-
PDF
- Split View
-
Views
-
Cite
Cite
Elena Castellano, Roberto Attanasio, Laura Gianotti, Flora Cesario, Francesco Tassone, Giorgio Borretta, Forearm DXA Increases the Rate of Patients With Asymptomatic Primary Hyperparathyroidism Meeting Surgical Criteria, The Journal of Clinical Endocrinology & Metabolism, Volume 101, Issue 7, 1 July 2016, Pages 2728–2732, https://doi.org/10.1210/jc.2016-1513
Close - Share Icon Share
A reduction in bone mineral density (BMD) is common in primary hyperparathyroidism (PHPT), above all at cortical sites. Guidelines for the management of asymptomatic PHPT (aPHPT) recommend a BMD evaluation at the lumbar spine, hip, and forearm. Surgery is recommended for patients with a T-score less than or equal to −2.5 at any of these sites. However, a BMD evaluation at the forearm is not routinely performed.
To evaluate the impact of measuring forearm BMD in the clinical management of aPHPT.
We retrospectively reviewed a prospective database of 172 patients with aPHPT, selecting the 116 patients in whom a dual x-ray absorptiometry (DXA) scan had been performed at all 3 sites.
Seventy-four out of 116 patients had a densitometric diagnosis of osteoporosis (OP) at any site, and the forearm was the only site involved in 13/74 (group A, 17.6% of osteoporotic patients and 11.2% of the whole aPHPT cohort). Patients belonging to group A were significantly older than the other aPHPT patients, whereas no difference was found in biochemical measurements. Six out of 13 patients belonging to group A (5.2% of the whole aPHPT cohort) fulfilled surgical criteria based only on a forearm T-score.
DXA at 3 sites revealed OP at the forearm, but not at the other sites, in 11.2% of aPHPT patients. Half of these cases met surgical criteria based on this one factor alone. These patients did not show any clinical (except age) or biochemical differences from the other patients. The implementation of forearm DXA increases the rate of patients with aPHPT meeting surgical criteria.
This study evaluated the impact of measuring forearm BMD in the clinical management of aPHPT, according to latest guidelines: the implementation of forearm DXA increases the rate of patients with aPHPT meeting surgical criteria.
A reduction in bone mineral density (BMD) is a common feature in primary hyperparathyroidism (PHPT) (1), also in its asymptomatic form (asymptomatic PHPT [aPHPT]) (2, 3).
PHPT mostly affects the cortical bone (1, 2). Low BMD is thus predominantly seen at sites rich in cortical bone, such as the distal forearm (1).
Guidelines for aPHPT management (4) indicate measuring BMD at multiple sites, namely the lumbar spine, hip, and forearm, and surgery is recommended for patients with a T-score of −2.5 or less at any of these sites.
Osteoporosis (OP) at any site is the most commonly met criterion among those indicated in the most recent guidelines (5). However, a BMD evaluation at the forearm is not routinely performed (6). Wood et al (6) reported distal forearm assessments in only 45% of a large surgical series submitted to a preoperative dual x-ray absorptiometry (DXA) scan, resulting in an underestimation of OP diagnoses. This is particularly significant for aPHPT, where an underestimation of OP can lead to surgical indications being missed (4).
To date, no data are available on the impact of omitting forearm DXA on the therapeutic management of aPHPT. We thus evaluated the role of forearm DXA assessments in a large series of aPHPT, as for additional OP diagnosis and surgical indications.
Materials and Methods
Design
A retrospective survey was performed on medical records of all patients diagnosed with aPHPT and attending our department for routine clinical care from January 1998 to December 2013.
Patients
Patients had been referred by general practitioners, primary care clinics, and subspecialty clinics.
Diagnosis of PHPT had been established by the presence of hypercalcemia and concomitant inappropriately raised serum PTH levels on at least 2 separate occasions (reference range for calcium and PTH levels, 8.4–10.2 mg/dL and 15–65 ng/L, respectively).
Patients diagnosed with multiple endocrine neoplasm, hyperparathyroidism-jaw tumor syndrome, and familial hypocalciuric hypercalcemia were excluded.
No patients had been taking calcium or vitamin D supplementations for at least 6 months.
