-
PDF
- Split View
-
Views
-
Cite
Cite
Livia Lamartina, S. Ippolito, M. Danis, F. Bidault, I. Borget, A. Berdelou, A. Al Ghuzlan, D. Hartl, P. Blanchard, M. Terroir, D. Deandreis, M. Schlumberger, E. Baudin, S. Leboulleux, Antiangiogenic Tyrosine Kinase Inhibitors: Occurrence and Risk Factors of Hemoptysis in Refractory Thyroid Cancer, The Journal of Clinical Endocrinology & Metabolism, Volume 101, Issue 7, 1 July 2016, Pages 2733–2741, https://doi.org/10.1210/jc.2015-4391
Close - Share Icon Share
Antiangiogenic tyrosine kinase inhibitors (TKIs) are the mainstay of advanced thyroid cancer (TC) treatment. Concern is rising about TKI-related toxicity.
To determine the incidence and to investigate the risk factors of hemoptysis in TC patients during TKI treatment.
We analyzed consecutive TC patients treated with TKI in our center between 2005 and 2013 and performed an independent review of computed tomography scan images for airway invasion assessment. Occurrence of grade 1–2 or grade 3–5 hemoptysis according to Common Terminology Criteria for Adverse Events version 4.03 and risk factors for hemoptysis were investigated.
A total of 140 patients (89 males; median age, 52 y) with medullary (56%), differentiated (33%), and poorly differentiated (11%) TC were enrolled. Thyroidectomy±neck dissection was performed in 123 patients and neck/mediastinum external-beam radiotherapy in 41 (32% with therapeutic purpose and 68% with adjuvant purpose). Patients received from 1 to 4 lines of TKI (median 1). Median follow-up was 24 months. Airway invasion was found in 65 (46%) cases. Hemoptysis occurred in 9 patients: grade 1–2 in 7 cases (5%) and grade 3–5 in 2 (1.4%) cases (fatal in 1). Hemoptysis was associated with presence of airway invasion (P = .04), poorly differentiated pathology (P = .03), history of therapeutic external-beam radiotherapy (P = .003), and thyroidectomy without neck dissection (P = .02).
Airway invasion, poorly differentiated pathology, therapeutic external-beam radiotherapy, and thyroidectomy without neck dissection are associated with and increased risk of hemoptysis in TC patients during antiangiogenic TKI treatment. Further research is needed to confirm this data and to sort out interactions between these risk factors. A careful assessment of airway invasion is mandatory before TKI introduction.
Airway invasion, poorly differentiated pathology, therapeutic EBRT, and thyroidectomy without neck dissection are associated with and increased risk of hemoptysis in TC patients during antiangiogenic TKI treatment. Further research is needed to confirm this data and to sort out interactions between these risk factors.
The introduction of antiangiogenic tyrosine kinase inhibitors (TKIs) opened a new era in the treatment of advanced unresectable medullary thyroid cancer (TC) (MTC) and radioiodine refractory differentiated TC (DTC). Insights in molecular pathways of cancer tumorigenesis shed light on the role of specific oncogenes. Somatic mutations are present in about 96% of papillary TCs (PTCs) (1). Familiar MTCs that account for 25% of MTCs harbor germline Ret proto-oncogene (RET) mutations; somatic RET mutations are found in 30%–50% of sporadic MTC, and rat sarcoma viral oncogene homolog (RAS) mutations are found in a large proportion of tumors without RET mutation (2–4). Elucidation of the mechanisms of angiogenesis has led to the identification of therapeutic targets (eg, vascular endothelial growth factor [VEGF] pathway) (5). TKIs act as selective inhibitors of 1 or multiple kinases (eg, B-Raf proto-oncogene, serine/threonine kinase; vascular endothelial growth factor receptor; MET proto-oncogene, receptor tyrosine kinase; Fms related tyrosine kinase 3; KIT proto-oncogene receptor tyrosine kinase; RET; fibroblast growth factor receptor; or platelet derived growth factor receptor beta) with antiangiogenetic and antitumoral effects. Prolongation of progression-free survival and objective tumor response rates are significantly better with TKIs compared with placebo, even if complete responses are rare (6–10). However, antiangiogenic TKIs can disclose a wide spectrum of toxicities, sometimes serious and even lethal. An increased risk of bleeding is associated with antiangiogenic TKIs (11), and severe bleeding has been reported in almost all antiangiogenic TKI trials (6–10). Direct tumor vascular or respiratory and digestive tract invasion and high dose external-beam radiotherapy (EBRT) appear to increase the risk of serious bleeding with antiangiogenic TKI (12).
The objective of this study was to determine the incidence of hemoptysis and to investigate risk factors of hemoptysis in TC patients during TKI treatment.
Subjects and Methods
Patients
The files of all patients with advanced TC treated at Gustave Roussy with antiangiogenic TKIs between January 1st, 2005 and December 31st, 2013 were retrospectively reviewed. Inclusion criteria were: 1) a confirmed pathology report by our pathologist; 2) follow-up of more than or equal to 1 month after initiation of TKI treatment; and 3) images of a computed tomography (CT) scan performed within 3 months before treatment start available for radiologic review. Authorization for the anonymous use of clinical data was signed by all the patients. Treatments were given within the frame of clinical trials or off label. Active hemoptysis before antiangiogenic TKI was a contra indication to the treatment if occurring 3–12 weeks before treatment start, depending on the clinical trial. The interval of time allowed after the occurrence of hemoptysis before giving the drug off label was at the discretion of the physician. If anticoagulation was needed, only treatment with low molecular weight heparin was allowed. Age, sex, pathology, initial surgery, history of cytotoxic chemotherapy, and EBRT were recorded as well as history of TKI treatment. EBRT was recorded as being given either in a therapeutic setting in case of unresectable neck or mediastinal disease detected on CT scan or in an adjuvant setting when given after macroscopically complete neck surgery. The occurrence of mild (grade 1–2) or severe (grade 3–5) hemoptysis were collected according to Common Terminology Criteria for Adverse Events version 4 (CTCAE v 4.03) considering the following terms bronchopulmonary hemorrhage, laryngeal hemorrhage, and tracheal hemorrhage (13). Mild hemoptysis is defined as self-limiting minimal bleeding (grade 1) with no need for medical intervention or moderate bleeding (grade 2) for which medical intervention is indicated. Severe hemoptysis is defined as bleeding with need of active medical, radiological or surgical intervention (grade 3), life threatening bleeding (grade 4), or lethal bleeding (grade 5).
CT scan
Airway invasion mentioned in the routine scan reports was recorded. Furthermore, neck and chest CT scan performed before each line of TKI were reviewed by 2 senior radiologists (M.D. and F.B.), blinded to the clinical data, to assess a posteriori the presence of tumoral invasion of the larynx, trachea, or bronchi. Airway invasion for each segment analyzed was deemed present when at least one of the following CT criteria was fulfilled: deformation of the lumen at the level of the mass and focal irregularity, thickening of the mucosal portion adjacent to the mass or tumor in contact with 180° or more of the laryngeal, tracheal, or bronchial circumference (Figure 1).
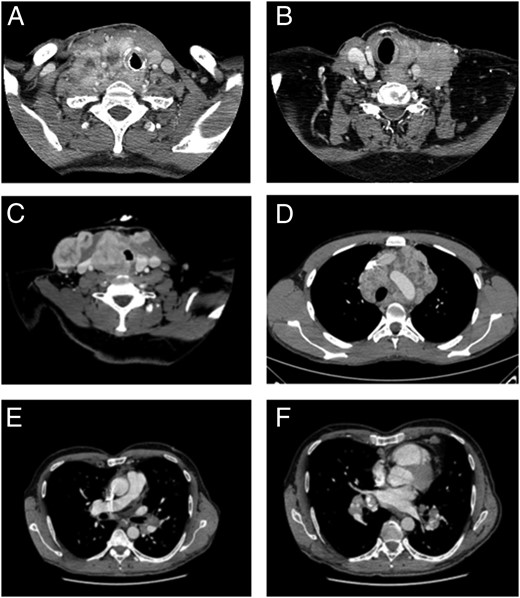
Airway invasion criteria.
