-
PDF
- Split View
-
Views
-
Cite
Cite
Maud Muchuweti, Lynet Nyamukonda, Larmet S Chagonda, Ashrrell R Ndhlala, Chipo Mupure, Mudadi Benhura, Total phenolic content and antioxidant activity in selected medicinal plants of Zimbabwe, International Journal of Food Science and Technology, Volume 41, Issue Supplement_1, August 2006, Pages 33–38, https://doi.org/10.1111/j.1365-2621.2006.01258.x
Close - Share Icon Share
Abstract
Seven commonly known medicinal plants from Zimbabwe were analysed for their antioxidant activity as well as their total phenolic content. The plant samples used in this study were Albizia amara, Elionurus muticus, Heteropyxis natalensis, Hoslundia opposita, Lippia javanica, Ocimum urticifolia and Warburgia salutaris. The plant samples were extracted using 70% ethanol. The 2,2-diphenyl-1-picrylhydrazyl radical assay was used to determine the antioxidant activity of the plant extracts, while the Folin–Ciocalteu method was used to determine the total phenolic content. The antioxidant activities of the plant extracts ranged from 95.84 ± 0.50% for E. muticus to 5.31 ± 4.47% for H. opposita. Total phenolics in the plant extract estimated as tannic acid equivalent (TAE) ranged from 0.098 ± 0.005 mg per 100 g for H. natalensis to 0.024 ± 0.006 TAE for H. opposita. There was a poor correlation (R = 0.522) between total phenolic content and antioxidant activity in the plant samples. The results indicate the presence of phenolic compounds as well as significant antioxidant activity.
Introduction
Numerous investigations have proved that medicinal herbs contain diverse classes of compounds such as polyphenols, tocopherols, alkaloids, tannins, carotenoids, terpenoids, etc. (Velioglu et al., 1998). Flavanols and other phenolic acids, tannins, lignans and lignin are especially common in the leaves, flowering tissue and woody parts such as the stem, bark and roots of plants. The flavanols and phenolic acids are particularly attractive, as they are known to exhibit various beneficial pharmacological properties such as vasoprotective, anticarcinogenic, antineoplastic, antiviral, anti-inflammatory, as well as antiallergic and antiproliferative activity on tumour cells (Kuhnau, 1976; Middleton & Kandaswami, 1992; Carr et al., 2000). Some of these properties have been related to the action of these compounds as antioxidants, free radical scavengers, quenchers of singlet and triplet oxygen and inhibitors of peroxidation. Antioxidant activity of phenolic compounds is correlated to some structure–activity relationships, such as redox properties and the number and arrangement of hydroxyl groups (Cotelle et al., 1996). Reactive oxygen species in the form of superoxide anion, hydrogen peroxide and hydroxyl radical are natural by-products of our body metabolism. They are dangerous, however, when present in excess, and can attack biological molecules such as lipids, proteins, enzymes, DNA and RNA, leading to tissue or cell injury associated with degenerative diseases (Pietta et al., 1998; Jung et al., 1999; Valentão et al., 2002). Thus, the occurrence of such oxidative damage may be a significant causative factor in the development of many chronic diseases, such as cancer and cardiovascular diseases (Lindley, 1998; Papas, 1999). Although the mammalian body has defence mechanisms to combat and reduce oxidative damage, epidemiological evidence indicates that the consumption of medicinal herbs and plants containing antioxidant phytonutrients – notably flavanols and other polyphenolics – is advantageous for our health.
Anthology of plants under study
Albizia amara tree is small and is about 3–5 m high with a wide, dense, round or umbrella-shaped canopy. On a nutritional basis, the plants are eaten by herbivores (cattle, sheep and goat) and may also be used as firewood. The Albizia species contain tannin and the bark is used as an astringent in diarrhoea and dysentery and internally to check uterine bleeding and the discharge in gonorrhoea as well as topically in ophthalmia and as wound dressing. The leaves may be used as an expectorant to treat common cold and is also an emetic. The Albizia species are well known for their insecticide properties as well as acting as a vermifuge (Dougall & Bogdan, 1958; Tredgold, 1986).
Elionurus muticus is commonly known as lemongrass and belongs to the hairy tridentgrass species. The vegetation type is dense, sour grassland.