Patients with no bone or kidney involvement, and without hypercalcemic symptoms, were classified as aPHPT. Regarding bone involvement, all patients had routinely undergone a radiographic evaluation of the skull and hands, looking for subtle signs of osteitis fibrosa cystica, such as subperiosteal resorption in fingers, salt and pepper mottling of the skull, or brown tumors. In terms of kidney involvement, patients were classified as symptomatic either if they complained of symptoms of nephro-urolithiasis or if stones (or calcinosis) were disclosed by routinely performed abdominal ultrasound (US) examination.
Among the 172 consecutive aPHPT patients, we selected the 116 patients who had undergone DXA at all 3 sites with reliable results (ie, degenerative disease or scoliosis as well as hardware problems, potentially leading to unreliable results, had been ruled out).
We retrospectively applied to all patients the criteria for surgery that were reported in the latest guidelines (4).
Methods
Serum total calcium and creatinine levels were assayed by automated analysis using colorimetric and enzymatic methods, whereas ionized serum calcium was analyzed by a specific probe after correction for pH.
Serum intact PTH concentrations were measured up to 2012 using a 2-site immunochemiluminometric assay (Immulite 2000; DPC) with an inter- and intraassay variation coefficient of 6.3%–8.8% and 4.2%–5.7%, respectively. Since 2012, serum intact PTH concentrations were measured using a third generation immunochemiluminometric assay (COBAS e411; Roche Diagnostics) with an inter- and intraassay variation coefficient of 3.1%–6.5% and 1.4%–3.2%, respectively.
Serum 25OH-vitamin D (25OHD) levels were measured by a RIA (DIAsource 25OH-Vit.D3-Ria-CT kit; DIAsource ImmunoAssays S.A.), with a detection limit of 0.6 ng/mL (1.5 nmol/L) and inter- and intraassay variation coefficient of 5.3% and 4.7%, respectively. Our laboratory periodically performs a quality control of every kit used with the quality control material provided by the manufacturer. Our laboratory is a member of the External Quality Assessment scheme for the estimation of 25OHD conducted by the QualiMedLab-CNR (Pisa, Italy), as a means of determining the accuracy of results.
BMD was measured at the lumbar spine (L2–L4), proximal femur, and distal third of the nondominant radius using the same instrument (DXA QDR-4500; Hologic) throughout the whole study period. Minor upgrades, above all in the reporting and duration of the procedure, did not significantly impact the results. Data are reported as absolute measurements (in grams per square centimeter).
This observational study was conducted in accordance with the Declaration of Helsinki. It was approved by the institutional review board and Ethical Committee of our institution. No informed consent was required for this study, because we only accessed a deidentified database retrospectively for analysis purposes. All data were collected as part of routine clinical and psychological procedures.
Statistical analysis
Variables were preliminarily tested for normal distribution with the Shapiro-Wilks W test and data were expressed as mean ± SD, or median and interquartile range as appropriate, according to the results. The Mann-Whitney U test and t test for unpaired samples were used to compare continuous variables with nonnormal and normal distribution, respectively. Differences in categorical variables were sought by χ2 or Fisher's test, as appropriate. The level of statistical significance was set at P < .05. Calculations were performed using Statistica for Windows 5.1 (Statsoft, Inc).
Results
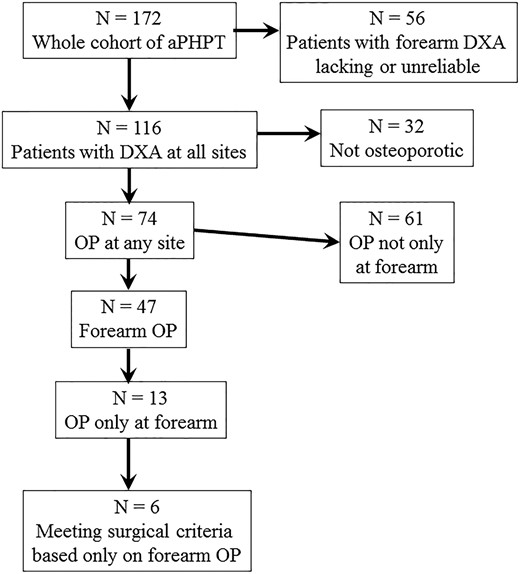
A total of 172 consecutive aPHPT patients were evaluated (Table 1). A total of 116 of these (67.4%), who fulfilled prespecified inclusion and exclusion criteria, were submitted to analysis. Figure 1 shows a diagram flow of the series.