A, Larynx tumor encirclement more than or equal to 180°. B, Larynx lumen deformation and wall thickening. C, Trachea lumen deformation and 180° tumor encirclement. D, Trachea 360° tumor encirclement. E, Bronchus lumen deformation (left main bronchus). F, Segmental bronchi 360° tumor encirclement (segmental bronchi of the inferior lobe of the left lung).
Follow-up protocol
Patients were monitored according to clinical trial protocol or according to good clinical practice and local guidelines. The therapeutic decision and follow-up strategy of the patients treated outside the frame of clinical trials were periodically discussed by the local thyroid tumor board. The follow-up protocol included at least: imaging assessments with CT scan every 3–6 months, regular physical examination, electrocardiogram, assessments of biomarkers, and kidney, liver, and bone marrow function.
Statistical analysis
Categorical variables are expressed as number and percentage, continuous variables as median and range. Differences for categorical variables were assessed with Fisher Exact test (Stat View 5.0.1; SAS, Inc). The significance level of P value was .05.
Results
Characteristics of the patients
The records of 10 patients among the 150 patients treated, and 4 treatment lines among 186 were excluded because of unavailable CT images. The study thus included 140 patients. The characteristics of the patients are reported in Table 1. Eighty-nine (64%) patients were males, the median age at TC diagnosis was 52 years (range, 16–80) and the median age at TKI initiation was 60 years (range, 23–86). Tumor pathology was MTC in 78 (56%) and DTC in 62 (44%), including 30 papillary, 16 follicular, and 16 poorly DTC (PDTC).
| Variable . | MTC, n = 78 (56%) . | DTC and PDTC, n = 62 (44%) . | Total, n = 140 . |
|---|---|---|---|
| Sex | |||
| Female | 22 (28%) | 29 (47%) | 51 (36%) |
| Male | 56 (72%) | 33 (53%) | 89 (64%) |
| Age | |||
| ≥45 | 41 (53%) | 54 (87%) | 95 (68%) |
| <45 | 37 (47%) | 8 (13%) | 45 (32%) |
| Histology | |||
| PTC | — | 30 (48%) | 30 (21%) |
| FTC | — | 16 (26%) | 16 (11%) |
| PDTC | — | 16 (26%) | 16 (11%) |
| Sporadic MTC | 56 (72%) | — | 56 (41%) |
| Hereditary MTC | 14 (18%) | — | 14 (10%) |
| MTC (RET status unknown) | 8 (10%) | — | 8 (6%) |
| T | |||
| T1 | 12 (15%) | 4 (6%) | 16 (11%) |
| T2 | 11 (14%) | 9 (15%) | 20 (14%) |
| T3 | 22 (29%) | 24 (39%) | 46 (33%) |
| T4 | 12 (15%) | 17 (27%) | 29 (21%) |
| Tx | 21 (27%) | 8 (13%) | 29 (21%) |
| N | |||
| N0 | 3 (4%) | 8 (13%) | 11 (8%) |
| N1 | 65 (83%) | 32 (52%) | 97 (69%) |
| Nx | 10 (13%) | 22 (35%) | 32 (23%) |
| Surgery | |||
| Yes, thyroidectomy | 7 (9%) | 19 (31%) | 26 (19%) |
| Yes, thyroidectomy + neck dissection | 59 (76%) | 38 (61%) | 97 (69%) |
| No | 12 (15%) | 5 (8%) | 17 (12%) |
| Radioiodine treatment | |||
| Yes | — | 49 (79%) | 49 (79%) |
| No | 13 (21%) | 13 (21%) | |
| Previous cytotoxic chemotherapy | |||
| Yes | 24 (31%) | 11 (18%) | 35 (25%) |
| No | 54 (69%) | 51 (82%) | 105 (75%) |
| EBRT | |||
| No | 54 (70%) | 45 (73%) | 99 (71%) |
| Yes, adjuvant | 19 (24%) | 9 (14%) | 28 (20%) |
| Yes, therapeutic | 5 (6%) | 8 (13%) | 13 (9%) |
| Variable . | MTC, n = 78 (56%) . | DTC and PDTC, n = 62 (44%) . | Total, n = 140 . |
|---|---|---|---|
| Sex | |||
| Female | 22 (28%) | 29 (47%) | 51 (36%) |
| Male | 56 (72%) | 33 (53%) | 89 (64%) |
| Age | |||
| ≥45 | 41 (53%) | 54 (87%) | 95 (68%) |
| <45 | 37 (47%) | 8 (13%) | 45 (32%) |
| Histology | |||
| PTC | — | 30 (48%) | 30 (21%) |
| FTC | — | 16 (26%) | 16 (11%) |
| PDTC | — | 16 (26%) | 16 (11%) |
| Sporadic MTC | 56 (72%) | — | 56 (41%) |
| Hereditary MTC | 14 (18%) | — | 14 (10%) |
| MTC (RET status unknown) | 8 (10%) | — | 8 (6%) |
| T | |||
| T1 | 12 (15%) | 4 (6%) | 16 (11%) |
| T2 | 11 (14%) | 9 (15%) | 20 (14%) |
| T3 | 22 (29%) | 24 (39%) | 46 (33%) |
| T4 | 12 (15%) | 17 (27%) | 29 (21%) |
| Tx | 21 (27%) | 8 (13%) | 29 (21%) |
| N | |||
| N0 | 3 (4%) | 8 (13%) | 11 (8%) |
| N1 | 65 (83%) | 32 (52%) | 97 (69%) |
| Nx | 10 (13%) | 22 (35%) | 32 (23%) |
| Surgery | |||
| Yes, thyroidectomy | 7 (9%) | 19 (31%) | 26 (19%) |
| Yes, thyroidectomy + neck dissection | 59 (76%) | 38 (61%) | 97 (69%) |
| No | 12 (15%) | 5 (8%) | 17 (12%) |
| Radioiodine treatment | |||
| Yes | — | 49 (79%) | 49 (79%) |
| No | 13 (21%) | 13 (21%) | |
| Previous cytotoxic chemotherapy | |||
| Yes | 24 (31%) | 11 (18%) | 35 (25%) |
| No | 54 (69%) | 51 (82%) | 105 (75%) |
| EBRT | |||
| No | 54 (70%) | 45 (73%) | 99 (71%) |
| Yes, adjuvant | 19 (24%) | 9 (14%) | 28 (20%) |
| Yes, therapeutic | 5 (6%) | 8 (13%) | 13 (9%) |
| Variable . | MTC, n = 78 (56%) . | DTC and PDTC, n = 62 (44%) . | Total, n = 140 . |
|---|---|---|---|
| Sex | |||
| Female | 22 (28%) | 29 (47%) | 51 (36%) |
| Male | 56 (72%) | 33 (53%) | 89 (64%) |
| Age | |||
| ≥45 | 41 (53%) | 54 (87%) | 95 (68%) |
| <45 | 37 (47%) | 8 (13%) | 45 (32%) |
| Histology | |||
| PTC | — | 30 (48%) | 30 (21%) |
| FTC | — | 16 (26%) | 16 (11%) |
| PDTC | — | 16 (26%) | 16 (11%) |
| Sporadic MTC | 56 (72%) | — | 56 (41%) |
| Hereditary MTC | 14 (18%) | — | 14 (10%) |
| MTC (RET status unknown) | 8 (10%) | — | 8 (6%) |
| T | |||
| T1 | 12 (15%) | 4 (6%) | 16 (11%) |
| T2 | 11 (14%) | 9 (15%) | 20 (14%) |
| T3 | 22 (29%) | 24 (39%) | 46 (33%) |
| T4 | 12 (15%) | 17 (27%) | 29 (21%) |
| Tx | 21 (27%) | 8 (13%) | 29 (21%) |
| N | |||
| N0 | 3 (4%) | 8 (13%) | 11 (8%) |
| N1 | 65 (83%) | 32 (52%) | 97 (69%) |
| Nx | 10 (13%) | 22 (35%) | 32 (23%) |
| Surgery | |||
| Yes, thyroidectomy | 7 (9%) | 19 (31%) | 26 (19%) |
| Yes, thyroidectomy + neck dissection | 59 (76%) | 38 (61%) | 97 (69%) |
| No | 12 (15%) | 5 (8%) | 17 (12%) |
| Radioiodine treatment | |||
| Yes | — | 49 (79%) | 49 (79%) |
| No | 13 (21%) | 13 (21%) | |
| Previous cytotoxic chemotherapy | |||
| Yes | 24 (31%) | 11 (18%) | 35 (25%) |
| No | 54 (69%) | 51 (82%) | 105 (75%) |
| EBRT | |||
| No | 54 (70%) | 45 (73%) | 99 (71%) |
| Yes, adjuvant | 19 (24%) | 9 (14%) | 28 (20%) |
| Yes, therapeutic | 5 (6%) | 8 (13%) | 13 (9%) |
| Variable . | MTC, n = 78 (56%) . | DTC and PDTC, n = 62 (44%) . | Total, n = 140 . |
|---|---|---|---|
| Sex | |||
| Female | 22 (28%) | 29 (47%) | 51 (36%) |
| Male | 56 (72%) | 33 (53%) | 89 (64%) |
| Age | |||
| ≥45 | 41 (53%) | 54 (87%) | 95 (68%) |
| <45 | 37 (47%) | 8 (13%) | 45 (32%) |
| Histology | |||
| PTC | — | 30 (48%) | 30 (21%) |
| FTC | — | 16 (26%) | 16 (11%) |
