-
PDF
- Split View
-
Views
-
Cite
Cite
Sigrun Halvorsen, Julinda Mehilli, Salvatore Cassese, Trygve S Hall, Magdy Abdelhamid, Emanuele Barbato, Stefan De Hert, Ingrid de Laval, Tobias Geisler, Lynne Hinterbuchner, Borja Ibanez, Radosław Lenarczyk, Ulrich R Mansmann, Paul McGreavy, Christian Mueller, Claudio Muneretto, Alexander Niessner, Tatjana S Potpara, Arsen Ristić, L Elif Sade, Henrik Schirmer, Stefanie Schüpke, Henrik Sillesen, Helge Skulstad, Lucia Torracca, Oktay Tutarel, Peter Van Der Meer, Wojtek Wojakowski, Kai Zacharowski, ESC Scientific Document Group , 2022 ESC Guidelines on cardiovascular assessment and management of patients undergoing non-cardiac surgery: Developed by the task force for cardiovascular assessment and management of patients undergoing non-cardiac surgery of the European Society of Cardiology (ESC) Endorsed by the European Society of Anaesthesiology and Intensive Care (ESAIC), European Heart Journal, Volume 43, Issue 39, 14 October 2022, Pages 3826–3924, https://doi.org/10.1093/eurheartj/ehac270
Close - Share Icon Share
All experts involved in the development of these guidelines have submitted declarations of interest. These have been compiled in a report and simultaneously published in a supplementary document to the guidelines. The report is also available on the ESC website www.escardio.org/Guidelines
 See the European Heart Journal online for supplementary data that include background information and detailed discussion of the data that have provided the basis of the guidelines.
See the European Heart Journal online for supplementary data that include background information and detailed discussion of the data that have provided the basis of the guidelines.
 Click here to access the corresponding ESC CardioMed chapters.
Click here to access the corresponding ESC CardioMed chapters.
Table of contents
1. Preamble 3832
2. Introduction 3834
2.1. What is new 3834
2.2. The magnitude of the problem 3839
2.3. Change in demographics 3840
2.4. Purpose 3840
2.5. The outcomes we want to prevent 3841
3. Clinical risk evaluation 3841
3.1. Surgery-related risk 3841
3.1.1. Timing of surgery 3842
3.2. Type of surgical approach 3842
3.2.1. Laparoscopy 3842
3.2.1.1. Vascular and endovascular procedures 3843
3.2.1.2. Video-assisted non-cardiac surgery 3843
3.3. Patient-related risk 3843
3.3.1. Initial assessment 3843
3.3.1.1. Patients aged <65 years without a history of cardiovascular disease or cardiovascular risk factors 3843
3.3.1.2. Patients aged ≥65 years or with cardiovascular risk factors 3843
3.3.1.3. Patients with established cardiovascular disease 3844
3.3.2. Patients with murmurs, chest pain, dyspnoea, or peripheral oedema 3845
3.3.2.1. Murmurs 3845
3.3.2.2. Chest pain 3845
3.3.2.3. Dyspnoea 3845
3.3.2.4. Peripheral oedema 3845
3.4. Timing of adequate risk evaluation 3846
3.5. Avoidance or allowance for surgery in the individual patient 3846
3.6. The patient perspective 3846
4. Pre-operative assessment tools 3847
4.1. Risk scores 3847
4.1.1. General risk calculators 3847
4.1.2. Frailty 3849
4.2. Functional capacity 3849
4.3. Electrocardiography 3850
4.4. Biomarkers 3850
4.5. Non-invasive and invasive procedures 3851
4.5.1. Resting transthoracic echocardiography 3851
4.5.2. Stress tests 3852
4.5.2.1. Exercise stress test 3852
4.5.2.2. Stress imaging 3852
4.5.3. Angiography 3853
4.5.3.1. Coronary computed tomography angiography 3853
4.5.3.2. Invasive coronary angiography 3853
5. General risk-reduction strategies 3854
5.1. Cardiovascular risk factors and lifestyle interventions 3854
5.2. Pharmacological 3854
5.2.1. Beta-blockers 3854
5.2.2. Amiodarone 3855
5.2.3. Statins 3855
5.2.4. Renin–angiotensin–aldosterone system inhibitors 3855
5.2.5. Calcium channel blockers 3855
5.2.6. Alpha-2 receptor agonists 3856
5.2.7. Diuretics 3856
5.2.8. Ivabradine 3856
5.2.9. Sodium–glucose co-transporter-2 inhibitors 3856
5.3. Peri-operative handling of antithrombotic agents 3857
5.3.1. Antiplatelets 3857
5.3.1.1. Single antiplatelet therapy 3857
5.3.1.2. Dual antiplatelet therapy 3860
5.3.1.3. De-escalation of antiplatelet effect 3862
5.3.1.4. Platelet function guided peri-operative management of antiplatelet therapy 3862
5.3.2. Oral anticoagulants 3863
5.3.2.1. Vitamin K antagonists 3863
5.3.2.1.1. Vitamin K antagonists in patients with mechanical heart valves 3863
5.3.2.1.2. Vitamin K antagonists for atrial fibrillation/venous thromboembolism 3864
5.3.2.1.3. Restarting vitamin K antagonists after invasive procedures or surgery 3864
5.3.2.1.4. Reversal of vitamin K antagonists 3864
5.3.2.2. Non-vitamin K antagonist oral anticoagulants 3864
5.3.2.2.1. Unplanned surgery in patients on non-vitamin K antagonist oral anticoagulants and reversal for emergency procedures 3864
5.3.2.2.2. Planned interventions in patients on non-vitamin K oral anticoagulants 3866
5.3.2.2.3. Bridging 3866
5.3.2.2.4. Laboratory testing before surgery 3866
5.3.2.2.5. Considerations for specific procedures 3867
5.3.2.2.6. When to restart non-vitamin K antagonist oral anticoagulants after interventions 3868
5.3.2.3. Combination therapy (antiplatelet and anticoagulant) 3868
5.4. Peri-operative thromboprophylaxis 3869
5.5. Patient blood management 3869
5.5.1. Pre-operative anaemia—diagnosis and treatment 3870
5.5.2. Bleeding and reduction of iatrogenic diagnostic/surgery-related blood loss 3870
5.5.3. Optimal blood component use with patient-centred clinical decision support 3871
6. Specific diseases 3871
6.1. Coronary artery disease 3871
6.1.1. Risk for patients with coronary artery disease 3871
6.1.2. Pre-operative risk assessment and management 3872
6.1.3. Revascularization strategies 3872
6.1.3.1. Chronic coronary syndromes 3872
6.1.3.2. Acute coronary syndromes 3872
6.2. Chronic heart failure 3874
6.2.1. Risk for patients with heart failure 3874
6.2.2. Pre- and post-operative management strategies 3874
6.2.3. Hypertrophic obstructive cardiomyopathy 3875
6.2.4. Patients with ventricular assist devices undergoing non-cardiac surgery 3875
6.3. Valvular heart disease 3875
6.3.1. Risk for patients with valvular heart disease 3875
6.3.2. Pre-operative management strategies and risk-reduction strategy 3876
6.3.2.1. Aortic valve stenosis 3876
6.3.2.2. Mitral valve stenosis 3877
6.3.2.3. Aortic valve regurgitation 3878
6.3.2.4. Mitral valve regurgitation 3878
6.3.2.5. Patients with prosthetic valve(s) 3878
6.3.2.6. Prophylaxis of infective endocarditis 3878
6.4. Known or newly diagnosed arrhythmias 3879
6.4.1. Peri-operative management—general measures 3879
6.4.2. Supraventricular arrhythmias 3879
6.4.3. Atrial fibrillation/flutter 3879
6.4.4. Ventricular arrhythmias 3879
6.4.5. Bradyarrhythmias 3881
6.4.6. Management of patients with cardiac implantable electronic devices 3881
6.5. Adult congenital heart disease 3882
6.6. Pericardial diseases 3883
6.7. Pulmonary disease and pulmonary arterial hypertension 3884
6.7.1. Pulmonary disease 3884
6.7.2. Pulmonary arterial hypertension 3884
6.8. Arterial hypertension 3885
6.9. Peripheral artery disease 3886
6.9.1. Peripheral artery disease and non-vascular non-cardiac surgery 3886
6.9.2. Peripheral artery disease and vascular non-cardiac surgery 3886
6.10. Cerebrovascular disease 3887
6.11. Renal disease 3887
6.12. Obesity 3888
6.13. Diabetes 3889
6.14. Cancer 3889
6.15. Coronavirus disease 2019 2019
7. Peri-operative monitoring and anaesthesia 3890
7.1. Peri-operative monitoring 3890
7.2. Anaesthesia 3891
7.2.1. Intra-operative haemodynamics 3891
7.2.2. Choice of anaesthetic agent 3892
7.3. Locoregional techniques 3892
7.4. Peri-operative goal-directed haemodynamic therapy 3893
7.5. Post-operative management 3893
8. Peri-operative cardiovascular complications 3893
8.1. Peri-operative myocardial infarction/injury 3894
8.2. Spontaneous myocardial infarction (after day 2) 3897
8.3. Takotsubo syndrome 3897
8.4. Acute heart failure 3897
8.5. Venous thromboembolism 3897
8.6. Atrial fibrillation and other relevant arrhythmias 3897
8.6.1. Prevention of post-operative atrial fibrillation 3897
8.6.2. Management of post-operative atrial fibrillation 3898
8.6.2.1. Rate and/or rhythm control 3898
8.6.2.2. Prevention of atrial fibrillation-related thromboembolic complications 3899
8.7. Peri-operative stroke 3899
9. Key messages 3900
10. Gaps in evidence 3900
11. Sex differences 3901
12. ‘What to do’ and ‘what not to do’ messages from the Guidelines 3901
13. Quality indicators 3906
14. Central illustration 3906
15. Supplementary data 3907
16. Data availability statement 3907
17. Author information 3907
18. Appendix 3907
19. References 3908
Tables of Recommendations
Recommendation Table 1 — Recommendations for selection of surgical approach and impact on risk 3843
Recommendation Table 2 — Recommendations for all patients scheduled for non-cardiac surgery 3845
Recommendation Table 3 — Recommendations for patients aged <65 years without signs, symptoms, or history of cardiovascular disease 3845
Recommendation Table 4 — Recommendations for pre-operative assessment in patients with previously unknown murmur, angina, dyspnoea, or peripheral oedema 3845
Recommendation Table 5 — Recommendations for patient information 3847
Recommendation Table 6 — Recommendations for pre-operative assessment of frailty and functional capacity 3849
Recommendation Table 7 — Recommendations for pre-operative risk assessment—electrocardiography and biomarkers 3851
Recommendation Table 8 — Recommendations for transthoracic echocardiography 3852
Recommendation Table 9 — Recommendations for stress imaging 3853
Recommendation Table 10 — Recommendations for coronary angiography 3853
Recommendation Table 11 — Recommendations for lifestyle and cardiovascular risk factors 3854
Recommendation Table 12 — Recommendations for pharmacological treatment 3856
Recommendation Table 13 — Recommendations for use of antiplatelet therapy in patients undergoing non-cardiac surgery 3862
Recommendation Table 14 — Recommendations for interruption and resumption of anticoagulants in patients undergoing non-cardiac surgery 3868
Recommendation Table 15 — Recommendations for thromboprophylaxis 3869
Recommendation Table 16 — Recommendations for intra- and post-operative complications associated with anaemia 3870
Recommendation Table 17 — Recommendations for intra- and post-operative complications associated with blood loss 3871
Recommendation Table 18 — Recommendations for intra- and post-operative complications associated with allogeneic blood transfusion 3871
Recommendation Table 19 — Recommendations for the timing of non-cardiac surgery and revascularization in patients with known coronary artery disease 3874
Recommendation Table 20 — Recommendations for management of heart failure in patients undergoing non-cardiac surgery 3875
Recommendation Table 21 — Recommendations for management of valvular heart disease in patients undergoing non-cardiac surgery 3878
Recommendation Table 22 — Recommendations for management of known or newly diagnosed arrhythmias 3880
Recommendation Table 23 — Recommendations for management of bradyarrhythmia and patients carrying cardiac implantable devices 3882
Recommendation Table 24 — Recommendations for management of patients with adult congenital heart disease undergoing non-cardiac surgery 3883
Recommendation Table 25 — Recommendations for pericardial diseases 3884
Recommendation Table 26 — Recommendations for patients with pulmonary arterial hypertension undergoing non-cardiac surgery 3885
Recommendation Table 27 — Recommendations for pre-operative management of hypertension 3886
Recommendation Table 28 — Recommendations for management of patients with peripheral artery disease and/or abdominal aortic aneurysm undergoing non-cardiac surgery 3887
Recommendation Table 29 — Recommendations for management of patients with suspected or established carotid artery disease undergoing non-cardiac surgery 3887
Recommendation Table 30 — Recommendations for management of patients with renal disease undergoing non-cardiac surgery 3888
Recommendation Table 31 — Recommendations for management of patients with obesity undergoing non-cardiac surgery 3888
Recommendation Table 32 — Recommendations for management of patients with diabetes mellitus undergoing non-cardiac surgery 3889
Recommendation Table 33 — Recommendations for peri-operative monitoring and anaesthesia 3893
Recommendation Table 34 — Recommendations for peri-operative cardiovascular complications 3899
List of tables
Table 1 Classes of recommendations 3833
Table 2 Levels of evidence 3833
Table 3 New concepts and sections in the current guidelines 3834
Table 4 What is new 3834
Table 4A New recommendations 3834
Table 4B Revised recommendations 3838
Table 5 Surgical risk estimate according to type of surgery or intervention 3842
Table 6 Risk score calculators 3848
Table 7 Pharmacokinetic and pharmacodynamic characteristics of antiplatelets 3857
Table 8 Pharmacokinetic and pharmacodynamic characteristics of oral anticoagulants 3858
Table 9 Bleeding risk according to type of non-cardiac surgery 3858
Table 10 Laboratory parameters for the diagnosis of absolute iron-deficiency anaemia 3870
Table 11 Peri-operative approach to patients with ventricular assist devices undergoing non-cardiac surgery 3875
Table 12 Peri-operative management of patients with arrhythmias 3880
Table 13 Risk stratification for non-cardiac surgery in adults with congenital heart disease 3883
Table 14 Patient-related and surgery-related factors to be considered when assessing peri-operative risk in patients with pulmonary arterial hypertension 3885
Table 15 Factors that could influence peri-operative risk during cancer surgery and preventive strategies 3890
List of figures
Figure 1 Total risk is an interaction of patient-related and surgery-related risk 3841
Figure 2 Pre-operative assessment before non-cardiac surgery 3844
Figure 3 Examples of questions and concerns expressed by patients 3847
Figure 4 Recommended measurements to assess and detect the risk of post-operative cardiac complications 3850
Figure 5 Recommendations for management of antiplatelet therapy in patients undergoing non-cardiac surgery 3859
Figure 6 P2Y12 inhibitor interruption after percutaneous coronary intervention before elective non-cardiac surgery 3860
Figure 7 Bridging with intravenous antiplatelet agents. ASA, acetylsalicylic acid; FU, follow-up; LD, loading dose; NCS, non-cardiac surgery; o.d., once a day 3861
Figure 8 Recommendations for management of oral anticoagulation therapy in patients undergoing non-cardiac surgery 3863
Figure 9 Peri-operative management of non-vitamin K antagonist oral anticoagulant according to the periprocedural risk of bleeding 3865
Figure 10 Timing of last non-vitamin K antagonist oral anticoagulant dose before elective NCS according to renal function 3866
Figure 11 Suggested strategy for potential reversal of non-vitamin K oral anticoagulants effect 3867
Figure 12 Management of patients with acute or chronic coronary syndrome scheduled for non-cardiac surgery 3873
Figure 13 Management of patients with severe aortic valve stenosis scheduled for non-cardiac surgery 3876
Figure 14 Management of patients with secondary mitral valve regurgitation scheduled for non-cardiac surgery 3877
Figure 15 Optimal location of return electrode during unipolar electrosurgery in patients with cardiac implantable electronic devices, depending on the surgery site 3882
Figure 16 Pathophysiological approach to address intra-operative hypotension 3892
Figure 17 Factors associated with peri-operative cardiovascular complications. SNS, sympathetic nervous system 3894
Figure 18 Differential diagnosis of elevated post-operative cardiac troponin concentrations 3895
Figure 19 Systematic work-up (aetiology) and therapy of peri-operative myocardial infarction/injury 3896
Figure 20 Prevention and management of post-operative atrial fibrillation 3898
Figure 21 Central illustration: the complex interplay between the intrinsic risk of surgery and the patient risk of peri-operative cardiovascular complications 3906
Abbreviations and acronyms
- AAA
Abdominal aortic aneurysm
- AAD
Antiarrhythmic drug
- ACEI
Angiotensin-converting-enzyme inhibitor
- ACHD
Adults with congenital heart disease
- ACS
Acute coronary syndrome
- ACS NSQIP
American College of Surgery National Surgical Quality Improvement Program
- AF
Atrial fibrillation
- AKI
Acute kidney injury
- aPTT
Activated partial thromboplastin time
- AR
Aortic valve regurgitation
- ARB
Angiotensin receptor blocker
- ARNI
Angiotensin receptor neprilysin inhibitor
- AS
Aortic valve stenosis
- ASA
Acetylsalicylic acid
- ASA–PS
American Society of Anesthesiology Physical Status
- ASCVD
Atherosclerotic cardiovascular disease
- AUB-HAS2
American University of Beirut (AUB)-HAS2
- AUC
Area under curve
- AVR
Aortic valve replacement
- BAV
Balloon aortic valvuloplasty
- BCSH
British Committee for Standards in Haematology
- b.i.d.
Bis in die (twice a day)
- BTKi
Bruton tyrosine kinase inhibitors
- BMI
Body mass index
- BMS
Bare metal stent
- BNP
B-type natriuretic peptide
- BP
Blood pressure
- b.p.m.
Beats per minute
- BSA
Body surface area
- CABG
Coronary artery bypass graft
- CAD
Coronary artery disease
- CARP
Coronary Artery Revascularization Prophylaxis (trial)
- CAS
Carotid artery stenting
- CASS
Coronary Artery Surgery Study
- CCB
Calcium channel blocker
- CCS
Chronic coronary syndrome
- CCTA
Coronary computed tomography angiography
- CEA
Carotid endarterectomy
- CHA2DS2-VASc
Congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, stroke, vascular disease, age 65–74 years, sex category (female)
- CI
Confidence interval
- CIED
Cardiac implantable electronic device
- CK
Creatinine kinase
- CKD
Chronic kidney disease
- CKD-EPI
Chronic Kidney Disease Epidemiology Collaboration
- Cmax
Maximum serum concentration
- CMR
Cardiac magnetic resonance
- COAPT
Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy for Heart Failure Patients with Functional Mitral Regurgitation (trial)
- COPD
Chronic obstructive pulmonary disease
- CORIDA
Per-procedural Concentration of Direct Oral Anticoagulants (trial)
- Coronary CTA VISION
Coronary Computed Tomographic Angiography and Vascular Events in Noncardiac Surgery Patients Cohort Evaluation (trial)
- COVID-19
Coronavirus disease 2019
- CPET
Cardiopulmonary exercise testing
- CRF
Cardiorespiratory fitness
- CRT
Cardiac resynchronization therapy
- CT
Computed tomography
- cTn T/I
Cardiac troponin T/I
- CTO
Chronic total occlusion
- CV
Cardiovascular
- CVD
Cardiovascular disease
- DAPT
Dual antiplatelet therapy
- DASI
Duke Activity Status Index
- DES
Drug-eluting stent
- DM
Diabetes mellitus
- DSE
Dobutamine stress echocardiography
- dTT
Diluted thrombin time
- EACTS
European Association for Cardio-Thoracic Surgery
- ECG
Electrocardiographic/electrocardiogram
- EDKA
Euglycaemic diabetic ketoacidosis
- eGFR
Estimated glomerular filtration rate
- EMI
Electromagnetic interference
- EORP
EURObservational Research Programme
- ESA
European Society of Anaesthesiology
- ESC
European Society of Cardiology
- ESH
European Society of Hypertension
- ESTS
European Society of Thoracic Surgeons
- ESVS
European Society for Vascular Surgery
- EuSOS
European Surgical Outcomes Study
- EVAR
Endovascular abdominal aortic aneurysm repair
- FDA
US Food and Drug Administration
- FFR
Fractional flow reserve
- FIIa
Factor IIa
- FOCUS
Focused cardiac ultrasound
- FXa
Factor Xa
- GDMT
Guideline-directed medical therapy
- GFR
Glomerular filtration rate
- HbA1c
Glycated haemoglobin
- HF
Heart failure
- HIP-ATTACK
HIP Fracture Accelerated Surgical TreaTment And Care tracK (trial)
- HR
Hazard ratio
- hs-cTn
High-sensitivity cardiac troponin
- i.v.
Intravenous
- ICA
Invasive coronary angiography
- ICD
Implantable cardioverter–defibrillator
- ICU
Intensive care unit
- ID
Iron deficiency
- IHD
Ischaemic heart disease
- INR
International normalized ratio
- ISCHEMIA
International Study of Comparative Health Effectiveness with Medical and Invasive Approaches (trial)
- iwFR
Instantaneous wave-free ratio
- KDIGO
Kidney Disease: Improving Global Outcomes
- LD
Loading dose
- LMWH
Low molecular weight heparin
- LOAD
Lowering the Risk of Operative Complications Using Atorvastatin Loading Dose (trial)
- LoE
Level of evidence
- LV
Left ventricular
- LVEF
Left ventricular ejection fraction
- LVESD
Left ventricular end-systolic diameter
- LVESDi
Left ventricular end-systolic dimension index
- MACE
Major adverse cardiovascular event
- MET
Metabolic equivalent
- METS
Measurement of Exercise Tolerance before Surgery (trial)
- MHV
Mechanical heart valve
- MI
Myocardial infarction
- MINS
Myocardial injury following non-cardiac surgery
- MR
Mitral valve regurgitation
- MS
Mitral valve stenosis
- NCS
Non-cardiac surgery
- NOAC
Non-vitamin K antagonist oral anticoagulant
- NSAID
Non-steroidal anti-inflammatory drug
- NSTE-ACS
Non-ST-segment elevation acute coronary syndrome
- NT-proBNP
N-terminal pro-B-type natriuretic peptide
- NYHA
New York Heart Association
- OAC
Oral anticoagulant
- o.d.
Omnie die (once a day)
- OR
Odds ratio
- OSA
Obstructive sleep apnoea
- PA
Pulmonary artery
- PAD
Peripheral artery disease
- PAH
Pulmonary arterial hypertension
- PAUSE
Perioperative Anticoagulant Use for Surgery Evaluation (trial)
- PBM
Patient Blood Management
- PCC
Prothrombin complex concentrate
- PCI
Percutaneous coronary intervention
- PE
Pulmonary embolism
- PMC
Percutaneous mitral commissurotomy
- PMI
Peri-operative myocardial infarction/injury
- POISE
PeriOperative ISchemic Evaluation Trial
- PPC
Prothrombin complex concentrate
- PT
Prothrombin time
- PVC
Premature ventricular contractions
- QI
Quality indicator
- RAAS
Renin−angiotensin−aldosterone system
- RBC
Red blood cell
- RCRI
Revised Cardiac Risk Index
- RCT
Randomized controlled trial
- RF
Radiofrequency
- rHuEPO
Recombinant human erythropoietin
- RR
Relative risk
- RV
Right ventricular
- SAPT
Single antiplatelet therapy
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2
- SAVR
Surgical aortic valve replacement
- SCD
Sudden cardiac death
- SGLT-2
Sodium–glucose co-transporter-2
- SORT
Surgical Outcome Risk Tool
- SPAP
Systolic pulmonary artery pressure
- STEMI
ST-segment elevation myocardial infarction
- SVT
Supraventricular tachycardia
- TAVI
Transcatheter aortic valve implantation
- TEE
Transoesophageal echocardiography
- TEER
Transcatheter edge-to-edge repair
- TIA
Transient ischaemic attack
- TTE
Transthoracic echocardiography
- UFH
Unfractionated heparin
- ULN
Upper limit of normal
- VAD
Ventricular assist device
- VATS
Video-assisted thoracic surgery
- VEGFi
Vascular endothelial grow factor inhibitor
- VF
Ventricular fibrillation
- VHD
Valvular heart disease
- VISION
Vascular Events in Noncardiac Surgery Patients Cohort Evaluation (trial)
- VKA
Vitamin K antagonist
- VKORC1
Vitamin K epoxide reductase complex 1
- VO2
Oxygen consumption
- VT
Ventricular tachycardia
- VTE
Venous thromboembolism
- WHA
World Health Assembly
- WPW
Wolff–Parkinson–White
1. Preamble
Guidelines summarize and evaluate available evidence, with the aim of assisting health professionals in proposing the best management strategies for an individual patient with a given condition. Guidelines and their recommendations should facilitate decision-making of health professionals in their daily practice. Guidelines, however, are not a substitute for the patient’s relationship with their practitioner. The final decisions concerning an individual patient must be made by the responsible health professional(s), based on what they consider to be the most appropriate in the circumstances. These decisions are made in consultation with the patient and caregiver as appropriate.
Guidelines are intended for use by health professionals. To ensure that all users have access to the most recent recommendations, the European Society of Cardiology (ESC) makes its guidelines freely available. The ESC warns readers that the technical language may be misinterpreted and declines any responsibility in this respect.
Many guidelines have been issued in recent years by the ESC. Because of their impact on clinical practice, quality criteria for the development of guidelines have been established in order to make all decisions transparent to the user. The recommendations for formulating and issuing ESC Guidelines can be found on the ESC website (https://www.escardio.org/Guidelines). The ESC Guidelines represent the official position of the ESC on a given topic and are regularly updated.
In addition to the publication of Clinical Practice Guidelines, the ESC carries out the EURObservational Research Programme of international registries of cardiovascular diseases and interventions, which are essential to assess diagnostic/therapeutic processes, use of resources, and adherence to guidelines. These registries aim to provide a better understanding of medical practice in Europe and around the world, and are based on high-quality data collected during routine clinical practice. Furthermore, the ESC develops sets of quality indicators (QIs)—which are tools to evaluate the level of implementation of the guidelines and may be used by the ESC, hospitals, healthcare providers, and professionals to measure clinical practice, and in educational programmes—alongside the key messages from the guidelines, to improve quality of care and clinical outcomes.
The members of this Task Force were selected by the ESC to represent professionals involved with the medical care of patients with this pathology. The selection procedure aimed to ensure that there is a representative mix of members, predominantly from across the whole of the ESC region and from relevant ESC Subspecialty Communities. Consideration was given to diversity and inclusion, notably with respect to gender and country of origin. A critical evaluation of diagnostic and therapeutic procedures was performed, including assessment of the risk–benefit ratio. The level of evidence and the strength of the recommendation of particular management options were weighed and scored according to pre-defined scales, as outlined below. The Task Force followed the ESC voting procedures. All recommendations subject to a vote achieved at least 75% among voting members.
The experts of the writing and reviewing panels provided declaration of interest forms for all relationships that might be perceived as real or potential sources of conflicts of interest. Their declarations of interest were reviewed according to the ESC declaration of interest rules and can be found on the ESC website (http://www.escardio.org/Guidelines) and have been compiled in a report and simultaneously published in a supplementary document to the guidelines. This process ensures transparency and prevents potential biases in the development and review processes. Any changes in declarations of interest that arose during the writing period were notified to the ESC and updated. The Task Force received its entire financial support from the ESC without any involvement from the healthcare industry.
The ESC CPG Committee supervises and coordinates the preparation of new guidelines. The Committee is also responsible for the approval process of these guidelines. The ESC Guidelines undergo extensive review by the CPG Committee and external experts, including a mix of members from across the whole of the ESC region and from relevant ESC Subspecialty Communities and National Cardiac Societies. After appropriate revisions, the guidelines are signed-off by all the experts involved in the Task Force. The finalized document is signed-off by the CPG Committee for publication in the European Heart Journal. The guidelines are developed after careful consideration of the scientific and medical knowledge and the evidence available at the time of their writing.
The task of developing the ESC Guidelines also includes creating educational tools and implementating programmes for the recommendations, including condensed pocket guidelines versions, summary slides, summary cards for non-specialists, and an electronic version for digital applications (smartphones, etc.). These versions are abridged and thus, for more detailed information, the user should always access the full text version of the guidelines, which is freely available via the ESC website and the European Heart Journal. The National Cardiac Societies of the ESC are encouraged to endorse, adopt, translate, and implement all ESC Guidelines. Implementation programmes are needed because it has been shown that the outcome of disease may be favourably influenced by the thorough application of clinical recommendations.
Health professionals are encouraged to take the ESC Guidelines fully into account when exercising their clinical judgment, and in determining and implementing preventive, diagnostic, or therapeutic medical strategies. However, the ESC Guidelines do not override, in any way whatsoever, the individual responsibility of health professionals to make appropriate and accurate decisions in considering each patient's health condition and in consulting with that patient or the patient’s caregiver where appropriate and/or necessary. It is also the health professional's responsibility to verify the rules and regulations applicable in each country to drugs and devices at the time of prescription and, where appropriate, to respect the ethical rules of their profession.
Off-label use of medication may be presented in these guidelines if a sufficient level of evidence shows that it can be considered medically appropriate to a given condition and if patients could benefit from the recommended therapy. However, the final decisions concerning an individual patient must be made by the responsible health professional, giving special consideration to:
the specific situation of the patient. In this respect, it is specified that, unless otherwise provided for by national regulations, off-label use of medication should be limited to situations where it is in the patient’s interest to do so, with regard to the quality, safety, and efficacy of care, and only after the patient has been informed and provided consent;
and country-specific health regulations, indications by governmental drug regulatory agencies, and the ethical rules to which health professionals are subject, where applicable.
2. Introduction
2.1. What is new
2.2. The magnitude of the problem
The annual volume of major surgery worldwide is estimated to be more than 300 million patients (about 5% of the world population), which is a 34% increase from 2004 to 2012.1,2 Nearly 74% of these operations are performed in countries spending substantial amounts on health care. When applied to European Union countries, which had an overall population of 448 million in 2020 (27 countries), this figure translates into a crude estimate of nearly 22 million major procedures annually.2
Nearly 85% of major operations are non-cardiac surgical procedures.3 In a recent report from the USA National Inpatient Sample database, nearly half of adults aged ≥45 years undergoing major non-cardiac surgery (NCS) presented with at least two cardiovascular (CV) risk factors, 18% had coronary artery disease (CAD), 4.7% had a history of stroke, and 7.7% had a modified Revised Cardiac Risk Index (RCRI) score ≥3 (range 0–6) in 2012–13. These prevalence rates show a substantial increase compared with the equivalent rates in 2008–09.4 In a large registry including 37 915 consecutive patients undergoing percutaneous coronary interventions (PCIs) with drug-eluting stent (DES), the rates of NCS after PCI were 11% and 24%, 1 and 3 years after PCI respectively. The cut-off ages at which NCS was more likely to occur within 1 and 3 years of PCI were 62 and 73 years respectively.5
The prevalence of comorbidities, the clinical condition of patients before surgery, and the urgency, magnitude, type, and duration of the surgical procedure determine the risk of peri-operative complications. In a recent cohort study of 40 000 patients aged ≥45 years undergoing inpatient NCS, one of seven experienced a major cardiac or cerebrovascular complication at 30 days.6 Cardiovascular complications can particularly occur in patients with documented or asymptomatic coronary heart disease, left ventricular (LV) dysfunction, valvular heart disease (VHD), and arrhythmias, who undergo surgical procedures that are associated with prolonged haemodynamic and cardiac stress. In the case of peri-operative myocardial ischaemia, three mechanisms are important: (i) oxygen supply–demand mismatch on the background of coronary artery stenosis that may become flow-limiting by peri-operative haemodynamic fluctuations; (ii) acute coronary syndrome (ACS) due to stress-induced erosion or rupture of a vulnerable atherosclerotic plaque in combination with pro-inflammatory and hypercoagulable states induced by surgery, and the haemodynamic distress resulting from fluid shifts and anaesthesia; and (iii) surgery-associated bleeding risk requiring interruption of antiplatelet therapies, which might lead to stent thrombosis among patients undergoing NCS after recent coronary stent placement. Left ventricular dysfunction and arrhythmias may occur for various reasons at all ages. Because the prevalence of CAD, VHD, heart failure, and arrhythmias increases with age, peri-operative CV mortality and morbidity are predominantly an issue in the adult population undergoing major NCS.