| . | Patients . | Normal Range . |
|---|---|---|
| Age (y) | 63.9 ± 12.9 | |
| Females (n, %) | 144 (83.7%) | |
| PTH (ng/L) | 123 [111] | 15–65 |
| s-Calcium (mg/dL) | 10.9 ± 0.8 | 8.4–10.2 |
| Ionized calcium (mmol/L) | 1.4 ± 0.3 | 1.13–1.32 |
| Urinary calcium (mg/24 h) | 227.9 ± 162.9 | 100–300 |
| 25OHD (ng/mL) | 27 [23.3] | >20 |
| Creatinine (mg/dL) | 0.9 ± 0.3 | 0.6–1. 2 |
| Distal third radius BMD (g/cm2) | 0.43 ± 0.22 | |
| Lumbar spine BMD (g/cm2) | 0.83 ± 0.42 | |
| Hip BMD (g/cm2) | 0.72 ± 0.37 |
| . | Patients . | Normal Range . |
|---|---|---|
| Age (y) | 63.9 ± 12.9 | |
| Females (n, %) | 144 (83.7%) | |
| PTH (ng/L) | 123 [111] | 15–65 |
| s-Calcium (mg/dL) | 10.9 ± 0.8 | 8.4–10.2 |
| Ionized calcium (mmol/L) | 1.4 ± 0.3 | 1.13–1.32 |
| Urinary calcium (mg/24 h) | 227.9 ± 162.9 | 100–300 |
| 25OHD (ng/mL) | 27 [23.3] | >20 |
| Creatinine (mg/dL) | 0.9 ± 0.3 | 0.6–1. 2 |
| Distal third radius BMD (g/cm2) | 0.43 ± 0.22 | |
| Lumbar spine BMD (g/cm2) | 0.83 ± 0.42 | |
| Hip BMD (g/cm2) | 0.72 ± 0.37 |
Data are expressed as mean ± SD when normally distributed, median and [IQR] when not normally distributed and as absolute number and percentage when categorical. BMD, bone mass density; IQR, interquartile range.
| . | Patients . | Normal Range . |
|---|---|---|
| Age (y) | 63.9 ± 12.9 | |
| Females (n, %) | 144 (83.7%) | |
| PTH (ng/L) | 123 [111] | 15–65 |
| s-Calcium (mg/dL) | 10.9 ± 0.8 | 8.4–10.2 |
| Ionized calcium (mmol/L) | 1.4 ± 0.3 | 1.13–1.32 |
| Urinary calcium (mg/24 h) | 227.9 ± 162.9 | 100–300 |
| 25OHD (ng/mL) | 27 [23.3] | >20 |
| Creatinine (mg/dL) | 0.9 ± 0.3 | 0.6–1. 2 |
| Distal third radius BMD (g/cm2) | 0.43 ± 0.22 | |
| Lumbar spine BMD (g/cm2) | 0.83 ± 0.42 | |
| Hip BMD (g/cm2) | 0.72 ± 0.37 |
| . | Patients . | Normal Range . |
|---|---|---|
| Age (y) | 63.9 ± 12.9 | |
| Females (n, %) | 144 (83.7%) | |
| PTH (ng/L) | 123 [111] | 15–65 |
| s-Calcium (mg/dL) | 10.9 ± 0.8 | 8.4–10.2 |
| Ionized calcium (mmol/L) | 1.4 ± 0.3 | 1.13–1.32 |
| Urinary calcium (mg/24 h) | 227.9 ± 162.9 | 100–300 |
| 25OHD (ng/mL) | 27 [23.3] | >20 |
| Creatinine (mg/dL) | 0.9 ± 0.3 | 0.6–1. 2 |
| Distal third radius BMD (g/cm2) | 0.43 ± 0.22 | |
| Lumbar spine BMD (g/cm2) | 0.83 ± 0.42 | |
| Hip BMD (g/cm2) | 0.72 ± 0.37 |
Data are expressed as mean ± SD when normally distributed, median and [IQR] when not normally distributed and as absolute number and percentage when categorical. BMD, bone mass density; IQR, interquartile range.