| PDTC | — | 16 (26%) | 16 (11%) |
| Sporadic MTC | 56 (72%) | — | 56 (41%) |
| Hereditary MTC | 14 (18%) | — | 14 (10%) |
| MTC (RET status unknown) | 8 (10%) | — | 8 (6%) |
| T | |||
| T1 | 12 (15%) | 4 (6%) | 16 (11%) |
| T2 | 11 (14%) | 9 (15%) | 20 (14%) |
| T3 | 22 (29%) | 24 (39%) | 46 (33%) |
| T4 | 12 (15%) | 17 (27%) | 29 (21%) |
| Tx | 21 (27%) | 8 (13%) | 29 (21%) |
| N | |||
| N0 | 3 (4%) | 8 (13%) | 11 (8%) |
| N1 | 65 (83%) | 32 (52%) | 97 (69%) |
| Nx | 10 (13%) | 22 (35%) | 32 (23%) |
| Surgery | |||
| Yes, thyroidectomy | 7 (9%) | 19 (31%) | 26 (19%) |
| Yes, thyroidectomy + neck dissection | 59 (76%) | 38 (61%) | 97 (69%) |
| No | 12 (15%) | 5 (8%) | 17 (12%) |
| Radioiodine treatment | |||
| Yes | — | 49 (79%) | 49 (79%) |
| No | 13 (21%) | 13 (21%) | |
| Previous cytotoxic chemotherapy | |||
| Yes | 24 (31%) | 11 (18%) | 35 (25%) |
| No | 54 (69%) | 51 (82%) | 105 (75%) |
| EBRT | |||
| No | 54 (70%) | 45 (73%) | 99 (71%) |
| Yes, adjuvant | 19 (24%) | 9 (14%) | 28 (20%) |
| Yes, therapeutic | 5 (6%) | 8 (13%) | 13 (9%) |
Initial treatment included total thyroidectomy with or without neck dissection in 123 (88%) patients, followed by radioactive iodine therapy in 49 of the 62 (79%) DTC patients. Before TKI initiation, neck and mediastinal EBRT was performed in 41 (29%) cases (24 MTC and 17 DTC). EBRT was performed 30 months (median) before the first TKI treatment (range, 3–196 mo) and was given in an adjuvant setting in 28 cases (67%) with a median dose of 60 Gy (range, 45–70 Gy) and in a therapeutic setting in 13 cases (33%) with a median dose of 60 Gy (range, 40–66). Finally, before TKI, 35 (34%) patients were treated with systemic chemotherapy with 5-fluorouracil and dacarbazin (9 cases) or 5-fluorouracil and streptozotocin (7 cases), doxorubicin and cisplatin (4 cases) or streptozocin (3 cases), platin salt and paclitaxel (2 cases) or etoposide (2 cases), gemcitabine and oxaliplatin (2 cases), or other regimens (6 cases).
Antiangiogenic TKI treatments
TKI treatments were administered as monotherapy after 4.8 years (median) from TC diagnosis (2 mo to 37 y). Seven drugs were used in the 140 patients for a total of 182 treatment lines. Treatment lines consisted in Vandetanib in 82 cases (45%), Sorafenib in 46 (25%), Sunitinib in 21 (12%), Cabozantinib in 12 (7%), Motesanib in 11 (6%), Lenvatinib in 9 (4.5%), and Aflibercept in 1 (0.5). Treatment lines are detailed in Table 2. None of the patients had a history of hemoptysis before the first line of TKI. Only 1 patient had a history of hemoptysis 25 days before the start of a second line TKI. Median follow-up after TKI initiation was 2 years (2 mo to 8 y). Median length of each TKI treatment line was 10.8 months (range, 0.1–95).
| TKI . | 1st Line of Treatment (n = 139) . | 2nd Line of Treatment (n = 38) . | 3rd Line of Treatment (n = 4) . | 4th Line of Treatment (n = 1) . | Total (n = 182) . | MTC (n = 100) . | DTC/PDTC (n = 82) . |
|---|---|---|---|---|---|---|---|
| Aflibercept | 1 (1%) | — | — | — | 1 (1%) | — | 1 (1%) |
| Cabozantinib | 10 (7%) | 1 (2%) | 1 (25%) | — | 12 (6%) | 12 (12%) | — |
| Lenvatinib | 6 (4%) | 3 (8%) | — | — | 9 (5%) | 3 (3%) | 6 (7%) |
| Motesanib | 11 (8%) | — | — | — | 11 (6%) | 9 (9%) | 2 (2%) |
| Sorafenib | 36 (26%) | 10 (26%) | — | — | 46 (25%) | 6 (6%) | 40 (50%) |
| Sunitinib | 5 (4%) | 12 (32%) | 3 (75%) | 1 (100%) | 21 (12%) | 6 (6%) | 15 (18%) |
| Vandetanib | 70 (50%) | 12 (32%) | — | — | 82 (45%) | 64 (64%) | 18 (22%) |
| TKI . | 1st Line of Treatment (n = 139) . | 2nd Line of Treatment (n = 38) . | 3rd Line of Treatment (n = 4) . | 4th Line of Treatment (n = 1) . | Total (n = 182) . | MTC (n = 100) . | DTC/PDTC (n = 82) . |
|---|---|---|---|---|---|---|---|
| Aflibercept | 1 (1%) | — | — | — | 1 (1%) | — | 1 (1%) |
| Cabozantinib | 10 (7%) | 1 (2%) | 1 (25%) | — | 12 (6%) | 12 (12%) | — |
| Lenvatinib | 6 (4%) | 3 (8%) | — | — | 9 (5%) | 3 (3%) | 6 (7%) |
| Motesanib | 11 (8%) | — | — | — | 11 (6%) | 9 (9%) | 2 (2%) |
| Sorafenib | 36 (26%) | 10 (26%) | — | — | 46 (25%) | 6 (6%) | 40 (50%) |
| Sunitinib | 5 (4%) | 12 (32%) | 3 (75%) | 1 (100%) | 21 (12%) | 6 (6%) | 15 (18%) |
| Vandetanib | 70 (50%) | 12 (32%) | — | — | 82 (45%) | 64 (64%) | 18 (22%) |
| TKI . | 1st Line of Treatment (n = 139) . | 2nd Line of Treatment (n = 38) . | 3rd Line of Treatment (n = 4) . | 4th Line of Treatment (n = 1) . | Total (n = 182) . | MTC (n = 100) . | DTC/PDTC (n = 82) . |
|---|---|---|---|---|---|---|---|
| Aflibercept | 1 (1%) | — | — | — | 1 (1%) | — | 1 (1%) |
| Cabozantinib | 10 (7%) | 1 (2%) | 1 (25%) | — | 12 (6%) | 12 (12%) | — |
| Lenvatinib | 6 (4%) | 3 (8%) | — | — | 9 (5%) | 3 (3%) | 6 (7%) |
| Motesanib | 11 (8%) | — | — | — | 11 (6%) | 9 (9%) | 2 (2%) |
| Sorafenib | 36 (26%) | 10 (26%) | — | — | 46 (25%) | 6 (6%) | 40 (50%) |
| Sunitinib | 5 (4%) | 12 (32%) | 3 (75%) | 1 (100%) | 21 (12%) | 6 (6%) | 15 (18%) |
| Vandetanib | 70 (50%) | 12 (32%) | — | — | 82 (45%) | 64 (64%) | 18 (22%) |
| TKI . | 1st Line of Treatment (n = 139) . | 2nd Line of Treatment (n = 38) . | 3rd Line of Treatment (n = 4) . | 4th Line of Treatment (n = 1) . | Total (n = 182) . | MTC (n = 100) . | DTC/PDTC (n = 82) . |
|---|---|---|---|---|---|---|---|
| Aflibercept | 1 (1%) | — | — | — | 1 (1%) | — | 1 (1%) |
| Cabozantinib | 10 (7%) | 1 (2%) | 1 (25%) | — | 12 (6%) | 12 (12%) | — |
| Lenvatinib | 6 (4%) | 3 (8%) | — | — | 9 (5%) | 3 (3%) | 6 (7%) |
| Motesanib | 11 (8%) | — | — | — | 11 (6%) | 9 (9%) | 2 (2%) |
| Sorafenib | 36 (26%) | 10 (26%) | — | — | 46 (25%) | 6 (6%) | 40 (50%) |
| Sunitinib | 5 (4%) | 12 (32%) | 3 (75%) | 1 (100%) | 21 (12%) | 6 (6%) | 15 (18%) |
| Vandetanib | 70 (50%) | 12 (32%) | — | — | 82 (45%) | 64 (64%) | 18 (22%) |
Airway invasion
The routine scan reports described airway invasion in 31 (22%) patients. The independent CT scan review described airway invasion at the moment of TKI introduction in 65 (46%) patients, corresponding to 77 (42%) TKI treatment lines. According to each treatment line, the airway invasion was present in the larynx in 19 (10%) cases, in the trachea in 50 (27.4%) cases, and in the bronchus in 42 (23%) cases. Signs of invasion of subsegmental bronchi were present in 46 (25%) cases, all of which were associated with either tracheal, bronchial or laryngeal invasion. The radiologic criteria of invasion of each segment are detailed in Supplemental Table 1.