In Europe, recent systematic data on the annual number and type of operations, and on patient outcomes are unfortunately lacking. Additionally, data definitions vary, as do data quantity and quality. Based on the estimates outlined above, nearly 6.6 million procedures are performed annually in European patients with CAD, peripheral artery disease (PAD), and cerebrovascular disease who are at high risk of CV complications. In a 7 day cohort study, the European Surgical Outcomes Study (EuSOS) group investigated the outcomes of NCS in 498 hospitals across 27 European nations and the UK; up to 8% of patients undergoing NCS required critical care admission, while in-hospital mortality ranged 1.4–21.5% (mean 4.0%), depending on safety precautions.7 In a recent prospective study of 2265 high-risk patients undergoing NCS in Switzerland, one out of five developed major adverse events within 365 days.8 When applied to the population in European Union countries, these figures translate into at least 660 000 major cardiac or cerebrovascular complications occurring annually due to NCS procedures.
The 2022 ESC Guidelines on cardiovascular assessment and management of patients undergoing NCS focus on the pre-operative CV risk assessment and peri-operative management of patients in whom cardiovascular disease (CVD) is a potential source of complications during NCS.
2.3. Change in demographics
Within the next 30 years, the ageing of the population will have a major impact on peri-operative patient management. Patients undergoing NCS are older than the rest of the population. Furthermore, it is estimated that by 2030, one-fifth of individuals aged >75 years will undergo surgery each year. In addition, between 2018 and 2050, the number of people in Europe aged 75–84 years is projected to increase by ∼60%. The total number of surgical procedures may increase even faster because of the greater need for interventions with increasing age. Demographics of patients undergoing surgery show trends towards increasing numbers of elderly patients and increasing numbers of patients with comorbidities, particularly CVDs. Thus, adults aged ≥75 years have a greater risk of peri-operative major adverse cardiovascular events (MACEs) (9.5% vs. 4.8% for younger adults [P < 0.001]).9 However, age per se seems to be responsible for a small increase in the risk of complications; greater risks are associated with urgency and significant CV, pulmonary, and renal disease.
2.4. Purpose
As many years have passed and new evidence has become available since the publication of the 2014 ESC/European Society of Anaethesiology (ESA) Guidelines on non-cardiac surgery: cardiovascular assessment and management,10 the ESC has decided to revise the guidelines on NCS. These new guidelines are based on the 2014 edition, but all sections have been revised or rewritten, and several new sections have been added. Some of the old recommendations are unchanged or have been revised, and new recommendations have been added.
These guidelines are intended for physicians, healthcare workers, and collaborators involved in the pre-operative, operative, and post-operative care of patients undergoing NCS. The objective is to endorse a standardized and evidence-based approach to peri-operative CV management. The guidelines recommend a stepwise evaluation of the patient that integrates clinical risk factors and test results with the estimated stress of the planned surgical procedure and the risks involved with the discontinuation of drugs. This results in an individualized risk assessment, with the opportunity of initiating medical therapy, coronary interventions, and specific surgical and anaesthetic techniques, or withholding medical therapy, in order to optimize the patient’s peri-operative condition. Further, it should be discussed in which institutions (specialized small hospital vs. tertiary care) the NCS will be performed. It is important that patients’ values and preferences with respect to the benefits and risks of surgery are taken into consideration, and that patients are involved in the decisions. This is particularly important when it comes to decisions about undergoing elective surgery or not, the timing of surgery, and choice of surgical and anaesthetic techniques.
Compared with non-surgical settings, randomized controlled trials (RCTs) are scarce in this field. However, since the publication of the 2014 ESC/ESA Guidelines on non-cardiac surgery: cardiovascular assessment and management there has been a significant increase in RCTs that are relevant in this setting. When no trials are available on a specific CV management regimen in the surgical setting, data from the non-surgical setting may be extrapolated and similar recommendations made, but with different levels of evidence.
These guidelines have the potential to improve peri- and post-operative outcomes and highlight the existence of a clear opportunity for improving the quality of care. Following the publication of these updated guidelines on NCS, their effects on outcomes should be monitored. The objective evaluations of the quality of the assessments and the outcomes are described in quality indicators (Section 13).
2.5. The outcomes we want to prevent
The recommendations in these guidelines are intended to prevent peri-operative CV morbidity and mortality, for example: peri-operative myocardial infarction/injury (PMI), stent thrombosis, acute heart failure (HF), haemodynamically relevant arrhythmias, pulmonary embolism (PE), ischaemic stroke, and death. It is also important to prevent bleeding complications, especially associated with antithrombotic treatment, since bleeding is associated with an increased risk of MI and death.6,11–13
3. Clinical risk evaluation
Cardiovascular morbidity and mortality in patients undergoing NCS are determined by two main factors: patient-related risk and type of surgery or procedure, including the circumstances under which it takes place (experience of institution, elective vs. emergency procedure).14 The risk may be reduced by an adequate pre-operative evaluation and proper selection of type and timing of the surgical procedure (Figure 1).
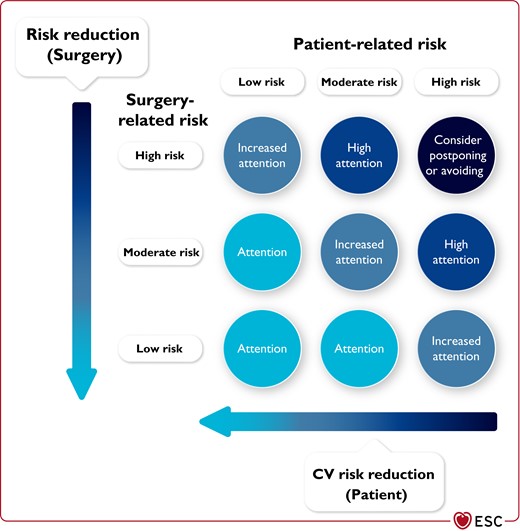
Total risk is an interaction of patient-related and surgery-related risk.
Ideally, the total risk should be as close as possible to the lower left corner, by choosing surgery/procedure/anaesthesia/institution with the lowest possible risk along with efforts to mitigate the patient’s CV risk.
3.1. Surgery-related risk
The surgery-related risk is determined by the type and duration of the surgery, and the urgency of the procedure or intervention. The type of anaesthesia and anaesthetic drugs may also influence the risk of complications in patients at intermediate to high cardiac risk undergoing NCS (see Section 7).15 The surgical risk estimate is a broad approximation of 30 day risk of CV death, MI, and stroke, which only takes into account the specific surgical intervention without considering the patient’s comorbidities (Table 5).10,16
| A new flowchart for general assessment of patients before NCS. |
| A new section on pre-operative assessment of patients with newly detected murmurs, dyspnoea, oedema, or angina. |
| A new section on the patient perspective. |
| A new section on assessment of frailty. |
| A revised and expanded focus on use of biomarkers in NCS |
| A revised and expanded section on peri-operative management of antiplatelet therapy. |
| A revised and expanded section on peri-operative management of oral anticoagulants. |
| A new section on peri-operative thromboprophylaxis. |
| A dedicated section on patient blood management. |
| A new section on management of cardiovascular risk in patients with cancer undergoing NCS. |
| A small section on NCS in patients with recent COVID-19. |
| A new section on diagnosis and management of post-operative complications during NCS. |
| A new flowchart for general assessment of patients before NCS. |
| A new section on pre-operative assessment of patients with newly detected murmurs, dyspnoea, oedema, or angina. |
| A new section on the patient perspective. |
| A new section on assessment of frailty. |
| A revised and expanded focus on use of biomarkers in NCS |
| A revised and expanded section on peri-operative management of antiplatelet therapy. |
| A revised and expanded section on peri-operative management of oral anticoagulants. |
| A new section on peri-operative thromboprophylaxis. |
| A dedicated section on patient blood management. |
| A new section on management of cardiovascular risk in patients with cancer undergoing NCS. |
| A small section on NCS in patients with recent COVID-19. |
| A new section on diagnosis and management of post-operative complications during NCS. |
COVID-19, coronavirus 2019; NCS, non-cardiac surgery
| A new flowchart for general assessment of patients before NCS. |
| A new section on pre-operative assessment of patients with newly detected murmurs, dyspnoea, oedema, or angina. |
| A new section on the patient perspective. |
| A new section on assessment of frailty. |
| A revised and expanded focus on use of biomarkers in NCS |
| A revised and expanded section on peri-operative management of antiplatelet therapy. |
| A revised and expanded section on peri-operative management of oral anticoagulants. |
| A new section on peri-operative thromboprophylaxis. |
| A dedicated section on patient blood management. |
| A new section on management of cardiovascular risk in patients with cancer undergoing NCS. |
| A small section on NCS in patients with recent COVID-19. |
| A new section on diagnosis and management of post-operative complications during NCS. |
| A new flowchart for general assessment of patients before NCS. |
| A new section on pre-operative assessment of patients with newly detected murmurs, dyspnoea, oedema, or angina. |
| A new section on the patient perspective. |
| A new section on assessment of frailty. |
| A revised and expanded focus on use of biomarkers in NCS |
| A revised and expanded section on peri-operative management of antiplatelet therapy. |
| A revised and expanded section on peri-operative management of oral anticoagulants. |
| A new section on peri-operative thromboprophylaxis. |
| A dedicated section on patient blood management. |
| A new section on management of cardiovascular risk in patients with cancer undergoing NCS. |
| A small section on NCS in patients with recent COVID-19. |
| A new section on diagnosis and management of post-operative complications during NCS. |
COVID-19, coronavirus 2019; NCS, non-cardiac surgery
| Low surgical risk (<1%) . | Intermediate surgical risk (1–5%) . | High surgical risk (>5%) . |
|---|---|---|
|
|
|
| Low surgical risk (<1%) . | Intermediate surgical risk (1–5%) . | High surgical risk (>5%) . |
|---|---|---|
|
|
|
CAS, carotid artery stenting; CEA, carotid endarterectomy; CV, cardiovascular; MI, myocardial infarction; VATS, video-assisted thoracic surgery.
Surgical risk estimate is a broad approximation of 30 day risk of CV death, MI, and stroke that takes into account only the specific surgical intervention, without considering the patient’s comorbidities.
Adapted from data in Glance et al., Muller et al., Bendixen et al., and Falcoz et al.18–23
| Low surgical risk (<1%) . | Intermediate surgical risk (1–5%) . | High surgical risk (>5%) . |
|---|---|---|
|
|
|
| Low surgical risk (<1%) . | Intermediate surgical risk (1–5%) . | High surgical risk (>5%) . |
|---|---|---|
|
|
|
CAS, carotid artery stenting; CEA, carotid endarterectomy; CV, cardiovascular; MI, myocardial infarction; VATS, video-assisted thoracic surgery.
Surgical risk estimate is a broad approximation of 30 day risk of CV death, MI, and stroke that takes into account only the specific surgical intervention, without considering the patient’s comorbidities.
Adapted from data in Glance et al., Muller et al., Bendixen et al., and Falcoz et al.18–23
Any surgical procedure may increase the level of cortisol and catecholamines as stress responses due to tissue injury and inflammation, and neuro–endocrine and sympathovagal imbalance. Changes in body core temperature, blood loss, and fluid shifts may cause a rise in vascular resistance as well as hypotension,17 leading to imbalance between myocardial oxygen demand and delivery. Bleeding, transfusion of blood products, tissue injury, and inflammatory response may affect the coagulation system, inducing a prothrombotic state.
3.1.1. Timing of surgery
In general, acute procedures carry a higher risk of complications than elective procedures. Uniform timing definitions are unfeasible, as the time spans may vary between diseases. These guidelines use the timing definitions below.
Immediate: surgery/intervention should be performed without any delay to save life or organ function.
Urgent: surgery/intervention should be performed without unnecessary delay to save life, limb, or organ function.
Time-sensitive: surgery/intervention should be performed as soon as possible as there is a time-dependent risk of losing limb or organ function, or increased risk of complications. Cancer surgery is typically time-sensitive, as is carotid surgery to prevent stroke in a symptomatic case. The time window for time-sensitive surgery will vary depending on the underlying disease.
Elective: surgery/intervention can be performed electively (not further defined) without significant risk of losing limb, or organ function, or increased risks of complications.
Many factors affect outcomes when comparing acute or time-sensitive vs. elective surgery: the general condition of the patient vs. the stage of the acute illness, and how far it has progressed. The best interests of the patient should be considered before deciding on treatment, informed consent to management should be obtained, if at all possible, and decisions should be clearly recorded.24
The degree of urgency should also be considered (i.e. does the procedure need to be performed outside working hours or can it wait until the next day?). In general, competences and supportive functions are not always present in the evenings or during the night; thus, an overall evaluation of what best serves the patient is necessary. The optimal timing of NCS should be discussed within the multidisciplinary team, including an anaesthesiologist, in order to achieve optimized anaesthesia for each patient (see Section 7).
3.2. Type of surgical approach
New surgical techniques have been introduced to replace open surgery and to reduce the overall risk for the patient.
3.2.1. Laparoscopy
Laparoscopic procedures, compared with open surgical procedures, have the advantage of causing less tissue trauma and intestinal paralysis, resulting in less incisional pain, better post-operative pulmonary function, significantly fewer wall complications, and diminished post-operative fluid shifts related to bowel paralysis.25 However, the pneumoperitoneum required for these procedures results in elevated intra-abdominal pressure and a reduction in venous return. Typical physiological sequelae are secondary to increased intra-abdominal pressure and absorption of the gaseous medium used for insufflation.
While healthy individuals on controlled ventilation typically tolerate pneumoperitoneum, patients with CVD, some types of adults with congenital heart disease (ACHD), and obese patients may experience adverse consequences.26 Pneumoperitoneum and Trendelenburg position result in increased mean arterial pressure, central venous pressure, mean pulmonary artery pressure, pulmonary capillary wedge pressure, and systemic vascular resistance impairing cardiac function.27,28 Therefore, compared with open surgery, the CV risk in patients with CVD is not necessarily reduced in patients undergoing laparoscopy, and both should be evaluated in the same way. This is especially true in patients undergoing interventions for morbid obesity, but also in other types of surgery, considering the risk of conversion to an open procedure.29,30 Superior short-term outcomes of laparoscopic vs. open procedures have been reported, depending on type of surgery, operator experience, and hospital volume; however, few studies provide direct measures of cardiac complications.31–33 The benefit of laparoscopic procedures is probably greater in elderly patients, with reduced length of hospital stay, intra-operative blood loss, incidence of post-operative pneumonia, time to return of normal bowel function, incidence of post-operative cardiac complications, and wound infections.34
3.2.1.1. Vascular and endovascular procedures
Endovascular abdominal aortic aneurysm repair (EVAR) is a procedure using femoral artery access only, and is therefore associated with lower operative mortality and morbidity than open repair. It minimizes the surgical risk in simultaneous surgery for the treatment of abdominal aortic aneurysm (AAA) and a non-cardiac disorder, and shortens the time delay from the treatment of AAA and the non-cardiac disorder in patients undergoing two-phase surgery.35–37 The early gain in mortality from EVAR procedures is lost after 3–4 years, compared with open surgical treatment, due to general morbidity (especially CV mortality) of AAA patients.
Various vascular and non-vascular NCS procedures bear different operative risks. While aortic and infra-inguinal vascular surgical procedures are both regarded as high-risk procedures, their risk can be modified by adequate peri-operative measures.38 For patients undergoing treatment of femoropopliteal artery disease, an endovascular-first approach may be advisable in case of additional significant comorbidity. A meta-analysis of studies comparing open surgery with PCI for the treatment of femoropopliteal arterial disease showed that femoral bypass surgery was associated with higher morbidity (odds ratio [OR] 2.93; 95% confidence interval [CI], 1.34–6.41) but similar mortality at 30 days compared with endovascular treatment.39
3.2.1.2. Video-assisted non-cardiac surgery
Video-assisted thoracic surgery (VATS) is supported by a trial showing fewer peri-operative complications and a better quality of life in the first year following surgery for stage 1 lung cancer compared with anterolateral thoracotomy.20 Also, a large propensity matched study conducted by the European Society of Thoracic Surgeons (ESTS) showed fewer post-operative complications following VATS compared with open thoracotomy.21 Overall, the benefits seem greatest in patients with reduced functional lung capacity.
Recommendations for selection of surgical approach and impact on risk
 |
 |
Recommendations for selection of surgical approach and impact on risk
 |
 |
3.3. Patient-related risk
3.3.1. Initial assessment
Patient-related risk is determined by patient’s age, the presence or absence of CV risk factors (e.g. smoking, hypertension, diabetes, dyslipidaemia, family disposition)40 or established CV disease, and comorbidities.41
Identification of patients at risk of CV complications is of paramount importance to choice of therapy when non-surgical options are available, or when the type of surgery or anaesthesia impacts the risk of complications. When emergency surgery is needed, the evaluation must necessarily be limited; however, most clinical circumstances allow a systematic approach.
As an initial assessment, it is recommended that all patients scheduled for NCS are evaluated by accurate history and physical examination, with special emphasis on CV risk factors, established CV disease, and comorbidities.40 It is also recommended to measure standard laboratory tests (e.g. haemoglobin and renal function) in all patients undergoing intermediate- to high-risk surgery. Based on this information, further assessment of patient-related risk can proceed depending on the surgery-related risk, as shown in Figure 2. It is recommended to perform an electrocardiogram (ECG), assess the functional capacity, and/or measure biomarkers (cardiac troponins and/or N-terminal pro-B-type natriuretic peptide [NT-proBNP]/B-type natriuretic peptide [BNP]) depending on the patient-related and surgery-related risk (Figure 2). Detailed information on available tools for risk assessment, their prognostic ability, and indications to perform them is given in Section 4. More details on pre-operative management of patients with specific CV diseases are given in Section 6.
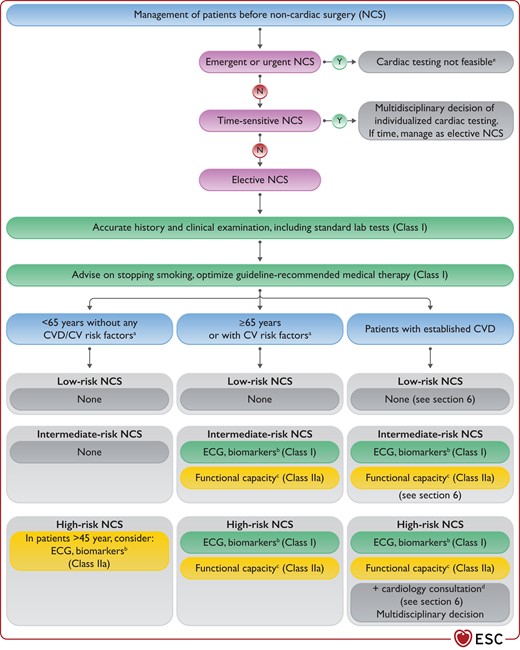
Pre-operative assessment before non-cardiac surgery.
CV, cardiovascular; CVD, cardiovascular disease; ECG, electrocardiogram; N, no; NCS, non-cardiac surgery. Y, yes; aCV risk factors: hypertension, smoking, dyslipidaemia, diabetes, family history of CVD. bBiomarkers: hs-cTn T/I (Class I) and/or BNP/NT-proBNP (Class IIa). If pathological, consult a cardiologist. cFunctional capacity based on Duke Activity Status Index (DASI) or the ability to climb two flights of stairs. dFor diagnostic and therapeutic efforts to be considered, see Section 6. eClose follow-up after intervention and subsequent management of heart disease are advised.
3.3.1.1. Patients aged <65 years without a history of cardiovascular disease or cardiovascular risk factors
Patients aged <65 years without signs, symptoms, or history of CVD or CV risk factors are considered to be of low risk, and can proceed to low- and moderate-risk surgery without additional pre-operative risk assessment.41 Before high-risk surgery, ECG and biomarkers should be considered (see Sections 4.3 and 4.4).42
Patients without signs or symptoms of CVD, but with a family history of genetic cardiomyopathy (i.e. dilatated, hypertrophic, arrhythmic, or restrictive cardiomyopathy, or LV non-compaction) should be evaluated with an ECG and an echocardiographic examination to rule out the presence of the disease, irrespective of the age.43 No specific data are available in the literature regarding risk of family members without the phenotype; however, they are at risk of developing the disease, which may be subclinical at the time of the NCS.43
3.3.1.2. Patients aged ≥65 years or with cardiovascular risk factors
Patients who are aged ≥65 years and patients with risk factors for CVD—such as hypertension, dyslipidaemia, or smoking—have an increased risk of having undetected CVD. The SCORE2 risk-prediction tool can be used to estimate their 10 year CVD risk outside the setting of NCS.40 Patients who are aged ≥65 years and patients with risk factors for CVD also have an increased risk of peri-operative complications during NCS.41,44 These patients need additional assessment before intermediate- and high-risk surgery (Figure 2) and optimal treatment of risk factors. This is also the case for patients with other diseases known to be associated with a high risk of concomitant undetected or known CVD (Sections 6.8 and 6.11–6.14).
3.3.1.3. Patients with established cardiovascular disease
The surgical procedure has the potential to aggravate the disease and increase morbidity and mortality in patients with established CVD. This may be preventable by implementing appropriate CV risk stratification prior to NCS and individually tailoring peri-operative therapy to reduce the risk.45 If time allows, it is also recommended to optimize guideline-recommended treatment of the disease before NCS. See Section 6 for a detailed discussion of risk assessment and management of patients with known CVD.
Recommendations for all patients scheduled for non-cardiac surgery
 |
 |
Recommendations for all patients scheduled for non-cardiac surgery
 |
 |
Recommendations for patients aged <65 years without signs, symptoms, or history of cardiovascular disease
 |
 |
Recommendations for patients aged <65 years without signs, symptoms, or history of cardiovascular disease
 |
 |
3.3.2. Patients with murmurs, chest pain, dyspnoea, or peripheral oedema
Patients without known CVD and scheduled for elective or acute NCS are often referred to a cardiologist because of symptoms or signs that may be caused by CVD. Murmurs, chest pain, dyspnoea, and oedema may suggest severe CVD, but may also be caused by non-cardiac disease. Thus, the medical history, family history, and risk factors have to be obtained and considered. The patient’s physical capacity should be assessed. The need for further evaluation of the patient should be decided according to the risk of the planned procedure or surgery.
3.3.2.1. Murmurs
In a patient with a heart murmur, but without any symptoms of CVD, the value of performing an echocardiogram is not well-established and consensus is missing.54–56 However, if a heart murmur suggesting clinically significant pathology is present before high-risk NCS, it is recommended to perform an echocardiogram, even in patients without any symptoms of CVD. Old age or increased NT-proBNP may increase the pre-test probability of haemodynamically significant but asymptomatic valvular disease. If the patient with the murmur also has symptoms of CVD, an echocardiogram is indicated before all NCS. The pre-operative setting is challenging, as the need for NCS and the risk of CVD have to be considered as independent factors. Thus, an echocardiogram may be useful in risk stratification for some patients, but whether it would improve outcome is uncertain. It is important to bear in mind that the time delay when performing additional but unnecessary examinations may worsen the patient’s prognosis.57 It has also been discussed that a focused cardiac ultrasound (FOCUS) could replace auscultation in general in the pre-operative evaluation of patients.58 While cardiac auscultation has severe limitations,59,60 the value of performing a FOCUS as a standard pre-operative evaluation remains uncertain. Cardiac auscultation should not be replaced by FOCUS.
3.3.2.2. Chest pain
Patients scheduled for NCS may also present with previously unrecognized symptoms suggestive of CAD. The disease leading to the need for NCS may aggravate a subclinical CAD, or the patient may have a concomitant undetected CAD. In an elective setting, if the symptoms are suggestive of CAD, the guidelines for CAD patients in the non-surgical setting should be followed (see Sections 4.5.3 and 6.1.2). If immediate, urgent, or time-sensitive NCS is needed, the time for and access to adequate diagnostic tools may be limited. However, ECG and troponins can be used to detect or exclude ACS (see Sections 4.3 and 4.4).
3.3.2.3. Dyspnoea
Dyspnoea is a symptom of a wide range of diseases and conditions. In a large series of patients, self-reported dyspnoea identified a subgroup of otherwise asymptomatic patients at increased risk of death from CVD and any cause.61 In the diagnostic work-up to find the reason for dyspnoea, spirometry, D-dimer, NT-proBNP/BNP, arterial blood gases, and transthoracic echocardiography (TTE) have diagnostic utility61 but limited specificity. If NT-proBNP/BNP is elevated, an echocardiogram should be performed. If NT-proBNP/BNP is not elevated, other reasons for dyspnoea should be explored.
3.3.2.4. Peripheral oedema
Increased hydrostatic pressure leading to oedema is a feature of a wide range of CV diseases, but an upright position is also a common cause of oedema. There is a spectrum of other diseases that can result in peripheral oedema not listed here.
Recommendations for pre-operative assessment in patients with previously unknown murmur, angina, dyspnoea, or peripheral oedema
 |
 |
 |
 |
Recommendations for pre-operative assessment in patients with previously unknown murmur, angina, dyspnoea, or peripheral oedema
 |
 |
 |
 |
3.4. Timing of adequate risk evaluation
Pre-operative CV assessment should be performed prior to surgery, ideally at the time when the decision for NCS has been made. Accurate estimates of the risks and benefits of surgery is a prerequisite for informed decision-making by both physicians and patients about the appropriateness of surgery. These estimates should also help in guiding surgical (endovascular/endoscopic vs. open approach) and monitoring (intermediate care, screening for CV complications) approaches, and help to detect an unexpectedly high CV risk.47 Therefore, the prognostic value of pre-operative CV risk assessment is much higher in elective vs. immediate or urgent surgery. Explicit communication of peri-operative CV risk, on the basis of the expected event rates,47 and risk communication tools such as the A to Z Inventory of Decision Aids (https://decisionaid.ohri.ca/AZinvent.php) are recommended.
3.5. Avoidance or allowance for surgery in the individual patient
In the clinical setting it can be difficult to decide whether CVD represents a contraindication to NCS. In general, the risk for the patient if not operated on must be considerably higher than the risk of the treatment. Ideally, an unstable cardiac patient should be stabilized before NCS, but waiting can be detrimental for acute surgical disease. No definite list can be made for which cardiac disease is a clear contraindication to NCS, but in patients with severe HF (New York Heart Association [NYHA] class IV), cardiogenic shock, severe pulmonary hypertension, or patients with severe frailty (see Section 4.1.2 for frailty assessment), high-risk NCS should probably be avoided. Life expectancy and quality of life should also be taken into consideration. However, the decision should be made after discussions between the surgeon, anaesthesiologist, cardiologist, and also a geriatrician for elderly patients, along with the patient and relatives.
3.6. The patient perspective
Patients with established CVD may face concerns about their underlying disease and current CV medication, co-ordination between the surgical team and their cardiologist (examples provided in Figure 3), and the potential excessive risk compared with the expected outcome of the surgery. Time should be allowed to address concerns and to provide evidence-based information on the risk–benefit trade-offs and the surgical treatment options (including non-surgical or ‘do nothing’ alternatives) to ensure informed consent, and to allow patients to engage in shared decision-making with the aim of supporting the best decision. The team needs to understand the patient’s concerns and expectations about the treatment and short- and long-term goals, as the risk-benefits of the intervention may not be aligned with patient preferences and wishes. Communicating in plain language (oral and written) and targeting communication to fit the individual level of health literacy is pivotal. Several studies have indicated a relatively high prevalence of limited health literacy in patients with CVD (e.g. with HF),62 and limited health literacy is associated with adverse outcomes.63 An example of a patient information sheet to be used in the communication with patients is given in the Supplementary data, Table S1.
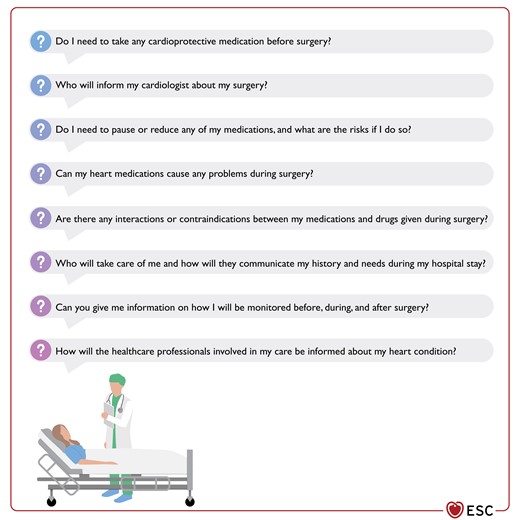
Recent systematic reviews and meta-analyses have focused on shared decision-making in the field of surgery across disease areas.64–67 In general, shared decision-making positively impacts decisional conflicts, knowledge gained, satisfaction, and decisional anxiety (although cultural variations may exist).67 In the breast cancer/endocrine and urology specialties, decision-making and communication aids appear to be effective methods for supporting patients’ involvement in decision-making when undergoing elective surgery. Moreover, educational information, provided through interactive multimedia, computer, or on DVD, used prior to the surgical consultation could enhance the decision-making process in addition to face-to-face communication.66
In Europe, the prevalence of pre-operative anxiety among patients undergoing surgical procedures varies from 27–80%.68 Although a certain level of anxiety in patients must be expected, peri-operative anxiety is associated with worse surgical outcomes and longer recovery,69–72 which highlights the importance of pre-surgical assessment and, in some patients, treatment of anxiety. Factors associated with pre-operative anxiety are complex and include, among others, age, sex, educational level, type of surgery, and fear of post-operative complications or the outcome.68 Psychological reactions in patients undergoing high- or medium–high-risk procedures and/or patients with previous negative experiences of NCS may warrant particular attention. Concerns and fears expressed by patients and relatives should be taken seriously. A number of reviews and meta-analyses have summarized the effects of interventions on surgical outcomes in abdominal, cardiac, and orthopaedic surgery, which may also be applicable to patients with CV conditions in these settings.73–75
4. Pre-operative assessment tools
4.1. Risk scores
4.1.1. General risk calculators
Several risk indices have been developed based on multivariable analyses of observational data and have been validated during the last decade (Table 6).47,49,76 Most risk calculators integrate both patient-related and surgery-related risk factors, but none of them include biomarkers among their variables. Calculators for most of the commonly used risk indices are available online (Table 6). The risk calculators can be used in addition, or as an alternative, to the assessment of surgery-related and patient-related risk factors described in Section 3.3. The Task Force decided against recommending one specific risk score. The Task Force also decided that the selection criteria for further pre-operative testing should be clinical criteria, and not based on a specific score.
| . | Revised Cardiac Risk Index (RCRI) (1999)a . | Surgical Risk Calculator (2011) . | The American College of Surgery National Surgical Quality Improvement Program (ACS NSQIP) (2013) . | Surgical Outcome Risk Tool (SORT) (2014) . | The American University of Beirut (AUB)-HAS2 Cardiovascular Risk Index (2019)b . |
|---|---|---|---|---|---|
| Variables | Ischaemic heart disease Cerebrovascular disease History of congestive heart failure Insulin therapy for diabetes Serum creatinine level ≥2 mg/dL High-risk surgery (each assigned 1 point) | Age ASA–PS grade Pre-operative dependent functional status Creatinine >1.5 mg/dL Type of surgery | Age Sex Functional status Emergency case ASA class Current steroid use Ascites within 30 days Systemic sepsis within 48 h Ventilator dependence Disseminated cancer Diabetes Hypertension on treatment Congestive HF Dyspnoea Current smoker History of severe COPD Dialysis Acute renal failure Body mass index Surgery code | ASA–PS grade Urgency of surgery High-risk surgical specialty Surgical severity (from minor to complex major) Cancer Age ≥65 years or over | History of Heart disease Symptoms of Heart disease (angina or dyspnoea) Age ≥75 years Anaemia (haemoglobin <12 g/dL) Vascular Surgery Emergency Surgery (2 H, 2 A and 2 S) (each assigned 1 point) |
| Score range | Score 1; risk 6.0% (4.9–7.4) Score 2; risk 10.1% (8.1–10.6) Score ≥3; risk 15% (11.1–20.0) | Absolute risk: 0–100% | Absolute risk: 0–100% | Absolute risk: 0–100% | Low risk (score 0–1); (0.3 and 1.6%)c Intermediate risk (score 2–3); (7.1 and 17%)c High risk (score >3); (>17%)c |
| Outcome | 30 day MI, cardiac arrest, death | Intra-operative and 30 day MI or cardiac arrest | Serious complications and any complications at 30 days | 30 day mortality | 30 day death, MI, or stroke |
| Derivation population | 1422 | 211 410 | 1 414 006 | 11 219 | 3284 |
| Validation population | Externally validated in various surgical populations | 257 385 | Externally validated in various surgical populations | 22 631 | 1 167 414 |
| Model performance (AUC) | 0.68–0.76 | 0.81–0.85 | 0.73 | 0.81–0.92 | 0.82 |
| Interactive calculator | https://www.mdcalc.com/revised-cardiac-risk-index-pre-operative-risk | http://www.surgicalriskcalculator.com/miorcardiacarrest | https://riskcalculator.facs.org | http://www.sortsurgery.com |
| . | Revised Cardiac Risk Index (RCRI) (1999)a . | Surgical Risk Calculator (2011) . | The American College of Surgery National Surgical Quality Improvement Program (ACS NSQIP) (2013) . | Surgical Outcome Risk Tool (SORT) (2014) . | The American University of Beirut (AUB)-HAS2 Cardiovascular Risk Index (2019)b . |
|---|---|---|---|---|---|
| Variables | Ischaemic heart disease Cerebrovascular disease History of congestive heart failure Insulin therapy for diabetes Serum creatinine level ≥2 mg/dL High-risk surgery (each assigned 1 point) | Age ASA–PS grade Pre-operative dependent functional status Creatinine >1.5 mg/dL Type of surgery | Age Sex Functional status Emergency case ASA class Current steroid use Ascites within 30 days Systemic sepsis within 48 h Ventilator dependence Disseminated cancer Diabetes Hypertension on treatment Congestive HF Dyspnoea Current smoker History of severe COPD Dialysis Acute renal failure Body mass index Surgery code | ASA–PS grade Urgency of surgery High-risk surgical specialty Surgical severity (from minor to complex major) Cancer Age ≥65 years or over | History of Heart disease Symptoms of Heart disease (angina or dyspnoea) Age ≥75 years Anaemia (haemoglobin <12 g/dL) Vascular Surgery Emergency Surgery (2 H, 2 A and 2 S) (each assigned 1 point) |
| Score range | Score 1; risk 6.0% (4.9–7.4) Score 2; risk 10.1% (8.1–10.6) Score ≥3; risk 15% (11.1–20.0) | Absolute risk: 0–100% | Absolute risk: 0–100% | Absolute risk: 0–100% | Low risk (score 0–1); (0.3 and 1.6%)c Intermediate risk (score 2–3); (7.1 and 17%)c High risk (score >3); (>17%)c |
| Outcome | 30 day MI, cardiac arrest, death | Intra-operative and 30 day MI or cardiac arrest | Serious complications and any complications at 30 days | 30 day mortality | 30 day death, MI, or stroke |
| Derivation population | 1422 | 211 410 | 1 414 006 | 11 219 | 3284 |
| Validation population | Externally validated in various surgical populations | 257 385 | Externally validated in various surgical populations | 22 631 | 1 167 414 |
| Model performance (AUC) | 0.68–0.76 | 0.81–0.85 | 0.73 | 0.81–0.92 | 0.82 |
| Interactive calculator | https://www.mdcalc.com/revised-cardiac-risk-index-pre-operative-risk | http://www.surgicalriskcalculator.com/miorcardiacarrest | https://riskcalculator.facs.org | http://www.sortsurgery.com |
AUC, area under curve; ASA–PS, American Society of Anesthesiology Physical Status; COPD, chronic obstructive pulmonary disease; HF, heart failure; MI, myocardial infarction; RCRI, Revised Cardiac Risk Index.