The selected 116 patients were not statistically different from the remaining 56 aPHPT patients in terms of demographic and biochemical parameters (Table 2).
| . | Included in the Analysis (n = 116) . | Excluded From Analysis (n = 56) . | Statistical Significance . |
|---|---|---|---|
| Age (y) | 63.7 ± 11.7 | 63.9 ± 14.9 | n.s. |
| Females (n, %) | 97 (83.6%) | 47 (84%) | n.s. |
| PTH (ng/L) | 123 [103] | 120 [130] | n.s. |
| s-Calcium (mg/dL) | 10.9 ± 0.8 | 11.01 ± 0.9 | n.s. |
| Ionized calcium (mmol/L) | 1.4 ± 0.2 | 1.45 ± 0.3 | n.s. |
| Urinary calcium (mg/24 h) | 214.4 ± 155 | 255 ± 182 | n.s. |
| 25OHD (ng/mL) | 27 [21] | 24.5 [21.5] | n.s. |
| Creatinine (mg/dL) | 0.8 ± 0.2 | 0.9 ± 0.3 | n.s. |
| Distal third radius BMD (g/cm2) | 0.43 ± 0.17 | 0.46 ± 0.23 | n.s. |
| Lumbar spine BMD (g/cm2) | 0.83 ± 0.29 | 0.77 ± 0.31 | n.s. |
| Hip BMD (g/cm2) | 0.72 ± 0.29 | 0.66 ± 0.2 | n.s. |
| . | Included in the Analysis (n = 116) . | Excluded From Analysis (n = 56) . | Statistical Significance . |
|---|---|---|---|
| Age (y) | 63.7 ± 11.7 | 63.9 ± 14.9 | n.s. |
| Females (n, %) | 97 (83.6%) | 47 (84%) | n.s. |
| PTH (ng/L) | 123 [103] | 120 [130] | n.s. |
| s-Calcium (mg/dL) | 10.9 ± 0.8 | 11.01 ± 0.9 | n.s. |
| Ionized calcium (mmol/L) | 1.4 ± 0.2 | 1.45 ± 0.3 | n.s. |
| Urinary calcium (mg/24 h) | 214.4 ± 155 | 255 ± 182 | n.s. |
| 25OHD (ng/mL) | 27 [21] | 24.5 [21.5] | n.s. |
| Creatinine (mg/dL) | 0.8 ± 0.2 | 0.9 ± 0.3 | n.s. |
| Distal third radius BMD (g/cm2) | 0.43 ± 0.17 | 0.46 ± 0.23 | n.s. |
| Lumbar spine BMD (g/cm2) | 0.83 ± 0.29 | 0.77 ± 0.31 | n.s. |
| Hip BMD (g/cm2) | 0.72 ± 0.29 | 0.66 ± 0.2 | n.s. |
Comparison between patients included in the analysis and the others. Normally distributed data are expressed as mean ± SD, not normally distributed ones as median and [IQR], categorical ones as absolute number and percentage. BMD, bone mass density; IQR, interquartile range.
