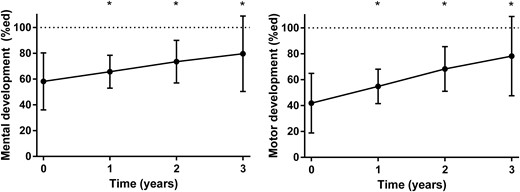
Mental and motor development during 3 years of GH treatment presented as means with 95% confidence interval. Significant changes compared with baseline are indicated with an asterisk. The dashed lines represent the maximum score of 100%.
The course of mental and motor development during 3 years of GH was not significantly different between boys and girls and between infants with a deletion or an mUPD (all P > 0.17). A younger age at start of GH treatment was significantly associated with a greater improvement in mental development (β = −5.5, SE = 1.9, P < 0.01) and motor development (β = −7.8, SE = 2.1, P < 0.001). Head circumference increased significantly from −1.0 SD score at baseline to 0.1 SD score after 3 years (P < 0.01), but this change in head circumference was not significantly associated with the course of mental and motor development (P = 0.77 and P = 0.88, respectively). Neither the change in height SD score nor the change in LBM% significantly influenced motor development during 3 years of GH (P = 0.33 and P = 0.99, respectively).
Discussion
In this study, we assessed psychomotor development during 3 years of GH in a large group of 63 infants and toddlers with PWS with a median age of 1 year at start of GH treatment. Infants and toddlers with PWS demonstrated delayed mental and motor development compared with healthy reference subjects, but, because psychomotor development in children with PWS significantly increased during 3 years of GH treatment, the disparity between children with PWS and healthy reference subjects decreased. Furthermore, the younger the age at start of GH treatment, the greater the increase in psychomotor development.
Mental and motor development increased during all 3 years of GH treatment. Hence, psychomotor development kept on improving, raising the mental and motor potential of infants with PWS. We previously found a significant improvement in mental and motor development after 1 year of GH treatment compared with randomized untreated controls in 29 infants with PWS with a median (IQR) age of 2.0 (1.3 to 3.0) years (7). In a subsequent study in 20 infants, the combination of GH and physical training resulted in a clinically relevant improvement in motor development (10). Myers et al. (8) reported a trend toward improved mobility and stability in infants and toddlers with PWS after 1 year of GH (n = 14) compared with randomized control subjects (n = 11) and more progression in language and cognitive development (n = 7 vs n = 5). The authors noted that it was difficult to quantify differences between groups because of small groups and large within-group variance.
Our data show a significant improvement in mental and motor development during 3 years of GH in very young children with PWS. However, part of this increase may be explained by spontaneous improvement of hypotonia with age and early start of physical therapy, as has been suggested based on a study comparing the effect of 1 year of GH or coenzyme Q10 in 26 infants and toddlers with PWS (17). Because all children with PWS in the Netherlands are treated with GH from a young age, we could not compare psychomotor development of GH-treated infants with that of infants without GH treatment.
We acknowledge that a randomized control trial would be the first-choice design to investigate the longer-term effects of GH on mental and motor development in infants with PWS, but it would be unethical to withhold GH for 3 years, knowing the positive effects of GH on numerous outcomes in children with PWS. We do not expect that parents and/or clinicians anticipating positive results influenced the results of this study because the results are based on BSID-II tests carried out by an independent psychologist. Additionally, this study was performed in very young children with PWS, and the likelihood of them deliberately influencing the results of the BSID-II is negligible.
We have previously shown that mental and motor development decreased in infants who were not treated with GH, whereas psychomotor development significantly increased in those who were treated with GH. Thus, it is likely that psychomotor development would have remained similar or would have decreased if children were not treated with GH (7). This shows that GH treatment plays an important role in psychomotor development in infants with PWS, aside from the spontaneous improvement with age. Six patients also participated in our previous study, but, compared with the 63 patients included in this study, this low number did not unduly influence the results presented in this paper. Moreover, the present data of a larger group confirm the preliminary findings of the first study in 29 infants with PWS (7).