The RCRI was updated January 2019.
The percentages relate to general surgeries.50
| . | Revised Cardiac Risk Index (RCRI) (1999)a . | Surgical Risk Calculator (2011) . | The American College of Surgery National Surgical Quality Improvement Program (ACS NSQIP) (2013) . | Surgical Outcome Risk Tool (SORT) (2014) . | The American University of Beirut (AUB)-HAS2 Cardiovascular Risk Index (2019)b . |
|---|---|---|---|---|---|
| Variables | Ischaemic heart disease Cerebrovascular disease History of congestive heart failure Insulin therapy for diabetes Serum creatinine level ≥2 mg/dL High-risk surgery (each assigned 1 point) | Age ASA–PS grade Pre-operative dependent functional status Creatinine >1.5 mg/dL Type of surgery | Age Sex Functional status Emergency case ASA class Current steroid use Ascites within 30 days Systemic sepsis within 48 h Ventilator dependence Disseminated cancer Diabetes Hypertension on treatment Congestive HF Dyspnoea Current smoker History of severe COPD Dialysis Acute renal failure Body mass index Surgery code | ASA–PS grade Urgency of surgery High-risk surgical specialty Surgical severity (from minor to complex major) Cancer Age ≥65 years or over | History of Heart disease Symptoms of Heart disease (angina or dyspnoea) Age ≥75 years Anaemia (haemoglobin <12 g/dL) Vascular Surgery Emergency Surgery (2 H, 2 A and 2 S) (each assigned 1 point) |
| Score range | Score 1; risk 6.0% (4.9–7.4) Score 2; risk 10.1% (8.1–10.6) Score ≥3; risk 15% (11.1–20.0) | Absolute risk: 0–100% | Absolute risk: 0–100% | Absolute risk: 0–100% | Low risk (score 0–1); (0.3 and 1.6%)c Intermediate risk (score 2–3); (7.1 and 17%)c High risk (score >3); (>17%)c |
| Outcome | 30 day MI, cardiac arrest, death | Intra-operative and 30 day MI or cardiac arrest | Serious complications and any complications at 30 days | 30 day mortality | 30 day death, MI, or stroke |
| Derivation population | 1422 | 211 410 | 1 414 006 | 11 219 | 3284 |
| Validation population | Externally validated in various surgical populations | 257 385 | Externally validated in various surgical populations | 22 631 | 1 167 414 |
| Model performance (AUC) | 0.68–0.76 | 0.81–0.85 | 0.73 | 0.81–0.92 | 0.82 |
| Interactive calculator | https://www.mdcalc.com/revised-cardiac-risk-index-pre-operative-risk | http://www.surgicalriskcalculator.com/miorcardiacarrest | https://riskcalculator.facs.org | http://www.sortsurgery.com |
| . | Revised Cardiac Risk Index (RCRI) (1999)a . | Surgical Risk Calculator (2011) . | The American College of Surgery National Surgical Quality Improvement Program (ACS NSQIP) (2013) . | Surgical Outcome Risk Tool (SORT) (2014) . | The American University of Beirut (AUB)-HAS2 Cardiovascular Risk Index (2019)b . |
|---|---|---|---|---|---|
| Variables | Ischaemic heart disease Cerebrovascular disease History of congestive heart failure Insulin therapy for diabetes Serum creatinine level ≥2 mg/dL High-risk surgery (each assigned 1 point) | Age ASA–PS grade Pre-operative dependent functional status Creatinine >1.5 mg/dL Type of surgery | Age Sex Functional status Emergency case ASA class Current steroid use Ascites within 30 days Systemic sepsis within 48 h Ventilator dependence Disseminated cancer Diabetes Hypertension on treatment Congestive HF Dyspnoea Current smoker History of severe COPD Dialysis Acute renal failure Body mass index Surgery code | ASA–PS grade Urgency of surgery High-risk surgical specialty Surgical severity (from minor to complex major) Cancer Age ≥65 years or over | History of Heart disease Symptoms of Heart disease (angina or dyspnoea) Age ≥75 years Anaemia (haemoglobin <12 g/dL) Vascular Surgery Emergency Surgery (2 H, 2 A and 2 S) (each assigned 1 point) |
| Score range | Score 1; risk 6.0% (4.9–7.4) Score 2; risk 10.1% (8.1–10.6) Score ≥3; risk 15% (11.1–20.0) | Absolute risk: 0–100% | Absolute risk: 0–100% | Absolute risk: 0–100% | Low risk (score 0–1); (0.3 and 1.6%)c Intermediate risk (score 2–3); (7.1 and 17%)c High risk (score >3); (>17%)c |
| Outcome | 30 day MI, cardiac arrest, death | Intra-operative and 30 day MI or cardiac arrest | Serious complications and any complications at 30 days | 30 day mortality | 30 day death, MI, or stroke |
| Derivation population | 1422 | 211 410 | 1 414 006 | 11 219 | 3284 |
| Validation population | Externally validated in various surgical populations | 257 385 | Externally validated in various surgical populations | 22 631 | 1 167 414 |
| Model performance (AUC) | 0.68–0.76 | 0.81–0.85 | 0.73 | 0.81–0.92 | 0.82 |
| Interactive calculator | https://www.mdcalc.com/revised-cardiac-risk-index-pre-operative-risk | http://www.surgicalriskcalculator.com/miorcardiacarrest | https://riskcalculator.facs.org | http://www.sortsurgery.com |
AUC, area under curve; ASA–PS, American Society of Anesthesiology Physical Status; COPD, chronic obstructive pulmonary disease; HF, heart failure; MI, myocardial infarction; RCRI, Revised Cardiac Risk Index.
The RCRI was updated January 2019.
The percentages relate to general surgeries.50
The RCRI estimates the risk of 30 day mortality, MI, or cardiac arrest, and is based on six variables.46,47 It has been validated in several countries and is easy to use.47 A score of 0 indicates a 4% risk of 30 day mortality, MI, or cardiac arrest; a score of 1 indicates a 6% risk; a score of 2, 10%; and a score of ≥3, 15%.47
The American College of Surgery National Surgical Quality Improvement Program (ACS NSQIP) developed an interactive risk calculator providing an estimate of the absolute 30 day probability of serious complications and any complications compared with the average patient.76 Evaluated in the US surgical database, the ACS NSQIP model performed better than the RCRI, but an external validation in the Philippines found both to have excellent discriminative abilities for predicting any MACE.48 The RCRI can be used without a web connection, whereas the ACS NSQIP is procedure-specific and is only available on the web. For clinical use, the RCRI is more accessible, but the ACS NSQIP offers procedure-specific absolute risk estimates, which are valuable in patient-guided decision-making. In vascular surgery, both risk calculators have shown moderate accuracy with an area under curve (AUC) of 0.64 (95% CI, 0.57–0.70) for ACS NSQIP and 0.60 (95% CI, 0.54–0.65) for RCRI, due to underestimation of the risk of MI.76a Attempts to generate procedure-specific vascular calculators have not given better predictions in validation cohorts.77
The Surgical Outcome Risk Tool (SORT) estimates 30 day mortality after NCS based on the American Society of Anesthesiologists Physical Status (ASA–PS) grade, urgency of surgery, surgical speciality and severity, cancer, and age ≥65 years. In the validation study, combining subjective assessment with the SORT was significantly better than using either alone.78,79 The Surgical Risk Calculator is another tool that predicts intra-operative and 30 day risk of MI or cardiac arrest based on age, ASA–PS grade, pre-operative dependent functional status, creatinine, and type of surgery.80
The American University of Beirut (AUB)-HAS2 Cardiovascular Risk Index is the most recently developed index to assess 30 day event risk (death, MI, or stroke), and stratifies patients undergoing NCS into low (score 0–1), intermediate (score 2–3), and high risk (score >3) based on six data elements (see Table 6); scores >3 denote a post-operative event rate of >10%.49 The AUB-HAS2 Index has been tested in a broad spectrum of surgical subpopulations and demonstrated superior discriminatory power compared with the commonly utilized RCRI (Table 6).50,51,81
There is significant variability in the predicted risk of cardiac complications using different risk-prediction tools; none can be disqualified with current evidence.82
4.1.2. Frailty
Frailty is an age-related, multidimensional state of decreased physiological reserve that results in diminished resiliency, loss of adaptive capacity, and increased vulnerability to stressors.83,84 The peri-operative evaluation of elderly patients (>70 years) who require elective intermediate- or high-risk NCS should include frailty screening, which has proven to be an excellent predictor of unfavourable health outcomes in the older surgical population.
Frailty has a relevant impact on mortality and MI risk but does not add to risk estimation derived from the ACS NSQIP calculator, as frailty is associated with variables already in the main model. By adding six variables, the ACS NSQIP predicts the risk of post-operative delirium, functional decline, need of a new mobility aid, or pressure ulcer.85 The use of this broader-inclusive score identifies cases that profit the most by involving a geriatrician in the pre- and post-operative team.86,87 A measure of frailty informs the patient and surgeon about further life expectancy and the chance of post-operative delirium, dependency of mobility support, and need of nursing home or other care support after planned surgery.
Of the available screening tools for frailty, the Frailty Index and the Frail Phenotype are the most commonly recommended.88,89 Of note, the Frailty Index includes cognitive testing, while both scores assess physical function.90,91 A simpler approach is offered by the Clinical Frailty Scale, which relies on information from the history taking. The Clinical Frailty Scale has been validated against the Frailty Index.88 For cognitive screening to incorporate with the Frailty Index, Mini-Cog© is a simple and fast screening tool validated for pre-operative screening92 (Supplementary data, Figure S1, and Tables S2 and S3).
Once a diagnosis of frailty is confirmed, the prognosis of a frail patient can be improved by shared decision-making between at least a treating physician (e.g. surgeon), anaesthesiologist, geriatrician, the patient, and the patient’s relatives. During the shared decision-making process, a careful discussion with a frail patient about goals of care could help them to have realistic expectations and make better informed decisions before surgery. After a shared decision to go ahead with a planned NCS, multimodal pre-habilitation programmes—including exercise, nutrition, and psychological interventions—could potentially improve the peri-operative prognosis of frail patients by an individualized approach tailored to the patient’s baseline functional status, comorbidities, and cognitive/psychological function.90
4.2. Functional capacity
Quantifying functional capacity has been a pivotal step in pre-operative cardiac risk assessment.10 Although the validity of interview-based assessment of functional capacity has been questioned,93 a recent large prospective cohort study of high-risk patients undergoing NCS found self-reported inability to climb two flights of stairs added incremental value to the 30 day cardiac event rate when added to the RCRI.94
Metabolic equivalents (METs) <4 have long been considered to indicate poor functional capacity; however, studies using METs have been based on subjective interviews and not shown proven value. In the Measurement of Exercise Tolerance before Surgery (METS) study, the Duke Activity Status Index (DASI) (https://www.mdcalc.com/duke-activity-status-index-dasi#evidence) had a more precise estimation of cardiac risk than subjectively assessed functional capacity, improving risk estimation using RCRI.95 A DASI score <34 was associated with increased odds of 30 day death or MI.96 From the DASI score, METs can be calculated as VO2 max (maximal oxygen consumption)/3.5; where VO2 max (mL/kg/min) = 0.43 × DASI + 9.6. Furthermore, cardiopulmonary exercise testing (CPET) did not predict 30 day mortality, post-operative MI, or cardiac arrest.94,95 It should be noted that a relatively low number of primary outcome events limited the statistical power of the analysis.
Recommendations for pre-operative assessment of frailty and functional capacity
 |
 |
 |
 |
Recommendations for pre-operative assessment of frailty and functional capacity
 |
 |
 |
 |
4.3. Electrocardiography
The 12-lead ECG is a widely available, simple, and inexpensive tool that is able to semi-quantitatively assess cardiac risk (e.g. Q waves indicative of previous MI), and detect unknown CV conditions requiring therapy (e.g. atrial fibrillation [AF] or AV-block).97–99 It is recommended to obtain a pre-operative 12-lead ECG in patients who are aged ≥65 years or have known CVD, CV risk factors, or symptoms suggestive of cardiac disorders, and scheduled to undergo intermediate- or high-risk surgery. It is not recommended to routinely obtain a pre-operative ECG in low-risk patients undergoing low-risk NCS.100
Comparison with previous ECG recordings is helpful whenever relevant abnormalities are identified. Pre-operative recording of ECG also enables identification of intra- and post-operative ECG changes.
4.4. Biomarkers
As the peri-operative risk for cardiac complications depends on the presence and extent of cardiac disease, widely available and simple biomarkers that detect and quantify essential prognostic aspects of cardiac involvement may aid in the evaluation. High-sensitivity cardiac troponin T/I (Hs-cTn T/I) quantifies myocardial injury, and BNP and NT-proBNP quantify haemodynamic cardiac wall stress (Figure 4). Both Hs-cTn T/I and BNP/NT-proBNP complement clinical assessment and ECG in risk prediction.9,52,53,101–103 Hs-cTn T/I and, to a lesser extent, BNP/NT-proBNP concentrations are higher in patients with stress-induced myocardial ischaemia vs. those without, and very low hs-cTn T/I concentrations achieve a very high negative predictive value to rule out myocardial ischaemia.104–107
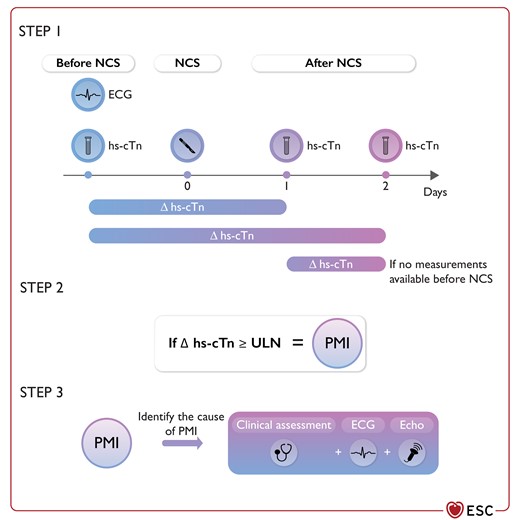
Recommended measurements to assess and detect the risk of post-operative cardiac complications.
ECG, electrocardiogram; hs-cTn, high-sensitivity cardiac troponin; PMI, peri-operative myocardial infarction/injury; ULN, upper limit of normal. In patients scheduled to undergo intermediate- or high-risk surgery, pre-operative risk assessment is complemented by ECG, hs-cTn, and BNP/NT-proBNP. An absolute increase in hs-cTn concentration of more than the ULN on days 1 or 2 after surgery compared to the pre-operative level is defined as PMI.109–111 In the absence of a pre-operative hs-cTn T/I concentration, a very high hs-cTn T/I concentration on day 1 (e.g. more than five-times the ULN) or a relevant change from day 1 to day 2 (absolute increase or decrease more than the ULN vs. day 1) would also achieve a reliable diagnosis of PMI. Detection of PMI should trigger ECG recording and detailed clinical evaluation for PMI work-up and therapy. The differential diagnosis of PMI according to the fourth universal definition of MI is discussed in Section 8. The ESC 0/1/2 h algorithm has not been validated for the peri-operative setting and cannot be used here.
Several large prospective studies have shown that both hs-cTn T/I and BNP/NT-proBNP have high and incremental prognostic value for peri-operative cardiac complications, including CV death, cardiac arrest, acute HF, and tachyarrhythmias. In a cohort of nearly 1000 subjects undergoing major elective NCS, individuals with pre-operative hs-cTn T concentrations of >14 ng/L had an in-hospital mortality of 6.9% vs. 1.2% in patients with hs-cTn T concentrations ≤14 ng/L (P < 0.001; AUC 0.81).53 In a large prospective cohort study including 10 402 patients from 16 centres, NT-proBNP improved risk predication beyond the RCRI.52 Among 1923 patients undergoing NCS, NT-proBNP outperformed both RCRI and echocardiographic parameters in the prediction of peri-operative CV events.103 Overall, hs-cTn T/I and BNP/NT-proBNP seem to have comparable accuracy in the prediction of cardiac complications.52,53,98–103,108 However, Hs-cTn T/I has four advantages over BNP/NT-proBNP: (i) it is more widely available; (ii) it is less expensive; (iii) if normal, it enables acute MI to be ruled out in the preceding days; and (iv) availability of pre-operative hs-cTn T/I concentration enables accurate diagnosis of PMI on Day 1 after surgery.109–111 See Section 8 for more details on diagnosis and treatment of PMI.
B-type natriuretic peptide/NT-proBNP has two advantages. First, if elevated, evidence from randomized controlled screening studies performed outside the peri-operative setting has supported the concept that BNP/NT-proBNP-triggered cardiac work-up and intensification of therapy improve outcomes.112,113 Second, HF is a frequently undiagnosed condition in the elderly population most often undergoing NCS.47,114 Interpreting BNP/NT-proBNP concentrations as quantitative markers of HF with evolving rule-in cut-offs may facilitate detection of HF, optimal intra-operative monitoring, and initiation or optimization of HF therapy after surgery.114
To date, there is insufficient evidence in support of other CV biomarkers for this specific indication.115,116
Recommendations for pre-operative risk assessment—electrocardiography and biomarkers
 |
 |
 |
 |
Recommendations for pre-operative risk assessment—electrocardiography and biomarkers
 |
 |
 |
 |
4.5. Non-invasive and invasive procedures
4.5.1. Resting transthoracic echocardiography
In large retrospective cohorts, routine pre-operative TTE before high-risk NCS did not reduce the risk of post-operative MACE or provide more information than clinical risk models.120–122 Poor exercise tolerance, abnormal ECG, suspected new or significant CVDs without follow-up within the last 90 days, unexplained dyspnoea, or coexisting clinical risk factors are appropriate indications for TTE.123,124 Pre-operative TTE provides information on three main risk markers for post-operative cardiac events: LV dysfunction, VHDs, and cardiomyopathies. Left ventricular systolic dysfunction is an important predictor of post-operative HF.125 However, low ventricular ejection fraction is a borderline independent predictor of major post-operative CV complications.126–128
Pre-operative FOCUS examination—with a hand-held ultrasound device for the assessment of murmurs, haemodynamic instability, ventricular function, and dyspnea—may impact patient management by improving the diagnostic accuracy of clinical assessment, and help to triage candidates for standard TTE, plan surgery and anaesthesia technique, and with post-operative monitoring.129–131 However, current evidence remains mostly confined to uncontrolled or retrospective observational studies with no clear benefits on the outcome, despite a favourable impact on peri-operative management.130,132 In a multicentre randomized trial, preliminary results showed that pre-operative FOCUS significantly reduced all-cause mortality.133 Notably, because of the lack of spectral Doppler capabilities, the FOCUS examination is only accurate for assessing main structural and functional abnormalities.
Patients with diastolic dysfunction are usually old, more hypertensive, obese, diabetic, and likely to have AF or chronic renal disease. Several studies with different clinical end-points have underlined the association of diastolic dysfunction with post-operative adverse events, including pulmonary oedema, AF, and mortality.134–138 A meta-analysis including 3876 patients undergoing NCS found pre-operative diastolic dysfunction to be an independent risk factor for pulmonary oedema, congestive HF, and MI after surgery.139 However, a recent retrospective study, including 7312 patients, showed no association between the degree of diastolic dysfunction and in-hospital mortality or hospital length of stay in NCS patients.140 Awareness of diastolic dysfunction or high LV filling pressure (e.g. pulmonary hypertension, left atrial volume, E/e′ ratio) seems necessary to optimize peri-operative patient management; however, evidence does not support screening for diastolic dysfunction.
4.5.2. Stress tests
4.5.2.1. Exercise stress test
Physical exercise, using a treadmill or bicycle ergometer, provides an estimate of functional capacity, evaluates blood pressure (BP) and heart rate response, and detects myocardial ischaemia through pathological ST-segment changes with poor sensitivity (61–73%) and specificity (60–80%).146 An exercise stress test alone should only be considered a valuable alternative to diagnose obstructive CAD if non-invasive imaging tests are unavailable.146 An exercise stress test is of no diagnostic value in patients with pre-existing ST-segment abnormalities (i.e. left bundle branch block, paced rhythm, Wolff–Parkinson–White [WPW] syndrome, ≥0.1 mV ST-segment depression on resting ECG, or taking digitalis). In addition, an exercise test is unsuitable for patients with limited exercise capacity, owing to their inability to reach their target heart rate. Therefore, an exercise stress test alone should only be considered a valuable alternative to diagnose obstructive CAD if non-invasive imaging tests are unavailable, or for assessing functional capacity when clinical history is ambiguous.146
4.5.2.2. Stress imaging
The use of stress imaging is appropriate for risk assessment in patients with clinical risk factors and poor functional capacity.147,148 The choice of the test is driven by local expertise. Selection, optimal, and safe performance of stress imaging should comply with related guidelines and recommendations.146,148,149 Stress imaging is not recommended in patients undergoing urgent surgery or with an unstable clinical condition. Evidence on the role of stress imaging for peri-operative risk prediction and patient management is largely based on inducible ischaemia by pharmacological stress testing, although no evidence indicates the superiority of pharmacological stress to exercise stress imaging in patients who are able to perform an adequate level of physical exercise. Several studies and meta-analyses have consistently defined clinical utility of pharmacological stress imaging for peri-operative risk assessment in patients undergoing NCS.150–154 Although RCTs related to post-operative outcome are lacking, there are large-scale prospective studies showing a risk-adjusted association of stress imaging results with peri-operative cardiac complications.155–157
Studies and meta-analyses have demonstrated similar prognostic value of stress echocardiography and myocardial perfusion imaging for peri-operative risk assessment with slightly higher negative predictive value of stress echocardiography,152 but the overall accuracy varies with ischaemic heart disease (IHD) prevalence.151 A moderate-to-large perfusion defect on either test is highly sensitive for post-operative cardiac events.152,158 Normal stress imaging exams without resting abnormalities have high negative predictive value.159,160 However, positive predictive value of stress imaging for peri-operative cardiac events is relatively low and requires confirmation by other tests.150,152,161
In a recent retrospective study including 4494 patients, dobutamine stress echocardiography (DSE) provided modest incremental predictive value for peri-operative CV complications over clinical variables and was found to be useful as part of a stepwise approach in the risk stratification of patients undergoing intermediate- to high-risk NCS.157 The strongest predictors of post-operative adverse events determined so far are significant ischaemia (more than four ventricular segments) during DSE, ischaemic threshold (60% of age-predicted maximal heart rate), and a history of congestive HF.162,163
A negative DSE without resting wall motion abnormality has excellent negative predictive value, even when target heart rate cannot be achieved despite an aggressive DSE regimen.164 In asymptomatic patients if functional capacity is unknown, stress echocardiography also enables dynamic evaluation of LV systolic and diastolic function; valvular diseases such as aortic valve stenosis (AS), mitral valve stenosis (MS), and hypertrophic obstructive cardiomyopathy; and pulmonary hypertension 165 However, the role of DSE for risk estimation in non-ischaemic heart diseases before NCS has yet to be studied.
Myocardial perfusion imaging is particularly suitable if patients have poor acoustic windows for DSE. Meta-analyses of patients undergoing major NCS have demonstrated that, compared with fixed defects, reversible perfusion defects were associated with higher risk of cardiac death or non-fatal MI. The risk of cardiac events correlates with the extent of reversible perfusion abnormalities (severe: >20% of myocardium). Normal myocardial perfusion imaging in high-risk patients identifies a low-risk subgroup comparable with those without clinical risk factors for adverse cardiac outcomes.154,159,160
Stress cardiac magnetic resonance (CMR) imaging and late gadolinium enhancement are also accurate tools for detection of IHD and prognostication.166
4.5.3. Angiography
4.5.3.1. Coronary computed tomography angiography
Coronary computed tomography angiography (CCTA) is recommended as an initial test for diagnosing CAD in stable patients with a low clinical likelihood or no previous diagnosis of CAD, and characteristics associated with a high likelihood of good image quality.146 In addition, CCTA is recommended as an alternative to invasive coronary angiography (ICA) for excluding non-ST-segment elevation acute coronary syndrome (NSTE-ACS) when there is low-to-intermediate likelihood of CAD, and when cardiac troponin and/or ECG are normal or inconclusive.98 The practical utility of CCTA is reduced when a high coronary calcium score is present.167
In patients undergoing NCS, the role of pre-operative CCTA to rule out CAD has been investigated in small- to medium-sized observational studies. The Coronary Computed Tomographic Angiography and Vascular Events in Noncardiac Surgery Patients Cohort Evaluation (Coronary CTA VISION) trial prospectively investigated the incremental predictive value of CCTA over RCRI in 955 patients with a history of or risk factors for CAD, or a history of congestive HF undergoing NCS.168 Coronary computed tomography angiography improved the risk estimation for the primary outcome of post-operative CV death and non-fatal MI within 30 days, although CCTA was associated with more than five times inappropriate risk overestimations among patients not experiencing the primary outcome. The predictive value of CCTA further improved when associated with non-invasive functional testing, such as myocardial perfusion imaging, with a positive and negative predictive value of 50% (95% CI, 21–79) and 100% (95% CI, 79–100), respectively.161
Coronary computed tomography angiography associated with additional functional assessment of coronary stenosis with fractional flow reserve (FFR) with computed tomography (CT) was able to identify functionally severe coronary stenosis in 57% of the asymptomatic patients with no history of cardiac disease undergoing carotid endarterectomy (CEA).169 In 135 asymptomatic patients with no history of cardiac disease undergoing peripheral vascular surgery, pre-operative FFR with CT facilitated the identification of functionally severe coronary stenosis in 53% of the patients. These patients benefited from further revascularization, with a 1 year lower rate of CV death and MI.170
4.5.3.2. Invasive coronary angiography
There is a lack of information from RCTs relating to the usefulness of ICA in patients scheduled for NCS. Adopting an ICA assessment may also cause an unnecessary and unpredictable delay in an already planned surgical intervention, and adding an independent procedural risk to the overall risk. Despite the fact that CAD may be present in a significant number of patients requiring NCS, indications for pre-operative coronary angiography and revascularization are similar to angiography indications in the non-surgical setting.98,146,171 Pre-operative treatment of patients with myocardial ischaemia, either medically or with intervention, is recommended.
5. General risk-reduction strategies
5.1. Cardiovascular risk factors and lifestyle interventions
Control of CV risk factors—including BP, dyslipidaemia, and diabetes—is important before NCS. For pre-operative management of BP and diabetes, see Sections 6.8 and 6.13, respectively.
While lifestyle modifications before intervention reduce the risk of several peri-operative complications, the impact on CV complications has not been adequately explored. Of the lifestyle changes recommended before surgery, smoking cessation is the best documented in RCTs. Smoking has been associated with a higher rate of post-operative complications at 30 days.173,174 Reviews of RCTs have shown an effect of smoking cessation up to 6 months post-operatively, with a clear reduction in any post-operative complications by hazard ratio (HR) 0.42 (95% CI, 0.27–0.65), particularly wound infections (HR, 0.43; 95% CI, 0.21–0.85).173,175 Regarding timing of cessation, reviews of observational studies have shown consistent associations with better surgical outcome for cessation >4 weeks before surgery, with each additional week resulting in a further improvement of 19%.176–178
Pre-operative exercise programmes have only been tested in small RCTs, and recent reviews have shown a relative risk (RR) reduction in post-operative complications of 67% (RR, 0.33; 95% CI, 0.17–0.61).179 Referral to a pre-operative exercise programme may be considered for patients scheduled for major or complex elective surgery.176,179,180 Weight reduction of obese patients immediately prior to surgery is not recommended.
Recommendations for lifestyle and cardiovascular risk factors
 |
 |
Recommendations for lifestyle and cardiovascular risk factors
 |
 |
5.2. Pharmacological
5.2.1. Beta-blockers
Beta-blockers reduce myocardial oxygen consumption by reducing contractile force and heart rate. Beta-blockers are also effective antiarrhythmic agents. In addition, some beta-blockers such as metoprolol have an effect on acute inflammatory responses by inhibiting neutrophil hyperactivation in acute settings.184 These properties mean that beta-blockers have been some of the most frequently tested cardioprotective agents in patients undergoing NCS. Several RCTs have evaluated the effects of peri-operative beta-blockade on clinical end-points in patients with different risk profiles (see Supplementary data, Section 3.1.1.). Type, dosing and titration, timing of initiation, duration of beta-blocker therapy, type of surgery, and risk profile of subjects significantly differ between studies, making comparisons complex.
The question about pre-surgery initiation of beta-blockers has been a matter of intense controversy (see Supplementary data, Section 3.1.1.1). The largest and latest trial on the topic, the Perioperative Ischemic Evaluation (POISE-1) trial, enrolled 8351 patients with or at risk of atherosclerotic disease, and not on beta-blockers before NCS. Patients were randomized to extended-release metoprolol succinate 200 mg daily or placebo 185 Treatment was initiated 2–4 h before surgery and maintained for 30 days. The primary outcome (composite of CV death, non-fatal MI, and non-fatal cardiac arrest) was significantly lower in the metoprolol arm (5.8% vs. 6.9% [P = 0.04]). Metoprolol was associated with significant reductions in MIs, coronary revascularizations, and AF. However, the incidences of all-cause death, stroke, and clinically significant hypotension or bradycardia were significantly higher in the metoprolol arm. Post hoc analysis showed that hypotension carried the greatest attributable risk of death and stroke 186 The high dose of extended metoprolol might have played a role in the adverse events seen at follow-up.
