-
PDF
- Split View
-
Views
-
Cite
Cite
S K Vimala Bharathi, M Maria Leena, J A Moses, C Anandharamakrishnan, Zein-based anti-browning cling wraps for fresh-cut apple slices, International Journal of Food Science and Technology, Volume 55, Issue 3, March 2020, Pages 1238–1245, https://doi.org/10.1111/ijfs.14401
Close - Share Icon Share
Abstract
The usage of cling wraps is emerging as an easy and cost-effective approach to protect fresh-cut fruits and vegetables from dust, whilst improving visual appeal on retail counters. This study focused on developing an alternate, protein-based packaging material as a food grade cling wrap for food packaging applications. Zein-based cling wraps were produced, and their physical and mechanical characteristics were evaluated and compared with conventionally used chitosan biopolymer films and commercial synthetic polymer films. Antioxidant potential of the prepared films was studied, and the effectiveness of the developed films as anti-browning cling wraps was evaluated using studies conducted on fresh-cut apple slices at ambient conditions. Anti-browning effects were in par with polymeric counterparts; however, zein cling wraps could better prevent weight loss in apple slices. Zein-based films can be adopted as biodegradable food grade cling wraps as an alternative to chitosan and synthetic polymeric materials.
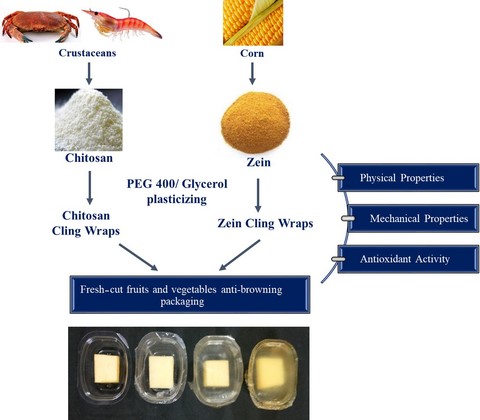
Introduction
It is well known that fresh-cut apples, potatoes and pears are prone to browning, when exposed to atmospheric conditions. To prevent enzymatic browning, several approaches including the use of chemical inhibitors (Zhou et al., 2018), edible coatings (Sharma & Rao, 2015), nanomaterials (Muñoz-Pina et al., 2018), non-thermal treatments (Lei et al., 2018) and modified atmosphere packaging (Ghidelli et al., 2015) have been adopted. This research focused on the development of a novel bio-based food grade packaging film that can be used as a cling wrap, whilst providing anti-browning effects.
Cling wrapping is a common approach linked to fresh-cut fruits and vegetables. It is reported that almost 5.52 million Americans use more than 10 rolls of plastic wrap along a 6-month period (NHCS, 2019). These wraps serve single-use only, and the amount of waste generated by such packaging materials and their impact on environment are significant. Recently, several key administrative bodies are taking initiatives to reduce single-use plastics, whilst promoting bio-based alternatives (EU, 2018). To the best of our knowledge, till date, there is no report on zein-based biopolymeric cling wraps. Understanding its significance, this work reports the development of zein-based cling wraps for food packaging applications and has compared the potential of the developed film in preventing browning in cut apple slices, with chitosan, a biopolymer from animal origin, with proven anti-browning characteristics. With increasing need to shift to bio-based approaches, the results of this study will prove significant.
Chitosan, the second most abundant polysaccharide, is known for its film-forming property, biodegradability, biocompatibility, antimicrobial property and importantly, for its mechanical properties, as they show similarity to medium chain commercial polymers (Xing et al., 2015). Recently, chitosan-based active packaging films incorporated with laponite immobilised silver nanoparticles have been developed for extending the storage life of litchi (Wu et al., 2018). Though chitosan films have proven applications in food packaging, majorly being of animal origin, its usage for fresh-cut fruits and vegetables remains questionable.
Zein is an aqueous, alcoholic, soluble protein extracted from corn gluten meal. Several food packaging applications of zein were explored, owing to its lower water vapour permeability and heat sealing property (Cho et al., 2010). Zein has been explored for use as packaging film coatings, antioxidant material, emulsifier and chewing gum base (Mehta & Trivedi, 2015; Kashiri et al., 2017; Santos et al., 2018). To overcome brittleness, a major concern in zein, optimised levels of plasticisers are used.