This plant has not been extensively studied and so not much is known about its medicinal properties, although it is known to contain essential oils. Economically, this grass is used for grazing purposes. Lemon grass is known to be sudorific and has febrifuge (reduction in fever) properties (Boose & Holt, 1999).
Heteropyxis natalensis is also called the lavender tree and belongs to the Heteropyxidaceae or lavender or tea family. Lavender tree has droopy foliage.
The tree has several medicinal uses. The leaves and roots of this plant are used medicinally to treat worms in stock. African healers prescribe inhaling steam from the roots to heal nose bleeding. The roots and leaves may also be used in the treatment of mental disorders. The fresh leaves may also be used during weaning (Tredgold, 1986).
Hoslundia opposita is a herb belonging to the mint family, which can be grown in gardens. It is widespread throughout tropical Africa and is therefore not indigenous to Zimbabwe. The roots of the plant are used as an antiseptic for wounds and cuts, to treat colds, as a purgative or laxative, to treat sore throat and in gonorrhoea. The leaf and leaf sap are used for convulsions, especially those caused by epilepsy, sore eyes, conjunctivitis, jaundice as well as stomach pain and vertigo. The leaves and flowers are especially used as an antidote for snake bites as well as for preventing them. The decoction of the leaves may also be used to treat ringworm as well as parasitic skin diseases (Codd, 1985; Pooley, 1998).
Lippia javanica is multistemmed, 1–2 m high and is also called the fever tree or lemon tree. This plant is well known medicinally to many African tribes and many avid herbalists. Different parts (leaves, twigs and roots) of the plant are used for different reasons. A strong tea infusion is used in the treatment of colds, coughs and bronchial problems. The plant is also used by the Xhosa people in South Africa to disinfect meat that has been infected with anthrax. The herb is effective against fever, especially in the case of malaria, influenza and measles and may be used as a prophylactic against lung infections (Van Wyk et al., 1997).
The smoke of the herb has proved to be very effective if inhaled in case of asthma and chronic cough. The strong infusion of the leaves may be used as a carminative, anticolic, anticatarrh and as an emetic. Applying the cooled decoction of the leaves as a lotion treats skin disorders such as rashes, stings and bites (Van Wyk et al., 1997).
Ocimum urticifolia belongs to the native species of basil. The essential oils obtained from the leaves and stems are used for intestinal and uterine colic as well as a febrifuge. Decoction of the plant may be used as an antiseptic in wound dressing or may be taken orally to reduce fever. A strong tea infusion has been known to regulate the menstrual cycle (Bassole et al., 2005).
Warburgia salutaris tree is commonly known as the pepper bark tree.
The plant is generally used to treat many ailments. The leaves are used as an expectorant or smoked to treat common cold. When the leaves are ground and dried to create snuff, they may be used to clear sinuses (Kubo et al., 1976, 1977; Rabe & Van Staden, 2000).
The aims and objectives of the study were to estimate the total phenolic content of selected medicinal plants, to evaluate the antioxidant activity and compare the total phenolic content and antioxidant activities of the medicinal plants and determine whether a correlation exists between them.
Materials and methods
Plants studied include A. amara (leaves and stems), E. muticus (whole plant), H. natalensis (leaves and stems), H. opposita (leaves and stems), Lippia javanicum (leaves and stems), O. urticifolia (leaves and stems) and W. salutaris (leaves and stems). The freshly cut plants were sorted out, dried for a week in the sun and later used for investigations.
Extraction
The dried plant extracts were milled using a mortar and pestle. Two-step extraction was done by shaking 5 g of the milled sample with 30 mL of 70% ethanol for 2 h. The extracts were filtered and concentrated in a Buchi rotary evaporator (R-114) (Sibata Scientific Technology, Tokyo, Japan) at 40 °C. The ethanolic extracts were prepared in triplicate.
Evaluation of antioxidant activity
Radical scavenging activity of plant extracts against stable 2,2-diphenyl-1-picrylhydrazyl hydrate (DPPH) was determined spectrophotometrically. When DPPH reacts with an antioxidant compound that can donate hydrogen, it is reduced. The changes in colour from deep violet to light yellow were measured at 515 nm on a u.v./visible light spectrophotometer. The assay was performed using a modified method described by Braca et al. (2001) to denote the hydrogen-donating ability of the crude extract. A volume of 3 mL of 0.004% DPPH methanol solution was used. The reaction was started by the addition of 0.1 mL (1 mg mL−1) of sample. The bleaching of DPPH was monitored at 517 nm, at room temperature for 30 min. The inhibition percentage (IP) of the DPPH radical was calculated as follows:
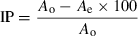
where Ao is the absorbance without the extract and Ae the absorbance with the extract.