| . | Included in the Analysis (n = 116) . | Excluded From Analysis (n = 56) . | Statistical Significance . |
|---|---|---|---|
| Age (y) | 63.7 ± 11.7 | 63.9 ± 14.9 | n.s. |
| Females (n, %) | 97 (83.6%) | 47 (84%) | n.s. |
| PTH (ng/L) | 123 [103] | 120 [130] | n.s. |
| s-Calcium (mg/dL) | 10.9 ± 0.8 | 11.01 ± 0.9 | n.s. |
| Ionized calcium (mmol/L) | 1.4 ± 0.2 | 1.45 ± 0.3 | n.s. |
| Urinary calcium (mg/24 h) | 214.4 ± 155 | 255 ± 182 | n.s. |
| 25OHD (ng/mL) | 27 [21] | 24.5 [21.5] | n.s. |
| Creatinine (mg/dL) | 0.8 ± 0.2 | 0.9 ± 0.3 | n.s. |
| Distal third radius BMD (g/cm2) | 0.43 ± 0.17 | 0.46 ± 0.23 | n.s. |
| Lumbar spine BMD (g/cm2) | 0.83 ± 0.29 | 0.77 ± 0.31 | n.s. |
| Hip BMD (g/cm2) | 0.72 ± 0.29 | 0.66 ± 0.2 | n.s. |
| . | Included in the Analysis (n = 116) . | Excluded From Analysis (n = 56) . | Statistical Significance . |
|---|---|---|---|
| Age (y) | 63.7 ± 11.7 | 63.9 ± 14.9 | n.s. |
| Females (n, %) | 97 (83.6%) | 47 (84%) | n.s. |
| PTH (ng/L) | 123 [103] | 120 [130] | n.s. |
| s-Calcium (mg/dL) | 10.9 ± 0.8 | 11.01 ± 0.9 | n.s. |
| Ionized calcium (mmol/L) | 1.4 ± 0.2 | 1.45 ± 0.3 | n.s. |
| Urinary calcium (mg/24 h) | 214.4 ± 155 | 255 ± 182 | n.s. |
| 25OHD (ng/mL) | 27 [21] | 24.5 [21.5] | n.s. |
| Creatinine (mg/dL) | 0.8 ± 0.2 | 0.9 ± 0.3 | n.s. |
| Distal third radius BMD (g/cm2) | 0.43 ± 0.17 | 0.46 ± 0.23 | n.s. |
| Lumbar spine BMD (g/cm2) | 0.83 ± 0.29 | 0.77 ± 0.31 | n.s. |
| Hip BMD (g/cm2) | 0.72 ± 0.29 | 0.66 ± 0.2 | n.s. |
Comparison between patients included in the analysis and the others. Normally distributed data are expressed as mean ± SD, not normally distributed ones as median and [IQR], categorical ones as absolute number and percentage. BMD, bone mass density; IQR, interquartile range.
Seventy-four out of 116 patients (64%) had OP, according to DXA results, with data at the different sites detailed in Table 3.
| . | Lumbar DXA . | Forearm DXA . | Hip DXA . |
|---|---|---|---|
| T score (mean ± SD) | −2.65 ± 3.25 | −2.6 ± 4.08 | −2.9 ± 2.87 |
| Patients with OP at this site (n, %) | 52 (45%) | 47 (41%) | 34 (29%) |
| Patients with OP only at this site (n, %) | 16 (13.8%) | 13 (11.2%) | 6 (5.2%) |
| . | Lumbar DXA . | Forearm DXA . | Hip DXA . |
|---|---|---|---|
| T score (mean ± SD) | −2.65 ± 3.25 | −2.6 ± 4.08 | −2.9 ± 2.87 |
| Patients with OP at this site (n, %) | 52 (45%) | 47 (41%) | 34 (29%) |
| Patients with OP only at this site (n, %) | 16 (13.8%) | 13 (11.2%) | 6 (5.2%) |
| . | Lumbar DXA . | Forearm DXA . | Hip DXA . |
|---|---|---|---|
| T score (mean ± SD) | −2.65 ± 3.25 | −2.6 ± 4.08 | −2.9 ± 2.87 |
| Patients with OP at this site (n, %) | 52 (45%) | 47 (41%) | 34 (29%) |
| Patients with OP only at this site (n, %) | 16 (13.8%) | 13 (11.2%) | 6 (5.2%) |
| . | Lumbar DXA . | Forearm DXA . | Hip DXA . |
|---|---|---|---|
| T score (mean ± SD) | −2.65 ± 3.25 | −2.6 ± 4.08 | −2.9 ± 2.87 |
| Patients with OP at this site (n, %) | 52 (45%) | 47 (41%) | 34 (29%) |
| Patients with OP only at this site (n, %) | 16 (13.8%) | 13 (11.2%) | 6 (5.2%) |
Forty-seven out of 74 (63.5%) osteoporotic patients had OP at the forearm. Of these, 13 had a T-score lower than −2.5 at the forearm only, and were identified as group A. These patients represented 11.2% (13/116) of the whole cohort of aPHPT patients, 17.6% (13/74) of the osteoporotic subgroup, and 27.7% (13/47) of the patients with forearm OP.