Hemoptysis occurrence
Nine (6%) patients experienced hemoptysis, their characteristics are listed in Table 3. Maximal grade of hemoptysis was mild (grade 1–2) in 7 cases (3 follicular TC [FTC], 3 PDTC, and 1 MTC), severe (grade 3–4) in 1 MTC case, and fatal (grade 5) in 1 PTC case. Grade 1–2 hemoptysis occurred after a median time of 2 months (range, 1–8) from initiation of TKI treatment, during the first line treatment in 6 cases or second line treatment in 1 case. Grade 3–4 hemoptysis occurred after 13 months of treatment during a first line treatment. Fatal grade 5 hemoptysis occurred after 19 days of treatment during a second line treatment. The TKIs involved were Vandetanib in 3 cases, Sorafenib in 2 cases, Motesanib in 1 case, Sunitinb in 1 case, Cabozantinib in 1 case, and Lenvatinib in 1 case. The patient who experienced fatal hemoptysis had a grade 1 hemoptysis between 2 treatment lines, 25 days before the introduction of the second line treatment. In all but 2 patients, the TKI treatment was discontinued after hemoptysis. The only 2 patients who continued the treatment had experienced a single episode of grade 1 hemoptysis that did not recur. Two of the 9 patients who experienced hemoptysis were under full anticoagulation therapy for a medical history of pulmonary embolism in the first and of atrial fibrillation in the other.
| Patient . | Histology . | TKI . | Treatment Line . | Hemoptysis Grade . | Time Span TKI Start, Hemoptysis . | Treatment Discontinuation . | Airway Invasion . | Surgery . | EBRT . |
|---|---|---|---|---|---|---|---|---|---|
| 1 | FTC | Sorafenib | 1st | 1–2 | 6 months | Yes | No | TT | Therapeutic |
| 2a | FTC | Motesanib | 1st | 1–2 | 8 months | Yes | No | TT + ND | Adjuvant |
| 3 | FTC | Sunitinib | 2nd | 1–2 | 2 months | No | Yes | TT | No |
| 4 | PDTC | Vandetanib | 1st | 1–2 | 2 months | Yes | Yes | TT + ND | Therapeutic |
| 5a | MTC | Vandetanib | 1st | 1–2 | 2 months | Yes | Yes | TT | Adjuvant |
| 6 | PDTC | Sorafenib | 1st | 1–2 | 1 month | No | Yes | TT + NDb | Therapeutic |
| 7 | PDTC | Vandetanib | 1st | 1–2 | 2 months | Yes | Yes | TT | No |
| 8 | MTC | Cabozantinib | 1st | 3–4 | 13 months | Yes | Yes | TT + ND | No |
| 9 | PTC | Lenvatinib | 2nd | 5 | 1 month | Yes | Yes | TT | Therapeutic |
| Patient . | Histology . | TKI . | Treatment Line . | Hemoptysis Grade . | Time Span TKI Start, Hemoptysis . | Treatment Discontinuation . | Airway Invasion . | Surgery . | EBRT . |
|---|---|---|---|---|---|---|---|---|---|
| 1 | FTC | Sorafenib | 1st | 1–2 | 6 months | Yes | No | TT | Therapeutic |
| 2a | FTC | Motesanib | 1st | 1–2 | 8 months | Yes | No | TT + ND | Adjuvant |
| 3 | FTC | Sunitinib | 2nd | 1–2 | 2 months | No | Yes | TT | No |
| 4 | PDTC | Vandetanib | 1st | 1–2 | 2 months | Yes | Yes | TT + ND | Therapeutic |
| 5a | MTC | Vandetanib | 1st | 1–2 | 2 months | Yes | Yes | TT | Adjuvant |
| 6 | PDTC | Sorafenib | 1st | 1–2 | 1 month | No | Yes | TT + NDb | Therapeutic |
| 7 | PDTC | Vandetanib | 1st | 1–2 | 2 months | Yes | Yes | TT | No |
| 8 | MTC | Cabozantinib | 1st | 3–4 | 13 months | Yes | Yes | TT + ND | No |
| 9 | PTC | Lenvatinib | 2nd | 5 | 1 month | Yes | Yes | TT | Therapeutic |
Abbreviations: ND, neck dissection; TT, total thyroidectomy.
Patients under full anticoagulation therapy.
The patient underwent total thyroidectomy and lymph node neck dissection and laryngectomy for invasive poorly DTC.