Evaluation of total phenolic compounds
The total phenolic compounds was determined by a slightly modified Folin–Ciocalteu method (1927). The Folin–Ciocalteu assay provides a simple, inexpensive, quick and useful estimate of total phenolics.
Extracts were prepared at a concentration of 0.1 mg mL−1. Hundred microlitres of the extract were transferred into a test tube and 750 μL of Folin–Ciocalteu reagent (previously diluted tenfold with distilled water, the mixture should be golden green and discarded if it is olive green) were added and mixed. The mixture was allowed to stand at room temperature for 5 min. A volume of 0.75 mL of 6% (w/v) sodium carbonate (6 g made up to 100 mL using distilled water) was added to the mixture and then mixed gently. A blank was also made by mixing water and the reagents. After allowing the mixture to stand at room temperature for 40 min, the absorbance was read at 725 nm using a u.v./visible spectrophotometer. The experiment was carried out in triplicate and a standard deviation was obtained for all results. The standard calibration curve was plotted using tannic acid and the results expressed as tannic acid equivalents (TAE) milligrams per 100 mg extract.
Statistical analysis
Statistical test was used at the 95% confidence interval (P < 0.05). A two-way test was used and the sample was rejected if −1.96 < Z > 1.96. The formula applied by Microsoft excel is
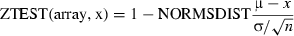
where μ is the mean of sample, δ the standard deviation of the sample and NORMSDIST was equal to 1.96 at the 95% confidence interval.
Results
Phytochemicals, especially phenolics are suggested to be the major bioactive compounds for health benefits. Plant extracts, which contain different classes of polyphenols, are very attractive not only in modern phytotherapy but also in the food industry. Therefore, in this study, we investigated the total phenolic content and antioxidant properties of ethanolic plant extracts of selected medicinal plants.
Percentage yield
The percentage yield of plant extracts ranged from 4.683% to 15.99%. The maximum percentage yield being obtained for L. javanica, as shown in Table 1. The percentage yields decreased in the following order: L. javanica > A. amara > H. natalensis > H. opposita > O. urticifolia > W. salutaris > E. muticus. The percentage yields provided adequate samples for the DPPH assay and the Folin–Ciocalteu assay.
Percentage yield, total phenolic content and medicinal plant extracts and β-Carotene
| . | Plant extract yield (%)a . | TAE mg per 100 g . | Percentage inhibition . |
|---|---|---|---|
| β-Carotene | – | 0.14 ± 0.024 | 98.84 ± 6.6 |
| Elionurus muticus | 4.68 ± 0.21 | 0.076 ± 0.003 | 51.86 ± 1.32 |
| Albizia amara | 15.90 ± 0.8 | 0.077 ± 0.005 | 95.84 ± 0.50 |
| Heteropyxis natalensis | 14.71 ± 0.31 | 0.096 ± 0.005 | 29.65 ± 0.38 |
| Ocimum urticifolia | 11.16 ± 0.23 | 0.024 ± 0.006 | 5.31 ± 0.48 |
| Warburgia salutaris | 10.07 ± 0.4 | 0.065 ± 0.018 | 74.4 ± 1.18 |
| Lippia javanicum | 15.99 ± 0.64 | 0.064 ± 0.008 | 29.87 ± 4.11 |
| Hoslundia opposita | 13.33 ± 0.32 | 0.054 ± 0.008 | 92.57 ± 1.0 |
| . | Plant extract yield (%)a . | TAE mg per 100 g . | Percentage inhibition . |
|---|---|---|---|
| β-Carotene | – | 0.14 ± 0.024 | 98.84 ± 6.6 |
| Elionurus muticus | 4.68 ± 0.21 | 0.076 ± 0.003 | 51.86 ± 1.32 |
| Albizia amara | 15.90 ± 0.8 | 0.077 ± 0.005 | 95.84 ± 0.50 |
| Heteropyxis natalensis | 14.71 ± 0.31 | 0.096 ± 0.005 | 29.65 ± 0.38 |
| Ocimum urticifolia | 11.16 ± 0.23 | 0.024 ± 0.006 | 5.31 ± 0.48 |
| Warburgia salutaris | 10.07 ± 0.4 | 0.065 ± 0.018 | 74.4 ± 1.18 |
| Lippia javanicum | 15.99 ± 0.64 | 0.064 ± 0.008 | 29.87 ± 4.11 |
| Hoslundia opposita | 13.33 ± 0.32 | 0.054 ± 0.008 | 92.57 ± 1.0 |
ag extract per 100 g of plant material.