Several meta-analyses, systematic reviews, and observational studies have also been reported (see Supplementary data, Table S4).187–189 Overall, initiation of beta-blockers before NCS was not associated with a net clinical benefit in most analyses, but they might be beneficial in patients with high CV risk profiles or who are undergoing high-risk surgical interventions (including vascular interventions).188,190–192 When oral beta-blockade is initiated in CAD patients who undergo NCS, the use of atenolol or bisoprolol as a first choice may be considered.190,193–195
In patients who are on chronic beta-blocker therapy before surgery, it is recommended to maintain these in the peri-operative period. Increased mortality following pre-operative beta-blocker withdrawal has been reported in five observational studies.190,196–199 Interruption of this therapy for >2 days post-operatively may double the risk of AF.200
Post-operative tachycardia should initially lead to treatment of the underlying cause— such as hypovolaemia, pain, blood loss, or infection—rather than simply increasing the beta-blocker dose. When beta-blockers are indicated, the optimal duration of the peri-operative beta-blockade cannot be derived from randomized trials.
According to a meta-analysis of RCTs including 14 967 patients, beta-blockers can reduce the risk of post-operative AF after NCS;201 however, this comes at the cost of an increased risk of bradycardia, hypotension, and stroke.187
The ultra-short-acting beta-blockers esmolol and landiolol have the theoretical advantages of very fast onset of effects and short half-lives. Notably, landiolol may lower BP to a lower extent than esmolol. Evidence of prevention of AF with landiolol after NCS is less robust and inconsistent than in the context of cardiac surgery.202–205 The timing of the initiation of beta-blockers to prevent AF remains unclear, with most prophylactic regimens using short-acting agents being started intra-operatively.187
5.2.2. Amiodarone
Amiodarone is the most frequently used agent for prevention of post-operative AF,206 with a risk reduction of 58% in NCS in a meta-analysis evaluating different antiarrhythmic drugs (AADs), but may induce relevant non-cardiac side effects.201 In another meta-analysis, amiodarone (oral or intravenous [i.v.]) and beta-blockers were equally effective in reducing post-operative AF.207 In another prospective RCT, a combination of beta-blocker plus amiodarone outperformed beta-blockers alone in reducing post-operative AF.208 It should be noted that the two latter studies were undertaken in patients undergoing cardiac surgery.
Overall, while preventive amiodarone seems to reduce the incidence of AF, the clinical benefits associated with its routine use are unclear.
5.2.3. Statins
Despite the wide-ranging use of statins in patients undergoing surgery, RCTs assessing the effects of initiating statin therapy during the peri-operative period are scarce. This should be viewed separately from patients already on statin therapy. The long-term use of statins in patients with CVD or high risk of CVD is well-established.40
Observational data suggest a potential benefit of statins in the peri-operative phase. In a large, retrospective, and observational cohort, which included 180 000 veterans undergoing NCS, the use of statins on the day of or the day after surgery was associated with a reduction in mortality (RR, 0.82; 95% CI, 0.75–0.89).209 Similar results were seen in a retrospective cohort study using hospital discharge and pharmacy records.210 Although both studies used propensity matching to reduce bias, these analyses are prone to confounding, especially when discharge and pharmacy records are used. As such, RCTs give a more reliable effect estimation, for example: the Lowering the Risk of Operative Complications Using Atorvastatin Loading Dose (LOAD) trial studied 648 statin-naïve patients, of whom 24% had a history of CVD and 49% had diabetes.211 In this randomized, placebo-controlled trial, patients received a loading dose of atorvastatin 80 mg within 18 h before surgery followed by 40 mg daily for 7 days. Use of atorvastatin did not reduce the risk of major events (all-cause mortality, non-fatal MI, or stroke at 30 days [HR, 0.87; 95% CI, 0.60–1.26; P = 0.46]). However, the trial was underpowered to draw definite conclusions. In addition, several meta-analyses have shown ambiguous results and most studies are of a limited size with less than 100 patients.212,213
Therefore, routine peri-operative initiation of statin therapy is not recommended. However, in patients in whom statin use is already indicated, treatment should be considered peri-operatively, particularly in patients scheduled for high-risk surgery (e.g. vascular surgery).
5.2.4. Renin–angiotensin–aldosterone system inhibitors
Data on peri-operative use of renin–angiotensin–aldosterone system (RAAS) inhibitors are inconclusive. The majority of studies suggest that continued use of RAAS inhibitors is associated with a higher risk of peri-operative hypotension and, as a consequence, higher use of vasopressors and inotropes. Furthermore, intra-operative hypotension and its duration is associated with end-organ damage, including kidney injury, myocardial damage, and stroke.214 In a small trial of 275 subjects, randomized to either continuation of their angiotensin-converting-enzyme inhibitors (ACEIs) or omission of the final pre-operative ACEI dose, patients randomized to omission of the last dose before surgery experienced intra-operative hypotension less frequently (76/137 [55%] vs. 95/138 [69%]) and vasopressor use was less likely.215 On the other hand, post-operative hypertension was more frequent in the omission group. Furthermore, in an observational cohort study consisting of 4802 patients undergoing NCS and using an ACEI or angiotensin receptor blocker (ARB), discontinuation of these drugs in the 24 h before surgery was associated with a lower risk of intra-operative hypotension (adjusted RR, 0.80; 95% CI, 0.72–0.93; P < 0.001), and associated with a reduction in the composite end-point consisting of all-cause mortality, stroke, and MI (adjusted RR, 0.82; 95% CI, 0.70–0.96; P = 0.01);216 8% of the patients in this cohort were diagnosed with HF, in whom RAAS inhibitors are the cornerstone of medical therapy. A systematic review, including nine studies (five RCTs and four cohort studies), revealed that withholding ACEI/ARB on the morning of surgery was not associated with mortality or MACE;217 however, it did confirm that withholding therapy was associated with less intra-operative hypotension (OR, 0.63; 95% CI, 0.47–0.85). If an ACEI/ARB is withheld prior to NCS, it should be restarted as soon as possible in order to prevent unintended long-term omission. No data on peri-operative effects of angiotensin receptor neprilysin inhibitors (ARNI) exist, but hypotension is more common compared with patients on ACEI.218
Some important RCTs in this field are ongoing: the impact of renin–angiotensin system inhibitors continuation vs. discontinuation on outcome after major surgery trial STOPorNOT219 (NCT03374449), and the POISE-3 trial (NCT03505723) are both assessing a hypotension-avoidance strategy vs. a hypertension-avoidance strategy on the risk of vascular death and major vascular events in patients who are followed for 30 days after NCS.
5.2.5. Calcium channel blockers
The effects of calcium channel blockers (CCBs) on the balance between myocardial oxygen supply and demand makes them theoretically suitable for risk-reduction strategies. The relevance of randomized trials assessing the peri-operative effects of CCBs is limited by their small size, lack of risk stratification, and the absence of systematic reporting of cardiac death and MI. A meta-analysis pooled 11 randomized trials totalling 1007 patients.220 Treatment with CCBs significantly reduced the number of episodes of myocardial ischaemia and supraventricular tachycardia (SVT) in the pooled analyses. However, the decrease in mortality and MI reached statistical significance only when both end-points were combined (RR, 0.35; 95% CI, 0.08–0.83; P = 0.02). In contrast, a matched case-control study of 1000 patients undergoing acute or elective aortic aneurysm surgery suggested that dihydropyridine use was independently associated with an increased incidence of peri-operative mortality.221 These observational data may be biased by the indications for the use of CCBs. In patients already on CCBs, particularly in those with vasospastic angina, it is recommended to continue CCBs during the peri-operative period, but withholding the dose on the day of surgery in order to avoid post-operative hypotension.
5.2.6. Alpha-2 receptor agonists
Alpha-2 receptor agonists reduce post-ganglionic noradrenaline output and might therefore reduce catecholamine surge during surgery. The European Mivazerol trial randomized 1897 patients with IHD who underwent intermediate- or high-risk NCS.222 Mivazerol did not decrease the incidence of death or MI in the whole population. However, it did decrease the incidence of death in a subpopulation of 904 patients undergoing vascular surgery.222 The international Peri-Operative ISchemic Evaluation 2 (POISE-2) trial randomized 10 010 patients undergoing NCS to clonidine or placebo.223 Clonidine did not reduce the rate of death or non-fatal MI in general or in patients undergoing vascular surgery (RR, 1.08; 95% Cl, 0.93–1.26; P = 0.29), but it did increase the risk of clinically important hypotension (RR, 1.32; 95% Cl, 1.24–1.40; P < 0.001) and non-fatal cardiac arrest (RR, 3.20; 95% Cl, 1.17–8.73; P = 0.02).
5.2.7. Diuretics
Diuretics are frequently used in patients with hypertension or HF. In general, therapy for treatment of hypertension should be continued to the day of surgery and resumed orally when possible. However, the benefit for continuing diuretics as antihypertensive therapy is unclear, and alternative antihypertensive agents may be considered. In HF, the dosage of diuretics should be adjusted well in advance for an optimal fluid balance before surgery, and to avoid fluid retention or dehydration.
The possibility of electrolyte disturbance should be considered in any patient receiving diuretics. Hypokalaemia is reported to occur in up to 36% of patients undergoing surgery (mostly NCS).224,225 Special attention should be given to patients prone to developing arrhythmias. Any electrolyte disturbance, especially hypokalemia and hypomagnesaemia, should be corrected in due time before surgery. Acute pre-operative repletion in asymptomatic patients may be associated with more risks than benefits; thus, minor asymptomatic electrolyte disturbances should not delay acute surgery.
In the peri-operative period, volume status in patients with HF should be carefully monitored and optimized by loop diuretics or fluids. However, retrospective data suggest that intra-operative prescription of diuretics may increase the risk of acute kidney injury (AKI) after NCS.226
5.2.8. Ivabradine
Heart rate is an independent and modifiable risk factor for periprocedural MI (and maybe death) after NCS. Ivabradine is a negative chronotropic agent without associated hypotensive effect, and is therefore a possible alternative to beta-blockers. However, there are few studies about the value of ivabradine for high-risk patients undergoing NCS.227 The small (78 patients) PeRi-OperaTivE CardioproTection With Ivabradine in Non-cardiac Surgery (PROTECTIN) (NCT04436016) trial is ongoing.
5.2.9. Sodium–glucose co-transporter-2 inhibitors
The use of sodium–glucose co-transporter-2 (SGLT-2) inhibitors is increasing, due to proven CV benefits for patients with type-2 diabetes mellitus (DM) and a beneficial effect on outcomes for patients with HF and renal insufficiency. Euglycaemic diabetic ketoacidosis (EDKA) is a rare but serious complication. While the incidence was not significantly increased with SGLT-2 inhibitors in RCTs, several case reports indicate that EDKA may occasionally occur after (non-cardiac) surgery in patients on SGLT-2 inhibitors.228 A systematic review indicated that precipitating factors include diabetes medication changes, diet modifications, and intercurrent illnesses.229 The US Food and Drug Administration (FDA) recommends interrupting SGLT-2 inhibitor therapy for at least 3–4 days before scheduled surgery and to be vigilant for symptoms related to EDKA, prompting measurement of ketones.
5.3. Peri-operative handling of antithrombotic agents
Management of patients taking antithrombotic agents and needing surgery or an invasive procedure should consider patient- and procedure-related risk of bleeding and thrombosis. Furthermore, the pharmacokinetic and pharmacodynamic characteristics of the antithrombotic drugs in use must be considered (Tables 7 and 8). The risk of bleeding associated with different types of interventions is shown in Table 9. Risk estimation and decision-making in patients requiring long-term antithrombotic therapy is challenging, since relevant associations exist between peri-operative antithrombotic management, bleeding, thrombotic events (MI and stroke), and mortality.6,11–13 Thus, interdisciplinary risk assessment ahead of the intervention is crucial, in order to classify the patient-related ischaemic and bleeding risks (e.g. cardiologist, neurologist, vascular specialist, and haematologist), and the surgical risk (surgeon and anaesthesiologist). Information on timing of intervention by indicated duration of antithrombotic therapy should be communicated with the patient and treating general physician.
| . | ASA . | Clopidogrel . | Prasugrel . | Ticagrelor . | Cangrelor . | Eptifibatide . | Tirofiban . |
|---|---|---|---|---|---|---|---|
| Target (type of blockade) | COX-1 (irreversible) | P2Y12 (irreversible) | P2Y12 (irreversible) | P2Y12 (reversible) | P2Y12 (reversible) | GPIIB/IIIa (reversible) | GPIIB/IIIa (reversible) |
| Application | Oral | Oral | Oral | Oral | i.v. | i.v. | i.v. |
| Time to Cmax | 0.5–1.0 h | 2 h (after 600 mg LD)a | 0.5 h (after 60 mg LD)a | 0.5 h (after 180 mg LD)a | 2 min | 5 min | 5 min |
| Prodrug | No | Yes | Yes | No | No | No | No |
| Bioavailability (%) | ∼50 | ∼50 | 80 | 36 | 100 | 100 | 100 |
| Drug interactions | NSAIDs (in particular ibuprofen + naproxen) | CYP3A4, CYP3A5, or CYP2C19 inhibitors or inducers | CYP3A4/A5 and CYP2B6 inhibitor | CYP3A4 inducers or inhibitors | None | None | None |
| Plasma half-life | 20 min | 0.5–1 h (active metabolite) | 0.5–1 h (active metabolite) | 6–12 h | 3–6 min | 2.5–2.8 h | 1.2–2 h |
| Duration of action after last dose | 7–10 days | 3–10 daysb | 7–10 daysb | 3–5 days | 1–2 h | 4 h | 8 h |
| Renal clearance of the active metabolite (%) | NR | NR | NR | NR | 58 | ∼50 | 65 |
| Dose regimen | o.d. | o.d. | o.d. | b.i.d. | Bolus, infusion | Bolus, infusion | Bolus, infusion |
| . | ASA . | Clopidogrel . | Prasugrel . | Ticagrelor . | Cangrelor . | Eptifibatide . | Tirofiban . |
|---|---|---|---|---|---|---|---|
| Target (type of blockade) | COX-1 (irreversible) | P2Y12 (irreversible) | P2Y12 (irreversible) | P2Y12 (reversible) | P2Y12 (reversible) | GPIIB/IIIa (reversible) | GPIIB/IIIa (reversible) |
| Application | Oral | Oral | Oral | Oral | i.v. | i.v. | i.v. |
| Time to Cmax | 0.5–1.0 h | 2 h (after 600 mg LD)a | 0.5 h (after 60 mg LD)a | 0.5 h (after 180 mg LD)a | 2 min | 5 min | 5 min |
| Prodrug | No | Yes | Yes | No | No | No | No |
| Bioavailability (%) | ∼50 | ∼50 | 80 | 36 | 100 | 100 | 100 |
| Drug interactions | NSAIDs (in particular ibuprofen + naproxen) | CYP3A4, CYP3A5, or CYP2C19 inhibitors or inducers | CYP3A4/A5 and CYP2B6 inhibitor | CYP3A4 inducers or inhibitors | None | None | None |
| Plasma half-life | 20 min | 0.5–1 h (active metabolite) | 0.5–1 h (active metabolite) | 6–12 h | 3–6 min | 2.5–2.8 h | 1.2–2 h |
| Duration of action after last dose | 7–10 days | 3–10 daysb | 7–10 daysb | 3–5 days | 1–2 h | 4 h | 8 h |
| Renal clearance of the active metabolite (%) | NR | NR | NR | NR | 58 | ∼50 | 65 |
| Dose regimen | o.d. | o.d. | o.d. | b.i.d. | Bolus, infusion | Bolus, infusion | Bolus, infusion |
ASA, acetylsalicylic acid; b.i.d., twice a day; Cmax, maximum serum concentration; i.v., intravenous; LD, loading dose; NR, non-relevant; o.d., once a day.
Time to Cmax for may be delayed by 8 h or more following a dose of opiate.
Depending on response status.
| . | ASA . | Clopidogrel . | Prasugrel . | Ticagrelor . | Cangrelor . | Eptifibatide . | Tirofiban . |
|---|---|---|---|---|---|---|---|
| Target (type of blockade) | COX-1 (irreversible) | P2Y12 (irreversible) | P2Y12 (irreversible) | P2Y12 (reversible) | P2Y12 (reversible) | GPIIB/IIIa (reversible) | GPIIB/IIIa (reversible) |
| Application | Oral | Oral | Oral | Oral | i.v. | i.v. | i.v. |
| Time to Cmax | 0.5–1.0 h | 2 h (after 600 mg LD)a | 0.5 h (after 60 mg LD)a | 0.5 h (after 180 mg LD)a | 2 min | 5 min | 5 min |
| Prodrug | No | Yes | Yes | No | No | No | No |
| Bioavailability (%) | ∼50 | ∼50 | 80 | 36 | 100 | 100 | 100 |
| Drug interactions | NSAIDs (in particular ibuprofen + naproxen) | CYP3A4, CYP3A5, or CYP2C19 inhibitors or inducers | CYP3A4/A5 and CYP2B6 inhibitor | CYP3A4 inducers or inhibitors | None | None | None |
| Plasma half-life | 20 min | 0.5–1 h (active metabolite) | 0.5–1 h (active metabolite) | 6–12 h | 3–6 min | 2.5–2.8 h | 1.2–2 h |
| Duration of action after last dose | 7–10 days | 3–10 daysb | 7–10 daysb | 3–5 days | 1–2 h | 4 h | 8 h |
| Renal clearance of the active metabolite (%) | NR | NR | NR | NR | 58 | ∼50 | 65 |
| Dose regimen | o.d. | o.d. | o.d. | b.i.d. | Bolus, infusion | Bolus, infusion | Bolus, infusion |
| . | ASA . | Clopidogrel . | Prasugrel . | Ticagrelor . | Cangrelor . | Eptifibatide . | Tirofiban . |
|---|---|---|---|---|---|---|---|
| Target (type of blockade) | COX-1 (irreversible) | P2Y12 (irreversible) | P2Y12 (irreversible) | P2Y12 (reversible) | P2Y12 (reversible) | GPIIB/IIIa (reversible) | GPIIB/IIIa (reversible) |
| Application | Oral | Oral | Oral | Oral | i.v. | i.v. | i.v. |
| Time to Cmax | 0.5–1.0 h | 2 h (after 600 mg LD)a | 0.5 h (after 60 mg LD)a | 0.5 h (after 180 mg LD)a | 2 min | 5 min | 5 min |
| Prodrug | No | Yes | Yes | No | No | No | No |
| Bioavailability (%) | ∼50 | ∼50 | 80 | 36 | 100 | 100 | 100 |
| Drug interactions | NSAIDs (in particular ibuprofen + naproxen) | CYP3A4, CYP3A5, or CYP2C19 inhibitors or inducers | CYP3A4/A5 and CYP2B6 inhibitor | CYP3A4 inducers or inhibitors | None | None | None |
| Plasma half-life | 20 min | 0.5–1 h (active metabolite) | 0.5–1 h (active metabolite) | 6–12 h | 3–6 min | 2.5–2.8 h | 1.2–2 h |
| Duration of action after last dose | 7–10 days | 3–10 daysb | 7–10 daysb | 3–5 days | 1–2 h | 4 h | 8 h |
| Renal clearance of the active metabolite (%) | NR | NR | NR | NR | 58 | ∼50 | 65 |
| Dose regimen | o.d. | o.d. | o.d. | b.i.d. | Bolus, infusion | Bolus, infusion | Bolus, infusion |
ASA, acetylsalicylic acid; b.i.d., twice a day; Cmax, maximum serum concentration; i.v., intravenous; LD, loading dose; NR, non-relevant; o.d., once a day.
Time to Cmax for may be delayed by 8 h or more following a dose of opiate.
Depending on response status.
| . | Warfarin . | Phenprocoumon . | Apixaban . | Dabigatran . | Edoxaban . | Rivaroxaban . |
|---|---|---|---|---|---|---|
| Target (type of blockade) | VKORC1 | VKORC1 | FXa | FIIa | FXa | FXa |
| Application | Oral | Oral | Oral | Oral | Oral | Oral |
| Time to Cmax | 2–6 h | 1.52 h ± 1.52 | 3–4 h | 1.25–3 h | 1–2 h | 2–4 h |
| Prodrug | No | No | No | Yes | No | No |
| Bioavailability (%) | >95 | 100 | 50 | 6.5 | 62 | 80–100 |
| Drug interactions | CYP2C9, CYP2C19, CYP2C8, CYP2C18, CYP1A2, CYP3A4, vitamin K | CYP2C9, CYP2C8, vitamin K | CYP3A4 inhibitors or inductors, P-glycoprotein inhibitors or inductors | P-glycoprotein inhibitors or inductors | P-glycoprotein inhibitors | CYP3A4 inhibitors or inductors, P-glycoprotein inhibitors or inductors |
| Plasma half-life | 36–48 h | ∼100 h | 12 h | 12–14 h | 6–11 h | 7–11 h (11–13 h in the elderly) |
| Duration of action after last dose | ∼5 days | ∼7 days | 24 h | 24 h | 24 h | 24 h |
| Renal clearance of the active metabolite (%) | Non-renal | Non-renal | 27 | 85 | 37–50 | 33 |
| Dose regimen | Adjusted according to INR | Adjusted according to INR | b.i.d. | b.i.d. | o.d. | o.d./b.i.d. |
| . | Warfarin . | Phenprocoumon . | Apixaban . | Dabigatran . | Edoxaban . | Rivaroxaban . |
|---|---|---|---|---|---|---|
| Target (type of blockade) | VKORC1 | VKORC1 | FXa | FIIa | FXa | FXa |
| Application | Oral | Oral | Oral | Oral | Oral | Oral |
| Time to Cmax | 2–6 h | 1.52 h ± 1.52 | 3–4 h | 1.25–3 h | 1–2 h | 2–4 h |
| Prodrug | No | No | No | Yes | No | No |
| Bioavailability (%) | >95 | 100 | 50 | 6.5 | 62 | 80–100 |
| Drug interactions | CYP2C9, CYP2C19, CYP2C8, CYP2C18, CYP1A2, CYP3A4, vitamin K | CYP2C9, CYP2C8, vitamin K | CYP3A4 inhibitors or inductors, P-glycoprotein inhibitors or inductors | P-glycoprotein inhibitors or inductors | P-glycoprotein inhibitors | CYP3A4 inhibitors or inductors, P-glycoprotein inhibitors or inductors |
| Plasma half-life | 36–48 h | ∼100 h | 12 h | 12–14 h | 6–11 h | 7–11 h (11–13 h in the elderly) |
| Duration of action after last dose | ∼5 days | ∼7 days | 24 h | 24 h | 24 h | 24 h |
| Renal clearance of the active metabolite (%) | Non-renal | Non-renal | 27 | 85 | 37–50 | 33 |
| Dose regimen | Adjusted according to INR | Adjusted according to INR | b.i.d. | b.i.d. | o.d. | o.d./b.i.d. |
b.i.d., twice a day; Cmax, maximum serum concentration; FIIa, factor IIa; FXa, factor Xa; INR, International normalized ratio; LD, loading dose; NOAC, non-vitamin K antagonist oral anticoagulant; o.d., once a day; VKORC1, vitamin K epoxide reductase complex 1.
| . | Warfarin . | Phenprocoumon . | Apixaban . | Dabigatran . | Edoxaban . | Rivaroxaban . |
|---|---|---|---|---|---|---|
| Target (type of blockade) | VKORC1 | VKORC1 | FXa | FIIa | FXa | FXa |
| Application | Oral | Oral | Oral | Oral | Oral | Oral |
| Time to Cmax | 2–6 h | 1.52 h ± 1.52 | 3–4 h | 1.25–3 h | 1–2 h | 2–4 h |
| Prodrug | No | No | No | Yes | No | No |
| Bioavailability (%) | >95 | 100 | 50 | 6.5 | 62 | 80–100 |
| Drug interactions | CYP2C9, CYP2C19, CYP2C8, CYP2C18, CYP1A2, CYP3A4, vitamin K | CYP2C9, CYP2C8, vitamin K | CYP3A4 inhibitors or inductors, P-glycoprotein inhibitors or inductors | P-glycoprotein inhibitors or inductors | P-glycoprotein inhibitors | CYP3A4 inhibitors or inductors, P-glycoprotein inhibitors or inductors |
| Plasma half-life | 36–48 h | ∼100 h | 12 h | 12–14 h | 6–11 h | 7–11 h (11–13 h in the elderly) |
| Duration of action after last dose | ∼5 days | ∼7 days | 24 h | 24 h | 24 h | 24 h |
| Renal clearance of the active metabolite (%) | Non-renal | Non-renal | 27 | 85 | 37–50 | 33 |
| Dose regimen | Adjusted according to INR | Adjusted according to INR | b.i.d. | b.i.d. | o.d. | o.d./b.i.d. |
| . | Warfarin . | Phenprocoumon . | Apixaban . | Dabigatran . | Edoxaban . | Rivaroxaban . |
|---|---|---|---|---|---|---|
| Target (type of blockade) | VKORC1 | VKORC1 | FXa | FIIa | FXa | FXa |
| Application | Oral | Oral | Oral | Oral | Oral | Oral |
| Time to Cmax | 2–6 h | 1.52 h ± 1.52 | 3–4 h | 1.25–3 h | 1–2 h | 2–4 h |
| Prodrug | No | No | No | Yes | No | No |
| Bioavailability (%) | >95 | 100 | 50 | 6.5 | 62 | 80–100 |
| Drug interactions | CYP2C9, CYP2C19, CYP2C8, CYP2C18, CYP1A2, CYP3A4, vitamin K | CYP2C9, CYP2C8, vitamin K | CYP3A4 inhibitors or inductors, P-glycoprotein inhibitors or inductors | P-glycoprotein inhibitors or inductors | P-glycoprotein inhibitors | CYP3A4 inhibitors or inductors, P-glycoprotein inhibitors or inductors |
| Plasma half-life | 36–48 h | ∼100 h | 12 h | 12–14 h | 6–11 h | 7–11 h (11–13 h in the elderly) |
| Duration of action after last dose | ∼5 days | ∼7 days | 24 h | 24 h | 24 h | 24 h |
| Renal clearance of the active metabolite (%) | Non-renal | Non-renal | 27 | 85 | 37–50 | 33 |
| Dose regimen | Adjusted according to INR | Adjusted according to INR | b.i.d. | b.i.d. | o.d. | o.d./b.i.d. |
b.i.d., twice a day; Cmax, maximum serum concentration; FIIa, factor IIa; FXa, factor Xa; INR, International normalized ratio; LD, loading dose; NOAC, non-vitamin K antagonist oral anticoagulant; o.d., once a day; VKORC1, vitamin K epoxide reductase complex 1.
| Surgery with minor bleeding risk . | Surgery with low bleeding risk (infrequent or with low clinical impact) . | Surgery with high bleeding risk (frequent or with significant clinical impact) . |
|---|---|---|
|
|
|
| Surgery with minor bleeding risk . | Surgery with low bleeding risk (infrequent or with low clinical impact) . | Surgery with high bleeding risk (frequent or with significant clinical impact) . |
|---|---|---|
|
|
|
AAA, abdominal aortic aneurysm.
Adapted from Steffel et al.240
| Surgery with minor bleeding risk . | Surgery with low bleeding risk (infrequent or with low clinical impact) . | Surgery with high bleeding risk (frequent or with significant clinical impact) . |
|---|---|---|
|
|
|
| Surgery with minor bleeding risk . | Surgery with low bleeding risk (infrequent or with low clinical impact) . | Surgery with high bleeding risk (frequent or with significant clinical impact) . |
|---|---|---|
|
|
|
AAA, abdominal aortic aneurysm.
Adapted from Steffel et al.240
5.3.1. Antiplatelets
5.3.1.1. Single antiplatelet therapy
In patients taking aspirin for primary prevention, the risk of ischaemic events is low and aspirin can be withdrawn prior to NCS. Permanent discontinuation should be considered post-operatively in low- and moderate-risk atherosclerotic cardiovascular disease (ASCVD) risk patients and/or in patients with high bleeding risk based on negative/neutral trials and the recommendations for primary prevention of CVD in the 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice.40,241
Due to the better risk–benefit ratio, aspirin has an established role for the long-term prevention of new cardiovascular events in patients with established CVD.242 The POISE-2 trial is the largest, randomized, placebo-controlled trial of peri-operative aspirin in patients undergoing NCS.243 The trial randomized 10 010 patients undergoing NCS with established CVD, or who were at increased CV risk, to aspirin or placebo. Patients were stratified according to whether they had not been taking aspirin before the study or were already on aspirin; 33% of the patients had known vascular disease (23% CAD, 9% PAD, and 5% stroke). Aspirin did not reduce the rates of death or non-fatal MI at 30 days (7.0% vs. 7.1% in the placebo group [HR, 0.99; 95% CI, 0.86–1.15; P = 0.92]). Major bleeding was more common in the aspirin group than in the placebo group (4.6% vs. 3.8% [HR, 1.23; 95% CI, 1.01–1.49; P = 0.04]). The primary outcome results were similar, irrespective of whether or not patients had been taking aspirin before the study, and were also similar in patients with and without vascular disease.
In a post hoc analysis of 470 patients (<5%) who had undergone previous PCI, aspirin use was associated with a significant reduction in death or MI (HR, 0.50; 95% CI, 0.26–0.95; P = 0.036) and MI alone (HR, 0.44; 95% CI, 0.22–0.87; P = 0.021), while the risk of major or life-threatening bleeding was not significantly increased in this setting.244 Although the analysis carries several limitations, it supports the perception that the ischaemic benefit of peri-operative aspirin use outweighs the bleeding risk in patients with previous PCI. Thus, among patients with previous PCI, in the absence of a very high bleeding risk, low-dose aspirin should be continued during the peri-operative period.
In patients undergoing transcatheter aortic valve implantation (TAVI) who have no other indication for oral anticoagulant (OAC) therapy, low-dose aspirin has been recommended as standard therapy by recent guidelines based on an RCT.245,246 There are no randomized data available assessing the withdrawal vs. continuation of aspirin in patients after TAVI on aspirin alone undergoing NCS.
If the bleeding risk outweighs the potential CV benefit, aspirin should be discontinued. For patients with high peri-operative bleeding risk (e.g. undergoing spinal surgery or certain neurosurgical or ophthalmological operations) aspirin should be discontinued for at least 7 days.
On rare occasions, chronic coronary syndrome (CCS) patients might be on clopidogrel monotherapy due to the results of recent trials247 and the recommendations of the 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation;98 therefore, periprocedural management of clopidogrel-based single antiplatelet therapy (SAPT) is required. Consensus has been reached that a short interruption of P2Y12 inhibitor monotherapy is recommended in patients at high risk of bleeding.
Patients treated with P2Y12 inhibitor monotherapy as part of a de-escalation strategy after PCI/ACS, or due to a recent stroke, PAD, or aspirin intolerance, might require peri-operative management of this monotherapy.248–250 A careful interdisciplinary evaluation of peri-operative bleeding vs. ischaemic risk is warranted in these situations, and individual decisions based on the peri-operative bleeding and ischaemic risk (e.g. surgery under P2Y12 monotherapy, switching to aspirin, short interruption, or bridging in the peri-operative phase) may be applicable, although evidence for these different regimens is missing. It should be recognized that the effects of ticagrelor or clopidogrel monotherapy on haemostasis are considerably less than when they are combined with aspirin.