Essentially, cling wrapping films must be flexible and easily mouldable when in contact with a relatively rigid material. To improve flexibility and mouldability, plasticisers such as polyethylene glycol (PEG) and glycerol can be used. These are less toxic in nature, biocompatible and biodegradable (Suyatma et al., 2005; Domjan et al., 2009). Additionally, FDA has approved PEG and glycerol as food additives that can be permitted for direct addition into food used for human consumption (FDA, 2018). Hence, the objective of this study was to explore the potential of a protein (zein) as cling wraps and to compare it with a chitosan cling wraps for food packaging applications. For this purpose, plasticiser levels and biopolymer concentrations were optimised. Importantly, to evaluate its scope for food packaging applications, the developed bio-based cling wraps were compared with commercially available polymeric cling wraps. Overall, we hypothesise that a completely biodegradable and food grade cling wrapping material can be developed using biopolymers plasticised with suitable plasticisers. Additionally, bio-based cling wraps with antioxidant property can prevent enzymatic browning of some food materials.
Materials and methods
Materials
Zein (88%–96% protein concentration, product code – W555025, CAS No: 9010-66-6) was procured from Sigma-Aldrich (St. Louis, MO, USA). Chitosan (medium molecular weight, degree of deacetylation ≥75%, viscosity: 200–800 cps, product code – GRM9358; CAS No: 9012-76-4) was procured from HiMedia Laboratories Pvt. Ltd., India. Polyethylene glycol 400 (PEG 400) and glycerol were procured from Merck Life Science Private Limited, India. Commercial poly-vinyl chloride-based cling wraps (Soft wrap, ALKA Life Styles Pvt. Ltd, India) and apples (var. red delicious) of uniform size, colour and ripeness, without visible injury or infection, were selected from a local wholesale distributor at Thanjavur, India.
Biopolymer film formulation and manufacturing
To identify the appropriate plasticiser for chitosan and zein, cling wraps were prepared by incorporating different concentrations of glycerol and PEG 400 based on preliminary trials. For chitosan, 30%, 40% and 50%, and for zein, 30%, 35% and 40% of plasticisers (PEG 400 and glycerol) were used. Whilst other higher concentrations resulted in stickiness, lower concentrations did not show plasticising effect as required for a cling wrap. Based on preliminary trials, it was also understood that glycerol gives better plasticising effects on chitosan than PEG 400, at the same concentration. In view of improved plasticising effects, 50% of PEG 400 was taken for all studies. Glycerol-incorporated zein cling wraps resulted in a greasy and unappealing appearance, compared to the glossy appearance of PEG 400-incorporated zein cling wraps. Also, zein cling wraps with glycerol as plasticiser were brittle. So, a minimum concentration of 30% glycerol was used for all studies. Above this concentration, glycerol-incorporated zein films became fragile.
To prepare chitosan cling wraps, chitosan solution was prepared by stirring 1.5% (w/v) of chitosan in 2% (v/v) acetic acid solution for 24 h (solution pH 3.70). Then, this solution was filtered using a cheese cloth to remove undissolved debris. Different concentrations (30%, 40% and 50% w/w of chitosan) of glycerol were added to chitosan solution and stirred for 30 min at 60 °C. Similarly, zein wraps were prepared by stirring 15% (w/v) of zein in 80% (v/v) aqueous ethanol for 30 min (solution pH 5.45). This was followed by the addition of different concentrations (30%, 35% and 40% w/w of zein) of PEG 400. Then, the solution was stirred continuously for 1.5 h. Cling wrap films were obtained by casting 20 mL of biopolymer solutions on the horizontal glass tray (20 cm × 20 cm) and allowed to dry until sufficient drying (24 h for chitosan, 8 h for zein films) at controlled condition (30 ± 2 °C and 57 ± 5% RH). For easy peeling of zein films, zein solution was casted on polyethylene terephthalate sheet coated glass plates. Films were stored at ambient condition for 48 h before analysis. In this article, cling wraps are symbolised with alpha-numeric coding, with the first alphabet representing the biopolymer (chitosan/zein) and the second alphabet representing the plasticiser. Last set of numbers denotes the concentration (Table S1).
Characterisation of prepared cling wraps
Moisture content of cling wraps was determined as a measure of loss in weight of film after drying at 105 °C for 24 h. To calculate the total soluble matter, weight of 2 cm × 2 cm cling wraps obtained after drying at 105 °C for 24 h was considered as initial dry mass of the film and the method described by Hromis et al. (2015) was adopted. Similarly, swelling degree, film thickness (Insize digital micrometer (3109-25S, Insize, India); sensitivity 0.01 mm) and film density were calculated. Density was obtained as average of at least five values.
Optical properties
Transparency of the cling wrap is an important parameter and was measured at the visible region (380–700 nm) with a sampling interval of 1 nm using UV-vis spectrophotometer (UV-1800, Shimadzu, Japan). Measurements were done by placing appropriately cut wraps in the shape of the cuvette with blank cuvettes as the reference (Saha et al., 2018). Opacity values were calculated from absorbance at 600 nm (A600) using Eqn (1).
