-
PDF
- Split View
-
Views
-
Cite
Cite
Elizabeth M Winter, Natasha M Appelman-Dijkstra, Parathyroid Hormone–Related Protein–Induced Hypercalcemia of Pregnancy Successfully Reversed by a Dopamine Agonist, The Journal of Clinical Endocrinology & Metabolism, Volume 102, Issue 12, 1 December 2017, Pages 4417–4420, https://doi.org/10.1210/jc.2017-01617
Close - Share Icon Share
Abstract
Parathyroid hormone–related protein (PTH-rP)–induced hypercalcemia or pseudophyperparathyroidism during pregnancy is a condition that can result in serious fetal and maternal complications. Among others, breast tissue might be the cause of this PTH-rP production, in which case medical treatment is possible, as we describe in this case.
A 32-year-old woman presented in the 15th week of pregnancy with massive enlargement of breasts and abdominal pain due to severe hypercalcemia, hypercalciuria, and suppressed PTH. Hematological and solid malignancy were excluded. PTH-rP was found to be fourfold to eightfold increased, which is pathological even for pregnancy term. PTH-rP is produced in mammarian tissue as well as in placental tissue, in reaction to prolactin receptor activation. Prolactin hypersensitivity of breast tissue can cause excessive PTH-rP production during pregnancy.
Dopamine agonists were applied to decrease prolactin.
Calcium levels normalized, and PTH-rP levels became undetectable with bromocriptin treatment. A full-term healthy baby was born without disorders of calcium homeostasis, neither directly after birth nor 2 years after follow-up. After delivery, dopamine agonists could be tapered without recurrence of hypercalcemia.
Pseudohyperparathyroidism of pregnancy was caused by increased sensitivity of mammarian tissue for prolactin, which could be treated medically, preventing emergency mastectomy.
Case Presentation
A 32-year-old woman with unremarkable medical history presented in the 15th week of a second pregnancy with abdominal pain, constipation, weight loss, polyuria, and polydipsia for several weeks. As during her previous pregnancy, she again experienced enormous breast augmentation. At physical examination, her body mass index was 21.8 kg/m2 with large and tender breasts, cup size H (D before pregnancy). Laboratory investigation showed a corrected calcium of 3.37 mmol/L (reference 2.15–2.55), with suppressed parathyroid hormone (PTH; <0.3 pmol/L; reference 2–8) and severe hypercalciuria (19 mmol/24 h; reference 2.5–8.0), for which treatment with isotonic saline was started. Vitamin D was low (36 nmol/L; reference 50–250). Phosphate, thyroid, kidney, and liver function were within reference range. PTH-related protein (PTH-rP) appeared to be increased, with values of 2.7 and 5.5 pmol/L (reference <0.7 for nonpregnant women), which is high even after correction for pregnancy term (1). Blood was collected in chilled EDTA-plasma tubes and processed immediately according to guidelines. Because PTH-rP measurements are known to be difficult in terms of assay performance, in the Netherlands, PTH-rP is centrally measured using an immunoradiometric assay from Diagnostic Systems Laboratories, and samples were drawn at multiple days to ensure that high and low values were confirmed. Malignant PTH-rP production was ruled out by thorough physical and radiographic investigations. It was hypothesized that the PTH-rP increase with concomitant hypercalcemia was related to the extreme breast enlargement, also called gigantomastia of pregnancy (2), due to hypersensitivity of the breast tissue to prolactin. We argued that reduction of prolactin level by dopamine agonists could reduce PTH-rP production and thereby lower calcium levels. Although prolactin was normal for pregnancy term (57 μg/mL) (1), this was considered feasible because gigantomastia of pregnancy concerns hypersensitivity rather than absolute hyperprolactinemia. The patient and her partner were fully informed on the initiation of dopamine agonist. Although not registered for this extremely rare condition, dopamine agonists, specifically bromocriptin, are considered safe during pregnancy and used, for instance, in pregnant patients with a prolactinoma. Bromocriptin, in quickly increasing dosage to 2.5 mg three times per day, was started at 18 weeks of gestation (arrow in Fig. 1), in addition to the saline infusions. This resulted in a decrease of prolactin followed by stabilization (Fig. 1B), in contrast to the expected physiologic rise during pregnancy (1). PTH-rP diminished within 3 weeks to undetectable levels, immediately followed by normalization of calcium (Fig. 1A). Shortly after start of treatment, patient reported less tender breasts, and breast growth ceased. Saline infusions were stopped at week 32 of gestation, without recurrence of hypercalcemia. Fetal status was closely monitored; no complications were noticed. At 39 weeks of gestation, a healthy baby was born, without any disorders of calcium homeostasis, neither immediately after birth nor in the following 2 years. Medication was continued after labor to compensate for PTH-rP produced by breast tissue after delivery. PTH-rP levels remained undetectable, and prolactin and calcium remained normal, even after discontinuation of medication. Because breast size did not considerably decrease, as expected, elective reconstructive surgery was performed 6 months after delivery (∼1100 g of normal breast tissue was resected).