| Patient . | Histology . | TKI . | Treatment Line . | Hemoptysis Grade . | Time Span TKI Start, Hemoptysis . | Treatment Discontinuation . | Airway Invasion . | Surgery . | EBRT . |
|---|---|---|---|---|---|---|---|---|---|
| 1 | FTC | Sorafenib | 1st | 1–2 | 6 months | Yes | No | TT | Therapeutic |
| 2a | FTC | Motesanib | 1st | 1–2 | 8 months | Yes | No | TT + ND | Adjuvant |
| 3 | FTC | Sunitinib | 2nd | 1–2 | 2 months | No | Yes | TT | No |
| 4 | PDTC | Vandetanib | 1st | 1–2 | 2 months | Yes | Yes | TT + ND | Therapeutic |
| 5a | MTC | Vandetanib | 1st | 1–2 | 2 months | Yes | Yes | TT | Adjuvant |
| 6 | PDTC | Sorafenib | 1st | 1–2 | 1 month | No | Yes | TT + NDb | Therapeutic |
| 7 | PDTC | Vandetanib | 1st | 1–2 | 2 months | Yes | Yes | TT | No |
| 8 | MTC | Cabozantinib | 1st | 3–4 | 13 months | Yes | Yes | TT + ND | No |
| 9 | PTC | Lenvatinib | 2nd | 5 | 1 month | Yes | Yes | TT | Therapeutic |
| Patient . | Histology . | TKI . | Treatment Line . | Hemoptysis Grade . | Time Span TKI Start, Hemoptysis . | Treatment Discontinuation . | Airway Invasion . | Surgery . | EBRT . |
|---|---|---|---|---|---|---|---|---|---|
| 1 | FTC | Sorafenib | 1st | 1–2 | 6 months | Yes | No | TT | Therapeutic |
| 2a | FTC | Motesanib | 1st | 1–2 | 8 months | Yes | No | TT + ND | Adjuvant |
| 3 | FTC | Sunitinib | 2nd | 1–2 | 2 months | No | Yes | TT | No |
| 4 | PDTC | Vandetanib | 1st | 1–2 | 2 months | Yes | Yes | TT + ND | Therapeutic |
| 5a | MTC | Vandetanib | 1st | 1–2 | 2 months | Yes | Yes | TT | Adjuvant |
| 6 | PDTC | Sorafenib | 1st | 1–2 | 1 month | No | Yes | TT + NDb | Therapeutic |
| 7 | PDTC | Vandetanib | 1st | 1–2 | 2 months | Yes | Yes | TT | No |
| 8 | MTC | Cabozantinib | 1st | 3–4 | 13 months | Yes | Yes | TT + ND | No |
| 9 | PTC | Lenvatinib | 2nd | 5 | 1 month | Yes | Yes | TT | Therapeutic |
Abbreviations: ND, neck dissection; TT, total thyroidectomy.
Patients under full anticoagulation therapy.
The patient underwent total thyroidectomy and lymph node neck dissection and laryngectomy for invasive poorly DTC.
Risk factors for hemoptysis
The risk factors of hemoptysis are listed in Tables 4 and 5 and consisted in PDTC pathology, therapeutic EBRT, type of initial surgery, and airway invasion on CT scan. No association was found with sex or previous cytotoxic chemotherapy.
Risk Factors of Hemoptysis Based on CT Scan Findings Before Every Treatment Line
| Variable . | Any Hemoptysis/All Treatment Lines . | Univariate Analysis (Fisher Exact Test) P Value . | OR (95% CI) . |
|---|---|---|---|
| Airway invasion (any segment) | |||
| No | 2/105 (2%) | 1 | |
| Yes | 7/77 (9%) | .04 | 5.1 (0.93–51.8) |
| Airway invasion (any segment) | |||
| No | 2/105 (2%) | 1 | |
| 1 segment | 3/46 (7%) | .32 | 3.3 (0.4–41.2) |
| 2 segments | 4/28 (14%) | .02 | 8.4 (1.1–97.8) |
| 3 segments | 0/3 (0%) | 1 | — |
| Airway lumen deformation (any segment) | |||
| No | 6/154 (4%) | 1 | |
| Yes | 3/28 (11%) | .14 | 2.9 (0.4–14.8) |
| Airway wall thickening (any segment) | |||
| No | 5/147 (3%) | 1 | |
| Yes | 4/35 (11%) | .07 | 3.6 (0.7–18) |
| Airway encirclement ≥180° (any segment) | |||
| No | 6/129 (5%) | .72 | 1 |
| Yes | 3/53 (6%) | 1.2 (0.2–6) | |
| Laryngeal invasion | |||
| No | 4/145 (3%) | 1 | |
| Yes | 3/19 (16%) | .03 | 6.5 (0.9–42.2) |
| Not available | 2/18 (11%) | .13 | 4.3 (0.4–33.1) |
| Laryngeal invasion | |||
| No | 4/145 (3%) | 1 | |
| Yes, 1 sign | 1/10 (10%) | .29 | 3.8 (0.1–44.9) |
| Yes, 2 signs | 2/8 (25%) | .03 | 11.3 (0.9–99.9) |
| Yes 3 signs | 0/1 (0%) | 1 | — |
| Not available | 2/18 (11%) | .13 | 4.3 (0.4–33.1) |
| Tracheal invasion | |||
| No | 4/132 (3%) | 1 | |
| Yes | 5/50 (10%) | .11 | 3.5 (0.7–18.6) |
| Tracheal invasion | |||
| No | 4/132 (3%) | 1 | |
| Yes, 1 sign | 3/32 (9%) | .12 | 3.5 (0.7–18.6) |
| Yes, 2 signs | 2/13 (15%) | .09 | 5.7 (0.5–45.2) |
| Yes 3 signs | 0/5 (0%) | 1 | — |
| Bronchi invasion | |||
| No | 6/137 (4%) | 1 | |
| Yes | 3/42 (7%) | .44 | 1.7 (0.3–8.3) |
| Not available | 0/3 (0%) | 1 | — |
| Bronchi invasion | |||
| No | 6/137 (4%) | 1 | |
| Yes, 1 sign | 3/38 (8%) | .41 | 1.9 (0.3–9.3) |
| Yes, 2 signs | 0/1 (0%) | 1 | — |
| Yes 3 signs | 0/3 (0%) | 1 | — |
| Not available | 0/3 (0%) | 1 | — |
| Variable . | Any Hemoptysis/All Treatment Lines . | Univariate Analysis (Fisher Exact Test) P Value . | OR (95% CI) . |
|---|---|---|---|
| Airway invasion (any segment) | |||
| No | 2/105 (2%) | 1 | |
| Yes | 7/77 (9%) | .04 | 5.1 (0.93–51.8) |
| Airway invasion (any segment) | |||
| No | 2/105 (2%) | 1 | |
| 1 segment | 3/46 (7%) | .32 | 3.3 (0.4–41.2) |
| 2 segments | 4/28 (14%) | .02 | 8.4 (1.1–97.8) |
| 3 segments | 0/3 (0%) | 1 | — |
| Airway lumen deformation (any segment) | |||
| No | 6/154 (4%) | 1 | |
| Yes | 3/28 (11%) | .14 | 2.9 (0.4–14.8) |
| Airway wall thickening (any segment) | |||
| No | 5/147 (3%) | 1 | |
| Yes | 4/35 (11%) | .07 | 3.6 (0.7–18) |
| Airway encirclement ≥180° (any segment) | |||
| No | 6/129 (5%) | .72 | 1 |
| Yes | 3/53 (6%) | 1.2 (0.2–6) | |
| Laryngeal invasion | |||
| No | 4/145 (3%) | 1 | |
| Yes | 3/19 (16%) | .03 | 6.5 (0.9–42.2) |
| Not available | 2/18 (11%) | .13 | 4.3 (0.4–33.1) |
| Laryngeal invasion | |||
| No | 4/145 (3%) | 1 | |
| Yes, 1 sign | 1/10 (10%) | .29 | 3.8 (0.1–44.9) |
| Yes, 2 signs | 2/8 (25%) | .03 | 11.3 (0.9–99.9) |
| Yes 3 signs | 0/1 (0%) | 1 | — |
| Not available | 2/18 (11%) | .13 | 4.3 (0.4–33.1) |
| Tracheal invasion | |||
| No | 4/132 (3%) | 1 | |
| Yes | 5/50 (10%) | .11 | 3.5 (0.7–18.6) |
| Tracheal invasion | |||
| No | 4/132 (3%) | 1 | |
| Yes, 1 sign | 3/32 (9%) | .12 | 3.5 (0.7–18.6) |
| Yes, 2 signs | 2/13 (15%) | .09 | 5.7 (0.5–45.2) |
| Yes 3 signs | 0/5 (0%) | 1 | — |
| Bronchi invasion | |||
| No | 6/137 (4%) | 1 | |
| Yes | 3/42 (7%) | .44 | 1.7 (0.3–8.3) |
| Not available | 0/3 (0%) | 1 | — |
| Bronchi invasion | |||
| No | 6/137 (4%) | 1 | |