Percentage yield, total phenolic content and medicinal plant extracts and β-Carotene
| . | Plant extract yield (%)a . | TAE mg per 100 g . | Percentage inhibition . |
|---|---|---|---|
| β-Carotene | – | 0.14 ± 0.024 | 98.84 ± 6.6 |
| Elionurus muticus | 4.68 ± 0.21 | 0.076 ± 0.003 | 51.86 ± 1.32 |
| Albizia amara | 15.90 ± 0.8 | 0.077 ± 0.005 | 95.84 ± 0.50 |
| Heteropyxis natalensis | 14.71 ± 0.31 | 0.096 ± 0.005 | 29.65 ± 0.38 |
| Ocimum urticifolia | 11.16 ± 0.23 | 0.024 ± 0.006 | 5.31 ± 0.48 |
| Warburgia salutaris | 10.07 ± 0.4 | 0.065 ± 0.018 | 74.4 ± 1.18 |
| Lippia javanicum | 15.99 ± 0.64 | 0.064 ± 0.008 | 29.87 ± 4.11 |
| Hoslundia opposita | 13.33 ± 0.32 | 0.054 ± 0.008 | 92.57 ± 1.0 |
| . | Plant extract yield (%)a . | TAE mg per 100 g . | Percentage inhibition . |
|---|---|---|---|
| β-Carotene | – | 0.14 ± 0.024 | 98.84 ± 6.6 |
| Elionurus muticus | 4.68 ± 0.21 | 0.076 ± 0.003 | 51.86 ± 1.32 |
| Albizia amara | 15.90 ± 0.8 | 0.077 ± 0.005 | 95.84 ± 0.50 |
| Heteropyxis natalensis | 14.71 ± 0.31 | 0.096 ± 0.005 | 29.65 ± 0.38 |
| Ocimum urticifolia | 11.16 ± 0.23 | 0.024 ± 0.006 | 5.31 ± 0.48 |
| Warburgia salutaris | 10.07 ± 0.4 | 0.065 ± 0.018 | 74.4 ± 1.18 |
| Lippia javanicum | 15.99 ± 0.64 | 0.064 ± 0.008 | 29.87 ± 4.11 |
| Hoslundia opposita | 13.33 ± 0.32 | 0.054 ± 0.008 | 92.57 ± 1.0 |
ag extract per 100 g of plant material.
Antioxidant activity
The DPPH assay was used to measure the antioxidant activity of the prepared plant extracts. Unlike laboratory-generated free radicals, such as the hydroxyl radical and superoxide anion, DPPH radical has the advantage of being unaffected by certain side reactions, such as metal ion chelation, and enzyme inhibition, brought about by various additives. A freshly prepared sample of DPPH exhibits a deep purple colour, with maximum absorbance at 517 nm. The purple color generally fades or disappears when an antioxidant is present in the medium. Thus, antioxidant molecules can quench DPPH free radicals (i.e. by providing hydrogen atoms or by electron donation, conceivably by free radical attack) and convert them to a pale yellow or bleached product (i.e. 2,2-diphenyl-1-hydrazine or a substituted analogous of hydrazine), resulting in a decrease in absorbance at 517 nm (Yamaguchi et al., 1998). Hence, the more rapidly the absorbance decreases, the more potent is the antioxidant activity of the extract, in terms of hydrogen-atom-donating capacity. Caution must, however, be exercised when interpreting such results, as the reactions that DPPH radical elicits are not as simple and as straightforward. One cannot arbitrarily assume that the decrease in absorbance is solely attributed to the antioxidant donating a hydrogen atom or an electron to DPPH. Nevertheless, the ‘DPPH test’ is a commonly employed assay in antioxidant studies and offers a rapid technique in which to screen for antioxidant activity.