The remaining 103 aPHPT patients were referred to as group B. As detailed in Table 4, patients belonging to group A were significantly older than those in group B. In group A, PTH levels were also higher and 25OHD were lower than in group B; however, this difference was not statistically significant. No significant difference was found in BMD values at the other sites.
| . | Groups . | Statistical Significance . | |||
|---|---|---|---|---|---|
| A (n = 13) . | B (n = 103) . | C (n = 61) . | A vs B . | A vs C . | |
| Age (y) | 71 ± 7.7 | 62.7 ± 11.8 | 65 ± 10.6 | 0.016 | n.s. |
| Females (n, %) | 11 (84.6%) | 86 (83.5%) | 52 (85.3%) | n.s. | n.s. |
| PTH (ng/L) | 183 [113] | 117 [87] | 130 [120] | n.s. | n.s. |
| s-Calcium (mg/dL) | 10.9 ± 0.9 | 10.9 ± 0.8 | 10.9 ± 0.75 | n.s. | n.s. |
| Ionized calcium (mmol/L) | 1.4 ± 0.1 | 1.4 ± 0.2 | 1.4 ± 0.2 | n.s. | n.s. |
| Urinary calcium (mg/24 h) | 203.8 ± 127.7 | 215.9 ± 159.3 | 229 ± 157 | n.s. | n.s. |
| 25OHD (ng/mL) | 18 [18] | 28 [22.25] | 24 [20] | n.s. | n.s. |
| Creatinine (mg/dL) | 0.9 ± 0.2 | 0.8 ± 0.2 | 0.84 ± 0.2 | n.s. | n.s. |
| Distal third radius BMD (g/cm2) | 0.40 ± 0.11 | 0.45 ± 0.11 | 0.40 ± 0.12 | n.s. | n.s. |
| Lumbar spine BMD (g/cm2) | 0.9 ± 0.26 | 0.82 ± 0.14 | 0.78 ± 0.12 | n.s. | 0.012 |
| Hip BMD (g/cm2) | 0.70 ± 0.11 | 0.72 ± 0.15 | 0.69 ± 0.14 | n.s. | n.s. |
| . | Groups . | Statistical Significance . | |||
|---|---|---|---|---|---|
| A (n = 13) . | B (n = 103) . | C (n = 61) . | A vs B . | A vs C . | |
| Age (y) | 71 ± 7.7 | 62.7 ± 11.8 | 65 ± 10.6 | 0.016 | n.s. |
| Females (n, %) | 11 (84.6%) | 86 (83.5%) | 52 (85.3%) | n.s. | n.s. |
| PTH (ng/L) | 183 [113] | 117 [87] | 130 [120] | n.s. | n.s. |
| s-Calcium (mg/dL) | 10.9 ± 0.9 | 10.9 ± 0.8 | 10.9 ± 0.75 | n.s. | n.s. |
| Ionized calcium (mmol/L) | 1.4 ± 0.1 | 1.4 ± 0.2 | 1.4 ± 0.2 | n.s. | n.s. |
| Urinary calcium (mg/24 h) | 203.8 ± 127.7 | 215.9 ± 159.3 | 229 ± 157 | n.s. | n.s. |
| 25OHD (ng/mL) | 18 [18] | 28 [22.25] | 24 [20] | n.s. | n.s. |
| Creatinine (mg/dL) | 0.9 ± 0.2 | 0.8 ± 0.2 | 0.84 ± 0.2 | n.s. | n.s. |
| Distal third radius BMD (g/cm2) | 0.40 ± 0.11 | 0.45 ± 0.11 | 0.40 ± 0.12 | n.s. | n.s. |
| Lumbar spine BMD (g/cm2) | 0.9 ± 0.26 | 0.82 ± 0.14 | 0.78 ± 0.12 | n.s. | 0.012 |
| Hip BMD (g/cm2) | 0.70 ± 0.11 | 0.72 ± 0.15 | 0.69 ± 0.14 | n.s. | n.s. |
| . | Groups . | Statistical Significance . | |||
|---|---|---|---|---|---|