5.3.1.2. Dual antiplatelet therapy
P2Y12 inhibitors in addition to aspirin are recommended for patients after PCI.98,146 The frequency of major NCS in the first year after PCI is ∼4%; most frequently orthopaedic, abdominal, and vascular surgery.251 Other observational data report cumulative incidences of NCS after PCI for 30 days, 6 months, and 1 year of 1%, 5%, and 9%, respectively.252
Observational studies have reported a substantial rate of MACE—including cardiac death, MI, and stent thrombosis—ranging between 2–8%251,253,254 in PCI patients undergoing NCS, with a more than two-fold increased risk compared with non-stented patients.255,256 The proportion of risk attributable to underlying CVD or stent implantation remains uncertain.254 Risk factors for MACE after NCS are: time from PCI to surgery, with the highest risk in the first month; primary PCI for ST-segment elevation myocardial infarction (STEMI); dual antiplatelet therapy (DAPT) interruptions/discontinuation; and lesion characteristics, including ostial and distal lesions.252,257–259 Urgency of surgery is a further risk factor. The ESC/ESA classification of NCS is a validated tool with which to predict the impact of the type of surgery on MACE.16
A meta-analysis of observational data indicated that discontinuation of clopidogrel for at least 5 days reduced the risk of re-operation for major bleeding by 50%, without increasing the risk of MACE or death.260 Other observational data indicate an increase in MACE with brief DAPT interruptions.261 However, these non-randomized data may have been biased by the type and urgency of surgery.260 Of note, the prognosis of stent thrombosis appears to be worse than for de novo coronary occlusion (and depends on the site of stent deployment), and premature interruption of DAPT in patients with recent coronary stent implantation is the strongest predictor for stent thrombosis.
The preferred management of patients on DAPT due to PCI is to delay elective NCS until completion of the full course of DAPT (6 months after elective PCI and 12 months after ACS).98,146 However, several recent trials have indicated that shortening DAPT duration to 1–3 months after implantation of modern DES is associated with acceptable rates of MACE and stent thrombosis in low- and moderate-risk patients. Based on these newer data, it is recommended to delay time-sensitive NCS until a minimum of 1 month of DAPT treatment has been effectuated. In high-risk CV patients, for example due to an ACS, a DAPT duration of at least 3 months should be considered before time-sensitive NCS. See Figure 5 for the recommended duration of DAPT before time-sensitive NCS. Once the P2Y12 inhibitor has been discontinued, surgery should be performed while the patient is still on aspirin.
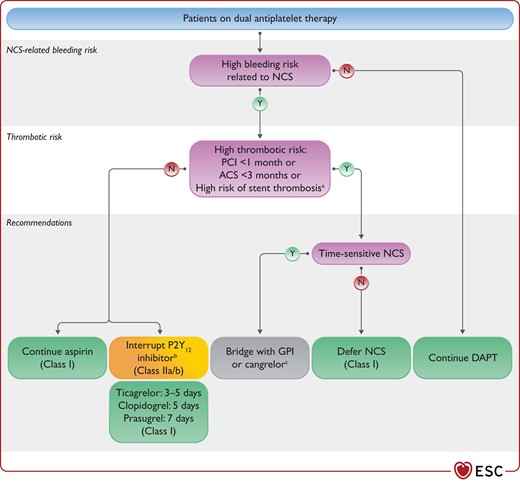
Recommendations for management of antiplatelet therapy in patients undergoing non-cardiac surgery.
ACS, acute coronary syndrome; DAPT, dual antiplatelet therapy; GPI, glycoprotein IIb/IIIa inhibitors; PCI, percutaneous coronary intervention; N, no; NCS, non-cardiac surgery. Y, yes; aHigh risk of peri-operative stent thrombosis defined by at least one of the following: history of stent thrombosis under antiplatelet therapy, reduced left ventricular ejection fraction (<40%), poorly controlled diabetes, severely impaired renal function/haemodialysis, recent complex PCI (i.e. severely calcified lesion, left main PCI, chronic total occlusion, bifurcational/crush technique, bypass graft PCI), or stent malapposition/residual dissection. bTiming of resumption after interdisciplinary risk assessment as soon as possible (within 48 h) after surgery. cFor dosing, see Figure 7.
Indications for long-term DAPT have recently emerged. Long-term DAPT (beyond 1 year) with clopidogrel, prasugrel, or ticagrelor in addition to aspirin should be considered in patients with high ischaemic risk, and may be considered in patients with moderate ischaemic risk, both in the absence of increased risk of major or life-threatening bleeding.98 When NCS is required, discontinuation of P2Y12 inhibitors is recommended for 3–7 days (depending on the P2Y12 inhibitor) for these additional indications for DAPT.
5.3.1.3. De-escalation of antiplatelet effect
The management of antiplatelet therapy in patients who have undergone recent PCI and are scheduled for NCS should be discussed by the surgeon and cardiologist, so that the balance between the risk of life-threatening surgical bleeding on antiplatelet therapy—best understood by the surgeon—and the risk of life-threatening stent thrombosis due to premature DAPT discontinuation—best understood by the cardiologist—can be considered (Figure 5 and Figure 6). An increased risk of MACE as a consequence of (major) bleeding also needs to be taken into consideration when balancing risk.
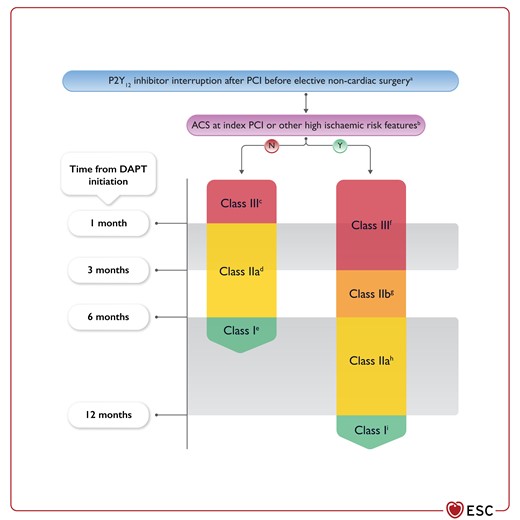
P2Y12 inhibitor interruption after percutaneous coronary intervention before elective non-cardiac surgery.
LoE, level of evidence; MI, myocardial infarction; N, no; PCI, percutaneous coronary intervention. Y, yes; aAvailability of 24 h cath-lab service is suggested in case of major surgery within 6 months in non-ACS/non-high-risk patients and within 12 months in ACS/high-risk patients. bHigh risk of peri-operative stent thrombosis defined by at least one of the following: history of recurrent MI, history of stent thrombosis under antiplatelet therapy, reduced left ventricular ejection fraction (<40%), poorly controlled diabetes, severely impaired renal function/haemodialysis, recent complex PCI (i.e. severely calcified lesion, left main PCI, chronic total occlusion, bifurcational/crush technique, bypass graft PCI), stent malapposition/residual dissection. cClass III LoE C. dClass IIa LoE B 250,265, 266,267. eClass I LoE A 268,146. fClass III LoE B 269. gClass IIb LoE B 270, 271. hClass IIa LoE B 272,273,274, 275, 276,277,278. iClass I LoE A 279, 280, 281, 98.
When time-sensitive surgery cannot be postponed and be performed with the recommended DAPT on board, de-escalation or shortening of DAPT is recommended. This may encompass either a switch from the more potent P2Y12 inhibitors prasugrel or ticagrelor to clopidogrel, or cessation of aspirin and use of prasugrel or ticagrelor monotherapy. If neither of these options is deemed to be sufficient, premature discontinuation of the P2Y12 inhibitor may be considered. If discontinuation is required, ticagrelor needs to be withheld for 3–5 days, clopidogrel for 5 days, and prasugrel for 7 days prior to surgery.262–264
Whenever possible, in patients with an indication for DAPT, surgery should be performed without discontinuation of aspirin. Aspirin might be discontinued as a last measure only with very high bleeding risk and a comparably low ischaemic risk. However, such surgical procedures should be performed in hospitals where 24/7 catheterization laboratories are available so as to treat patients immediately in case of peri-operative ischaemic events.
Although generally not recommended, bridging with i.v. compounds (eptifibatide/tirofiban or cangrelor) might be applicable in rare cases when DAPT cannot be interrupted before NCS (e.g. in patients with very high risk of stent thrombosis, history of recurrent MI, recent PCI) (see Figure 5 and Figure 7).282
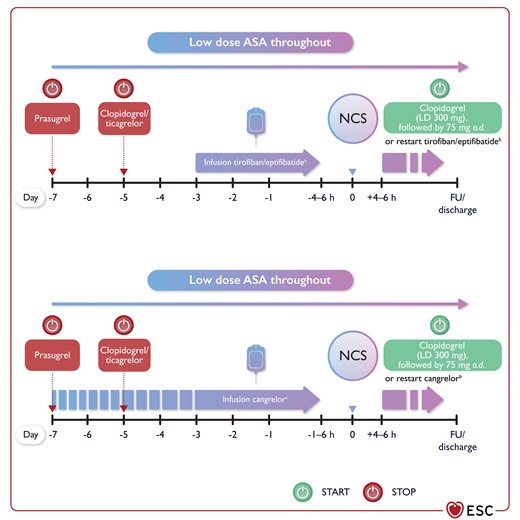
Bridging with intravenous antiplatelet agents. ASA, acetylsalicylic acid; FU, follow-up; LD, loading dose; NCS, non-cardiac surgery; o.d., once a day.
aTirofiban: 0.1 µg/kg/min; if creatinine clearance <50 mL/min, adjust to 0.05 µg/kg/min. Eptifibatide: 2.0 µ/kg/min; if creatinine clearance is <50 mL/min, adjust to 1.0 µg/kg/min. bUntil oral P2Y12 inhibitor therapy is possible. cInitiate within 72 h from P2Y12 inhibitor discontinuation at a dose of 0.75 μg/kg/min for a minimum of 48 h and a maximum of 7 days.
For patients receiving antiplatelet therapy, who have excessive or life-threatening peri-operative bleeding, transfusion of platelets is recommended as a bail-out strategy. However, ticagrelor and its active metabolite may also inhibit aggregation of transfused platelets. Experimental data indicate that administration of albumin binds ticagrelor and reduces its inhibitory effect on platelet aggregation.283 A monoclonal antibody fragment (PB2452) for neutralizing ticagrelor is in development but is not yet clinically available.284
5.3.1.4. Platelet function guided peri-operative management of antiplatelet therapy
Platelet function testing has several theoretical advantages in the peri-operative setting, including: (i) the identification of patients on antiplatelet therapy who are at increased risk of surgery-related bleeding; (ii) individualized timing of elective surgery after antiplatelet therapy cessation; and (iii) guiding therapy in bleeding complications.285–287 However, neither the optimal assay nor a universal cut-off value associated with bleeding has been defined and validated in patients undergoing NCS.
Recommendations for use of antiplatelet therapy in patients undergoing non-cardiac surgery
 |
 |
Recommendations for use of antiplatelet therapy in patients undergoing non-cardiac surgery
 |
 |
5.3.2. Oral anticoagulants
Approximately one in four patients taking anticoagulant therapy will require a surgical or invasive procedure within 2 years.290 Peri-operative management of oral anticoagulant (OAC) therapy depends on surgery- and patient-related factors and the specific OAC agent (vitamin K antagonist [VKA] or a non-vitamin K antagonist oral anticoagulant [NOAC]). See Figure 8 for a summary of recommendations for the management of OACs in patients undergoing NCS.
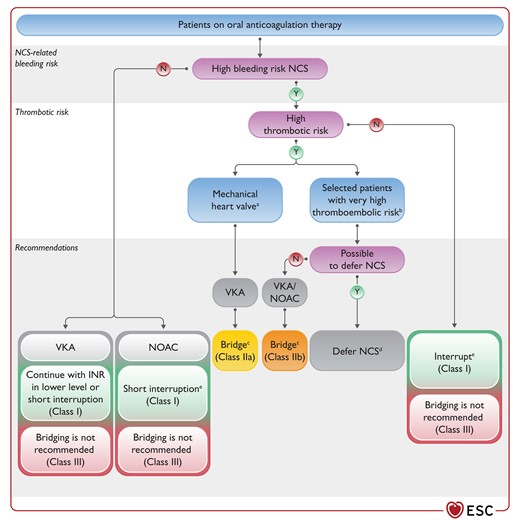
Recommendations for management of oral anticoagulation therapy in patients undergoing non-cardiac surgery.
CHA2DS2-VASc, congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, stroke, vascular disease, age 65–74 years, sex category (female); N, no; NCS, non-cardiac surgery; NOAC, non-vitamin K antagonist oral anticoagulant; VKA, vitamin K antagonist; VTE, venous thromboembolism. Y, yes; aMechanical aortic valve replacement (AVR) and any thromboembolic risk factor (atrial fibrillation, previous thromboembolism, severe left ventricular dysfunction, hypercoagulable state), or older-generation mechanical AVR, or a mechanical mitral valve replacement. bRecent stroke <3 months, high risk of VTE recurrences (e.g. antithrombin 3 deficiency or protein C and/or S deficiency), left ventricular apex thrombus, atrial fibrillation with a very high stroke risk. cBridging with unfractionated heparin or low molecular weight heparin. dE.g. >3 months after stroke/VTE. eFor NOAC management during NCS, see Figures 9 and 10.
Surgery-related factors include urgency of the intervention and the procedure-related bleeding risk (reflecting both the risk of bleeding occurrence and the risk of adverse outcome if bleeding occurs) (see Table 8). Procedures where mechanical compression is unfeasible carry a high risk of serious bleeding complications.
Patient-related factors include age, individual thrombotic risk, history of bleeding complications, renal function, concomitant medication, comorbidity, etc. Patients requiring a reversal agent need careful monitoring of haemostatic parameters and evaluation of thrombotic and bleeding risk in the peri-operative phase, as reversal might be insufficient or prothrombotic rebound might occur. In the latter case, an interdisciplinary decision should be made with regard to early resumption of anticoagulation treatment.
5.3.2.1. Vitamin K antagonists
Three drugs are currently used: warfarin (half-life 36–48 h), acenocoumarol (half-life 12 h), and phenprocoumon (half-life 100 h).
Vitamin K antagonists in patients with mechanical heart valves
Maintenance of therapeutic international normalized ratio (INR) is crucial for patients with mechanical heart valves (MHVs). Minor surgical procedures and procedures where bleeding is easily controlled can be performed without VKA interruption. The INR should be monitored and maintained at the lower level of the therapeutic range. Major surgical procedures needing INR ≤1.5 require VKA interruption, and heparin bridging should be considered. However, the evidence to support bridging therapy is limited and derived from cohort studies with poor or no comparator groups.291 Furthermore, the current-generation mechanical prosthetic valves in the aortic position are associated with a lower risk of thromboembolism compared with the older ones.291 Randomized controlled trials of bridging vs. no bridging therapy for patients with AF who do not have an MHV have shown higher risk of bleeding without a change in incidence of thromboembolic events, and increasing concerns have been raised that bridging therapy exposes patients to higher bleeding risks without reducing the risk of thromboembolism.292,293 The recently published PERI-OP trial compared bridging therapy vs. placebo in patients with either an MHV, AF, or atrial flutter who required interruption of OAC therapy for surgery, and found no significant benefit for post-operative dalteparin bridging to prevent major thromboembolism.294 The results were consistent for the AF (n = 1166) and MHVs groups (n = 350). Therefore, in patients with MHVs with a low risk of thromboembolism (e.g. mechanical bileaflet aortic valve in patients with sinus rhythm), bridging may not be needed. In patients with MHVs with a high risk of thromboembolism (mechanical aortic valve replacement [AVR] and any thromboembolic risk factor, or an older-generation mechanical AVR, or a mechanical mitral or tricuspid valve replacement), bridging with heparin should be considered during the peri-operative time interval when the INR is subtherapeutic (Figure 8). In all situations, the risks of bleeding should be weighed against the benefits of thromboembolism prevention.
Intravenous unfractionated heparin (UFH) is the only heparin treatment approved for bridging in patients with MHVs. Subcutaneous low molecular weight heparin (LMWH), although used off-label, has supplanted the use of UFH as a bridging therapy, owing to the lower incidence of thrombocytopenia, greater convenience, more predictable dose–response relationship, and significant cost saving resulting from outpatient administration. A meta-analysis of nine studies of 1042 patients with MHVs showed no differences between LMWH and UFH in the risks of thromboembolic events or major bleeding events.295 When LMWH is used, it should be given at a therapeutic dose twice a day and adjusted for renal impairment, when applicable. Anti-factor Xa (FXa) activity monitoring with target levels from 0.5–1.0 U/mL may be useful when the dosage may be difficult to determine (e.g. in patients with renal dysfunction or obesity). Vitamin K antagonist bridging strategies are shown in Supplementary data, Figure S2.
Vitamin K antagonists for atrial fibrillation/venous thromboembolism
In patients using VKA for AF or venous thromboembolism (VTE), invasive procedures with a low bleeding risk can be performed without VKA interruption.296–299 The INR should be monitored and maintained at the lower level of the therapeutic range. When interruption is necessary due to high bleeding risk procedures, the BRIDGE trial in AF patients showed that 3–5 days of warfarin interruption without bridging was superior to heparin bridging, having the same incidence of arterial and venous thromboembolism and significantly lower incidence of major bleeding.292
Bridging therapy may be considered for patients with a high thrombotic risk (i.e. AF with CHA2DS2-VASc (congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, stroke, vascular disease, age 65–74 years, sex category [female]) score >6, recent cardioembolic stroke <3 months, or high risk of VTE recurrence, weighing the risk of bleeding against the risk of thromboembolism291,294,300 (see Supplementary data, Figure S3).
Restarting vitamin K antagonists after invasive procedures or surgery
Patients who have interrupted VKA treatment before surgery should restart the OAC 12–24 h after the invasive procedure, if the bleeding is well-controlled and gastric and intestinal reabsorption have been re-established. The restarting dose should be the maintenance dose plus a boosting dose of 50% for 2 days. Patients managed with bridging therapy should start LMWH or UFH together with VKA 24 h after surgery, if the bleeding is well-controlled and maintained, until the INR has reached the therapeutic range. In patients undergoing surgery with a high bleeding risk, therapeutic dose LMWH should be delayed for 48–72 h after haemostasis has been secured.
Reversal of vitamin K antagonists
Reversal of VKA can be managed with vitamin K, prothrombin complex concentrates (PPCs), and plasma administration. Vitamin K (from 2–10 mg depending on the INR value) can be used orally, with a predictable reduction in INR in 18–24 h or i.v. (in 25–50 mL normal saline over 15–30 min) for more rapid INR reduction (4–6 h). It should be noted that coagulation factors can still be below normal despite INR normalization, which means that bleeding risk might not yet be normalized. In patients needing reversal for immediate major surgery, PPCs or plasma should be used. Four-factor PPCs are the preferred option301 and are dosed on the basis of INR and body weight (INR 2–4 at 25 U/kg, INR 4–6 at 35 U/kg, INR >6 at 50 U/kg, with a maximum dose of 5000 U at 100 kg of body weight). When four-factor PPCs are unavailable, three-factor PPCs or plasma may be used. Patients requiring a reversal agent need careful monitoring of haemostatic parameters and evaluation of thrombotic and bleeding risks in the peri-operative phase, as reversal might be insufficient or a prothrombotic rebound might occur. In the latter case, an interdisciplinary decision should be made with regard to early resumption of anticoagulation treatment.
5.3.2.2. Non-vitamin K antagonist oral anticoagulants
Four drugs are currently used: dabigatran (factor IIa inhibitor), apixaban, rivaroxaban, and edoxaban (FXa inhibitors). The pharmacokinetic and pharmacodynamic characteristics of these drugs are shown in Table 8.
Unplanned surgery in patients on non-vitamin K antagonist oral anticoagulants and reversal for emergency procedures
When an urgent surgical intervention is required, it is recommended that NOAC therapy is immediately interrupted. Peri-operative management of NOAC therapy in specific procedural settings and suggested strategies for potential reversal of NOAC anticoagulant effect are shown in Figures 9–1199,240,302 (see Supplementary data, Table S5).
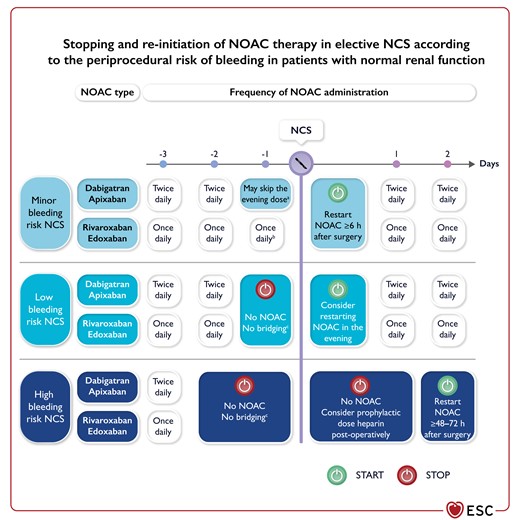
Peri-operative management of non-vitamin K antagonist oral anticoagulant according to the periprocedural risk of bleeding.
NCS, non-cardiac surgery; NOAC, non-vitamin K antagonist oral anticoagulant. aIn patients/circumstances favouring NOAC accumulation (e.g. renal dysfunction, older age, concomitant medication), the NOAC should be paused 12–24 h earlier. bIn patients on rivaroxaban or edoxaban taking the dose in the evening, the evening dose may be skipped. cNOACs have predictable weaning of the anticoagulant effect. Owing to the increase in bleeding risk associated with bridging, it is generally not recommended to use bridging in patients taking NOACs. Very few circumstances when bridging with heparin may be considered in patients taking a NOAC include high thromboembolic risk conditions, such as: 1) patients with a recent (within 3 months) thromboembolic event (stroke, systemic embolism, or VTE); 2) patients who experienced a thromboembolic event during previous interruption of NOAC therapy.
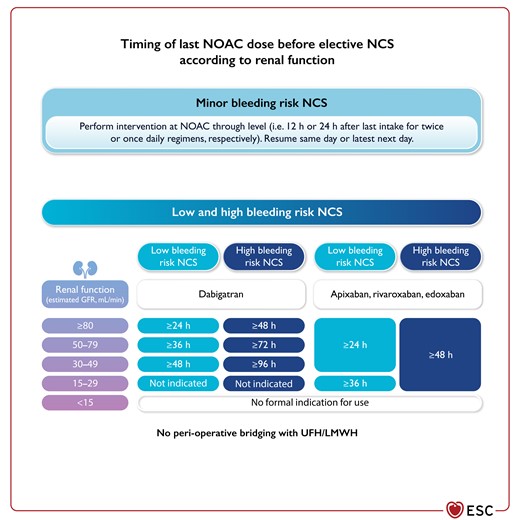
Timing of last non-vitamin K antagonist oral anticoagulant dose before elective NCS according to renal function.
GFR, glomerular filtration rate; LMWH, low molecular weight heparin; NCS, non-cardiac surgery; NOAC, non-vitamin K antagonist oral anticoagulant; UFH, unfractionated heparin.
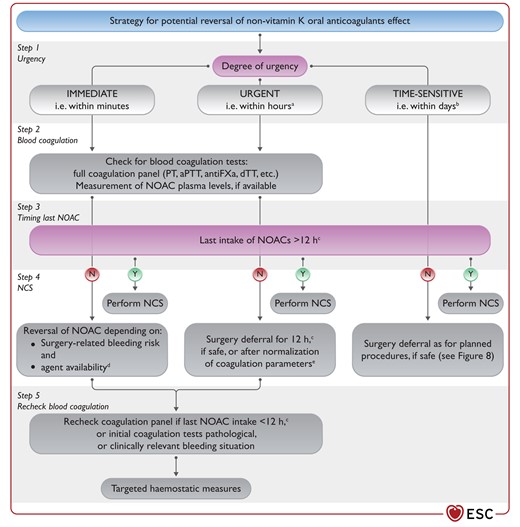
Suggested strategy for potential reversal of non-vitamin K oral anticoagulants effect.
aPTT, activated partial thromboplastin time; dTT, diluted thrombin time; FXa, factor Xa; N, no; NOAC, non-vitamin K antagonist oral anticoagulant; PT, prothrombin time; UFH, unfractionated heparin. Y, yes; aConditions that are potentially life-threatening or that may threaten the survival of limb or organ. bConditions that can be managed and procedure delayed for several days. c>24 h in case of significantly reduced renal function (i.e. eGFR <50 mL/min). dIf specific reversal agent is unavailable, consider non-specific haemostatic agents (prothrombin complex concentrate [PCC] or activated PCC [aPCCs]). Idarucizumab has only been tested in patients undergoing urgent surgery. Andexanet has not been tested in patients requiring urgent surgery. Andexanet binds all FXa inhibitors (including UFH) non-specifically. eUpon re-check.
Whereas the open-label prospective trial testing the specific reversal agent idarucizumab in patients on dabigatran enrolled participants experiencing acute major bleeding or requiring urgent surgical intervention,303 the trial with the reversal agent andexanet alpha for FXa inhibitors included only patients with acute major bleeding under therapy, but not those requiring urgent surgery.304 However, the off-label use of andexanet alpha in life-threatening situations requiring an immediate intervention may be considered, bearing in mind that andexanet alpha non-specifically binds all FXa inhibitors, which may have important implications for further treatment, including the administration of UFH or LMWH.240 When specific reversal agents are unavailable, prothrombin complex concentrate (PCC) or activated PCC should be considered, although there is a lack of evidence on their efficacy and safety for emergency procedures in patients taking a NOAC.290,305 Performing immediate or urgent surgery under general rather than spinal anaesthesia is prudent, in order to reduce the risk of epidural haematoma.
Prior to unplanned surgery, the full panel of coagulation blood tests (see Supplementary data, Table S6) should be obtained in order to assess the patient’s coagulation status. The indication for reversal (and/or non-specific haemostatic) agents is primarily governed by the patient’s clinical presentation, but initial assessment of coagulation status may have important implications for treatment in the next few hours or days. Specific coagulation tests such as diluted thrombin time (dTT) or ecarin clotting assay for dabigatran and antiFXa chromogenic assays for FXa inhibitors, and the assessment of NOAC plasma levels, may help in interpreting routine coagulation tests and waning of anticoagulant effect.240
Planned interventions in patients on non-vitamin K oral anticoagulants
Invasive surgical interventions may require temporary discontinuation of NOAC therapy, while many less-invasive procedures with a relatively low risk of bleeding may be performed under minimally interrupted or uninterrupted NOAC therapy (Figure 9).240
Bridging
In patients taking a NOAC, peri-operative bridging using heparin or LMWH was associated with increased risk of bleeding without reduction in thromboembolic events.290,306–308 Therefore, when NOAC interruption is required for surgery, bridging is not recommended, except in a few high thrombotic risk circumstances (see Figure 9). However, post-operative thromboprophylaxis with LMWH should be considered in patients in whom NOAC therapy cannot be quickly restarted. In patients receiving bridging with LMWH, monitoring of antiFXa activity and dose adjustment to a target level of 0.5–1.0 U/mL may be considered.
Laboratory testing before surgery
Pre-operative assessment of anticoagulation status in patients on a NOAC undergoing planned surgery provides a direct assessment of residual drug concentration. Shorter NOAC interruption time intervals in patients undergoing low-risk procedures may result in mildly or moderately elevated NOAC levels, as seen in the Perioperative Anticoagulant Use for Surgery Evaluation (PAUSE) trial,309 whereas creatinine clearance <50 mL/min, standard NOAC dose (compared with reduced dose), body weight <70 kg, and female sex were associated with elevated NOAC levels among patients undergoing high-risk surgery. The use of amiodarone, verapamil, or diltiazem was also associated with elevated pre-operative NOAC levels in the prospective Per-procedural Concentration of Direct Oral Anticoagulants (CORIDA) trial.310 Importantly, elevated NOAC levels were not found to be independently associated with bleeding complications.309,310
The evidence base for modifying the duration of pre-operative NOAC interruption time intervals according to residual NOAC plasma levels is unavailable, and ‘safe’ plasma levels of NOACs for different procedures are largely unknown. The time-based NOAC interruption (Figure 9) appears safe in most patients undergoing surgery.311,312 When NOACs are interrupted for >72 h, the likelihood of any residual NOAC plasma levels is very low.309,310
Considerations for specific procedures
Before interventions that carry a very high risk of bleeding—such as spinal or epidural anaesthesia, or lumbar puncture requiring intact haemostasis---interruption of NOACs for up to five half-lives (i.e. 3 days for FXa inhibitors or 4–5 days for dabigatran) should be considered, whereas NOACs can usually be restarted 24 h after the intervention.313,314
Dental procedures are generally considered to be associated with minor bleeding risk, and adequate local haemostasis is usually easily achieved. Hence, most dental procedures can be performed in an outpatient setting, with uninterrupted NOAC (or a single skipped dose) and using specific local haemostatic measures (such as the application of oxidized cellulose or absorbable gelatine sponge, sutures, tranexamic acid mouthwash or compressive gauze). Most professional statements on dental surgery advise uninterrupted NOAC, but these recommendations are mostly based on expert consensus, while some studies are currently ongoing.315–317
When to restart non-vitamin K antagonist oral anticoagulants after interventions
In general, NOAC can be restarted 6–8 h after interventions with rapid and complete haemostasis. When the bleeding risk with full-dose anticoagulation resumption outweighs the risk of thromboembolic events, therapeutic anticoagulation may be postponed to >48–72 h after the procedure, using prophylactic post-operative thromboprophylaxis until the resumption of full-dose NOAC is deemed safe (Figure 9).240 Post-operative heparin administration should also be considered in patients unable to take oral therapy. Off-label use of reduced-dose NOACs to attenuate the risk of post-operative bleeding is not recommended, as there is no evidence informing such an approach.
5.3.2.3. Combination therapy (antiplatelet and anticoagulant)
In general, according to the 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS), dual antithrombotic therapy should be adopted in most patients with AF and a recent PCI.99 Elective surgery should be postponed until the period when antiplatelet therapy can be safely discontinued in combination therapy (6 months after elective PCI or 12 months after ACS).268 Peri-operative handling of NOACs should follow the above recommendations (Figures 9 and 10). In urgent/emergency surgery with high bleeding risk, operative measures to reduce bleeding and/or reversal strategies of anticoagulation might be applied. In patients receiving combination therapy for other indications (e.g. TAVI and AF), according to recent trial results, antiplatelet therapy can be safely discontinued before NCS.318 In patients receiving low-dose OACs as part of a vascular protection strategy, rivaroxaban should be paused for at least 24 h before surgery and resumed according to the post-operative bleeding risk.
Recommendations for interruption and resumption of anticoagulants in patients undergoing non-cardiac surgery
 |
 |
 |
 |
Recommendations for interruption and resumption of anticoagulants in patients undergoing non-cardiac surgery
 |
 |
 |
 |
5.4. Peri-operative thromboprophylaxis
Trends show that the case-fatality of peri-operative VTE has declined over the past few decades.319,320 Its causal relationship with preventable mortality has been challenged by a recent meta-analysis.321 Thus, peri-operative VTE should be regarded as a marker of increased mortality risk rather than a causal factor. Careful pre-operative assessment is essential to identify patients with increased VTE risk who might benefit from peri-operative thromboprophylaxis. Procedure-related (e.g. type of surgery and likelihood of post-operative immobilization) and patient-related factors contribute to the risk of VTE. For non-orthopaedic surgical patients at low risk of VTE, mechanical methods of VTE prophylaxis (graduated compression stockings, intermittent pneumatic compression, or venous foot pump) rather than pharmacologic prophylaxis or no prophylaxis are recommended. Patients with CV disease (e.g. patients with recent MI or HF) have increased risk of peri-operative VTE.322 The Caprini score has been developed for risk stratification,323 and validated in different surgical settings (see Supplementary data, Table S7).324–327
Thromboprophylaxis should be considered for patients with moderate (i.e. 5–8 points) and high scores (i.e. ≥9 points). Thromboprophylaxis should be initiated during the hospital stay until 12 h before NCS and continued post-operatively based on individual risk assessment for bleeding. In most cases, thromboprophylaxis should be continued until the patient becomes fully mobilized or until hospital discharge (usually up to 10 days). Extended pharmacological VTE prophylaxis beyond discharge is not routinely recommended in most non-orthopaedic surgical patients. Although there are insufficient data regarding thromboprophylaxis after cancer surgery (particularly major abdominal and/or pelvic surgery for cancer), the consensus is to extend treatment duration with preferred use of LMWH for 3–4 weeks. Decisions on prophylaxis in populations for which the Caprini score has not been validated (such as orthopaedic surgery) should be based on individual and procedure-specific risk factors. Among those, a previous VTE is the strongest risk predictor (see Supplementary data, Table S7).328 For special situations and populations (e.g. neurosurgery, elderly, obese), please refer to available specific practice guidelines.329–332
Large phase 3 and phase 4 studies comparing NOACs with LMWH have shown similar results regarding efficacy and safety after major orthopaedic surgery.333 The usual time period for thromboprophylaxis after total knee and hip arthroplasty was up to 14 days and 35 days in RCTs, respectively,334–339 but large-scale data suggest safety of foreshortening the duration restricted to the hospital stay after fast-track surgery.340 Recent practice guidelines and a meta-analysis suggest a rationale for the use of aspirin as thromboprophylaxis in modern elective total hip and knee arthroplasty.341,342 However, there is a need for more adequately powered clinical trials with appropriate end-points comparing aspirin with other pharmacological methods. Aspirin should not be used as the sole initial agent for VTE prophylaxis, but switching to aspirin following a short course (e.g. 5 days) of rivaroxaban may be suitable for selected low-risk patients.343 It is recommended to implement patient care programmes—including post-operative mobilization, electronic prophylaxis recommendations, and teaching sessions regarding daily use of thromboprophylaxis—as these have been shown to be beneficial in reducing the risk of post-operative VTE complications.344
5.5. Patient blood management
Major surgery is associated with a high risk of peri-operative blood loss. Preferred treatment of acute anaemia related to peri-operative blood loss is transfusion of allogenic blood products. However, a large body of evidence indicates that inappropriate transfusion of red blood cells (RBCs) may be associated with inherent complications and impaired surgical outcome. Therefore, it is important to identify at-risk patients pre-operatively and manage peri-operative bleeding in any patients undergoing major surgery.