Surface colour of the film was evaluated using a spectrophotometer (ColorQuest XE, Hunter Lab, USA) in reflectance specular included mode, with illumination D65 and observer angle of 10o, and was considered as an average of five readings. White coloured standard tile (L* = 92.82; a* = −1.57; b* = 3.87) was used as the background and standard. Total colour difference (ΔE*) was calculated using Eqn (2).
where , and represent the difference in colour value in terms of lightness, greenness to redness, and blueness to yellowness of a standard white plate, and cling wraps, respectively.
Water vapour permeability
Moisture barrier properties of the prepared cling wraps are expressed in terms of water vapour permeability (WVP) and represented in g.mm.kPa−1 m−2 day−1. Gravimetrically, it was determined by ASTM E96 method. Measuring cups were covered with the film and sealed airtight, and 100% RH was maintained inside the cup using distilled water. On the other side of the film, 0% RH was created using silica gel. Weight lost from the cup was recorded over a period of 24 h at intervals of 2 h for the initial 6 h, followed by measurements at intervals of 6 h. A graph was plotted between loss in weight and time. The linear regression from points of weight loss versus time at constant rate period was divided by the area of the film to calculate water vapour transmission rate (WVTR). Using water vapour transmission rate (WVTR), WVP was calculated using Eqn (3) (Sobral et al., 2001).
where L is the film thickness (mm) and ΔP is the partial water vapour pressure difference (kPa) between the two sides of the film. The thickness of the films was measured using digital micrometer (3109-25S, Insize, India) having a sensitivity of 0.01 mm. Average of thickness at various portions of the film was considered.
Mechanical properties
The effect of plasticiser concentration on mechanical properties of developed cling wraps was analysed by measuring ultimate tensile strength (UTS) and ultimate strain (US). These tests were performed as per ASTM D882-10 in Tinius Olsen H50KL (Tinius Olsen Limited, UK). Cling wraps were cut in dimensions of 10 mm width and 100 mm length, and the initial grip was maintained as 50 mm. Crosshead speed was set to be 2 mm min−1. At least 5 replications were done for each film.
Antioxidant activity
Antioxidant activity of cling wraps was performed using 2, 2-diphenyl-picrylhydrazyl (DPPH) radical scavenging assay. Briefly, 5 mg of cling wraps was taken in individual tubes containing 3 mL of 10−4M DPPH solution prepared in methanol. This was incubated in dark under room temperature for 60 min. Absorbance was measured at 517 nm against pure methanol using UV/vis spectrophotometer, and DPPH solution was considered as the control. DPPH radical scavenging activity was calculated using Eqn (4).
where AC is the absorbance of the control and AS is the absorbance of sample.
Effect of developed cling wraps on cut apple slices
Wrapping of container containing apple slices
Mild washing, rinsing and drying of apples were done, followed by peeling and coring. Then, the apples were manually cut as cubes. Soon after dicing, the pieces were placed in a container and the container was wrapped with the chitosan and zein films. To compare the effect of the developed cling wraps on quality retention, two controls were considered as follows: apple slices exposed to ambient conditions, and apple slices wrapped with commercial PVC cling wraps. Then, analyses were carried out at ambient condition (30 ± 2 °C room temperature and 57 ± 5% relative humidity) for 24 h at 4 h intervals.
Effect of cling wraps on surface colour and weight loss
Surface colour of cut apple slices was evaluated using a reflectance spectrophotometer (ColorQuest XE, Hunter Lab, USA) in the method explained in earlier section. Weight losses in apple slices were evaluated by measuring the initial weight of fresh-cut apples and the weights at different intervals of time. Weights were measured using a digital balance with the precision of ± 0.001 g. Loss in weight was represented as percentage of weight loss (Attia et al., 2011).
Statistical analysis
All the experiments were carried out in triplicates. Data were analysed using one-way analysis of variance (Anova) using SPSS software to determine the differences among the samples at significance level P ≤ 0.05. Differences were analysed using LSD post hoc tests.
Results and discussion
Effect of different plasticiser concentration on the properties of cling wraps
Moisture content
The moisture content of cling wraps was observed to increase with increase in plasticiser concentration (Table 1). Maximum value of 31.63 ± 4.66% moisture content was observed in chitosan films with 50% glycerol. Increase in moisture content was due to the addition of hydrophilic plasticisers. Moreover, zein-based films showed comparatively lesser moisture content than chitosan-based films. This is due to the presence of high content of non-polar amino acids that makes zein films hydrophobic (Malhotra et al., 2015).