| A (n = 13) . | B (n = 103) . | C (n = 61) . | A vs B . | A vs C . | |
| Age (y) | 71 ± 7.7 | 62.7 ± 11.8 | 65 ± 10.6 | 0.016 | n.s. |
| Females (n, %) | 11 (84.6%) | 86 (83.5%) | 52 (85.3%) | n.s. | n.s. |
| PTH (ng/L) | 183 [113] | 117 [87] | 130 [120] | n.s. | n.s. |
| s-Calcium (mg/dL) | 10.9 ± 0.9 | 10.9 ± 0.8 | 10.9 ± 0.75 | n.s. | n.s. |
| Ionized calcium (mmol/L) | 1.4 ± 0.1 | 1.4 ± 0.2 | 1.4 ± 0.2 | n.s. | n.s. |
| Urinary calcium (mg/24 h) | 203.8 ± 127.7 | 215.9 ± 159.3 | 229 ± 157 | n.s. | n.s. |
| 25OHD (ng/mL) | 18 [18] | 28 [22.25] | 24 [20] | n.s. | n.s. |
| Creatinine (mg/dL) | 0.9 ± 0.2 | 0.8 ± 0.2 | 0.84 ± 0.2 | n.s. | n.s. |
| Distal third radius BMD (g/cm2) | 0.40 ± 0.11 | 0.45 ± 0.11 | 0.40 ± 0.12 | n.s. | n.s. |
| Lumbar spine BMD (g/cm2) | 0.9 ± 0.26 | 0.82 ± 0.14 | 0.78 ± 0.12 | n.s. | 0.012 |
| Hip BMD (g/cm2) | 0.70 ± 0.11 | 0.72 ± 0.15 | 0.69 ± 0.14 | n.s. | n.s. |
| . | Groups . | Statistical Significance . | |||
|---|---|---|---|---|---|
| A (n = 13) . | B (n = 103) . | C (n = 61) . | A vs B . | A vs C . | |
| Age (y) | 71 ± 7.7 | 62.7 ± 11.8 | 65 ± 10.6 | 0.016 | n.s. |
| Females (n, %) | 11 (84.6%) | 86 (83.5%) | 52 (85.3%) | n.s. | n.s. |
| PTH (ng/L) | 183 [113] | 117 [87] | 130 [120] | n.s. | n.s. |
| s-Calcium (mg/dL) | 10.9 ± 0.9 | 10.9 ± 0.8 | 10.9 ± 0.75 | n.s. | n.s. |
| Ionized calcium (mmol/L) | 1.4 ± 0.1 | 1.4 ± 0.2 | 1.4 ± 0.2 | n.s. | n.s. |
| Urinary calcium (mg/24 h) | 203.8 ± 127.7 | 215.9 ± 159.3 | 229 ± 157 | n.s. | n.s. |
| 25OHD (ng/mL) | 18 [18] | 28 [22.25] | 24 [20] | n.s. | n.s. |
| Creatinine (mg/dL) | 0.9 ± 0.2 | 0.8 ± 0.2 | 0.84 ± 0.2 | n.s. | n.s. |
| Distal third radius BMD (g/cm2) | 0.40 ± 0.11 | 0.45 ± 0.11 | 0.40 ± 0.12 | n.s. | n.s. |
| Lumbar spine BMD (g/cm2) | 0.9 ± 0.26 | 0.82 ± 0.14 | 0.78 ± 0.12 | n.s. | 0.012 |
| Hip BMD (g/cm2) | 0.70 ± 0.11 | 0.72 ± 0.15 | 0.69 ± 0.14 | n.s. | n.s. |
No significant difference was found between group A and the remaining OP patients (61, group C) regarding all the evaluated parameters, except lumbar BMD (Table 4).
Six out of 13 patients in group A (46%, and 5.2% of the whole aPHPT cohort) fulfilled the criteria for surgery based on forearm BMD alone.
Discussion
Our study shows that routine forearm DXA led to a diagnosis of OP in an additional 11.2% of patients with aPHPT. Half of these patients thus met the surgical criteria (4) based on forearm DXA alone.