A hallmark study including >200 000 major surgical patients showed that even mild anaemia significantly increased the risk of morbidity—including respiratory, urinary, wound, septic, and thromboembolic complications—and mortality across all age groups.345 Moreover, Baron and colleagues analysed >39 000 surgical patients and showed that anaemia was significantly associated with increased mortality rate, hospital length of stay, and post-operative admission to intensive care.346 Up to 48% of surgical patients suffer from anaemia, and therefore anaemia should be considered to be a risk factor any time during hospitalization.347 Von Heymann and colleagues analysed 4494 cardiac surgical patients and showed that pre-operative anaemia and intra-operative transfusion were independently associated with decreased long-term survival.348 In addition, long-term survival was decreased by 50% in anaemic patients receiving blood transfusion compared with those without blood transfusion.
Anaemia may contribute to myocardial ischaemia, particularly if CAD is present. Iron deficiency (ID) is the underlying cause of anaemia in ∼50% of all cases.347 It was recently shown that ID was associated with increased risk of 90 day mortality both in patients with (4–14%) and without (2–5%) anaemia.349 In addition, the incidence of serious adverse events, major cardiac and cerebrovascular events, allogenic blood transfusion requirements, and length of stay were increased in patients with ID.
Based on the possibility of preserving the patients’ own blood resources and to enable safe handling of donor blood, the World Health Assembly (WHA) has endorsed the Patient Blood Management (PBM) approach (WHA63.12). Patient Blood Management is a patient-centred and multidisciplinary approach to manage anaemia, minimize iatrogenic blood loss and bleeding, and harness tolerance to anaemia in an effort to improve patient outcome.350–355 A comprehensive PBM programme addressing all three PBM pillars was associated with reduced transfusion need of RBC units, and lower complication and mortality rates.350
5.5.1. Pre-operative anaemia—diagnosis and treatment
A serum ferritin level <30 ng/mL, transferrin saturation <20%, and/or microcytic hypochromic red cells (mean corpuscular volume <80 fl, mean corpuscular haemoglobin <27 g/dL) are indicative of ID. In the presence of inflammation or transferrin saturation <20%, a ferritin level of <100 ng/mL points to functional ID (iron sequestration) (Table 10).
Laboratory parameters for the diagnosis of absolute iron-deficiency anaemia
| Parameter . | Normal . | Iron deficiency . |
|---|---|---|
| Mean corpuscular haemoglobin (g/dL) | 28–33 | <27 |
| Mean cellular volume (fL) | 80–96 | <80 |
| Transferrin saturation (%) | 16–45 | <20 |
| Ferritin (ng/mL) | 18–360 | <30a |
| Reticulocytes haemoglobin (ng/mL) | 18–360 | <30 |
| Parameter . | Normal . | Iron deficiency . |
|---|---|---|
| Mean corpuscular haemoglobin (g/dL) | 28–33 | <27 |
| Mean cellular volume (fL) | 80–96 | <80 |
| Transferrin saturation (%) | 16–45 | <20 |
| Ferritin (ng/mL) | 18–360 | <30a |
| Reticulocytes haemoglobin (ng/mL) | 18–360 | <30 |
In cases of chronic kidney disease, chronic heart failure or infections, iron deficiency is diagnosed with ferritin level <100 ng/mL or transferrin saturation <20%.358
Laboratory parameters for the diagnosis of absolute iron-deficiency anaemia
| Parameter . | Normal . | Iron deficiency . |
|---|---|---|
| Mean corpuscular haemoglobin (g/dL) | 28–33 | <27 |
| Mean cellular volume (fL) | 80–96 | <80 |
| Transferrin saturation (%) | 16–45 | <20 |
| Ferritin (ng/mL) | 18–360 | <30a |
| Reticulocytes haemoglobin (ng/mL) | 18–360 | <30 |
| Parameter . | Normal . | Iron deficiency . |
|---|---|---|
| Mean corpuscular haemoglobin (g/dL) | 28–33 | <27 |
| Mean cellular volume (fL) | 80–96 | <80 |
| Transferrin saturation (%) | 16–45 | <20 |
| Ferritin (ng/mL) | 18–360 | <30a |
| Reticulocytes haemoglobin (ng/mL) | 18–360 | <30 |
In cases of chronic kidney disease, chronic heart failure or infections, iron deficiency is diagnosed with ferritin level <100 ng/mL or transferrin saturation <20%.358
Apart from compromised bone marrow function, most types of anaemia are correctable within a period of 2–4 weeks. Oral and i.v. iron therapy can be used to treat ID. Intravenous iron products consist of an iron core embedded in a carbohydrate shell, which influences the stability of the drug, for example: iron sucrose comprises a less stable shell, allowing a maximum dose of 200 mg per infusion, whereas ferric carboxymaltose, ferric derisomaltose, and ferumoxytol have a stable shell associated with slow iron release and allowing a higher dose. Administration of i.v. iron has been shown to effectively reverse anaemia in ID patients.356,357
Intravenous iron is efficacious and safe359 and should be used in patients in whom oral iron is not tolerated, or if surgery is planned in short notice after the diagnosis of ID. A prospective observational study of 1728 major surgical patients showed that the prevalence of ID was 50%, 46.3%, and 52.7% in patients with haemoglobin <8.0, 8.0–8.9, and 9.0–9.9 g/dL, respectively.357 Furthermore, all iron supplemented iron-deficient anaemic patients required fewer RBC transfusions during the post-operative period, and a reduced intra-operative transfusion rate was observed if iron was supplemented >7 days before surgery. In addition, the length of stay was significantly reduced by 2.8 days for iron supplemented patients. In the recent PREVENTT trial studying patients with anaemia undergoing major abdominal surgery, pre-operative iron transfusion failed to improve outcomes;360 however, due to a fault in the study design, all anaemic patients received i.v. iron but not all (∼50–70%) were suffering from ID.
Recombinant human erythropoietin (rHuEPO) has frequently been used together with iron supplementation to increase pre-operative haemoglobin concentrations. A recent Cochrane review found that the administration of rHuEPO + iron to anaemic patients prior to NCS, compared with control treatment, reduced the need for RBC transfusion and increased the haemoglobin concentration pre-operatively; however, there were no important differences in the risk of adverse events or mortality within 30 days, or in length of hospital stay.361 Well-designed, adequately powered RCTs are required to more precisely estimate the impact of this combined treatment.
Pre-operative management of patients with anaemia can be simplified by making use of standard operating procedures or algorithms in which thresholds for diagnosis and treatment are depicted.362 An example of such an algorithm can be found in the PBM programme363 (see Supplementary data, Figure S4) and in the British Committee for Standards in Haematology (BCSH) Guidelines on the Identification and Management of Pre-Operative Anaemia.364
Recommendations for intra- and post-operative complications associated with anaemia
 |
 |
Recommendations for intra- and post-operative complications associated with anaemia
 |
 |
5.5.2. Bleeding and reduction of iatrogenic diagnostic/surgery-related blood loss
Blood loss associated with laboratory testing can either cause or aggravate hospital-acquired anaemia, which is associated with increased length of stay and complications. In 1867 patients undergoing cardiac surgery, an average of 115 tests per patient were performed, with a cumulative median volume of 454 mL.365 A reduction in blood drawn for laboratory analyses can be achieved by lowering the frequency of sampling and using paediatric-size collection tube sizes, for example. To decrease blood loss, blood-saving bundles could be used (e.g. a closed-loop arterial blood sampling system, smaller sampling tubes, reduction of frequency of blood drawings, and sample numbers). Such a strategy decreased mean blood loss per intensive care unit (ICU) day from 43.3 mL to 15.0 mL (P < 0.001),366 mainly due to the introduction of closed-loop arterial blood sampling systems. In addition, units of transfused RBCs per 100 observation days decreased from 7 to 2.3 (P < 0.001).366
A reduction in surgery-related blood loss starts from the pre-operative stage, with appropriate cessation strategies for anticoagulation and antiplatelet therapy. Intra-operative approaches to avoid blood loss include: (i) advanced anaesthetic; (ii) advanced surgical techniques with meticulous haemostasis, such as minimally invasive surgery and laparoscopic surgery; (iii) judicious use of diathermy dissection; (iv) physician’s mindfulness regarding limiting blood loss; and (v) application of topical haemostatic agents.367–369
Adequate coagulation management to minimize blood loss needs to be a pre-condition before RBC transfusion is considered. In this respect, the use of a coagulation algorithm is recommended,370,371 encompassing pre-operative assessment,372 and ensuring basic conditions for haemostasis, reversal of anticoagulants, point-of-care diagnostics in bleeding patients, and optimized coagulation management with the use of clotting factor concentrates.373,374
Tranexamic acid is an antifibrinolytic agent that is widely used for prophylaxis and treatment of bleeding caused by hyperfibrinolysis. A meta-analysis including 129 trials encompassing more than 10 000 patients to assess the effect of tranexamic acid on blood transfusion showed that administration of tranexamic acid reduced allogeneic blood transfusion by 38% (P < 0.001).375 In the recent POISE-3 study, 9535 patients undergoing NCS were randomized to tranexamic acid (1 g i.v. bolus) or placebo at the start and end of surgery. The incidence of the primary efficacy outcome (composite bleeding outcome) at 30 days was significantly lower with tranexamic acid than with placebo (HR, 0.76; 95% CI, 0.67–0.87).376 With respect to the primary safety outcome (composite CV outcome), the results did not meet the non-inferiority criteria (HR, 1.02; 95% CI, 0.92–1.14; P = 0.04 for non-inferiority).
The use of (washed) cell recovery is highly recommended in surgical settings where blood loss is routinely or anticipated to be >500 mL, as it reduces the rate of exposure to allogeneic RBCs, risk of infection, and length of stay. A meta-analysis including 47 trials encompassing 3433 patients of all surgical disciplines showed that the use of washed cell salvage reduced the rate of exposure to allogeneic RBC transfusion by 39% (P < 0.001), risk of infection by 28% (P = 0.03), and length of stay by 2.31 days (P < 0.001).377
Recommendations for intra- and post-operative complications associated with blood loss
 |
 |
 |
 |
Recommendations for intra- and post-operative complications associated with blood loss
 |
 |
 |
 |
5.5.3. Optimal blood component use with patient-centred clinical decision support
In order to optimize utilization of allogeneic blood products and to ensure guideline-adherent transfusion strategies, computerized physician order-entry systems should be considered.390,391 For example, Kaserer and colleagues evaluated the effectiveness of a monitoring and feedback programme and compared transfusion rates of >210 000 patients before and after implementation;392 overall, transfusion of RBCs was reduced by 40%.
Informed consent should be obtained from patients prior to transfusion of allogeneic blood products. It is necessary to effectively communicate the risks and benefits of the various potential interventions to the patient. It may further be recommended that any transfusion of allogeneic blood products should be mentioned in the discharge summary. In addition, the patient’s own preferences and values should be considered when developing a medical plan.
Recommendations for intra- and post-operative complications associated with allogeneic blood transfusion
 |
 |
Recommendations for intra- and post-operative complications associated with allogeneic blood transfusion
 |
 |
6. Specific diseases
Patients with CVD have an increased risk of peri-operative CV complications.45 Both the risk of complications and the peri-operative management depend on the specific type of CVD.
6.1. Coronary artery disease
6.1.1. Risk for patients with coronary artery disease
The peri-operative risk of CV complications for patients with established CAD depends on the baseline CV risk, type of surgery, and degree of urgency of the NCS. Older patients have a higher risk than younger patients, and patients with a recent ACS have a higher risk than those with CCS. The presence of comorbidities may also influence the risk.
6.1.2. Pre-operative risk assessment and management
The diagnostic evaluation and pre-operative management of patients with CCS undergoing NCS are outlined in Section 4. In particular, the value of CCTA and ICA are discussed in Sections 4.5.3.1. and 4.5.3.2.
In patients in need of immediate NCS, the operation must be performed without further delay and the time for pre-operative assessment is limited.
In patients scheduled for elective NCS who present with an ACS, the management of ACS should follow the guidelines for ACS patients in the non-surgical setting.98,171 In such settings, it would be reasonable to consider treating the culprit lesion only before NCS. Potential changes in timing of surgery and the peri-operative management (e.g. type of surgery, anaesthesia, medical therapy, and peri-operative monitoring) should be considered.
In patients with known CAD, it is recommended to collect information regarding previous invasive and non-invasive diagnostic examinations, and therapeutic interventions for CAD, within a sufficient time interval before the NCS, ideally at the time the proposal for NCS is made.
6.1.3. Revascularization strategies
The indication for coronary revascularization depends on the clinical presentation of CAD (ACS vs. CCS), urgency, and cardiac risk of NCS. In general, there is clear evidence that routine revascularization improves outcomes in ACS patients and less support for such a strategy in patients with CCS. The process of decision-making related to revascularization in CCS should be individualized, in order to prioritize revascularization in case of involvement of a significant amount of ischaemic myocardium or refractory symptoms, while medical management is a valuable option in patients with less relevant CAD manifestations.
6.1.3.1. Chronic coronary syndromes
The rationale for coronary revascularization before NCS is to prevent peri-operative myocardial ischaemia leading to acute MI, haemodynamic instability, and arrhythmia. Data from autopsy studies following fatal peri-operative MI showed that more than two-thirds of the patients had significant left main or three-vessel disease.397 A retrospective registry based on the Coronary Artery Surgery Study (CASS) study found that coronary artery bypass graft (CABG) reduced the risk of peri-operative mortality and MI in patients undergoing major NCS, particularly in subjects with three-vessel disease and reduced left ventricular ejection fraction (LVEF).398 However, the evidence in support of routine prophylactic revascularization before NCS is based on relatively small clinical trials and retrospective registries unrepresentative of current clinical practice.
In the Coronary Artery Revascularization Prophylaxis (CARP) trial, 510 patients with CCS were randomized to either optimal medical therapy or coronary revascularization (surgical or percutaneous) before major vascular surgery.399 In this study, patients receiving coronary revascularization compared with those who were medically treated did not differ in terms of acute MI after 30 days (8.4% vs. 8.4%, respectively) and mortality after 2.7 years (22% vs. 23%, respectively). Of note, this study excluded patients with significant left main disease, while one-third of the patients presented with three-vessel disease.399 In another randomized trial, 426 patients without evidence of CAD and scheduled for CEA were randomized to either routine coronary angiography with provisional revascularization before CEA or CEA without previous coronary angiography. There were no significant differences between the treatment groups in all-cause mortality, acute MI, and stroke at 30 days.172 A subsequent meta-analysis including 3949 patients showed no clinical benefit associated with routine prophylactic revascularization before NCS.400 A recent retrospective analysis on 4414 patients undergoing total joint arthroplasty found that the risk of adverse CV events was increased in patients with CAD, regardless of coronary revascularization before surgery. However, in patients receiving coronary revascularization, the risk of adverse CV events decreased, as the interval between revascularization and total joint arthroplasty was >2 years.401
The lack of evidence in support of routine prophylactic revascularization in CCS does not preclude a decision-process based on individual risk–benefit assessment in patients with a significant amount of ischaemic myocardium (as in the case of left main disease) and/or with refractory symptoms. The International Study of Comparative Health Effectiveness with Medical and Invasive Approaches (ISCHEMIA) trial randomized 5179 patients with stable CAD and moderate or severe ischaemia to an initial invasive strategy (consisting of angiography plus revascularization, as appropriate) or to an initial strategy of medical therapy alone, with bail-out angiography if medical therapy failed.402 This trial did not find a significant difference in the primary composite end-point of death and MI between treatment groups. Interestingly, these neutral results were inapplicable to patients with severe left main disease, since these patients were excluded after a pre-randomization CCTA. It has yet to be demonstrated whether CCTA represents a valuable tool with which to select patients with stable CAD and/or moderate or severe ischaemia who might benefit from an initial invasive strategy. A large angiography-based registry of 9016 CCS patients with high-risk coronary anatomy (three-vessel disease with ≥70% stenosis in all three epicardial vessels or left main disease ≥50% stenosis) showed improved outcomes (all-cause mortality or MI) in patients undergoing revascularization (both for PCI or CABG) vs. conservative medical therapy (HR, 0.62; 95% CI, 0.58–0.66; P < 0.001).403
The 2018 ESC/EACTS Guidelines on myocardial revascularization and the 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes generally apply to this population of patients, as long as the NCS can be postponed long enough to allow safe discontinuation of DAPT.146,404 Similarly, the choice between PCI and CABG should follow the general rules outlined in the above-mentioned guidelines.268,404 The use of intravascular imaging for planning and optimization of PCI is encouraged.405,406
6.1.3.2. Acute coronary syndromes
There are no trials specifically addressing the strategy of revascularization in ACS patients scheduled for NCS. High- and very high-risk patients should be treated according to the 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation,98 with an early (<24 h) or immediate (<2 h) invasive strategy, respectively. In low-risk groups amenable to a selective invasive strategy, the decision-making should be consistent with the approach for CCS patients.
In a post hoc analyses of the HIP-ATTACK (HIP Fracture Accelerated Surgical TreaTment And Care tracK) trial, patients with increased baseline troponin before randomization showed lower risk of mortality with accelerated surgery (within 6 h from the diagnosis) vs. standard of care (HR, 0.38; 95% CI, 0.21–0.66).57
The selection of the type of revascularization (PCI or CABG) should be based on the coronary anatomy and complexity of atherosclerosis, and the presence of diabetes.268,404 When PCI is chosen, the use of a DES is recommended.407 In case of a life-threatening clinical condition requiring undeferrable NCS and concomitant ACS-STEMI with an indication for coronary revascularization, a minimalistic approach with plain balloon angioplasty and delayed stenting might be considered.408,409 Figure 12 shows a summary of diagnostic and therapeutic pathways in patients with CAD scheduled for NCS.
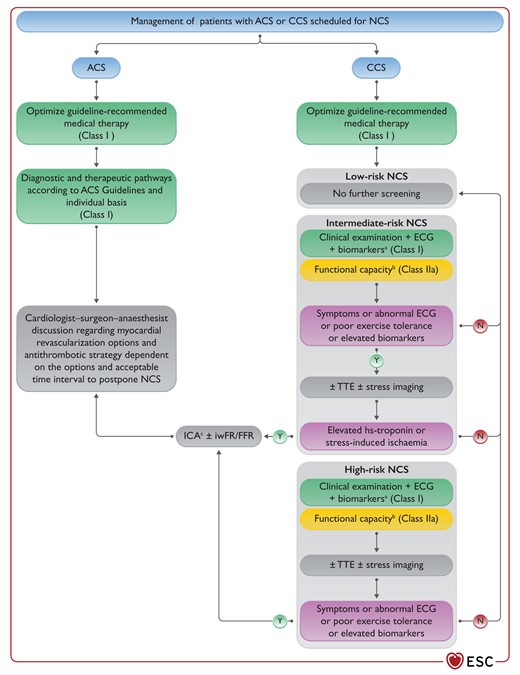
Management of patients with acute or chronic coronary syndrome scheduled for non-cardiac surgery.
ACS, acute coronary syndrome; BNP, B-type natriuretic peptide; CABG, coronary artery bypass graft; CAD, coronary artery disease; CCS, chronic coronary syndrome; ECG, electrocardiogram; FFR, fractional flow reserve; hs-cTn, high-sensitivity cardiac troponin; ICA, invasive coronary angiography; iwFR, instantaneous wave-free ratio; N, no; NCS, non-cardiac surgery; NT-proBNP, N-terminal proBNP; PCI, percutaneous coronary intervention; TTE, transthoracic echocardiography. Y, yes; The figure provides a schematic representation of diagnostic tools and therapy to be implemented according to surgical risk and underlying cardiac condition. aBiomarkers: hs-cTn T/I (Class I) ± BNP/NT-proBNP (Class IIa). bFunctional capacity based on Duke Activity Status Index (DASI) or the ability to climb two flights of stairs. cICA ± PCI/CABG on a case-by-case basis according to the Heart Team.
Recommendations for the timing of non-cardiac surgery and revascularization in patients with known coronary artery disease
 |
 |
Recommendations for the timing of non-cardiac surgery and revascularization in patients with known coronary artery disease
 |
 |
6.2. Chronic heart failure
6.2.1. Risk for patients with heart failure
Heart failure is an established risk factor for post-operative mortality across a broad range of surgical specialties.410–412 Several tools for risk calculations in patients undergoing NCS include HF as a predictor of adverse post-operative events.411,413
The risk of adverse post-operative events associated with HF depends on whether the LV systolic function is preserved or reduced, on the haemodynamic compensation, and on the presence of symptoms.414,415 In patients undergoing NCS, there is a risk of acute decompensated HF, with rapid onset or worsening of symptoms and/or signs of HF, precipitated by fluid accumulation and/or comorbid conditions.412
Patients with peri-operative acute or chronic HF are at increased risk of mortality during NCS. In a recently published analysis of 21 560 996 hospitalizations for NCS, the presence of any diagnosis of HF was associated with significantly higher in-hospital all-cause mortality compared with absence of HF (4.8% vs. 0.78%; adjusted OR, 2.15; 95% CI, 2.09–2.22).416 Among patients with a chronic HF diagnosis, peri-operative mortality was greater in those with acute exacerbation of chronic HF compared to those with compensated chronic HF. In a recent large-scale cohort study of individuals undergoing ambulatory surgery, the crude 90 day mortality was 2.0% among patients with HF and 0.4% among patients without HF.417 The crude risk of 30 day post-operative complications was 5.7% and 2.7%, respectively. Of note, the risk of mortality progressively increased with decreasing systolic function. It is not recommended to perform elective NCS in patients with decompensated HF.
The value of pre-operative assessment of LV function with TTE and measurement of natriuretic peptides (BNP or NT-proBNP) is discussed in Section 4.4. The TTE should not be older than 6 months, or performed just before NCS in the case of clinical worsening.
6.2.2. Pre- and post-operative management strategies
In order to reduce the risk of acute decompensation and the risk of mortality, optimal guideline-directed medical treatment of HF before scheduled NCS is recommended.412 Special attention should be given to the fluid balance, since high-volume infusion is often needed in the peri-operative period. Invasive monitoring of the arterial pressure aiming to obtain oximetric and metabolic parameters during NCS is frequently needed for intermediate- to high-risk NCS among HF patients. Furthermore, dynamic variables derived from the arterial pressure waveform (cardiac output, pulse pressure variation, stroke volume variation) are useful for guiding protocolled goal-directed therapy. Use of more invasive tools, such as right heart catheterization or transoesophageal echocardiography (TEE), might be considered on an individual patient level (see Section 7.1).
Baseline medication should be continued throughout the peri-operative period, in accordance with the recommendations provided in Section 5.2. It is recommended to perform ECG, measure biomarkers of myocardial injury (cTn T/I), and perform echocardiography to tailor the optimal treatment strategy in patients with acute decompensated HF post-operatively.
The management of patients with cardiac implantable electronic devices (CIEDs) undergoing NCS is discussed in Section 6.4.5. In patients with resynchronization devices (cardiac resynchronization therapy [CRT]), it is recommended to keep the device on to provide better haemodynamic stability.
6.2.3. Hypertrophic obstructive cardiomyopathy
Patients with hypertrophic cardiomyopathy with LV outflow tract obstruction (HOCM) have an increased risk of complications during NCS and require additional attention.418 It is recommended to perform TTE before NCS in order to determine the extent of the hypertrophy, obstruction, and diastolic function.419 Avoidance of prolonged pre-operative fasting and dehydration is important to maintain stroke volume and reduce the risk of increased obstruction. Furthermore, it is important to avoid vasodilating anaesthetic agents and maintain neutral fluid balance during the peri-operative period. The heart rate should be kept low (60–65 beats per minute [b.p.m.]) and AF should be avoided. Medication used to treat LV outflow tract obstruction should remain on board during NCS.
6.2.4. Patients with ventricular assist devices undergoing non-cardiac surgery
Ventricular assist devices (VADs) play an important role in the treatment of patients with end-stage HF who require a bridge to heart transplantation or as a permanent destination therapy. As the number of patients receiving VAD as destination therapy increases,420,421 the need for NCS in this specific subset of patients is expected to increase in years to come. Non-cardiac surgery should be performed in surgical centres that have access to VAD teams (Table 11).
Peri-operative approach to patients with ventricular assist devices undergoing non-cardiac surgery
| Pre-operative . | Intra-operative . | Post-operative . |
|---|---|---|
|
|
|
| Pre-operative . | Intra-operative . | Post-operative . |
|---|---|---|
|
|
|
CT, computed tomography; ECG, electrocardiogram; HF, heart failure; ICU, intensive care unit; PA, pulmonary artery; RV, right ventricular; TEE, transoesophageal echocardiography; VAD, ventricular assist device.
Adapted from Roberts et al.421
Peri-operative approach to patients with ventricular assist devices undergoing non-cardiac surgery
| Pre-operative . | Intra-operative . | Post-operative . |
|---|---|---|
|
|
|
| Pre-operative . | Intra-operative . | Post-operative . |
|---|---|---|
|
|
|
CT, computed tomography; ECG, electrocardiogram; HF, heart failure; ICU, intensive care unit; PA, pulmonary artery; RV, right ventricular; TEE, transoesophageal echocardiography; VAD, ventricular assist device.
Adapted from Roberts et al.421
Recommendations for management of heart failure in patients undergoing non-cardiac surgery
 |
 |
Recommendations for management of heart failure in patients undergoing non-cardiac surgery
 |
 |
6.3. Valvular heart disease
6.3.1. Risk for patients with valvular heart disease
Valvular heart disease increases the risk of peri-operative CV complications during NCS. The magnitude of risk is highly variable and depends on the severity of VHD and type of NCS. It is particularly increased in patients with obstructive valve pathology, for example symptomatic AS or MS, where peri-operative volume shifts and arrhythmia may lead to rapid decompensation.424
6.3.2. Pre-operative management strategies and risk-reduction strategy
Clinical and echocardiographic evaluation is recommended in all patients with known or suspected VHD who are scheduled for elective intermediate-or-high-risk NCS. Patients in whom mild-to-moderate VHD was diagnosed >1 year earlier should have clinical and echocardiographic re-assessment. Heart team discussion may be helpful in patients with significant VHD. The risks of valvular intervention and the risk of NCS-related complications should be estimated and communicated to the patient and the surgical team.
6.3.2.1. Aortic valve stenosis
The peri-operative risk associated with AS during NCS depends upon the presence of symptoms, stenosis severity, and coexisting cardiac disease (e.g. CAD, mitral insufficiency, or reduced LVEF). Severe symptomatic AS is a significant risk factor for post-operative MI and HF, and a predictor for 30 day and long-term mortality after NCS.425,426 Careful peri-operative management is essential in patients undergoing intermediate- and high-risk NCS, albeit the significance of AS in patients undergoing low-risk NCS might have been overemphasized in studies that pre-date the more recent advances in anaesthesia, surgical techniques, and post-operative management. Aortic valve replacement has been associated with reduced in-hospital and 30 day mortality and morbidity among patients with AS undergoing intermediate- to high-risk NCS.425,426 However, the decision regarding the timing of AVR in relation to NCS should be weighted according to the baseline risk profile and the risk associated with the NCS. The choice of surgical aortic valve replacement (SAVR) vs. TAVI should follow the 2021 ESC/EACTS Guidelines for the management of valvular heart disease245 and patient’s informed preference (Figure 13).
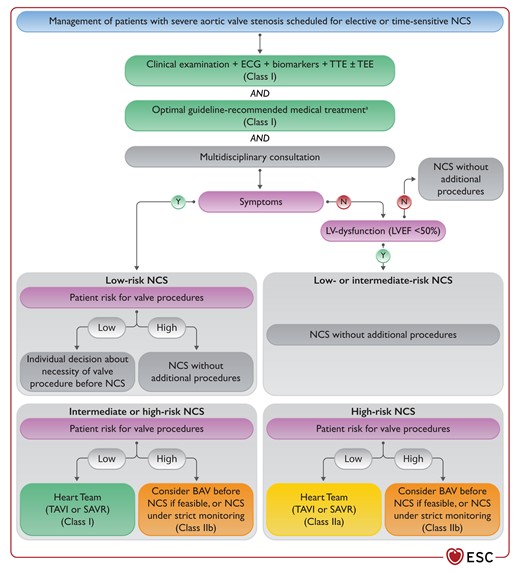
Management of patients with severe aortic valve stenosis scheduled for non-cardiac surgery.
BAV, balloon aortic valvuloplasty; ECG, electrocardiogram; LV, left ventricular; LVEF, left ventricular ejection fraction; N, no; NCS, non-cardiac surgery; SAVR, surgical aortic valve replacement; TAVI, transcatheter aortic valve implantation; TTE, transthoracic echocardiography; TEE, transoesophageal echocardiography. Y, yes; The figure provides a schematic representation of diagnostic assessment or therapy to be implemented according to surgical risk and underlying cardiac condition. aThis applies to treatment of complications (e.g. atrial fibrillation, heart failure). No medical therapy is recommended for aortic stenosis per se.
In patients with severe symptomatic AS, in whom NCS can be deferred, aortic valve intervention (SAVR or TAVI) is recommended before NCS. In patients requiring time-sensitive NCS, TAVI is a reasonable option.427 In patients with severe symptomatic AS in need of time-sensitive NCS in whom TAVI or SAVR are unfeasible, balloon aortic valvuloplasty (BAV) may be considered before NCS as a bridge to definitive aortic valve repair. Asymptomatic patients with severe AS and normal LVEF can safely undergo low- to intermediate-risk NCS, unless the NCS is associated with large volume shifts.245,428
6.3.2.2. Mitral valve stenosis
Non-cardiac surgery can be performed with a relatively low risk of complications in patients with mild MS (valve area >1.5 cm2) and in asymptomatic patients with moderate-to-severe MS (valve area ≤1.5 cm2) and systolic pulmonary artery pressure (SPAP) <50 mmHg on echocardiography.429 As transmitral gradients are flow-sensitive, tachycardia and fluid overload can cause pulmonary oedema during NCS. In this regard, arterial vasodilators should be avoided and surveillance for peri-operative AF is of paramount importance. Management of anticoagulation for patients with high thrombotic risk is discussed in Section 5.3.2. In asymptomatic patients with moderate-to-severe MS and SPAP >50 mmHg, and in symptomatic patients, the peri-operative risk of CV events is increased. In this case, a percutaneous mitral commissurotomy (PMC) should be considered before high-risk NCS. Otherwise, a multidisciplinary team should manage patients with moderate-to-severe MS who are ineligible for PMC, and NCS should be performed only if necessary. Intermediate-risk NCS may be performed in asymptomatic patients with severe MS with appropriate intra-operative and post-operative haemodynamic monitoring, if valve morphology is unsuitable for PMC.
6.3.2.3. Aortic valve regurgitation
In patients with mild-to-moderate aortic valve regurgitation (AR), NCS can be performed without additional risk. Patients with severe AR in whom valvular intervention is needed should be treated before intermediate-or-high-risk elective NCS (see Recommendation Table 21 and the 2021 ESC/EACTS Guidelines for the management of valvular heart disease).245
6.3.2.4. Mitral valve regurgitation
In patients with severe symptomatic mitral valve regurgitation (MR), the type of valve disease (primary or secondary) and LV function should be assessed. Patients with secondary MR, especially of ischaemic aetiology, are at increased risk of CV complications during NCS.430 Patients with severe symptomatic MR fulfilling the intervention criteria should be referred for valve treatment before intermediate- or high-risk elective NCS. In case of symptomatic moderate-to-severe secondary MR, patients meeting the Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy for Heart Failure Patients With Functional Mitral Regurgitation (COAPT) criteria should be considered for transcatheter edge-to-edge treatment before NCS (Figure 14).245,431 In patients with severe primary MR with symptoms or asymptomatic with LV dysfunction, valve repair is the recommended therapy (see Recommendation Table 21 and the 2021 ESC/EACTS Guidelines for the management of valvular heart disease245).