Baseline psychomotor development varied widely among infants and toddlers with PWS, with developmental scores ranging from 15% to 91% of healthy references. This variability was especially large in infants younger than 1 year of age. Infants with a higher motor developmental score had a higher mental developmental score, which could be related to the fact that some mental developmental milestones cannot be reached without reaching certain motor developmental milestones first. For example, infants with poor head stability will not be able to visually follow the voice of their parent(s) and/or caregiver(s).
It could be that, once infants and toddlers have reached certain motor milestones (e.g., sitting or walking independently), they are capable to better explore their environment, which enhances their mental development. This is supported by the significant correlation between the change in motor and mental development during 3 years of GH. Infants with an improvement in motor development would thus be more likely to improve their mental abilities (7, 17).
Brain development during infancy and early childhood is very important for cognitive functioning in the long term (18). We previously found that children with PWS had a trend toward smaller white matter volume, indicating reduced structural connectivity or aberrant myelinization, which may reflect a delay in brain maturation and underlie cognitive deficits in these children (19). GH receptors are expressed throughout the brain, and GH and IGF-I are expected to be involved in brain growth, development, and myelinization. Because serum IGF-I levels are low in children with PWS prior to starting GH treatment, GH might improve brain development by increasing IGF-I levels (20, 21).
We found that a younger age at start of GH treatment led to greater improvement in psychomotor development, suggesting that starting GH treatment early, in a critical period of neurodevelopment, could enhance psychomotor and cognitive development in the longer term (21, 22). One of our previous studies also reported a significant effect of age at start of GH on cognitive development in 50 prepubertal children (2). Another study in 42 children with PWS stated that if GH was started during infancy, GH had a greater effect on adaptive functioning (6). More studies are required to confirm these findings in subjects with PWS.
In contrast to our expectation, but in line with previous data (7), we did not find a significant association between psychomotor development and head circumference at baseline or during 3 years of GH treatment. Head circumference increased from low-normal to normal during GH, but the change in head circumference relative to the change in height was below 1, which means that height SD score increased more than head circumference SD score (data not shown). Thus, it seems that the neuroregulatory role of GH and insulin-like growth factor (IGF-I) in the central nervous system is more important for psychomotor development than the increase in head circumference (20, 23).
Our study investigated the effects of 3 years of GH treatment on psychomotor development. BSID-II is suitable for children with a developmental age between 0 and 3.5 years and can be compared with references using mental and motor index scores. Because infants and toddlers with PWS have delayed psychomotor development, their BSID-II results may only be described in terms of developmental age. Expressing this developmental age as a percentage of the expected mental and motor development for their age allowed us to evaluate psychomotor development during 3 years of GH despite their delay in psychomotor development (14). It is not possible to perform a study of the effects of more than 3 years of GH on mental and motor development with the BSID-II because most children will exceed the maximum developmental age during the first 3 years of GH treatment.
In summary, our study shows that, in a large group of infants and toddlers with PWS, mental and motor development increased significantly during 3 years of GH treatment, reducing the gap between infants with PWS and their healthy peers. Infants with lower baseline psychomotor development advanced more than infants with higher baseline psychomotor development. The increased awareness of PWS and the improved genetic tests have made it possible to diagnose PWS during early infancy. Because starting GH treatment at a younger age seems to result in better psychomotor development, our current clinical practice involves initiation of GH treatment in very young infants with PWS.
Abbreviations:
- BSID-II
Bayleys Scales of Infant Development II
- FM
fat mass
- IQR
interquartile range
- LBM
lean body mass
- mUPD
maternal uniparental disomy
- PWS
Prader-Willi syndrome
Acknowledgments
We thank all children and parents for their enthusiastic participation in this study; Mariëlle van Eekelen for all her work; and all collaborating pediatric endocrinologists, pediatricians, and other healthcare providers.
Financial Support: This study was supported by an independent research grant from Pfizer (to A.C.S.H.-K.). Pfizer was neither involved in conception or design of the study, nor in collection, analysis or interpretation of data, writing the manuscript, or decision to submit the manuscript for publication.
Disclosure Summary: A.C.S.H.-K. received an independent research grant from Pfizer. The remaining authors have nothing to disclose.