Moisture content, total soluble matter, swelling degree and film density of developed chitosan and zein cling wraps
| Cling wrap . | Moisture content (%) . | Total soluble matter (%) . | Swelling degree (%) . | Film density (g cm−3) . |
|---|---|---|---|---|
| CH 0 | 7.58 ± 3.28a,A | 16.05 ± 4.34a,A | 126.38 ± 14.58a,A | 0.63 ± 0.21a,A |
| CG 30 | 22.81 ± 2.43b,B | 16.97 ± 4.07a,AB | 105.37 ± 4.72b,B | 1.91 ± 0.15b,B |
| CG 40 | 29.37 ± 1.07c,C | 17.47 ± 3.09a,AB | 43.29 ± 4.01c,C | 2.61 ± 0.05c,C |
| CG 50 | 31.63 ± 4.66c,C | 21.19 ± 2.13a,B | 81.28 ± 1.71d,D | 2.60 ± 0.46c,C |
| ZN 0 | 2.98 ± 0.81a,A | 6.81 ± 1.90a,C | 3.98 ± 2.04a,E | 1.12 ± 0.48a,D |
| ZP 30 | 8.81 ± 4.30b,A | 27.52 ± 3.23b,D | 26.99 ± 6.67b,F | 1.77 ± 0.06b,B |
| ZP 35 | 9.46 ± 2.47b,A | 29.13 ± 1.99b,DE | 19.34 ± 0.28b,F | 1.68 ± 0.17b,B |
| ZP 40 | 13.28 ± 2.04b,D | 32.62 ± 2.32c,E | 6.73 ± 4.38a,E | 2.05 ± 0.19b,B |
| Cling wrap . | Moisture content (%) . | Total soluble matter (%) . | Swelling degree (%) . | Film density (g cm−3) . |
|---|---|---|---|---|
| CH 0 | 7.58 ± 3.28a,A | 16.05 ± 4.34a,A | 126.38 ± 14.58a,A | 0.63 ± 0.21a,A |
| CG 30 | 22.81 ± 2.43b,B | 16.97 ± 4.07a,AB | 105.37 ± 4.72b,B | 1.91 ± 0.15b,B |
| CG 40 | 29.37 ± 1.07c,C | 17.47 ± 3.09a,AB | 43.29 ± 4.01c,C | 2.61 ± 0.05c,C |
| CG 50 | 31.63 ± 4.66c,C | 21.19 ± 2.13a,B | 81.28 ± 1.71d,D | 2.60 ± 0.46c,C |
| ZN 0 | 2.98 ± 0.81a,A | 6.81 ± 1.90a,C | 3.98 ± 2.04a,E | 1.12 ± 0.48a,D |
| ZP 30 | 8.81 ± 4.30b,A | 27.52 ± 3.23b,D | 26.99 ± 6.67b,F | 1.77 ± 0.06b,B |
| ZP 35 | 9.46 ± 2.47b,A | 29.13 ± 1.99b,DE | 19.34 ± 0.28b,F | 1.68 ± 0.17b,B |
| ZP 40 | 13.28 ± 2.04b,D | 32.62 ± 2.32c,E | 6.73 ± 4.38a,E | 2.05 ± 0.19b,B |
Different lowercase and uppercase letters in the superscript mark significantly different mean values (P ≤ 0.05) within the same polymer and overall treatment (same column), respectively.