The antioxidant values (percentage inhibition) of the crude ethanolic extracts from the seven plant species were examined and compared with one another.
| . | Z test results (P < 0.05) . | ||
|---|---|---|---|
| 1 . | 2 . | 3 . | |
| Albizia amara | 0.977 | 0.13 | 0.193 |
| Elionurus muticus | 0.435 | 0.0501 | 0.965 |
| Heteropyxis natalensis | 0.91 | 0.0252 | 0.732 |
| Hoslundia opposita | 0.223 | 0.976 | 0.111 |
| Lippia javanica | 0.178 | 0.141 | 0.977 |
| Ocimum urticifolia | 0.0467 | 0.458 | 0.963 |
| Warburgia salutaris | 0.159 | 0.159 | 0.977 |
| β−Carotene | 0.208 | 0.12 | 0.977 |
| . | Z test results (P < 0.05) . | ||
|---|---|---|---|
| 1 . | 2 . | 3 . | |
| Albizia amara | 0.977 | 0.13 | 0.193 |
| Elionurus muticus | 0.435 | 0.0501 | 0.965 |
| Heteropyxis natalensis | 0.91 | 0.0252 | 0.732 |
| Hoslundia opposita | 0.223 | 0.976 | 0.111 |
| Lippia javanica | 0.178 | 0.141 | 0.977 |
| Ocimum urticifolia | 0.0467 | 0.458 | 0.963 |
| Warburgia salutaris | 0.159 | 0.159 | 0.977 |
| β−Carotene | 0.208 | 0.12 | 0.977 |
| . | Z test results (P < 0.05) . | ||
|---|---|---|---|
| 1 . | 2 . | 3 . | |
| Albizia amara | 0.977 | 0.13 | 0.193 |
| Elionurus muticus | 0.435 | 0.0501 | 0.965 |
| Heteropyxis natalensis | 0.91 | 0.0252 | 0.732 |
| Hoslundia opposita | 0.223 | 0.976 | 0.111 |
| Lippia javanica | 0.178 | 0.141 | 0.977 |
| Ocimum urticifolia | 0.0467 | 0.458 | 0.963 |
| Warburgia salutaris | 0.159 | 0.159 | 0.977 |
| β−Carotene | 0.208 | 0.12 | 0.977 |
| . | Z test results (P < 0.05) . | ||
|---|---|---|---|
| 1 . | 2 . | 3 . | |
| Albizia amara | 0.977 | 0.13 | 0.193 |
| Elionurus muticus | 0.435 | 0.0501 | 0.965 |
| Heteropyxis natalensis | 0.91 | 0.0252 | 0.732 |
| Hoslundia opposita | 0.223 | 0.976 | 0.111 |
| Lippia javanica | 0.178 | 0.141 | 0.977 |
| Ocimum urticifolia | 0.0467 | 0.458 | 0.963 |
| Warburgia salutaris | 0.159 | 0.159 | 0.977 |
| β−Carotene | 0.208 | 0.12 | 0.977 |
| . | Z test results (P < 0.05) . | ||
|---|---|---|---|
| 1 . | 2 . | 3 . | |
| Albizia amara | 0.0316 | 0.942 | 0.613 |
| Elionurus muticus | 0.0653 | 0.353 | 0.0971 |
| Heteropyxis natalensis | 0.159 | 0.159 | 0.977 |
| Hoslundia opposita | 0.941 | 0.0314 | 0.617 |
| Lippia javanica | 0.973 | 0.0745 | 0.316 |
| Ocimum urticifolia | 0.784 | 0.0235 | 0.885 |
| Warburgia salutaris | 0.0272 | 0.934 | 0.65 |
| β−Carotene | 0.953 | 0.546 | 0.037 |
| . | Z test results (P < 0.05) . | ||
|---|---|---|---|
| 1 . | 2 . | 3 . | |
| Albizia amara | 0.0316 | 0.942 | 0.613 |
| Elionurus muticus | 0.0653 | 0.353 | 0.0971 |
| Heteropyxis natalensis | 0.159 | 0.159 | 0.977 |
| Hoslundia opposita | 0.941 | 0.0314 | 0.617 |
| Lippia javanica | 0.973 | 0.0745 | 0.316 |
| Ocimum urticifolia | 0.784 | 0.0235 | 0.885 |
| Warburgia salutaris | 0.0272 | 0.934 | 0.65 |