| Yes, 1 sign | 3/38 (8%) | .41 | 1.9 (0.3–9.3) |
| Yes, 2 signs | 0/1 (0%) | 1 | — |
| Yes 3 signs | 0/3 (0%) | 1 | — |
| Not available | 0/3 (0%) | 1 | — |
Risk Factors of Hemoptysis Based on CT Scan Findings Before Every Treatment Line
| Variable . | Any Hemoptysis/All Treatment Lines . | Univariate Analysis (Fisher Exact Test) P Value . | OR (95% CI) . |
|---|---|---|---|
| Airway invasion (any segment) | |||
| No | 2/105 (2%) | 1 | |
| Yes | 7/77 (9%) | .04 | 5.1 (0.93–51.8) |
| Airway invasion (any segment) | |||
| No | 2/105 (2%) | 1 | |
| 1 segment | 3/46 (7%) | .32 | 3.3 (0.4–41.2) |
| 2 segments | 4/28 (14%) | .02 | 8.4 (1.1–97.8) |
| 3 segments | 0/3 (0%) | 1 | — |
| Airway lumen deformation (any segment) | |||
| No | 6/154 (4%) | 1 | |
| Yes | 3/28 (11%) | .14 | 2.9 (0.4–14.8) |
| Airway wall thickening (any segment) | |||
| No | 5/147 (3%) | 1 | |
| Yes | 4/35 (11%) | .07 | 3.6 (0.7–18) |
| Airway encirclement ≥180° (any segment) | |||
| No | 6/129 (5%) | .72 | 1 |
| Yes | 3/53 (6%) | 1.2 (0.2–6) | |
| Laryngeal invasion | |||
| No | 4/145 (3%) | 1 | |
| Yes | 3/19 (16%) | .03 | 6.5 (0.9–42.2) |
| Not available | 2/18 (11%) | .13 | 4.3 (0.4–33.1) |
| Laryngeal invasion | |||
| No | 4/145 (3%) | 1 | |
| Yes, 1 sign | 1/10 (10%) | .29 | 3.8 (0.1–44.9) |
| Yes, 2 signs | 2/8 (25%) | .03 | 11.3 (0.9–99.9) |
| Yes 3 signs | 0/1 (0%) | 1 | — |
| Not available | 2/18 (11%) | .13 | 4.3 (0.4–33.1) |
| Tracheal invasion | |||
| No | 4/132 (3%) | 1 | |
| Yes | 5/50 (10%) | .11 | 3.5 (0.7–18.6) |
| Tracheal invasion | |||
| No | 4/132 (3%) | 1 | |
| Yes, 1 sign | 3/32 (9%) | .12 | 3.5 (0.7–18.6) |
| Yes, 2 signs | 2/13 (15%) | .09 | 5.7 (0.5–45.2) |
| Yes 3 signs | 0/5 (0%) | 1 | — |
| Bronchi invasion | |||
| No | 6/137 (4%) | 1 | |
| Yes | 3/42 (7%) | .44 | 1.7 (0.3–8.3) |
| Not available | 0/3 (0%) | 1 | — |
| Bronchi invasion | |||
| No | 6/137 (4%) | 1 | |
| Yes, 1 sign | 3/38 (8%) | .41 | 1.9 (0.3–9.3) |
| Yes, 2 signs | 0/1 (0%) | 1 | — |
| Yes 3 signs | 0/3 (0%) | 1 | — |
| Not available | 0/3 (0%) | 1 | — |
| Variable . | Any Hemoptysis/All Treatment Lines . | Univariate Analysis (Fisher Exact Test) P Value . | OR (95% CI) . |
|---|---|---|---|
| Airway invasion (any segment) | |||
| No | 2/105 (2%) | 1 | |
| Yes | 7/77 (9%) | .04 | 5.1 (0.93–51.8) |
| Airway invasion (any segment) | |||
| No | 2/105 (2%) | 1 | |
| 1 segment | 3/46 (7%) | .32 | 3.3 (0.4–41.2) |
| 2 segments | 4/28 (14%) | .02 | 8.4 (1.1–97.8) |
| 3 segments | 0/3 (0%) | 1 | — |
| Airway lumen deformation (any segment) | |||
| No | 6/154 (4%) | 1 | |
| Yes | 3/28 (11%) | .14 | 2.9 (0.4–14.8) |
| Airway wall thickening (any segment) | |||
| No | 5/147 (3%) | 1 | |
| Yes | 4/35 (11%) | .07 | 3.6 (0.7–18) |
| Airway encirclement ≥180° (any segment) | |||
| No | 6/129 (5%) | .72 | 1 |
| Yes | 3/53 (6%) | 1.2 (0.2–6) | |
| Laryngeal invasion | |||
| No | 4/145 (3%) | 1 | |
| Yes | 3/19 (16%) | .03 | 6.5 (0.9–42.2) |
| Not available | 2/18 (11%) | .13 | 4.3 (0.4–33.1) |
| Laryngeal invasion | |||
| No | 4/145 (3%) | 1 | |
| Yes, 1 sign | 1/10 (10%) | .29 | 3.8 (0.1–44.9) |
| Yes, 2 signs | 2/8 (25%) | .03 | 11.3 (0.9–99.9) |
| Yes 3 signs | 0/1 (0%) | 1 | — |
| Not available | 2/18 (11%) | .13 | 4.3 (0.4–33.1) |
| Tracheal invasion | |||
| No | 4/132 (3%) | 1 | |
| Yes | 5/50 (10%) | .11 | 3.5 (0.7–18.6) |
| Tracheal invasion | |||
| No | 4/132 (3%) | 1 | |
| Yes, 1 sign | 3/32 (9%) | .12 | 3.5 (0.7–18.6) |
| Yes, 2 signs | 2/13 (15%) | .09 | 5.7 (0.5–45.2) |
| Yes 3 signs | 0/5 (0%) | 1 | — |
| Bronchi invasion | |||
| No | 6/137 (4%) | 1 | |
| Yes | 3/42 (7%) | .44 | 1.7 (0.3–8.3) |
| Not available | 0/3 (0%) | 1 | — |
| Bronchi invasion | |||
| No | 6/137 (4%) | 1 | |
| Yes, 1 sign | 3/38 (8%) | .41 | 1.9 (0.3–9.3) |
| Yes, 2 signs | 0/1 (0%) | 1 | — |
| Yes 3 signs | 0/3 (0%) | 1 | — |
| Not available | 0/3 (0%) | 1 | — |
| Variable . | Any Hemoptysis/All Patients . | Univariate Analysis (Fisher Exact Test) P Value . | OR (95% CI) . |
|---|---|---|---|
| Sex | |||
| Male | 4/89 (4%) | 1 | |
| Female | 5/51 (10%) | .28 | 2.3 (0.5–12.2) |
| Age | |||
| <45 y | 0/45 (0%) | 1 | |
| ≥45 y | 9/95 (9%) | .06 | — |
| Surgery of primary tumor | |||
| Yes, thyroidectomy | 5/26 (19%) | 1 | |
| Yes, thyroidectomy + neck dissection | 4/97 (4%) | .02 | 0.2 (0.0–0.9) |
| No | 0/17 (0%) | .13 | — |
| Histologic type | |||
| MTC | 2/78 (3%) | 1 | |
| DTC (papillary or follicular) | 4/46 (9%) | .19 | 3.7 (0.5–42.1) |
| PDTC | 3/16 (19%) | .03 | 8.7 (0.9–113.3) |
| Cytotoxic chemotherapy | |||
| No | 6/105 (6%) | 1 | |
| Yes | 3/35 (9%) | .69 | 1.5 (0.2–7.7) |
| External beam radiation | |||
| No | 3/99 (3%) | 1 | |
| Yes, adjuvant | 2/28 (7%) | .3 | 2.4 (0.2–22.3) |
| Yes, therapeutic | 4/13 (31%) | .003 | 13.5 (2–107.8) |
| Variable . | Any Hemoptysis/All Patients . | Univariate Analysis (Fisher Exact Test) P Value . | OR (95% CI) . |
|---|---|---|---|
| Sex | |||
| Male | 4/89 (4%) | 1 | |
| Female | 5/51 (10%) | .28 | 2.3 (0.5–12.2) |
| Age | |||
| <45 y | 0/45 (0%) | 1 | |
| ≥45 y | 9/95 (9%) | .06 | — |
| Surgery of primary tumor | |||
| Yes, thyroidectomy | 5/26 (19%) | 1 | |
| Yes, thyroidectomy + neck dissection | 4/97 (4%) | .02 | 0.2 (0.0–0.9) |
| No | 0/17 (0%) | .13 | — |
| Histologic type | |||
| MTC | 2/78 (3%) | 1 | |
| DTC (papillary or follicular) | 4/46 (9%) | .19 | 3.7 (0.5–42.1) |
| PDTC | 3/16 (19%) | .03 | 8.7 (0.9–113.3) |
| Cytotoxic chemotherapy | |||
| No | 6/105 (6%) | 1 | |
| Yes | 3/35 (9%) | .69 | 1.5 (0.2–7.7) |
| External beam radiation | |||
| No | 3/99 (3%) | 1 | |
| Yes, adjuvant | 2/28 (7%) | .3 | 2.4 (0.2–22.3) |
| Yes, therapeutic | 4/13 (31%) | .003 | 13.5 (2–107.8) |
| Variable . | Any Hemoptysis/All Patients . | Univariate Analysis (Fisher Exact Test) P Value . | OR (95% CI) . |
|---|---|---|---|
| Sex | |||
| Male | 4/89 (4%) | 1 | |