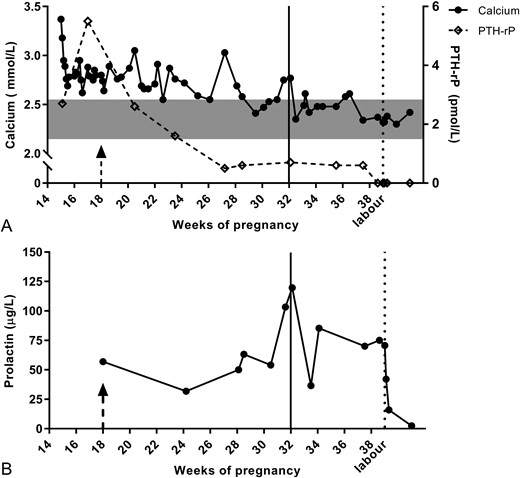
(A) Calcium, PTH-rP, and (B) prolactin values throughout pregnancy and after delivery at week 39. (A) Immediately at presentation, saline infusions were started, resulting in a decrease of calcium but not of PTH-rP. After introduction of dopamine agonist therapy (bromocriptin) at week 18 (arrow), PTH-rP values decreased, directly followed by a decline in calcium until normal values were reached. PTH-rP was hardly detectable from week 27 onward. Saline infusions were ended by week 32 (line) without recurrence of hypercalcemia. After labor (dotted line), calcium remained stable within normal range. (B) Prolactin levels stabilized with bromocriptin therapy rather than the physiological and expected rise in pregnancy. Few missed doses of bromocriptin around week 32 resulted in a transient rise in prolactin and calcium. Gray box represents normal ranges of serum calcium. Arrow indicates start of dopamine agonist therapy at week 18. Line indicates end of saline infusions. Dotted line indicates labor.
Discussion
PTH-rP–induced hypercalcemia, also called pseudohyperparathyroidism (3, 4), in pregnancy is a severe condition caused by mammarian or placental overproduction of PTH-rP, previously treated by acute mastectomy (4, 5) and caesarean section (6), respectively. However, as we describe in this case, conservative treatment with dopamine agonists can resolve the hypercalcemia.
A rise in calcium in reaction to PTH-rP production is a physiological response during pregnancy to contribute to the extra fetal need for calcium (7). During pregnancy, prolactin and PTH-rP, produced by mammarian and placental tissue, accompany normal calcium regulators PTH, calcitonin, and vitamin D. Prolactin stimulates breast tissue to release serotonin (5-hydroxytryptamine), which leads to PTH-rP production from epithelial cells (8). Through PTH-rP release, but also directly, prolactin stimulates receptor activator of nuclear factor κB ligand–induced bone resorption and calcium release (9, 10). The slight increase in calcium levels leads in the end to suppression of PTH. PTH-rP, however, also acts as an important growth factor for bone as well as for other organs in fetal and early life (7, 8, 10). This physiological PTH-rP production during pregnancy can overshoot when mammarian tissue is hypersensitive to prolactin, which can be accompanied by an excessive increase in breast size due to hyperproliferation, the so-called gigantomastia of pregnancy (2, 5). Because it is caused by prolactin hypersensitivity, enlargement stops after labor, but will return in next pregnancies (2), as in our case. Nephrocalcinosis was observed in our patient already within week 15 of her second pregnancy, which could only be explained by longstanding hypercalciuria during first pregnancy, as 15 weeks is too short to result in nephrocalcinosis.
Gigantomastia of pregnancy is mainly described with respect to mechanical problems such as pain and ulceration (2). Only few of the published cases report on high calcium and PTH-rP levels (3–5). It remains to be elucidated whether these laboratory values were not measured at all or were normal and not described. The first report mentioning hypercalcemia with massive mammary hyperplasia during pregnancy was treated by mastectomy, resulting in immediate resolution of hypercalcemia (4). The pathophysiologic mechanism was not known by that time. It was only later, after PTH-rP was cloned in 1988 and demonstrated in breast tissue of lactating mice, that the breast tissue of this patient was reinvestigated and PTH-rP activity was shown, leading to the conclusion of PTH-rP–induced hypercalcemia (5) or pseudohyperparathyroidism (3, 4). Bromocriptin has been described before to partly reduce mammary hypertrophy (2), but has never been described to treat PTH-rP–related hypercalcemia of pregnancy.
In our case, treatment with bromocriptin resulted in an arrest in breast growth and normalization of calcium levels after PTH-rP decline. This supports the hypothesis that the pseudohyperparathyroidism in this case is due to increased mammarian PTH-rP production by hypersensitivity of breast tissue to prolactin, leading to excessive mammarian growth and thus PTH-rP overproduction. However, placental lactogen cannot be ruled out completely because this production can also be modulated by dopamine agonists (3). Although placental PTH-rP production could have contributed, the remarkable gigantomastia suggests the breasts were the main source. Our patient demonstrated a direct relationship between bromocriptin and prolactin/calcium levels. After she had missed some doses of bromocriptin around week 32, prolactin and calcium rose and normalized with reinitiation of medication (Fig. 1).
In conclusion, PTH-rP–induced hypercalcemia/pseudohyperparathyroidism of pregnancy is a rare entity that can be caused by prolactin hypersensitivity of breast tissue, in which medical treatment with dopamine agonists can induce complete remission of hypercalcemia and result in a fully carried pregnancy with good outcome.
Abbreviations:
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
References