| β−Carotene | 0.953 | 0.546 | 0.037 |
| . | Z test results (P < 0.05) . | ||
|---|---|---|---|
| 1 . | 2 . | 3 . | |
| Albizia amara | 0.0316 | 0.942 | 0.613 |
| Elionurus muticus | 0.0653 | 0.353 | 0.0971 |
| Heteropyxis natalensis | 0.159 | 0.159 | 0.977 |
| Hoslundia opposita | 0.941 | 0.0314 | 0.617 |
| Lippia javanica | 0.973 | 0.0745 | 0.316 |
| Ocimum urticifolia | 0.784 | 0.0235 | 0.885 |
| Warburgia salutaris | 0.0272 | 0.934 | 0.65 |
| β−Carotene | 0.953 | 0.546 | 0.037 |
| . | Z test results (P < 0.05) . | ||
|---|---|---|---|
| 1 . | 2 . | 3 . | |
| Albizia amara | 0.0316 | 0.942 | 0.613 |
| Elionurus muticus | 0.0653 | 0.353 | 0.0971 |
| Heteropyxis natalensis | 0.159 | 0.159 | 0.977 |
| Hoslundia opposita | 0.941 | 0.0314 | 0.617 |
| Lippia javanica | 0.973 | 0.0745 | 0.316 |
| Ocimum urticifolia | 0.784 | 0.0235 | 0.885 |
| Warburgia salutaris | 0.0272 | 0.934 | 0.65 |
| β−Carotene | 0.953 | 0.546 | 0.037 |
Percentage inhibition
The percentage inhibition reached nearly 100% for the standard β-carotene – 98.84 ± 0.656%, E. muticus – 95.84 ± 0.504% and W. salutaris– 92.57 ± 1.004% as shown in Figure 2. This suggests that these extracts may contain higher concentrations of active compounds than those needed in the reaction for DPPH scavenging. In the follow-up work, it will be necessary to apply diluted samples of the extracts to provide better evidence. The final solution after reaction with DPPH radical always had some yellowish resultant colour, and therefore the absorption inhibition of extracts when compared with colourless methanol solution could not reach 100%. Generally, the percentage inhibition decreased in the following order: β−carotene > E. muticus > W. salutaris > L. javanica > A. amara > O. urticifolia >H. natalensis > H. opposita.
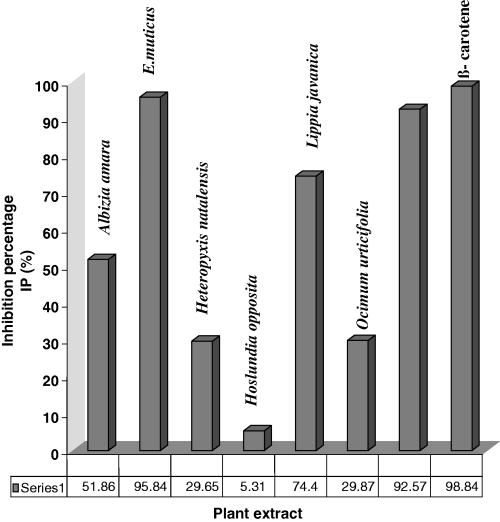
From the results shown in Table 1, it can be seen that all the values were in the range of Z, and hence all results were statistically significant.
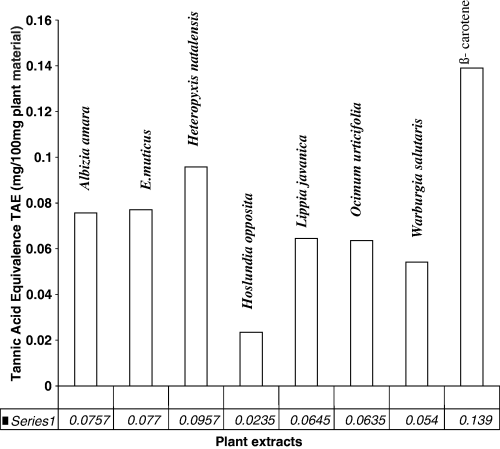
Folin–Ciocalteu method is used for the determination of total phenolic compounds. So far, phenolics constitute one of the major groups of compounds acting as primary antioxidants. Therefore, it was reasonable to determine their total content in selected medicinal plants. The content of phenolic compounds is expressed as milligrams tannic acid per 100 mg plant sample. The amounts of total phenolics in the studied medicinal plants are shown in Table 1. A high content was observed for H. natalensis (0.0957 ± 0.0241 TAE), followed by E. muticus (0.0770 ± 0.00527 TAE), A. amara (0.0757 ± 0.00333 TAE), L. javanica (0.0645 ± 0.0169 TAE), O. urticifolia (0.0635 ± 0.00826 TAE), W. salutaris (0.0540 ±0.00779 TAE) and H. opposita (0.0235 ± 0.00568 TAE).