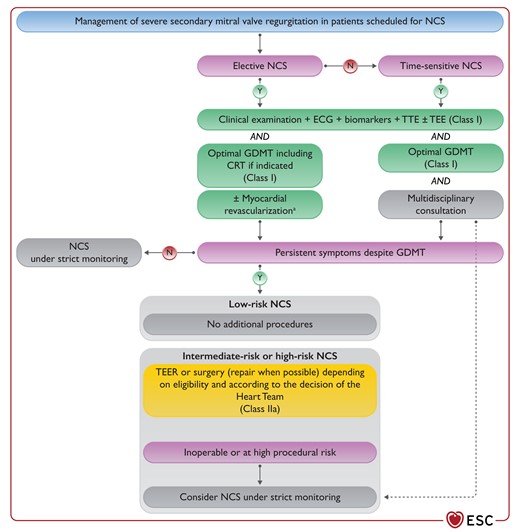
Management of patients with secondary mitral valve regurgitation scheduled for non-cardiac surgery.
ECG, electrocardiogram; GDMT, guideline-directed medical therapy; N, no; NCS, non-cardiac surgery; TTE, transthoracic echocardiography; TEE, transoesophageal echocardiography; TEER, transcatheter edge-to-edge repair. Y, yes; aCoronary angiography ± PCI/CABG on a case-by-case according to the expert team.
In patients with reduced LVEF and concomitant AR or MR, intra-operative haemodynamic monitoring, heart rate control, and careful fluid balance are crucial to avoid haemodynamic deterioration, especially during high-risk surgery.
Recommendations for management of valvular heart disease in patients undergoing non-cardiac surgery
 |
 |
 |
 |
Recommendations for management of valvular heart disease in patients undergoing non-cardiac surgery
 |
 |
 |
 |
6.3.2.5. Patients with prosthetic valve(s)
Patients who have undergone previous surgical correction of VHD and have a prosthetic valve can undergo NCS provided that there is no evidence of valve dysfunction. In current practice, the main problem is the need for a modification of the anticoagulation regimen in the peri-operative period; this is discussed in detail in Section 5.3.
6.3.2.6. Prophylaxis of infective endocarditis
Prophylaxis against infective endocarditis in patients requiring NCS should be consistent with the 2015 ESC Guidelines for the management of infective endocarditis.432
6.4. Known or newly diagnosed arrhythmias
Arrhythmias pose a significant burden to patients undergoing NCS, contributing to excessive morbidity and mortality.433,434
6.4.1. Peri-operative management—general measures
Arrhythmias—namely, supraventricular tachycardia (SVT) and ventricular tachycardia (VT)—may accompany acute surgical illness, but should not defer urgent surgical procedures, unless the arrhythmia is life-threatening. All patients with known arrhythmia undergoing elective surgery should have a 12-lead ECG performed pre-operatively and undergo a cardiology check-up. Prevention of potential arrhythmic triggers is crucial: electrolyte and acid-base imbalance, myocardial ischaemia (also caused by excessive blood loss and anaemia), and large volume shifts, which can provoke subsequent autonomic hyperactivity, should be avoided in the pre-, intra-, and post-operative periods. Patients with a systolic HF should receive optimal treatment, as such therapy reduces the risk of total mortality and sudden cardiac death (SCD).435 Patients already taking AADs should generally not stop taking these drugs. Patients at high-risk of malignant arrhythmias should have continuous ECG monitoring through the whole peri-operative period, with particular emphasis placed on patients who have had implantable cardioverter–defibrillators (ICDs) deactivated during NCS.
6.4.2. Supraventricular arrhythmias
Supraventricular arrhythmias do not usually cause deferral of surgery. In rare cases, presence of pre-excitation and AF conducted rapidly over an accessory pathway flag a patient at risk of SCD and may indicate the need for ablation, if surgery is not emergent.
Supraventricular premature beats usually do not require therapy. Identification and correction of potential triggers (electrolyte and acid-base imbalance, volume overload, etc.) are highly recommended. Peri-operative SVT typically responds well to vagal manoeuvres, or to a bolus of adenosine if unsuccessful. If SVT persists or reoccurs, i.v. beta-blockers, verapamil, or diltiazem can be used for rhythm conversion or temporal slowing of atrioventricular conduction.436 Prompt cardioversion should be performed in rare cases of haemodynamically unstable SVT. If prophylactic therapy is needed to prevent recurrent SVT, beta-blockers or non-dihydropyridine CCBs (verapamil, diltiazem) can be used, and flecainide/propafenone or amiodarone can be considered if ineffective. Rarely, when SVT recurs despite therapy or becomes incessant, ablation should be considered in patients undergoing high-risk, non-emergent surgery. Recently published results of an RCT confirmed the superiority of radiofrequency (RF) ablation over AADs for persistent atrioventricular nodal re-entry tachycardia. Large registries and meta-analyses have demonstrated efficacy and safety of RF ablation in WPW syndrome and other SVTs, with a single-procedure success rate of >90.437–441
6.4.3. Atrial fibrillation/flutter
The majority of patients with AF receive lifelong OAC therapy for the prevention of stroke and systemic embolism,99 and the peri-operative management of OAC therapy will depend on the type of surgery (see Section 5.3.2).99,240 Sometimes AF is asymptomatic442 and may be first detected on admission for surgery, or may first occur in the pre-operative period. The initial management of newly diagnosed AF includes prevention of thromboembolism, and symptom control, and should not be deferred awaiting consultation with a cardiologist.99 In patients with newly diagnosed AF who require systemic OAC therapy for stroke prevention, the choice of anticoagulant in the pre-operative period depends on the type of surgery (see Section 5.3.2). Optimal rate control (i.e. resting heart rate <110 b.p.m.)99 is mandatory in all patients with AF, whereas rhythm control (i.e. achieving and maintenance of sinus rhythm) in the pre-operative period may be considered only if symptoms persist despite optimal rate control.
Rate control can be achieved using beta-blockers or non-dihydropyridine CCBs (verapamil, diltiazem). Amiodarone can be used as first-line therapy in patients with HF, whereas digoxin is usually ineffective in high adrenergic conditions such as surgery. Pharmacological cardioversion of symptomatic recent-onset AF can be attempted using flecainide or propafenone; in patients without significant LV hypertrophy, LV systolic dysfunction, or IHD, the use of flecainide or propafenone results in prompt (3–5 h) and safe restoration of sinus rhythm in >50% of patients. Intravenous amiodarone administration has a limited and delayed effect but can slow the heart rate within 12 h; i.v. vernakalant is the most rapidly cardioverting drug, including patients with mild HF and/or IHD.99,443 Dofetilide is not used in Europe, and ibutilide is effective to convert atrial flutter to sinus rhythm.99,444 In patients with AF and haemodynamic instability, emergency cardioversion (most commonly electrical direct current cardioversion) is indicated.99 Alternatively, pharmacological cardioversion using i.v. AADs should be attempted, if consistent with the patient’s clinical status. Systemic OAC therapy to prevent thromboembolic events should be started as soon as possible.99 Post-operative AF is discussed in Section 8.6.
Management of atrial flutter follows the same principles as AF with respect to OAC therapy. Rate control is usually an initial approach in patients with atrial flutter;436 however, drugs that slow atrioventricular conduction (digoxin, beta-blockers, or non-dihydropyridine CCBs) are usually less effective than in AF. In patients with a high ventricular rate, electrical cardioversion is frequently needed.445–448 Amiodarone may be an alternative used to control rate, especially in HF or critically compromised patients.449 Dofetilide and ibutilide are effective in converting atrial flutter to sinus rhythm, whereas class IA and IC drugs and amiodarone are less efficient and should not be used.450–453
6.4.4. Ventricular arrhythmias
Premature ventricular contractions (PVC) and non-sustained VT are frequent in the general population and patients undergoing NCS. Specific clinical features have been suggested as predictors of increasing incidence of PVC.454 These arrhythmias have historically been considered benign; however, recent studies have suggested that they may be associated with an adverse outcome, even in patients with apparently normal hearts, especially if frequent (e.g. >10–20%).455–459 In patients with heart disease, the prognostic impact of PVC and non-sustained VT depends on type and extent of heart damage.460–466 In patients undergoing urgent NCS, they do not require treatment unless frequent and symptomatic. If haemodynamically compromising, up-titration of beta-blockers is recommended; amiodarone (300 mg i.v. bolus) should be considered if beta-blockers are not tolerated or contraindicated.467 Further diagnostics to rule out significant heart disease is necessary in patients awaiting elective NCS, especially if frequent, complex (non-sustained VT), symptomatic, or in those with a positive family history of SCD.
Polymorphic VT and ventricular fibrillation (VF) can be provoked by ischaemia, electrolyte imbalance, or may be manifestations of primary electrical disease, such as long-QT or Brugada syndrome. Monomorphic VT is often associated with the presence of scarred myocardium. Hence, peri-operative VT or VF in a patient awaiting surgery should lead to a diagnostic work-up to exclude severe ventricular dysfunction (see Section 4.5.1), and to rule out CAD requiring prompt revascularization (see Section 6.1) and other potential causes of arrhythmia (primary electrical disease, dyselectrolytaemia). Monomorphic VT in patients without overt structural or electrical heart disease (idiopathic VT, most commonly arising from outflow tract) is associated with good prognosis and may be left untreated or, if symptomatic, may be treated with beta-blockers, verapamil, or sodium channel blockers. Patients with haemodynamically compromising VT should undergo electrical cardioversion (after i.v. sedation, if conscious) and VF should be terminated with prompt defibrillation. Recurrent VT and VF in the setting of acute ischaemia may be effectively treated with beta-blockers and amiodarone, and myocardial revascularization in case of obstructive CAD.468 Up-titration of beta-blockers to the maximal tolerated doses can prevent arrhythmia recurrence.469
Haemodynamically stable, sustained VT should be cardioverted as a first-line treatment; i.v. procainamide or flecainide may be considered in patients without HF or myocardial ischaemia. In cases in which these drugs are unavailable, i.v. amiodarone can be used. In selected cases, when monomorphic VT recurs in patients with scarred myocardium with no reversible causes, despite optimal therapy, invasive electrophysiological study and ablation should be performed pre-operatively, if NCS can be deferred. After extensive endocardial VT ablation, treatment with an OAC for a limited period of time might be reasonable.470,471 A summary of diagnostic and therapeutic pathways in patients with SVT or VT is shown in Table 12.
| Type of arrhythmia . | SVT . | Idiopathic VT in structurally/functionally normal heart . | VT in structural heart disease . |
|---|---|---|---|
| Diagnostics |
|
|
|
| Acute management |
|
|
|
| Prevention of recurrence |
|
|
|
| Type of arrhythmia . | SVT . | Idiopathic VT in structurally/functionally normal heart . | VT in structural heart disease . |
|---|---|---|---|
| Diagnostics |
|
|
|
| Acute management |
|
|
|
| Prevention of recurrence |
|
|
|
AAD, antiarrhythmic drug; CCB, calcium channel blocker; CT, computer tomography; ECG, electrocardiogram; MRI, magnetic resonance imaging; NCS, non-cardiac surgery; OMT, optimal medical therapy; SVT, supraventricular tachycardia; TTE, transthoracic echocardiography; VT, ventricular tachycardia.
Before high-risk surgery.
High-sensitivity cardiac troponin T/I, and/or BNP/ N-terminal pro-BNP.
| Type of arrhythmia . | SVT . | Idiopathic VT in structurally/functionally normal heart . | VT in structural heart disease . |
|---|---|---|---|
| Diagnostics |
|
|
|
| Acute management |
|
|
|
| Prevention of recurrence |
|
|
|
| Type of arrhythmia . | SVT . | Idiopathic VT in structurally/functionally normal heart . | VT in structural heart disease . |
|---|---|---|---|
| Diagnostics |
|
|
|
| Acute management |
|
|
|
| Prevention of recurrence |
|
|
|
AAD, antiarrhythmic drug; CCB, calcium channel blocker; CT, computer tomography; ECG, electrocardiogram; MRI, magnetic resonance imaging; NCS, non-cardiac surgery; OMT, optimal medical therapy; SVT, supraventricular tachycardia; TTE, transthoracic echocardiography; VT, ventricular tachycardia.
Before high-risk surgery.
High-sensitivity cardiac troponin T/I, and/or BNP/ N-terminal pro-BNP.
Recommendations for management of known or newly diagnosed arrhythmias
 |
 |
 |
 |
Recommendations for management of known or newly diagnosed arrhythmias
 |
 |
 |
 |
6.4.5. Bradyarrhythmias
Temporary cardiac pacing during the peri-operative period should be limited to patients undergoing urgent NCS, if bradycardia is haemodynamically compromising despite i.v. chronotropic drugs, or provokes episodes of ventricular tachyarrhythmia.481 In patients undergoing elective NCS, surgery should be deferred if possible, and permanent pacemaker should be implanted, if indications for pacing are fulfilled.481 Prophylactic pacing in the settings of asymptomatic bifascicular block, with or without first-degree atrioventricular block, is generally not indicated, and chronotropic drugs (atropine, isoprenaline, adrenaline, or, alternatively, aminophylline, dopamine, or glucagon in beta-blocker or CCB overdosing) are usually effective. Patients with bifascicular bundle branch block or prolonged His-ventricular interval are at an increased risk of developing complete heart block.482,483 Equipment needed to perform emergent transcutaneous pacing and personnel able to perform such a procedure should be immediately available during NCS in patients with bifascicular block; alternatively, a permanent pacemaker may be implanted.481
6.4.6. Management of patients with cardiac implantable electronic devices
Patients with CIEDs can undergo NCS, pending adequate peri-operative device management. A pre-operative check should have been performed at least once within the 12 months preceding surgery for pacemaker patients and within 6 months for patients with ICD, in the absence of any malfunction (remote monitoring can also be used for check-ups).484,485 In pacing-dependent patients, patients with biventricular pacing for CRT, and ICD-recipients undergoing elective NCS associated with risk of electromagnetic interference (EMI) (e.g. involving the use of unipolar electrocoagulation, especially above the umbilicus), CIED check and reprogramming should be performed immediately before surgery. In pacemaker-dependent patients, devices should be reprogrammed to non-sensing or asynchronous pacing mode to protect against inhibition of the pacemaker. This can be performed in the majority of pacemaker models by placing a magnet over the pacemaker can.486,487 However, magnet mode in modern pacemakers, except Medtronic and Sorin/Livanova/Microport, is programmable and may not be asynchronous pacing; thus, magnet application is not a universal remedy against EMI-induced malfunction. Furthermore, asynchronous pacing may lead to pacing on the T wave, which can provoke VT/VF. However, the risk of clinically significant EMI is low487–489 and a practical solution would be to monitor the patient via plethysmography or an arterial line, and limit the use of electrocautery if pauses occur during ECG monitoring.
Patients with leadless pacemakers may safely undergo surgery, with precautions similar to patients with conventional pacemakers, avoiding EMI and after reprogramming the pacemaker into non-sensing mode in pacing-dependent patients (due to its intracardiac location and lack of a Hall-effect sensor/reed switch, this device cannot be temporarily reprogrammed to an asynchronous mode with a magnet applied over the body of the pacemaker).490,491
In patients with ICDs undergoing NCS with anticipated risk of EMI, arrhythmia detection or antiarrhythmic therapies through the device should be switched off before NCS,492 or a magnet should be put over the device.488,489,492 All modern ICDs will respond to magnet application by inhibiting antitachycardia therapy, while the brady pacing is left intact. Deactivation by programming mandates telemetry and cardioversion equipment until reactivation, which may be impractical. Furthermore, there is a risk that the patient is discharged without the device being reactivated. These factors would favour the use of a magnet instead of deactivation. In some patients where the device is inaccessible for magnet application, a magnet cannot be used and reprogramming is mandatory. From this point onwards, throughout the whole procedure until reactivation of the ICD, the patient should have continuous ECG monitoring, and personnel skilled in early detection of arrhythmias, defibrillation, and cardiopulmonary resuscitation manoeuvres should be present. As soon as possible after NCS, it is recommended that the ICD is checked and therapies switched on.485
Patients with subcutaneous ICD can undergo surgery after switching off the antiarrhythmic therapy or magnet application; however, if thoracic surgery is planned, especially with a median sternotomy, the surgeon should be aware of the presence of the ICD and the course of the subcutaneous electrode. This can prevent mechanical damage of the lead, the direct use of electrocautery on the electrode, or the placement of sternal wires in close proximity to the sensing electrodes.493
In pacing-dependent patients, patients with CRT, and ICD patients, EMI with the device should be avoided (e.g. with electrocautery). Use of bipolar electrocautery, short bursts of impulses limited to several seconds (<5 s), with the lowest effective energy, and operating with a pen or stylus away from the device (>15 cm) can minimize the risk of interference with the device. In the case of unipolar electrocoagulation, the electrosurgical unit should be connected in a way that keeps the current circuit away from the CIED can and electrodes. However, the manufacturer’s recommendations should be considered (usually recommending placement of the indifferent electrode on the opposite site of the body to the one operated on, but possibly close to the surgical site, on a well-vascularized, muscular area). Consequently, the indifferent return pad should be placed as far away from the CIED as possible, keeping the surgical site between the CIED and the return electrode (Figure 15).494–497
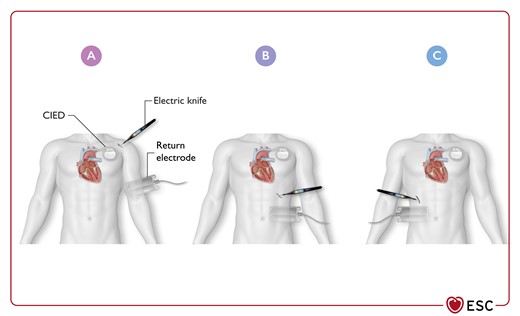
Optimal location of return electrode during unipolar electrosurgery in patients with cardiac implantable electronic devices, depending on the surgery site.
CIED, cardiac implantable electronic device. Use of bipolar electrocautery, short (<5 s) bursts of impulses, with the lowest effective energy, operating with pen or stylus away (>15 cm) from the device can minimize the risk of interference with the device. (A) Surgery site on ipsilateral site above CIED. (B) Surgery on ipsilateral site below CIED. (C) Surgery on contralateral site.494
In patients with implantable loop recorders (especially those not undergoing remote monitoring and regular downloads of the CIED memory), the device memory download is to be considered before procedures associated with possible EMI, or involving the anatomical location close to the device, to avoid misrecognizing and recording noise as arrhythmia or erasing the memory.498,499
Recommendations for management of bradyarrhythmia and patients carrying cardiac implantable devices
 |
 |
 |
 |
Recommendations for management of bradyarrhythmia and patients carrying cardiac implantable devices
 |
 |
 |
 |
6.5. Adult congenital heart disease
Adults with congenital heart disease (ACHD) account for >60% of the population with congenital heart disease (CHD).500,501 Accordingly, ACHD represent an increasing proportion of NCS admissions502 and might be at high risk of CV events.
Pre-operative risk assessment in ACHD needs to focus on the underlying disease, type of surgery, residua, and sequelae.503 Coexistence of HF, pulmonary hypertension, arrhythmia, hypoxaemia, damage to other organs, and endocarditis may considerably influence the baseline risk of these patients from no additional risk to very high risk of worse prognosis.503,504 Thus, original medical and surgical reports should be obtained along with current data, which should include symptoms, exercise capacity, oxygen saturation, laboratory values (BNP, haemoglobin, creatinine, etc.), and medication.
In a recent report, absolute mortality in ACHD patients undergoing NCS exceeded 4%.502 Mortality and peri-operative morbidity were greater in ACHD compared with a matched comparison cohort, and patients with severe ACHD had the highest mortality rate. It is well known that patients with pulmonary hypertension and with Eisenmenger syndrome have a higher risk of complications.505 A large registry confirmed these findings: patients with severe CHD had an increased risk of 30 day mortality, overall mortality, and reintubation, while patients with intermediate CHD had a moderate increase in overall mortality and risk of reintubation.506 Based on this study and recent guidelines,503 the classification in Table 13 is proposed for risk stratification.
Risk stratification for non-cardiac surgery in adults with congenital heart disease
| Minor risk | Patients with small, uncorrected defects, and no need for medication or any other treatment Patients with successfully corrected CHD with no symptoms, no relevant residua, and no need for medication |
| Intermediate risk | Patients with corrected or uncorrected conditions with residual haemodynamic abnormality, with or without medication |
| Severe risk | Patients with uncorrected cyanotic heart disease, pulmonary hypertension, other complex CHD, ventricular dysfunction requiring medication, and patients listed for heart transplantation |
| Minor risk | Patients with small, uncorrected defects, and no need for medication or any other treatment Patients with successfully corrected CHD with no symptoms, no relevant residua, and no need for medication |
| Intermediate risk | Patients with corrected or uncorrected conditions with residual haemodynamic abnormality, with or without medication |
| Severe risk | Patients with uncorrected cyanotic heart disease, pulmonary hypertension, other complex CHD, ventricular dysfunction requiring medication, and patients listed for heart transplantation |
CHD, congenital heart disease.
Risk stratification for non-cardiac surgery in adults with congenital heart disease
| Minor risk | Patients with small, uncorrected defects, and no need for medication or any other treatment Patients with successfully corrected CHD with no symptoms, no relevant residua, and no need for medication |
| Intermediate risk | Patients with corrected or uncorrected conditions with residual haemodynamic abnormality, with or without medication |
| Severe risk | Patients with uncorrected cyanotic heart disease, pulmonary hypertension, other complex CHD, ventricular dysfunction requiring medication, and patients listed for heart transplantation |
| Minor risk | Patients with small, uncorrected defects, and no need for medication or any other treatment Patients with successfully corrected CHD with no symptoms, no relevant residua, and no need for medication |
| Intermediate risk | Patients with corrected or uncorrected conditions with residual haemodynamic abnormality, with or without medication |
| Severe risk | Patients with uncorrected cyanotic heart disease, pulmonary hypertension, other complex CHD, ventricular dysfunction requiring medication, and patients listed for heart transplantation |
CHD, congenital heart disease.
A consultation by an ACHD specialist is necessary, especially in patients with intermediate or severe ACHD scheduled for intermediate- or high-risk NCS. It is recommended that elective surgery in intermediate- and severe-risk ACHD patients should take place in centres experienced in the management of ACHD patients. It is generally recommended to perform the least invasive procedures and anaesthesia with as low an impact on haemodynamics as possible.
Optimal peri-operative care in ACHD undergoing NCS starts with a proper pre-operative evaluation. Adults with congenital heart disease can present with multiorgan involvement (kidneys, liver, lung, and endocrine system), which should be considered during the diagnostic work-up.503 In many cases, patients with ACHD have a lifelong indication for OAC therapy or antithrombotic treatment, mainly due to arrhythmias or increased thromboembolic risk associated with specific ACHD. Oral anticoagulant therapy in the peri-operative phase should be re-evaluated on a case-by-case basis. The CHA2DS2-VASc score has not been validated in ACHD patients and should not be used in this group.
Continuous haemodynamic monitoring can be necessary in patients with ACHD and should include invasive BP monitoring, especially in cases with ACHD of moderate or severe complexity. It is worth mentioning that, according to the type of ACHD or surgery (e.g. coarctation of the aorta or following a Blalock-Taussig shunt), the location of the arterial line for continuous BP and gas exchange monitoring requires careful attention. In patients with persistent right-to-left shunts, air filters must be used for venous access. Ventilator management and extubation can be complicated by the presence of restrictive lung disease.507 Antibiotic prophylaxis for endocarditis should be given according to the 2015 ESC Guidelines for the management of infective endocarditis.432 Furthermore, post-operative care in an ICU with experience in handling ACHD patients is often necessary.
A prolonged monitoring period in this setting should be considered, with a special focus on arrhythmias and an optimal volume management, as it has been shown that up to 50% of adverse events were attributable to lapses in the post-operative monitoring and care.508 Two groups at special risk are patients with chronic cyanosis and those after the Fontan procedure. Chronic cyanosis is associated with multiorgan involvement. Furthermore, bleeding risk is higher due to multiple collaterals, platelet dysfunction, and alterations in the coagulation cascade.509 In patients with Eisenmenger syndrome, conditions that increase pulmonary vascular resistance such as hypothermia, metabolic acidosis, hypercapnia, and hypovolaemia must be avoided.509 This is also true for patients after the Fontan procedure, where venous return relies on low pulmonary pressures. If intra-abdominal pressure rises too high in these patients, venous return is drastically reduced, with a subsequent drop in cardiac output. These haemodynamic aspects should be carefully considered in cases of laparoscopic or open NCS.
Recommendations for management of patients with adult congenital heart disease undergoing non-cardiac surgery
 |
 |
Recommendations for management of patients with adult congenital heart disease undergoing non-cardiac surgery
 |
 |
6.6. Pericardial diseases
Active pericardial disease is infrequent at the time of NCS, but potentially life-threatening. The clarification of underlying aetiology is of utmost importance for peri-operative management (viral or bacterial infection, malignant, systemic autoimmune, metabolic, or autoreactive disease). The treatment of these conditions should follow the recommendations provided in the 2015 ESC Guidelines for the diagnosis and management of pericardial diseases.510
Acute pericarditis is a clear indication to postpone an elective surgical procedure. However, in cases of undeferrable NCS, attention is required regarding drug-to-drug interactions. The frequently used colchicine is predominantly metabolized through the liver, while renal excretion accounts for only 10–20%. Colchicine may increase sensitivity to central nervous system depressants and exert a respiratory depressant effect.511 Adverse events of peri-operative significance include diarrhoea, worsening renal failure, and, very rarely, bone marrow suppression, hepatotoxicity, paralysis, convulsions, and cardiopulmonary collapse. Immunosuppressive drugs such as steroids and interleukin-1 receptor antagonist agents suppress the immune system, and may increase the risk of infection and delay wound healing in the peri-operative phase.511
Imminent cardiac tamponade is an absolute contraindication for all surgical procedures, especially when a general anaesthesia is required.510,512 Before NCS, pericardial effusion should first be percutaneously drained, under local anaesthesia. In cases of small or moderate chronic pericardial effusion and constrictive pericarditis, attention should be paid to take measurements to increase cardiac pre-load. The pre-load should be optimized peri-operatively with i.v. fluids prior to general anaesthetic induction to facilitate ventricular filling. Manipulation and medication diminishing venous return to the heart should be avoided or minimized. Positive pressure ventilation might cause a dramatic decline in pre-load and should be avoided. If spontaneous breathing is not possible, ventilation with minimal inspiratory pressures (low tidal volumes, high respiratory rate) should be considered. Anaesthetics that minimize changes in heart rate, systemic vascular resistance, venous return, and myocardial contractility should be selected.189 Ketamine, a sympathetic stimulant that preserves spontaneous ventilation, is the drug of first choice. Combinations of opiates, benzodiazepines, and nitric oxide, with or without low doses of volatile anaesthetics, are acceptable for maintenance of anaesthesia. Muscle relaxants with minimal circulatory effects should be preferred, although the modest increase in heart rate observed with administration of pancuronium is also acceptable.
6.7. Pulmonary disease and pulmonary arterial hypertension
The coexistence of pulmonary disease in cardiac patients undergoing NCS may increase the operative risk. Pre-existing pulmonary disease has a significant impact on peri-operative risk, but the most common effect is an increase in the risk of post-operative pulmonary complications. These complications particularly occur after abdominal or thoracic surgery, and the risk seems to be increased in smokers. Certain respiratory conditions are associated with CV pathology and may require special cardiac risk assessment and management, in addition to dealing with the pulmonary disease per se. Three such conditions are chronic obstructive pulmonary disease (COPD), obesity hypoventilation syndrome, and pulmonary arterial hypertension (PAH).
6.7.1. Pulmonary disease
Chronic obstructive pulmonary disease is as a major cause of morbidity and mortality.513 Although patients with COPD have an increased risk of CVD, there is no evidence that COPD is related to a higher risk of peri-operative cardiac complications. However, post-operative pulmonary complications result in significant mortality and morbidity. Pre-operative evaluation, using specific post-operative pulmonary complication tools, can be used to stratify at-risk patients and enable optimal pre-operative and peri-operative management.514 In patients with COPD undergoing NCS, the pre-operative treatment goals are to optimize pulmonary function and minimize post-operative respiratory complications; this includes using the pre-operative period for education, including possible cessation of smoking (2 months before surgery), instruction concerning chest physiotherapy and lung expansion manoeuvres, muscular endurance training, and re-nutrition if required. Beta-adrenergic agonists and anticholinergic agents should be continued until the day of surgery in all symptomatic COPD patients with bronchial hyper-reactivity. In some cases, short-term systemic/inhaled steroids may be considered. Where there is active pulmonary infection, appropriate antibiotics should be administered for at least 10 days and, if possible, elective NCS should be deferred.515
Obesity hypoventilation syndrome is defined as the triad of obesity, daytime hypoventilation, and sleep-disordered breathing. Although distinct from simple obesity and sleep apnoea, it is estimated that 90% patients with obesity hypoventilation syndrome also have obstructive sleep apnoea (OSA). The prevalence of obesity hypoventilation syndrome is 0.15–3% of adults and 7–22% in patients undergoing bariatric surgery.516 Obesity hypoventilation syndrome is associated with even higher morbidity, including HF (and obesity-related cardiomyopathy), angina pectoris, pulmonary hypertension (30–88%) and cor pulmonale, and increased peri-operative mortality.516 Patients at high risk of obesity hypoventilation syndrome who are undergoing NCS should be referred for additional specialist investigation of sleep-disordered breathing and pulmonary hypertension, with pre-operative initiation of appropriate positive airway pressure therapy, and planning of peri-operative techniques (anaesthetic and surgical) and post-operative positive airway pressure management within an appropriate monitored environment.516,517
6.7.2. Pulmonary arterial hypertension
Pulmonary arterial hypertension is associated with increased morbidity and mortality in patients undergoing NCS.518 A meticulous pre-operative diagnostic work-up in this subset of patients should include assessment of functional status and severity of disease, in addition to comorbidities and the type of NCS. Echocardiography and right heart catheterization (if clinically indicated) are key components in the pre-operative work-up. The morbidity and mortality associated with PAH derive from the haemodynamic response of the right ventricle to acute increases in afterload.519
In patients with severe PAH, peri-operative mortality ranging between 3–18% has been reported, depending on the severity of the underlying disease, and the nature and urgency of the surgical procedure. Emergency procedures are also associated with a high risk of complications.520,521 Patient-related and surgery-related factors should be considered when assessing peri-operative risk in patients with PAH (Table 14).522 Owing to the potential for anaesthesia and surgery to be complicated by acute right HF and pulmonary hypertensive crisis, elective NCS in patients with PAH should be adequately discussed in a multidisciplinary team. Ideally, patients with PAH undergoing NCS should have optimal medical treatment before surgery and be managed in a centre experienced in PAH. Patients scheduled for NCS should be discussed by a pneumologist, cardiologist, surgeon, and an anaesthesiologist.523 The management of patients with PAH in the peri-operative setting should follow the recommendations provided in the 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension.524
Patient-related and surgery-related factors to be considered when assessing peri-operative risk in patients with pulmonary arterial hypertension
| Patient-related peri-operative risk factors in patients with PAH . | Surgery-related peri-operative risk factors in patients with PAH . |
|---|---|
|
|
| Patient-related peri-operative risk factors in patients with PAH . | Surgery-related peri-operative risk factors in patients with PAH . |
|---|---|
|
|
PAH, pulmonary arterial hypertension.
Adapted from Olsson et al.522
Patient-related and surgery-related factors to be considered when assessing peri-operative risk in patients with pulmonary arterial hypertension
| Patient-related peri-operative risk factors in patients with PAH . | Surgery-related peri-operative risk factors in patients with PAH . |
|---|---|
|
|
| Patient-related peri-operative risk factors in patients with PAH . | Surgery-related peri-operative risk factors in patients with PAH . |
|---|---|
|
|
PAH, pulmonary arterial hypertension.