Moisture content, total soluble matter, swelling degree and film density of developed chitosan and zein cling wraps
| Cling wrap . | Moisture content (%) . | Total soluble matter (%) . | Swelling degree (%) . | Film density (g cm−3) . |
|---|---|---|---|---|
| CH 0 | 7.58 ± 3.28a,A | 16.05 ± 4.34a,A | 126.38 ± 14.58a,A | 0.63 ± 0.21a,A |
| CG 30 | 22.81 ± 2.43b,B | 16.97 ± 4.07a,AB | 105.37 ± 4.72b,B | 1.91 ± 0.15b,B |
| CG 40 | 29.37 ± 1.07c,C | 17.47 ± 3.09a,AB | 43.29 ± 4.01c,C | 2.61 ± 0.05c,C |
| CG 50 | 31.63 ± 4.66c,C | 21.19 ± 2.13a,B | 81.28 ± 1.71d,D | 2.60 ± 0.46c,C |
| ZN 0 | 2.98 ± 0.81a,A | 6.81 ± 1.90a,C | 3.98 ± 2.04a,E | 1.12 ± 0.48a,D |
| ZP 30 | 8.81 ± 4.30b,A | 27.52 ± 3.23b,D | 26.99 ± 6.67b,F | 1.77 ± 0.06b,B |
| ZP 35 | 9.46 ± 2.47b,A | 29.13 ± 1.99b,DE | 19.34 ± 0.28b,F | 1.68 ± 0.17b,B |
| ZP 40 | 13.28 ± 2.04b,D | 32.62 ± 2.32c,E | 6.73 ± 4.38a,E | 2.05 ± 0.19b,B |
| Cling wrap . | Moisture content (%) . | Total soluble matter (%) . | Swelling degree (%) . | Film density (g cm−3) . |
|---|---|---|---|---|
| CH 0 | 7.58 ± 3.28a,A | 16.05 ± 4.34a,A | 126.38 ± 14.58a,A | 0.63 ± 0.21a,A |
| CG 30 | 22.81 ± 2.43b,B | 16.97 ± 4.07a,AB | 105.37 ± 4.72b,B | 1.91 ± 0.15b,B |
| CG 40 | 29.37 ± 1.07c,C | 17.47 ± 3.09a,AB | 43.29 ± 4.01c,C | 2.61 ± 0.05c,C |
| CG 50 | 31.63 ± 4.66c,C | 21.19 ± 2.13a,B | 81.28 ± 1.71d,D | 2.60 ± 0.46c,C |
| ZN 0 | 2.98 ± 0.81a,A | 6.81 ± 1.90a,C | 3.98 ± 2.04a,E | 1.12 ± 0.48a,D |
| ZP 30 | 8.81 ± 4.30b,A | 27.52 ± 3.23b,D | 26.99 ± 6.67b,F | 1.77 ± 0.06b,B |
| ZP 35 | 9.46 ± 2.47b,A | 29.13 ± 1.99b,DE | 19.34 ± 0.28b,F | 1.68 ± 0.17b,B |
| ZP 40 | 13.28 ± 2.04b,D | 32.62 ± 2.32c,E | 6.73 ± 4.38a,E | 2.05 ± 0.19b,B |
Different lowercase and uppercase letters in the superscript mark significantly different mean values (P ≤ 0.05) within the same polymer and overall treatment (same column), respectively.
Total soluble matter
Total soluble matter is an important property of a packaging film used for food applications. Total soluble matter of the cling wraps was positively correlated with plasticiser concentration (Table 1). As the concentration of hygroscopic plasticiser in the cling wraps was increasing, solubility increased. Highest total soluble matter was found to be 21.19 ± 2.13 and 32.62 ± 2.32 for CG 50 and ZP 40, respectively.
Swelling degree
Swelling degree is a key parameter to be measured for packaging materials that come in contact with high moisture foods. When the movement of water molecules is restricted by the polymer structure, degree of swelling decreases. Swelling degree of the developed cling wraps was found to decrease significantly with increasing concentration of plasticiser in both chitosan and zein films (Table 1). This could be due to leaching of higher amounts of plasticisers by the water during the swelling experiment. Similar results were obtained by Hermans et al. (2014) in glycerol plasticised and un-plasticised chitosan films. These authors reported that un-plasticised chitosan film have hydroxyl and amino groups that interact with water molecule present; with increase in plasticiser concentration, the hydrogen bond formed between the amino groups of chitosan and glycerol interacts to hinder uptake of water by the film. Since chitosan and zein contain amino groups, there is a possibility of similar interactions to occur (Yang et al., 2010; Zhang et al., 2011).
Film thickness and film density
Thickness of the developed cling wraps was found to be in the range of 57.5-72.5 μm. For chitosan cling wraps, average thickness values were found to be 63.5 ± 5.5 μm, whereas, for zein wraps, average thickness was 67.5 ± 7.5 μm. Film density was found to be in the range of 1.91 to 2.61 g cm−3 and 1.68 to 1.85 g cm−3 for chitosan and zein cling wraps, respectively (Table 1).
Optical properties
Transparency can be quantified as a measure of per cent transmittance; 100% transmittance indicates a completely transparent film. Addition of glycerol into zein cling wraps reduced film transparency significantly (Fig. 1). This is due to migration of glycerol to the surface of the zein film and was evident from surface stickiness of zein cling wraps. Though chitosan wraps were more transparent than zein cling wraps, zein cling wraps were more appealing due to its glossy surface. Also, addition of plasticisers did not significantly affect the transparency of both chitosan and zein cling wraps. In addition, better transparency was observed for glycerol-incorporated chitosan film, as compared to PEG 400-incorporated chitosan film, proving that glycerol can better suit chitosan cling wraps.