| Female | 5/51 (10%) | .28 | 2.3 (0.5–12.2) |
| Age | |||
| <45 y | 0/45 (0%) | 1 | |
| ≥45 y | 9/95 (9%) | .06 | — |
| Surgery of primary tumor | |||
| Yes, thyroidectomy | 5/26 (19%) | 1 | |
| Yes, thyroidectomy + neck dissection | 4/97 (4%) | .02 | 0.2 (0.0–0.9) |
| No | 0/17 (0%) | .13 | — |
| Histologic type | |||
| MTC | 2/78 (3%) | 1 | |
| DTC (papillary or follicular) | 4/46 (9%) | .19 | 3.7 (0.5–42.1) |
| PDTC | 3/16 (19%) | .03 | 8.7 (0.9–113.3) |
| Cytotoxic chemotherapy | |||
| No | 6/105 (6%) | 1 | |
| Yes | 3/35 (9%) | .69 | 1.5 (0.2–7.7) |
| External beam radiation | |||
| No | 3/99 (3%) | 1 | |
| Yes, adjuvant | 2/28 (7%) | .3 | 2.4 (0.2–22.3) |
| Yes, therapeutic | 4/13 (31%) | .003 | 13.5 (2–107.8) |
| Variable . | Any Hemoptysis/All Patients . | Univariate Analysis (Fisher Exact Test) P Value . | OR (95% CI) . |
|---|---|---|---|
| Sex | |||
| Male | 4/89 (4%) | 1 | |
| Female | 5/51 (10%) | .28 | 2.3 (0.5–12.2) |
| Age | |||
| <45 y | 0/45 (0%) | 1 | |
| ≥45 y | 9/95 (9%) | .06 | — |
| Surgery of primary tumor | |||
| Yes, thyroidectomy | 5/26 (19%) | 1 | |
| Yes, thyroidectomy + neck dissection | 4/97 (4%) | .02 | 0.2 (0.0–0.9) |
| No | 0/17 (0%) | .13 | — |
| Histologic type | |||
| MTC | 2/78 (3%) | 1 | |
| DTC (papillary or follicular) | 4/46 (9%) | .19 | 3.7 (0.5–42.1) |
| PDTC | 3/16 (19%) | .03 | 8.7 (0.9–113.3) |
| Cytotoxic chemotherapy | |||
| No | 6/105 (6%) | 1 | |
| Yes | 3/35 (9%) | .69 | 1.5 (0.2–7.7) |
| External beam radiation | |||
| No | 3/99 (3%) | 1 | |
| Yes, adjuvant | 2/28 (7%) | .3 | 2.4 (0.2–22.3) |
| Yes, therapeutic | 4/13 (31%) | .003 | 13.5 (2–107.8) |
The risk of hemoptysis was lower in patients with MTC (3%) compared with patients with DTC (9%) (P = .18, odds ratio [OR] = 3.7 [95% confidence interval (CI) 0.5–42.1]) and was highest in patients with PDTC (19%) (P = .03, OR = 8.7 [95% CI 0.9–113.3]). The patients who did not undergo EBRT had a lower risk of hemoptysis (3%) compared with patients who did undergo adjuvant EBRT (7%) (P = .3, OR = 2.4 [95% CI 0.2–22.3]) and patients who did undergo therapeutic EBRT carried the highest risk of hemoptysis (31%) (P = .003, OR = 13.5 [95% CI 2–107.8]). A more radical surgical procedure (total thyroidectomy and neck dissection) was associated with a lower risk (4%) of hemoptysis compared with thyroidectomy alone (18%) (P = .02, OR = 0.2 [95% CI 0.0–0.9]). Hemoptysis occurred only in patients aged 45 years or older, but no statistically significant association was found. No association was found between therapeutic EBRT and surgical procedure (P = .43, OR = 0.6 [95% CI 0.1–3.9]) or between initial extra thyroid extension (pT3 or pT4 primary TC) and surgical procedure (P = 1, OR = 1.1 [95% CI 0.4–2.8]). The risk of hemoptysis was studied based on the findings of the independent review of the images of the CT scan performed less than or equal to 3 months before each treatment line (Table 5). Considering each treatment line, a significant association between hemoptysis and the presence of airway invasion of at least 1 segment was found (P = .04, OR = 5.1 [95% CI 0.9–51.8]). The association was stronger when 2 airway segments were invaded by the tumor (P = .02, OR = 8.4 [1.1–97.8]). However, none of the 3 patients with evidence of 3 airway segment invasion on CT scan experienced hemoptysis. Looking at each airway segment and each criteria analyzed, only the invasion of the larynx (P = .03, OR = 6.4 [95% CI 0.9–42.1]) and laryngeal lumen deformation (P = .01, OR = 10.6 [95% CI 1.4–73.2]) were associated with hemoptysis. The risk of hemoptysis was of 3% in the absence of laryngeal invasion, 10% when 1 radiologic sign of laryngeal invasion was present (P = .29, OR = 3.8 [95% CI 0.1–44.9]), and 25% when 2 signs were present (P = .03, OR = 11.3 [95% CI 0.9–99.9]). No association was found between presence of airway invasion and therapeutic EBRT or surgical procedure (P = .8, OR = 1.3 [95% CI 0.4–5.3] and 0.7, OR = 0.8 [95% CI 0.3–2.1], respectively). Twelve out of 16 (75%) patients with PDTC also had evidence of airway invasion on CT scan compared with 53 out of 124 (43%) patients with DTC and MTC (P = .02, OR = 4.0 [1.1–17.9]).
Only 1 patient out of 54 patients with none of the risk factors identified experienced hemoptysis. The risk of hemoptysis was of 2% (1/60) (P = 1, OR 0.9 [0.0–71.8]), when 1 of the risk factors of hemoptysis was present; 16% (3/19) when 2 risk factors were present (P = .05, OR = 9.6 [0.7–529.3]) and 67% (4/6) when the 3 risk factors were present (P = .001, OR = 80.4 [5.5–5041.8]). Only 1 patient cumulated the 4 risk factors and he did not experienced hemoptysis.
Both high-grade hemoptysis occurred in male patients aged 45 years or older with airway invasion. Both patients had evidence of tumor invasion of 2 airway segments: trachea associated with bronchus in the first and larynx in the latter. No statistically significant association between high-grade hemoptysis and surgery, therapeutic EBRT, or pathology was found.
Discussion
Nine patients experienced hemoptysis of any grade during antiangiogenic TKI treatment with an overall hemoptysis rate of 6%. Hemoptysis was observed only in patients aged 45 years or older. The rates of hemoptysis of any grade (5%) and of grade 3–5 (1.4%) observed in our cohort are consistent with the rates observed in current literature (14). Hemoptysis occurred during the first line of treatment in all but 2 patients and within 2 months of initiation of treatment in almost 70% of the patients. Such an early onset of hemoptysis has been previously reported (15) and led to suspect an association with some risk factors present at baseline rather than a cumulative effect of the drugs. Indeed, 4 significant risk factors for hemoptysis were identified: PDTC pathology, therapeutic EBRT, surgical procedure, and evidence of airway invasion on CT scan.