Several studies have reported on the relationship between phenolic content and antioxidant activity. Some authors found a correlation between the phenolic content and the antioxidant activity, while others found no such relationship. Velioglu et al. (1998) reported a strong relationship between total phenolic content and antioxidant activity in certain plant products. Kähkönen et al. (1999) reported that no significant correlations could be found between the total phenolic content and the antioxidant activity of ninety-two plant extracts of the studied subgroups. Some authors proceeded to comment that different phenolic compounds show different colorimetric responses when using the Folin–Ciocalteu reagent. Similarly, the molecular antioxidant response to free radicals varies markedly, depending on the chemical structure and the oxidation conditions. Thus, the antioxidant activity of an extract cannot be predicted on the basis of its phenolic content.
In this study, the findings do not show a conclusive relationship between total phenolic content and antioxidant activity (Fig. 3). For example, H. natalensis had the second highest level of phenolic content but had the second lowest antioxidant IP, while H. opposita showed both the lowest phenolic content and the lowest antioxidant activity. This serves to show that H. opposita showed a correlation between the two variables, while H. natalensis showed no observed correlation. It can be observed that the total phenolic content in the analysed plant samples showed only low correlation with the antioxidant activity (R = 0.522). As results generally do not show whether there is correlation between antioxidant activity and total phenolic content, a large sample size would be required in future experiments to ascertain correlation.
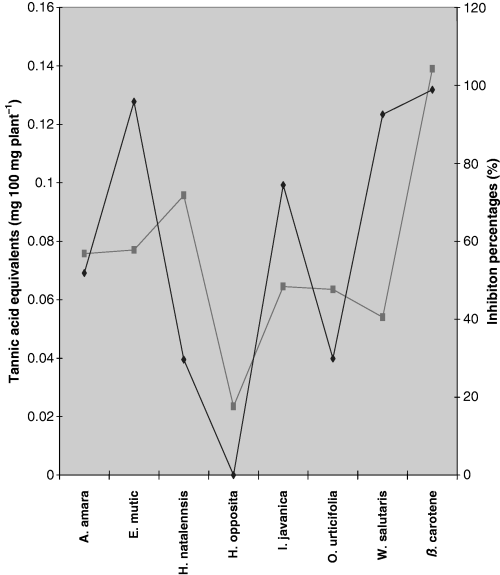
Heteropyxis natalensis had a very low antioxidant IP as shown in Figure 2 (29.65 ± 0.381%) but had one of the highest phenolic content levels (0.0957 ± 0.00505); on the contrary W. salutaris had the second lowest phenolic content (0.0540 ± 0.00799) but had one of the highest antioxidant activities (92.57 ± 1.004%) as shown in Figure 1. Although it is believed that the total number of hydroxyl groups present in the aromatic constituents of a plant extract, in part, offers better antioxidant properties, it is presumed that compounds present in ethanolic extracts belong to different classes of phenolics. These classes most likely have varying antioxidant strengths and that the synergistic effect of polyphenolics with one another and/or components present in an extract may contribute to the overall observed antioxidant activity (Shahidi et al., 1994). This may explain the anomalies that are experienced with W. salutaris and H. natalensis.
Conclusions
In this study, the ethanolic extracts of the seven plant species found in Zimbabwe were found to possess phenolics as well as antioxidant activity. The results gained in these assays provide simple data that make it possible to classify extracts according to their total phenolic content and antioxidant potential.
There is a need to characterise phenolic compounds present within each plant extracts, so as to assign different antioxidant activities, to ascertain whether phenolic structure affects antioxidant activity and also to determine whether synergism definitely occurs between certain phenolic compounds.
The therapeutic value of the plant extracts may be partly because of their antioxidant activity. Further studies on the absorption and effects of phytochemicals present in the plant extracts on antioxidant status in animal models are needed to evaluate their potential health benefits. More tests need to be carried out using a large number of plants to determine whether there is correlation between antioxidant activity and phenolic content.
The authors thank UZ Research Board and UN-IRNA for financial support and partnership.