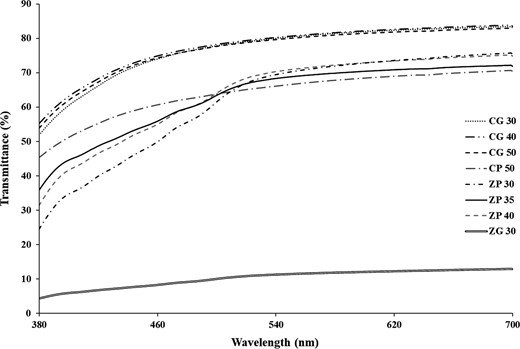
Transmittance spectra of developed chitosan and zein cling wraps as a function of time.
Though opacity values of cling wraps did not vary significantly with plasticiser content, addition of glycerol into zein films increased its opacity (Table 2). This is because of the cloudy appearance of the film due to the migration of glycerol to the surface. This results in the unappealing appearance in glycerol-added zein films. Thus, glycerol is not suitable for zein cling wraps. This was further proved by increased colour differences in glycerol-added zein films. Moreover, there was no evident colour difference in cling wraps with the increased concentration of plasticisers.
Optical properties of developed chitosan and zein cling wraps as a function of plasticiser concentration
| Cling wraps . | Film opacity (AU mm−1) . | Colour difference . | WVP (*10−2 g mm day−1 m−2 kPa−1) . | Antioxidant activity (%) . |
|---|---|---|---|---|
| CG 30 | 2.29 ± 0.12a,B | 9.44 ± 0.10a,B | 3.7 ± 0.51a,A | 21.24 ± 2.60a,B |
| CG 40 | 2.4 ± 0.26a,B | 9.94 ± 0.35a,B | 4.2 ± 0.96a,A | 20.46 ± 3.56a,B |
| CG 50 | 2.275 ± 0.10a,B | 9.82 ± 0.79a,B | 4.3 ± 1.17a,A | 19.96 ± 2.51a,B |
| CP 50 | 2.37 ± 0.35a,B | 9.89 ± 0.87a,B | – | – |
| ZP 30 | 2.51 ± 0.97b,B | 21.93 ± 1.08a,A | 5.01 ± 1.15b,B | 68.89 ± 0.99a,A |
| ZP 35 | 3.46 ± 1.63b,B | 18.52 ± 1.38b,A | 6.1 ± 0.06ab,A | 69.31 ± 1.33a,A |
| ZP 40 | 1.9 ± 1.55b,B | 21.65 ± 1.22a,A | 6.9 ± 0.15a,A | 65.19 ± 3.17b,A |
| ZG 30 | 17.53 ± 1.98a,A | 23.28 ± 0.96a,A | – | – |
| Cling wraps . | Film opacity (AU mm−1) . | Colour difference . | WVP (*10−2 g mm day−1 m−2 kPa−1) . | Antioxidant activity (%) . |
|---|---|---|---|---|
| CG 30 | 2.29 ± 0.12a,B | 9.44 ± 0.10a,B | 3.7 ± 0.51a,A | 21.24 ± 2.60a,B |
| CG 40 | 2.4 ± 0.26a,B | 9.94 ± 0.35a,B | 4.2 ± 0.96a,A | 20.46 ± 3.56a,B |
| CG 50 | 2.275 ± 0.10a,B | 9.82 ± 0.79a,B | 4.3 ± 1.17a,A | 19.96 ± 2.51a,B |
| CP 50 | 2.37 ± 0.35a,B | 9.89 ± 0.87a,B | – | – |
| ZP 30 | 2.51 ± 0.97b,B | 21.93 ± 1.08a,A | 5.01 ± 1.15b,B | 68.89 ± 0.99a,A |
| ZP 35 | 3.46 ± 1.63b,B | 18.52 ± 1.38b,A | 6.1 ± 0.06ab,A | 69.31 ± 1.33a,A |
| ZP 40 | 1.9 ± 1.55b,B | 21.65 ± 1.22a,A | 6.9 ± 0.15a,A | 65.19 ± 3.17b,A |
| ZG 30 | 17.53 ± 1.98a,A | 23.28 ± 0.96a,A | – | – |
Different lowercase and uppercase letters in the superscript mark significantly different mean values (P ≤ 0.05) within the same polymer and overall treatment (same column), respectively.