Our study presents some limitations. Its retrospective design led to incomplete data availability and given its rarity, the number of hemoptysis events is low precluding any multivariate analysis. A heterogeneous treatment and follow-up protocol was applied for the patients who were treated outside a clinical trial. Nevertheless, this heterogeneity may better reflect the problems that clinicians face in the real life. Finally, the study population included only patients referred to a TC referral center and this may have led to select patients with a more severe prognosis.
Hemoptysis were observed in our cohort with almost all the antiangiogenic TKI studied with no significant difference in the rates of hemoptysis observed. Some antiangiogenic TKI might be at higher risk of bleeding than other but given the small sample of patients, the number of treatment lines and the number of events, the establishment of a definite association between a particular antiangiogenic TKI and the risk of hemoptysis is impossible. As stated in previous reports (14) the risk of bleeding is most probably a class effect of all antiangiogenic TKIs. The pathogenesis of hemorrhagic side effects of antiangiogenic TKI probably rely on the inhibition of VEGF pathway that reduces the renewal capacity of the endothelium, especially after a trauma (16).
Hemoptysis was more frequent in case of PDTC (19%) compared with DTC (9%) and MTC (3%), in case of therapeutic EBRT (31%) compared with no EBRT (3%), and in the absence of neck dissection (19%) compared with a thyroidectomy with neck dissection (4%). EBRT given in an adjuvant setting was not associated to an increase risk of hemoptysis. Finally, the presence of airway invasion demonstrated with CT scan was associated with a 4- to 5-fold higher risk of hemoptysis in TC patients treated with antiangiogenic TKI (9% in case of airway invasion compared with 2% in the absence of airway invasion). The risk increased considerably with the presence of 2 (16%) and 3 (67%) risk factors. The 2 patients who experienced severe hemoptysis, had both evidence of airway invasion on CT scan. Only the patient in whom hemoptysis was fatal had a history of therapeutic neck and mediastinum EBRT and had had thyroidectomy without neck dissection as primary treatment. Multivariate analysis was not performed, however, no association was found between EBRT and pathology or surgical procedure or between the presence of airway invasion and EBRT or surgical procedure. Nevertheless, 12 out of 16 (75%) patients with PDTC also had evidence of airway invasion on CT scan compared with 53 out of 124 (43%) patients with DTC and MTC (P = .02, OR = 4.0 [1.1–17.9]). The association between PDTC and hemoptysis is probably related to the concomitant presence of airway invasion.
As previously reported (12), EBRT of neck and/or mediastinum was associated with an increased risk of hemoptysis during antiangiogenic TKI treatment (P = .02, OR 5.0 [95% CI 1.0–32.6]). EBRT may be a cause of vascular damage favoring hemoptysis (17). Therapeutic neck/mediastinum EBRT is commonly indicated in patients with unresectable tumor, which often disclose gross airway and/or vascular invasion. Therefore, the association between hemoptysis and therapeutic EBRT could be linked more to tumor aggressiveness or other factors than to the radiation method itself even if we did not observe any association between EBRT and the airway invasion on CT in this cohort. The absence of a homogenous EBRT protocol is a limitation of our study due to its retrospective design which is emphasize by the fact that the median dose of the EBRT was high (60 Gy) and comparable in both groups.
Airway invasion demonstrated on the CT scan is a risk factor of hemoptysis with an hemoptysis rate of 17% when 2 of the 3 segments (laryngeal, tracheal, or bronchi) are invaded. A cautious analysis of the CT scan regarding airway invasion is therefore necessary. Airway invasion observed in our study (46%) was more frequent than expected from the data in the literature (from 3.6% to up to about 23% with the highest proportion observed in tertiary referral centers) (18). The occurrence of airway invasion is though more frequent in patients eligible to receive antiangiogenic TKI treatment, compared with the whole TC population. Moreover, the diagnosis of airway invasion on CT scan may be difficult and requires radiologic expertise. A striking difference in the rate of airway invasion was noted when only the CT scan reports were evaluated (22%) and after an independent CT scan images review by expert radiologists (46%). Both these elements stress the need of an accurate evaluation of the presence of airway invasion as part of the work-up of TC patients before antiangiogenic TKI treatment start. The rate of airway invasion might even have been higher if routine endoscopic airway examination had been performed. The discussion in a multidisciplinary board may improve the collaboration between clinicians and radiologists that may help to assess this issue.
A more radical surgical procedure, thyroidectomy with neck dissection, was associated with a lesser risk (4%) of hemoptysis compared with thyroidectomy alone (19%) (P = .02, OR 5.1 [95% CI 1.0–28.1]). Airway invasion is more often due to the primary tumor but it can rely also on regional lymph node metastases in up to 12% of the patients (19). A more radical surgery may have prompted a more efficient tumor clearance avoiding airway invasion. Nevertheless, this hypothesis is not confirmed by pathology report data. Indeed, airway invasion and presence of extracapsular invasion (T3 or T4 tumor) on pathology report were not significantly different between patients who underwent thyroidectomy alone and those who underwent thyroidectomy and neck dissection (P = .82 and P = .83, respectively). Moreover, none the 17 patients who did not undergo surgery at all (for gross unresectable disease) experienced hemoptysis. Further studies are needed to clarify this issue and recommend for or against a more radical surgical approach.
Antiangiogenic TKI treatment has a substantial risk of adverse events (16) that can be severe and rarely even fatal (20). The use of these drugs should be limited to patients with progressive and/or threatening disease not eligible for other treatments (4, 21, 22). In these selected patients, TKIs have a significant impact on progression-free survival and represent the best alternative to supportive care. Our study provides a better insight into the risk factors of hemoptysis, one of the most common and potentially threatening adverse event of antiangiogenic TKIs in TC patients (16). A better evaluation of previous treatments and of airway status may improve the identification of the patients with higher risk of hemoptysis. The presence of any of the risk factors identified in this retrospective study and especially airway invasion should not be considered as a strict contra indication to antiangiogenic TKI in these patients as they represent, at the moment, the only effective treatment option. The frequency of hemoptysis was low in this study, and hemoptysis were mild in most cases however, it led to stop the treatment. Physicians should be aware of the risk factors of hemoptysis and educate patients to stop the drug and contact their doctor immediately in case of minimal hemoptysis. If other drugs with a different antineoplastic mechanism of action will become available, the occurrence of airway invasion will most probably impact on treatment choices. A history of hemorrhage is generally considered an important risk factor of other hemorrhagic events and was therefore an exclusion criterion in most antiangiogenic TKI clinical trials (8–10). In our series, the only patient who had grade 1 hemoptysis before antiangiogenic TKI start experienced fatal hemoptysis. Even if is not possible to draw conclusions from a single case, it seems reasonable to avoid antiangiogenic TKI use in patients with a recent history of bleeding.
Conclusion
EBRT in a therapeutic setting, PDTC pathology, less extensive surgical procedure and airway invasion are associated to an increased risk of hemoptysis in TC patients during antiangiogenic TKI treatment. Further studies with a broader population are needed to confirm our data and assess if the aforementioned factors are independent predictors of hemoptysis. Before antiangiogenic TKI introduction, a careful evaluation of airway should be undertaken and a close surveillance should be maintained thereafter. Future research may highlight the best treatment sequence for patients with airway invasion from TC.
Acknowledgments
L.L. contributed to this work as recipient of the PhD program of Biotechnologies and Clinical Medicine of the University of Rome, “Sapienza.”
Disclosure Summary: M.S. received research grants and honoraria from Amgen, Astra Zeneca, Bayer, Eisai, and Exelixis; S.L. received honoraria from Astra Zeneca, Bayer, and Eisai; L.L., S.I., M.D., F.B., I.B., A.B., A.A.G., D.H., P.B., M.T., D.D., E.B. have nothing to disclose.
L.L. and S.A. contributed equally to this work.
Abbreviations
- CI
confidence interval
- CT
computed tomography
- DTC
differentiated TC
- EBRT
external-beam radiotherapy
- FTC
follicular TC
- MTC
medullary TC
- OR
odds ratio
- PDTC
poorly DTC
- PTC
papillary TC
- RET
Ret proto-oncogene
- TC
thyroid cancer
- TKI
tyrosine kinase inhibitor
- VEGF
vascular endothelial growth factor.