Our study also shows that patients with OP at the forearm alone cannot be distinguished from other aPHPT patients based on demographic or biochemical features, except age.
Excess PTH increases bone turnover due to an expansion of the remodeling space and an increased endocortical resorption (7). The resulting high bone turnover is associated with a BMD reduction. The impact is different among various skeletal sites, with the preferential involvement of cortical bone (8).
In clinical practice, bone damage is currently evaluated by DXA (9). The most recent guidelines for aPHPT management (4) recommend performing DXA at all 3 sites, including, beyond lumbar spine and hip, the forearm, which best reflects cortical bone damage (10). PHPT is indeed the only disease in which forearm BMD is recommended according to the International Society for Clinical Densitometry (11). However, this recommendation is not routinely applied in real practice (6).
In 2012, Wood et al reported an underuse of preoperative forearm DXA assessments in a large surgical PHPT series (6). This assessment was performed in only 45% of their series, namely in 54% of patients evaluated by endocrinologists and in 17% of those seen by nonendocrinologists. One possible explanation is that forearm DXA is considered redundant whenever PHPT is diagnosed in patients with known OP at the routinely examined lumbar and femoral sites (11). In addition, forearm DXA assessments require a separate application form in some institutions, thus reducing its implementation (6).
Failing to measure BMD at the forearm may result in an underestimation of OP (9). In particular, in Wood et al's series (6) forearm DXA led to the diagnosis of OP in an additional 6.4% of patients. In accordance with Wood et al's data (6), we observed an increase in OP diagnoses due to forearm DXA. Importantly, however, the net increment was nearly twice as high in our series, namely 11.2%. In fact, our aPHPT sample is not entirely comparable with the surgical series of Wood et al (6), where symptomatic and asymptomatic patients were pooled. We hypothesize that the likelihood of diagnosing OP based on a forearm assessment alone is higher in aPHPT than in symptomatic patients. Another possible explanation is that most patients in our series underwent a forearm assessment.
To the best of our knowledge, our study is the first to evaluate the impact of forearm DXA in aPHPT, for both additional diagnoses of OP and additional surgical indications. The diagnosis of OP in aPHPT, besides leading to a more accurate clinical characterization of the disease, strongly impacts on the therapeutic decision, as it is included in surgical criteria (4). Our study thus provides further substantial information: forearm DXA assessments increased aPHPT patients fulfilling surgical indications by 5.2%. Therefore, the routine implementation of forearm DXA could change the therapeutic strategy in a nonnegligible proportion of patients.
The limitations of our study need to be taken into account.
First, the use of renal US might be regarded as questionable, because computerized tomography (CT) scanning is more sensitive at detecting nephrolithiasis. CT scanning is currently regarded as the gold standard for diagnosing renal stones, with sensitivity and specificity levels in symptomatic patients of 96% and 97%, respectively (12). The diagnostic performance of US is lower than CT, however, without the burden of exposure to radiation associated with CT (1–1.5 mSv) (13). In addition, US is a low-cost procedure and is widely available. Although US could lead to an underestimation of asymptomatic nephrolithiasis, we believe that it is suitable for assessing silent renal stones in PHPT as a potentially harmful but not life-threatening clinical problem.
In addition, there was a trend to higher PTH levels in group A patients vs the remaining aPHPT patients; however, this difference was not statistically significant. We cannot rule out that this difference would become significant if the sample was larger, thus suggesting a more active PHPT and, hence, a greater impact at the cortical site in the patients with OP at the forearm alone.
In conclusion, forearm DXA led to additional diagnoses of OP in 11.2% of patients, increasing the rate of surgical indications in aPHPT patients by 5.2%. As no other specific predictor of forearm damage is currently available, our results support and strengthen the recommendation of the current guidelines to systematically perform DXA at 3 sites in all PHPT patients (4).
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
Abbreviations
- aPHPT
asymptomatic PHPT
- BMD
bone mineral density
- CT
computerized tomography
- DXA
dual x-ray absorptiometry
- 25OHD
25OH-vitamin D
- PHPT
primary hyperparathyroidism
- OP
osteoporosis
- US
ultrasound.