Optical properties of developed chitosan and zein cling wraps as a function of plasticiser concentration
| Cling wraps . | Film opacity (AU mm−1) . | Colour difference . | WVP (*10−2 g mm day−1 m−2 kPa−1) . | Antioxidant activity (%) . |
|---|---|---|---|---|
| CG 30 | 2.29 ± 0.12a,B | 9.44 ± 0.10a,B | 3.7 ± 0.51a,A | 21.24 ± 2.60a,B |
| CG 40 | 2.4 ± 0.26a,B | 9.94 ± 0.35a,B | 4.2 ± 0.96a,A | 20.46 ± 3.56a,B |
| CG 50 | 2.275 ± 0.10a,B | 9.82 ± 0.79a,B | 4.3 ± 1.17a,A | 19.96 ± 2.51a,B |
| CP 50 | 2.37 ± 0.35a,B | 9.89 ± 0.87a,B | – | – |
| ZP 30 | 2.51 ± 0.97b,B | 21.93 ± 1.08a,A | 5.01 ± 1.15b,B | 68.89 ± 0.99a,A |
| ZP 35 | 3.46 ± 1.63b,B | 18.52 ± 1.38b,A | 6.1 ± 0.06ab,A | 69.31 ± 1.33a,A |
| ZP 40 | 1.9 ± 1.55b,B | 21.65 ± 1.22a,A | 6.9 ± 0.15a,A | 65.19 ± 3.17b,A |
| ZG 30 | 17.53 ± 1.98a,A | 23.28 ± 0.96a,A | – | – |
| Cling wraps . | Film opacity (AU mm−1) . | Colour difference . | WVP (*10−2 g mm day−1 m−2 kPa−1) . | Antioxidant activity (%) . |
|---|---|---|---|---|
| CG 30 | 2.29 ± 0.12a,B | 9.44 ± 0.10a,B | 3.7 ± 0.51a,A | 21.24 ± 2.60a,B |
| CG 40 | 2.4 ± 0.26a,B | 9.94 ± 0.35a,B | 4.2 ± 0.96a,A | 20.46 ± 3.56a,B |
| CG 50 | 2.275 ± 0.10a,B | 9.82 ± 0.79a,B | 4.3 ± 1.17a,A | 19.96 ± 2.51a,B |
| CP 50 | 2.37 ± 0.35a,B | 9.89 ± 0.87a,B | – | – |
| ZP 30 | 2.51 ± 0.97b,B | 21.93 ± 1.08a,A | 5.01 ± 1.15b,B | 68.89 ± 0.99a,A |
| ZP 35 | 3.46 ± 1.63b,B | 18.52 ± 1.38b,A | 6.1 ± 0.06ab,A | 69.31 ± 1.33a,A |
| ZP 40 | 1.9 ± 1.55b,B | 21.65 ± 1.22a,A | 6.9 ± 0.15a,A | 65.19 ± 3.17b,A |
| ZG 30 | 17.53 ± 1.98a,A | 23.28 ± 0.96a,A | – | – |
Different lowercase and uppercase letters in the superscript mark significantly different mean values (P ≤ 0.05) within the same polymer and overall treatment (same column), respectively.
Mechanical properties
As expected, film flexibility increased with increase in plasticiser concentration (Fig. 2a,b). On comparison between the plasticising effect of PEG 400 and glycerol on chitosan films, glycerol showed better plasticising effects, as essential for a cling wrap. Domjan et al. (2009) also proved that chitosan plasticised with PEG 400 films showed higher tensile strength than that with glycerol. Moreover, zein films showed better plasticising effect in the presence of PEG 400 and films prepared with glycerol were brittle (Fig. 2b). This is because, hydrogen and hydrophobic bonds result in brittle zein films (Malhotra et al., 2015). Addition of plasticiser improves film flexibility by interacting with these bonds. Glycerol migrates to the surface of the zein film, making it brittle. This makes it clear that PEG 400 can be used as better plasticiser for zein-based cling wraps.
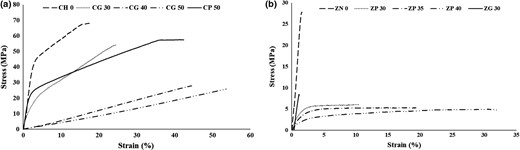
Stress vs strain for cling wraps as a function of plasticiser concentration a) chitosan cling wraps; b) zein cling wraps.
Water vapour permeability
Water vapour permeability of the developed cling wraps is shown in Table 2. With increase in plasticiser concentration, water vapour permeability of biopolymer films also increased. Addition of hydrophilic plasticisers increased permeability due to its hygroscopic nature. Also, reduction in structural density of the protein network with addition of plasticiser increases its permeability to water by increasing free volume, and thus, the mobility (Cuq et al., 1997). Water vapour permeability of commercial cling wraps was found to be almost 10 times lower than that of the developed bio-based films.
Antioxidant activity
Antioxidant activity property of the packaging material aids in maintaining the quality of the food material. From Table 2, it is evident that zein films showed higher antioxidant activity at all plasticiser concentrations than those of chitosan films. In general, free radical scavenging activity of chitosan is attributed to the presence of hydroxyl groups as well as amine groups (Yang et al., 2010). The scavenging activity of zein is due to the presence of amino acids. Major amino acids responsible for the antioxidant activity of zein are histidine, arginine, alanine, methionine, valine and leucine (Zhang et al., 2011). Moreover, no significant variation in the antioxidant activity of bio-based cling wraps was observed with increasing plasticiser concentration.
Effect of cling wraps on the surface colour of cut apple slices
Considering mouldability, flexibility, stickiness and other film characteristics such as water vapour permeability and tensile strength, CG 40 and ZP 35 were taken for further analysis (Fig. S1)
Surface colour
To eliminate the effect of other factors such as maturity stage and portion of the slice taken, changes in colour parameters, especially L* and a* values, were evaluated. Fig. 3a and b represents the degree of variation of surface colour of apple slices from fresh slices. In general, less deviation in lightness values of apple slices from those of fresh is considered to have lesser degree of browning. The deviation in lightness value is found to increase with storage time. On comparing the a* value of apple slices wrapped with various films, no considerable difference was observed in colour values of apple slices in containers wrapped with chitosan or zein cling wraps and commercial PVC film. Moreover, all the apple slices wrapped with films were superior in terms of surface color, compared to the apple slices exposed to ambient conditions. Restricted browning in commercial cling wraps may be attributed to the barrier properties of the material itself and, in case of biopolymer films, its antioxidant property.
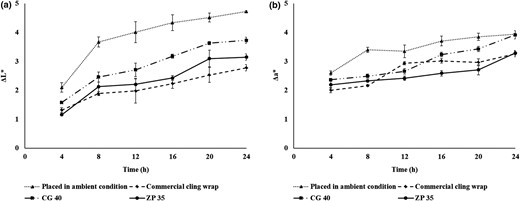
Effect of cling wraps on cut apple slices a) ΔL* values; b) Δa* values (CG 40–Chitosan plasticised with 40% glycerol; ZP 35–Zein plasticised with 35% PEG 400).
Weight loss
Moisture loss (%) in cut apple slices is represented as per cent weight loss (Fig 4). In general, apple slices wrapped inside films loose moisture to the environment due to differences in ambient RH and the RH developed inside the container (Mannozzi et al., 2018). As biopolymer films permeate relatively higher amount of moisture, weight loss was lower in commercial cling-wrapped apple slices, followed by zein and chitosan cling-wrapped apple slices. Though water vapour permeability of chitosan cling wraps was similar to that of zein cling wraps, higher weight loss in chitosan-wrapped apple slices was observed and this is correlated with its hydrophilic nature and the higher swelling degree of chitosan wraps. Apple slices exposed to ambient showed a significantly higher weight loss. In general, the packaging material allowing higher moisture loss induces higher shrinkage and microbial invasion. Thus, the weight loss studies suggest that zein films are superior to chitosan films from the perspective of maintaining the freshness of the apple slices.
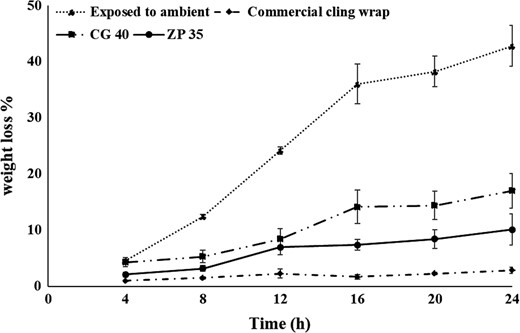
Weight loss of the apple slices as a function of time (CG 40–Chitosan plasticised with 40% glycerol; ZP 35–Zein plasticised with 35% PEG 400).
Conclusion
This research highlights the development of a novel zein-based film for cling wrapping of fresh-cut apples. Both zein and chitosan were used with PEG-400 and glycerol as plasticisers, their concentrations were optimised, and storage studies on fresh-cut apple slices, focusing on anti-browning properties, were done. Though water vapour permeability was higher as compared to conventional polymeric films, all other essential attributes for a cling wrap were found to be acceptable for the developed biopolymers. Interestingly, zein-based cling wraps were observed to be superior to chitosan-based cling wraps, in terms of maintaining the freshness of the apple slices as well as the antioxidant characteristics. This shows the prospects of using zein-based materials for cling wrapping applications.
Acknowledgment
The author M. Maria Leena acknowledges Department of Science and Technology, Government of India, for Women Scientist (SR/WOS-A/LS-386/2016) fellowship.
Conflict of interest
The authors declare no conflict of interest.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethical guidelines
Ethics approval was not required for this research.