Adapted from Olsson et al.522
Several novel therapies aimed at reducing pulmonary pressures are used pre-operatively in patients undergoing NCS. Of these therapies, endothelin receptor antagonists, phosphodiesterase inhibitors, and prostacyclin analogues are the most used.525 This medication should be continued during the peri-operative phase because therapy disruption may lead to a critical rebound of the PAH. Inhaled nitric oxide allows selective pulmonary vasodilatation with rapidity of action and it can be given to patients who develop worsening pulmonary hypertension post-operatively to maintain right ventricular (RV) function and haemodynamic stability.526
Recommendations for patients with pulmonary arterial hypertension undergoing non-cardiac surgery
 |
 |
Recommendations for patients with pulmonary arterial hypertension undergoing non-cardiac surgery
 |
 |
6.8. Arterial hypertension
The prevalence of arterial hypertension in adults in Europe is ∼30–45%.527 Of these patients, <40% have well-controlled BP (<140/90 mmHg). A large observational study has shown that patients with untreated hypertension 1 month before surgery had a 69% increased risk of 90 day post-operative mortality.183 Further, overall CV risk assessment, including the search for hypertension-mediated organ damage, is of paramount importance in hypertensive patients, and mandatory when there is newly detected BP elevation.528
Postponing surgery is usually not advised in patients with grade 1 or 2 hypertension. In contrast, in subjects with a systolic BP ≥180 mmHg and/or diastolic BP ≥110 mmHg, deferring the intervention until BP is under control is advisable, except for emergency surgery.236,527,529,530 It also seems important to avoid large peri-operative BP fluctuations. In a recent randomized trial among patients undergoing abdominal surgery, an individualized intra-operative treatment strategy with systolic BP values kept within a 10% difference from the pre-operative office measurement resulted in a reduced risk of post-operative organ dysfunction.528 In a meta-analysis including 130 862 patients undergoing surgery, intra-operative hypotension was associated with increased risk of morbidity (OR, 2.08; 95% CI, 1.56–2.77), mortality (OR, 1.94; 95% CI, 1.32–2.84), cardiac complications (OR, 2.44; 95% CI, 1.52–3.93), and AKI (OR, 2.68; 95% CI, 1.31–5.55).531 In patients with hypertension, hypoperfusion may occur at higher BP levels and peri-operative BP control should be tailored to pre-operative levels.528
In patients referred for elective NCS, control of BP should be prioritized, especially in patients with systolic BP >160 mmHg. The management of patients with hypertension in the pre-operative setting should follow the recommendation provided in the 2018 ESC/European Society of Hypertension (ESH) Guidelines for the management of arterial hypertension.529 These guidelines advocate an approach using RAAS inhibitors (in patients aged <70 years) or CCBs (in patients aged >70 years) as single therapy in moderate hypertension and both in combination where dual therapy is needed, adding a diuretic and an aldosterone antagonist if additional medication is needed for adequate control. Beta-blockers are restricted to patients where it is specifically indicated.529 In patients with hypertension and a clear indication for beta-blocker, third-generation beta-blockers—such as carvedilol, celiprolol, labetolol, and nebivolol—have superior antihypertensive effects compared with other beta-blockers and fewer adverse effects, but there are no RCTs reporting outcomes in hypertensive patients.529 In a large observational study, a beta-blocker prescription prior to NCS was associated with lower 30 day mortality in patients with three or four cardiac risk factors.188 However, for patients with no cardiac risk factors, the risk of death was significantly increased with beta-blockers.188,532
Most patients with stage 3 hypertension on the day of surgery will be classified as hypertensive urgencies. In these cases, the 2018 ESC/ESH Guidelines for the management of arterial hypertension recommend ACEIs, CCBs, or diuretics. Neither ACEIs nor diuretics are recommended on the day of surgery. The use of CCBs is supported by a meta-analysis of 11 studies of CCBs during NCS.220 Further, a study of 989 well-controlled patients with hypertension without hypertension-related organ damage, testing the use of fast-acting nasal nifedipine when stage 3 hypertension (systolic BP >180 and/or diastolic BP >110 mmHg) was diagnosed the day of NCS, found no difference in outcome between administration of nifedipine and surgery on the same day compared with deferral for hypertension control before resuming surgery.533 Immediate nifedipine treatment was associated with shorter hospital stays. As this was the first study testing the need for deferral of stage 3 hypertension, it challenged the need for this practice. For patients with hypertensive emergencies (systolic BP >180 and/or diastolic BP >110 mmHg and organ damage), the 2018 ESC/ESH Guidelines for the management of arterial hypertension recommend labetolol, nitroglycerin, nitroprusside, etc., according to the affected organ.529
The timing of administration of the antihypertensive drugs, and their continuation or discontinuation in the peri-operative period is discussed in Section 5.2.
Recommendations for pre-operative management of hypertension
 |
 |
Recommendations for pre-operative management of hypertension
 |
 |
6.9. Peripheral artery disease
Patients with peripheral artery disease (PAD) usually have advanced atherosclerotic disease affecting multiple vascular beds in varying degrees and have a worse prognosis compared with patients without PAD.534–538 Patients with PAD generally differ in their risk profiles, according to whether they undergo vascular or non-vascular NCS.
6.9.1. Peripheral artery disease and non-vascular non-cardiac surgery
Decisions on pre-operative treatment of pre-existing PAD and AAA in patients scheduled for non-vascular NCS should be made on an individual basis, taking into account symptoms and risks of surgery. Non-cardiac surgery should be prioritized in patients needing revascularization for PAD, but careful peri-operative monitoring of deterioration in lower extremity perfusion is warranted, particularly in those patients with chronic limb-threatening ischaemia (e.g. peripheral BP of ≤50–70 mmHg in the foot joint and ≤30–50 mmHg in the toes).539 For patients with AAA, pain control is essential to ensure stable BP, minimizing rupture risk. Patients with large AAA (i.e. >5 cm in diameter for women and >5.5 cm for men) should be evaluated for aortic aneurysm repair (preferably EVAR)540–542 before non-vascular NCS is planned, particularly in the case of malignant tumours, depending on the stage of malignant disease.
6.9.2. Peripheral artery disease and vascular non-cardiac surgery
The 2017 ESC Guidelines on the diagnosis and treatment of peripheral arterial diseases535 and the European Society for Vascular Surgery (ESVS) 2019 Clinical Practice Guidelines on the management of abdominal aorto-iliac artery aneurysms35 provide detailed evidence and recommendations on the screening of patients before vascular NCS and their treatment modality selection. Notably, there is randomized evidence against prophylactic coronary revascularization before major vascular surgery in CCS patients due to lack of benefit in improvement of peri-operative and long-term outcomes (2.7 years mean follow-up).399 Assessment of functional capacity might offer guidance to select candidates for cardiac assessment prior to major NCS, although severe walking impairment may challenge this test. The previously established risk predictive model to detect mortality in patients undergoing elective AAA repair may be helpful.543 Low-dose rivaroxaban plus aspirin initiated within 10 days after a lower limb revascularization procedure reduces post-operative thrombotic events (acute limb ischaemia, amputation, MI, ischaemic stroke, and CV death) in patients undergoing lower limb revascularization, whether it is carried out using an endovascular or open surgical approach.544 Handling of other co-drugs should follow the recommendations detailed in Section 5.2.
Recommendations for management of patients with peripheral artery disease and/or abdominal aortic aneurysm undergoing non-cardiac surgery
 |
 |
Recommendations for management of patients with peripheral artery disease and/or abdominal aortic aneurysm undergoing non-cardiac surgery
 |
 |
6.10. Cerebrovascular disease
Patients undergoing NCS should be questioned about previous neurological symptoms, and those with symptoms suggestive of transient ischaemic attack (TIA) or stroke in the preceding 6 months should undergo pre-operative neurological consultation and neurovascular and brain imaging, if appropriate. In the absence of dedicated studies addressing this issue, the criteria for carotid revascularization in symptomatic and asymptomatic patients is described in detail in the 2017 ESC Guidelines on the diagnosis and treatment of peripheral arterial diseases, in collaboration with the ESVS; these guidelines should also guide the management of patients with carotid disease who are undergoing NCS.535
In patients with symptomatic carotid disease, the benefit of carotid revascularization is particularly high in patients with recent (<3 months) TIA or stroke, and ≥70% carotid artery stenosis. Thus, carotid revascularization should be performed first and elective NCS should be postponed.545
The question as to whether patients with severe asymptomatic carotid occlusive disease who are undergoing elective major NCS require pre-operative carotid revascularization remains a matter of debate. Importantly, the purpose of carotid revascularization in this setting is more the long-term prevention of stroke than peri-operative stroke risk reduction; therefore, if carotid revascularization is indicated, this may be performed before or after the planned NCS. Independent of the revascularization strategy, patients with carotid artery stenosis benefit from aggressive CV risk-factor modification to prevent peri-operative myocardial ischaemia.
Recommendations for management of patients with suspected or established carotid artery disease undergoing non-cardiac surgery
 |
 |
Recommendations for management of patients with suspected or established carotid artery disease undergoing non-cardiac surgery
 |
 |
6.11. Renal disease
Renal disease is associated with several cardiac comorbidities, including hypertension, HF, CAD, and arrhythmias.546 Consistently, renal disease portends a significant increase in the post-operative risk of CV events, including MI, stroke, and progression of HF in patients undergoing NCS. For this reason, most risk indices for the quantification of pre-operative risk in patients undergoing NCS include renal function.
Chronic kidney disease (CKD) is defined as impaired kidney function or raised proteinuria, confirmed on two or more occasions at least 3 months apart. The kidney function can be assessed through the estimated glomerular filtration rate (eGFR) calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula, including sex, age, ethnic origin, and serum creatinine concentration. A cut-off glomerular filtration rate (GFR) value <60 mL/min/1.73 m2 significantly correlates with MACEs. Identification of cardiac patients at risk of worsening of renal function in the peri-operative phase of NCS is of paramount importance, in order to initiate supportive measures such as maintenance of adequate intravascular volume for renal perfusion and use of vasopressors.547
Patients with cardiac comorbidities are prone to develop AKI after major NCS, due to worsening of haemodynamic status associated with fluids or blood loss, and withdrawal or continuation of cardio-active therapies. The peri-operative management of patients undergoing NCS and treated with cardio-active drugs has been discussed in Section 5.2. Acute kidney injury reduces long-term survival in patients with normal baseline renal function.548 Of interest, ∼30–40% of all cases of AKI occur after surgery and the incidence of post-operative AKI ranges 18–47%. Risk factors for the development of post-operative AKI following NCS include cardiac (decompensated or chronic HF, hypertension, cardio-active drugs) and non-cardiac triggers (age, sex, emergent, and/or intraperitoneal surgery, mild pre-operative renal insufficiency, pre-operative creatinine elevation, CKD, and DM).549,550 The combination of a low cardiac output/high venous pressure and/or the administration of iodinated contrast media during diagnostic and operative procedures represent the most frequent causes of AKI in hospitalized cardiac patients, regardless of pre-existing impaired renal function. Contrast-induced AKI is defined as a rise in serum creatinine of 44 mmol/L (0.5 mg/dL) or a 25% relative rise from baseline at 48 h (or 5–10% at 12 h) following contrast administration. It occurs in up to 15% of patients with CKD who are undergoing radiographic procedures.551 Although most cases of contrast-induced AKI are self-limiting, with renal function returning to normal within 7 days of the procedure, these patients occasionally (0.5–12% of cases) develop overt renal failure associated with increased morbidity and mortality. To reduce the risk of contrast-induced AKI in subjects requiring contrast-enhanced radiography, the Kidney Disease: Improving Global Outcomes (KDIGO) Clinical Practice Guidelines recommend: pre-operative hydration with i.v. isotonic fluids; the use of the minimum volume of contrast media; and the use of low-osmolar or iso-osmolar contrast media, regardless of pre-existing renal disease.547 In the post-operative phase, kidney function should be monitored by applying established AKI scoring systems to raise awareness and enable early intervention.552
Recommendations for management of patients with renal disease undergoing non-cardiac surgery
 |
 |
Recommendations for management of patients with renal disease undergoing non-cardiac surgery
 |
 |
6.12. Obesity
The prevalence of people being overweight and having obesity is reaching epidemic proportions in Western countries557 and is the second leading cause of preventable death following tobacco use.558 Obesity is defined as a body mass index (BMI) of ≥30 kg/m2, morbid obesity as a BMI ≥35 kg/m2, and super-morbid obesity as a BMI ≥50 kg/m2. Obese individuals have a higher prevalence of CV risk factors and a higher risk of death,559 and are a population who are at increased risk of adverse events in the case of surgical procedures. There are specific recommendations for the pre-operative risk assessment of obese patients undergoing NCS, regardless of the presence of pre-existing cardiac conditions.560 However, while obesity accelerates the propensity for CVD, it seems that many types of CVD may have a better prognosis in the overweight population compared with their leaner counterparts, a phenomenon that is known as the ‘obesity paradox’.561,562 Similarly, in cases of NCS, mildly obese patients present lower mortality risk compared with underweight and normal weight patients, both post-operatively and at long-term follow-up.563 This finding may be related to the lower prevalence of PMI in mildly obese patients undergoing NCS.564
It has been suggested that cardiorespiratory fitness (CRF), rather than BMI, should be used to assess CV risk in obese patients. While the classification based on BMI is simple, highly reproducible, and widely adopted in clinical practice, it does not reflect fat distribution and body composition. Cardiorespiratory fitness refers to the ability of the circulatory and respiratory systems to supply oxygen to skeletal muscles during sustained physical activity, which is of paramount importance, especially in patients with cardiac diseases. The primary measure of CRF is VO2 max.565 A cohort study of nearly 10 000 patients with CAD followed for almost 15 years showed that those with relatively good CRF had favourable prognosis regardless of body composition;566 however, a lower CRF was found to be a major predictor of mortality, regardless of BMI.561,567 Whether specific optimization and/or treatment strategies might have a positive impact on the outcome of obese patients with pre-existing or newly diagnosed cardiac comorbidities and scheduled for NCS is a matter of ongoing controversy. Studies assessing the effect of weight loss interventions (low-energy diets with or without an exercise component) on clinical outcomes in patients undergoing NCS found inconsistent results in terms of peri-operative morbidity or mortality.568,569
Recommendations for management of patients with obesity undergoing non-cardiac surgery
 |
 |
Recommendations for management of patients with obesity undergoing non-cardiac surgery
 |
 |
6.13. Diabetes
Due to the progressive ageing of the population undergoing surgical procedures and the increasing prevalence of obesity worldwide, the prevalence of diabetes among patients undergoing NCS is expected to increase in years to come.4,571 Several studies have demonstrated that diabetic patients undergoing NCS have a higher prevalence of CAD than non-diabetic patients. Furthermore, patients with diabetes are more likely to have silent ischaemia because of altered neural pain pathways in the heart.572 For this reason, patients with diabetes appear to have a greater risk of post-operative myocardial ischaemia. Different reasons exist behind the relation between DM and increased peri-operative mortality in patients undergoing NCS. First, patients with diabetes are known to have more comorbidity and/or advanced CAD at the time of intervention. Second, diabetes is a clear risk factor for stroke. Diabetes is associated with post-operative congestive HF and wound infections. Many patients with diabetes have impaired renal function. The presence or a new diagnosis of impaired glucose metabolism in patients scheduled for NCS should follow the recommendations provided for the general population in the 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases,573 including increased awareness regarding possible subclinical organ damage.
The glycated haemoglobin (HbA1c) test should be performed in all patients with diabetes or impaired glucose metabolism scheduled for NCS, if this measurement has not been performed in the previous 3 months. There is evidence to support that pre-admission optimal treatment of hyperglycaemia in patients scheduled for elective NCS is effective in reducing the post-operative risk of CV events, including MI, stroke, and progression of HF.574 In contrast, no clear association has been shown between intra-operative blood glucose levels and the subsequent risk of surgical site infection, MI, stroke, and death in patients undergoing NCS.575 The risk of acidosis associated with metformin use is also debated.576 Nevertheless, repeated blood glucose monitoring on the day of surgery is recommended, with a general consensus to maintain peri-operative glucose levels <10.0 mmol/L without causing hypoglycaemia (target level 5.6–10.0 mmol/L). This can be achieved either with subcutaneous doses of rapid-acting insulin analogues or with i.v. insulin.577 Handling of SGLT2 inhibitors in relation to surgery has been discussed in Section 5.2.
Recommendations for management of patients with diabetes mellitus undergoing non-cardiac surgery
 |
 |
 |
 |
Recommendations for management of patients with diabetes mellitus undergoing non-cardiac surgery
 |
 |
 |
 |
6.14. Cancer
Due to their generally older age, cancer patients have a high prevalence of CV risk factors and CVD, being a population at increased risk of adverse events in cases of NCS. It is therefore important to optimize treatment of CV risk factors and known CVD before NCS, following the general and disease-specific recommendations provided in other sections of these guidelines. Furthermore, NCS may be particularly challenging in cancer patients because of previous administration of potentially cardiotoxic chemotherapy or fibrosis due to previous radiation. For example, the widely used anthracyclines have a dose-dependent relation with the incidence of HF and the use of trastuzumab can lead to important cardiotoxicity and should be taken into account in the pre-operative assessment. Furthermore, radiation therapy to areas that included the heart may lead to premature CAD and VHD; previous thoracic radiotherapy may predispose younger patients to heart disease who would otherwise not have an elevated risk. Post-operative AF is frequently observed in patients undergoing cancer surgery, with the highest incidence reported for lung surgery. Patients with cancer are at elevated risk of thrombosis due to both the disease itself and patient- and treatment-related factors. In a small study of patients undergoing planned open surgery for abdominal or pelvic cancer, enoxaparin prophylaxis for 4 weeks compared with 1 week reduced the incidence of thrombosis (4.8% in the enoxaparin group vs.12.0% in the placebo group; P = 0.02).582 Although later studies have shown somewhat conflicting results, the consensus is to recommend extending thromboprophylaxis after major abdominal and/or pelvic surgery for cancer to 4–5 weeks, with preferred use of LMWH.583 A summary of patient-related and cancer therapy-related factors that could influence peri-operative risk is shown in Table 15. Further information is available in the 2022 ESC Guidelines on cardio-oncology.584
Factors that could influence peri-operative risk during cancer surgery and preventive strategies
| . | Factors that could influence peri-operative risk during cancer surgery . | Preventive strategies . |
|---|---|---|
|
|
|
|
|
|
| . | Factors that could influence peri-operative risk during cancer surgery . | Preventive strategies . |
|---|---|---|
|
|
|
|
|
|
AF, atrial fibrillation; BTKi, Bruton tyrosine kinase inhibitors; CV, cardiovascular; CVD, cardiovascular disease; ECG, electrocardiogram; VEGFi, vascular endothelial grow factor inhibitor; VTE, venous thromboembolism.
Factors that could influence peri-operative risk during cancer surgery and preventive strategies
| . | Factors that could influence peri-operative risk during cancer surgery . | Preventive strategies . |
|---|---|---|
|
|
|
|
|
|
| . | Factors that could influence peri-operative risk during cancer surgery . | Preventive strategies . |
|---|---|---|
|
|
|
|
|
|
AF, atrial fibrillation; BTKi, Bruton tyrosine kinase inhibitors; CV, cardiovascular; CVD, cardiovascular disease; ECG, electrocardiogram; VEGFi, vascular endothelial grow factor inhibitor; VTE, venous thromboembolism.
6.15. Coronavirus disease 2019
Coronavirus disease 2019 (COVID-19) is an infectious disease caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). In a recent observational study, among 140 231 patients scheduled for NCS, 2.2% of patients had a pre-operative diagnosis of SARS-CoV-2 infection.585 Patients undergoing surgery with peri-operative or recent SARS-CoV-2 appear to be at increased risk of post-operative VTE and mortality compared with patients with no history of SARS-CoV-2 infection.586 Furthermore, the possible myocardial injury associated with COVID-19 infection might increase the intrinsic peri-operative risk of adverse cardiac events associated with surgical procedures.587,588
To date, there is no specific CV screening to be performed after COVID-19 infection in patients scheduled for NCS. The pre-operative evaluation of CV risk associated with NCS in this specific subset of patients should incorporate, beyond the general risk assessment outlined in these guidelines, the severity of COVID-19 disease, history of CV complications during COVID-19 infection, and functional capacity after recovery. This information is deemed of importance to further optimize timing of surgery and the treatment of COVID-19-associated cardiovascular conditions affecting the peri-operative risk of NCS.589
The optimal timing of NCS in patients with a history of COVID-19 infection is largely unknown. Generally, elective NCS should be postponed until complete recovery and optimization of coexisting comorbidities. Registry data of patients undergoing NCS after COVID-19 infection report increased risks of mortality and morbidity up to 7 weeks post-COVID diagnosis.586 Another study has reported greater risk of post-operative complications up to 8 weeks post-diagnosis.590 However, it should be noted that almost all available data come from study periods with zero to low prevalence of vaccination, and no robust data exist on patients recovering from the more recent Delta and Omicron variants.
A joint statement on elective surgery and anaesthesia for patients after COVID-19 infection from the American Society of Anaesthesiologists and Anaesthesia Patient Safety Foundation591 suggests delaying elective surgery for 7 weeks after a SARS-CoV-2 infection in unvaccinated patients who are asymptomatic at the time of surgery. The clinical course of COVID-19 infection, presence and intensity of cardiopulmonary symptoms during the acute and late phases, and pre-existing comorbidities should be taken into consideration. In addition, persistence of COVID-19 symptoms—such as fatigue, shortness of breath, and chest discomfort—should be given attention, as this condition is associated with higher post-operative mortality independent of the timing of COVID-19 diagnosis.588,589 The evidence is currently insufficient to make recommendations for those who become infected after vaccination.
7. Peri-operative monitoring and anaesthesia
7.1. Peri-operative monitoring
Despite the absence of prospective RCTs investigating the prognostic relevance of peri-operative monitoring, previous evidence clearly indicates that the routine use of monitoring improves safety of surgical procedures. Mandatory intra-operative monitoring of the CV and respiratory systems, temperature, neuromuscular transmission, and depth of anaesthesia is recommended.592,593
Routine CV monitoring includes ECG, automated non-invasive BP measurement at regular intervals, and peripheral oxygen saturation with pulse oximetry. Near-infrared spectroscopy has recently been introduced to assess regional tissue perfusion and oxygenation. In selected cases, more invasive monitoring techniques can be applied such as continuous arterial BP measurement via an arterial catheter and monitoring of cardiac output. Mean arterial pressure and heart rate remain stable, even with variation of up to 30% of total blood volume.594 Right heart catheterization can be used to continuously measure central venous pressure and/or pulmonary artery pressure, pulmonary artery wedge pressure (as a reflection of LV diastolic pressure), and cardiac output. However, these are static variables that do not reliably reflect the CV filling status, and have been shown to accurately guide fluid therapy in 50% of patients.595
While routine use of pulmonary catheterization is discouraged during NCS, using dynamic variables, such as stroke volume variation or pulse pressure variation, has become the gold standard. In addition, TEE is increasingly being used as an intra-operative monitoring technique in major surgery in cardiac compromised patients and during cardiac surgery. Decisions on the extent of peri-operative monitoring and implementation of specific strategies during NCS should always be based on an individual patient-directed assessment, taking into consideration the severity of surgery and the patient’s physical condition. Basic peri-operative monitoring of the respiratory system consists of pulse oximetry and capnography; both methods are non-invasive and easily applicable. Pulse oximetry enables in vivo measurement of arterial oxygen saturation, and capnometry measures end-tidal carbon dioxide concentration during inspiration and expiration; these monitors also provide information about the global haemodynamic status.
Patients, independent of the anaesthetic technique and information provided by the different monitoring systems, must be regularly controlled.596 In addition, blood loss and urine output should be checked, when appropriate, and overall clinical status.597 Special attention is required for the activation and setting of audible alarms, as inadequate use or failure to respond to intra-operative alarms may result in patient hazard and undesirable outcomes.598
7.2. Anaesthesia
The decision on the optimal peri-operative strategy should be based on close exchange of clinical information between anaesthesiologists, cardiologists, surgeons, and other relevant specialists. In addition, it is mandatory that any proposed strategy is presented to and discussed with the patient. An informed discussion with the patient describing the planned patient pathway and expectations during the pre-, peri-, and post-operative phases of care, and what to expect from staff and surroundings, should be given using a clear, concise, and simple description. The ESA published Pre-operative evaluation of adults undergoing elective noncardiac surgery: updated Guideline from the European Society of Anaesthesiology in 2018.560 This current section focuses on issues that are specifically important to patients with CV risk factors and diseases, taking into account the most recent developments in peri-operative management of these patients.
7.2.1. Intra-operative haemodynamics
Most anaesthetic techniques reduce sympathetic tone, leading to a decrease in venous return due to increased compliance of the venous system, vasodilatation, and decreased BP. Therefore, maintenance of adequate organ flow and perfusion pressure is of key importance in anaesthesiological management, especially in the CV-compromised patient. The importance of keeping stable peri-operative haemodynamics has been recognized for many years.599
In the past few years, several studies have focused on the relationship between intra-operative hypotension and post-operative patient outcome. A recent systematic review identified 42 studies looking at associations between various absolute and relative intra-operative hypotension definitions and post-operative adverse outcomes after NCS.214 The reported associations suggest that organ injury (myocardial injury, stroke, AKI) might occur when the mean arterial pressure decreases to <80 mmHg for ≥10 min, and that this risk increases with BP becoming progressively lower. However, most of the included studies had a retrospective observational design with a large variability in patient characteristics. In addition, the definitions of intra-operative hypotension varied widely across included studies. A recent study on the incidence of intra-operative hypotension as a function of the chosen cut-off definition described 48 different definitions of intra-operative hypotension. When applying these definitions to a cohort of 15 509 consecutive adult patients undergoing NCS under general anaesthesia, any episode of systolic BP <80 mmHg was found in 41% of the patients, and 93% of the patients had at least one episode of systolic BP >20% below baseline. The relation between threshold values from the literature and incidence of intra-operative hypotension showed a sigmoidal shaped cumulative incidence curve, with intra-operative hypotension occurrence frequencies varying from 5–99%.600 It seems that no universal target BP to define intra-operative hypotension can currently be defined. In addition, in studies on intra-operative hypotension, both the threshold to define hypotension and the method chosen to model intra-operative hypotension affected the association of intra-operative hypotension with outcome.601 As a consequence, different studies on intra-operative hypotension are uncomparable and clinical conclusions on reported results remain hazardous. A recent expert consensus statement concluded that intra-operative mean arterial pressures <60–70 mmHg are associated with myocardial injury, AKI, and death. These complications are a function of hypotension severity and duration.602 It remains to be established whether correction of intra-operative hypotension is also associated with improved post-operative outcome. To date, only one study has specifically addressed the question of whether an individualized BP management strategy reduces post-operative complications in a multicentre RCT including 292 patients. An individualized management strategy of targeting a systolic BP within 10% of the patient’s normal resting value resulted in significantly lower rates of post-operative organ dysfunction compared with standard practice (38.1% vs. 51.7%, respectively).528 These findings support the benefits of personalizing care, especially in surgical patients at high-risk of cardiac complications. It is important to underscore the importance of a physiopathological approach in understanding the underlying mechanisms of intra-operative hypotension, taking into account the extent and severity of the patient’s comorbidities; only then will a tailored treatment targeting the cause of intra-operative hypotension be possible (Figure 16).
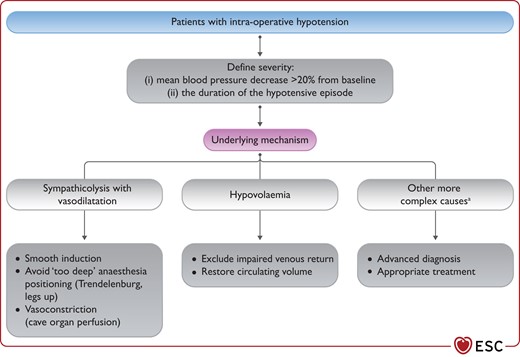
Pathophysiological approach to address intra-operative hypotension.
aE.g. peri-operative myocardial failure.
The severity of intra-operative hypotension is defined both by the threshold decrease from baseline and the duration of the hypotensive episode. The underlying mechanisms for intra-operative hypotension need to be identified: sympathicolysis with vasodilation, hypovolaemia, or other more complex causes such as peri-operative myocardial failure. Once the underlying mechanisms are identified, a targeted therapeutic strategy can be applied. This includes considering whether administration of specific chronic vasoactive medication such as ACEIs or ARBs should be interrupted 24 h prior to surgery.216 A detailed analysis of the strategies for the different chronic CV medication is discussed in Section 5. Of note, post-operative outcome is not only negatively influenced by the occurrence of intra-operative hypotension, but also by hypotensive events during the initial four post-operative days.603 For adult non-cardiac surgical patients, there is insufficient evidence to recommend a general upper limit of arterial pressure at which therapy should be initiated, although pressures >160 mmHg have been associated with myocardial injury and MI.602
Intra-operative tachycardia may adversely affect the myocardial oxygen balance and thus result in peri-operative myocardial injury. A retrospective analysis of 41 140 patients found that a heart rate ≥90 b.p.m. was associated with an increased risk of myocardial injury.604 These findings were similar to observations in the Vascular Events in Noncardiac Surgery Patients Cohort Evaluation (VISION) trial.605 An analysis of high-risk surgical patients found that an HR >87 b.p.m., recorded at rest before pre-operative cardiopulmonary exercise testing, was strongly associated with distinct CV phenotypes, which may explain the unintended, adverse consequences of non-personalized treatments aimed at reducing intra-operative tachycardia in isolation from other haemodynamic factors.606 Finally, a recent study evaluated the association between intra-operative tachycardia and a composite of post-operative myocardial injury and all-cause mortality. The major finding of this study was the lack of an association between intra-operative heart rate >90 b.p.m. and the composite outcome; HR >80 b.p.m. or >100 b.p.m. were also not associated with the composite outcome.607 Taken together, the assumed association between intra-operative tachycardia and adverse peri-operative outcome remains a subject of ongoing controversy. However, it seems advisable to consider intra-operative tachycardia as an indicator of haemodynamic impairment.
7.2.2. Choice of anaesthetic agent
The choice of the anaesthetic agent has been considered to be of little importance in terms of patient outcome, provided that vital functions are adequately supported. Evidence from surgical myocardial revascularization studies indicates that a volatile vs. i.v. anaesthetic regimen is associated with less post-operative troponin release without impact on clinical outcomes.599,608 A recent large multicentre randomized trial found a neutral effect on 12 month mortality associated with volatile vs. i.v. anaesthetic regimen.609 In NCS, incidence of post-operative cardiac events is not influenced by the choice of a volatile or an i.v. anaesthetic regimen.610
7.3. Locoregional techniques
The analgesic effects of neuraxial analgesia are well-established. The main peri-operative indications for epidural analgesia include major open abdominal surgery and thoracotomy. Possible additional benefits of epidural analgesia, such as accelerated recovery and decreased post-operative complications, remain a matter of debate.611,612
Neuraxial analgesia may induce sympathetic blockade. When reaching the thoracic dermatome level 4, a reduction in cardiac sympathetic drive may occur, with subsequent reduction in myocardial contractility, heart rate, and change in cardiac loading conditions. There are no studies specifically investigating the changes in outcomes related to neuraxial anaesthetic techniques in patients with cardiac disease. Cardiac patients often take various types of drugs that interfere with coagulation; therefore, care should be taken to ensure sufficient coagulation ability when neuraxial blocks are applied.613
Current research is focusing on alternatives for neuraxial analgesia with similar effects on peri-operative pain control in patients with cardiac comorbidities undergoing NCS; these include alternative analgesic techniques such as i.v. analgesia, continuous wound infiltration, paravertebral bloc, and selective nerve blocks.
7.4. Peri-operative goal-directed haemodynamic therapy
Goal-directed therapy aims to optimize CV performance, in order to achieve normal or even supranormal oxygen delivery to tissues, by optimizing pre-load and inotropic function using pre-defined haemodynamic targets. In contrast to clinical signs or arterial pressure-orientated standard therapy, goal-directed therapy is based on flow or fluid responsiveness of haemodynamic variables, such as stroke volume, response to fluid challenges, stroke volume or pulse pressure variation, or similar optimization of cardiac output. Goal-directed therapy was initially based on the use of a pulmonary artery catheter. Less-invasive techniques have recently been developed, including: transoesophageal Doppler, transpulmonary dilution techniques, and advanced pressure waveform analysis. Early goal-directed fluid therapy—in the right patient cohort and with a clearly defined protocol—has been shown to decrease post-operative mortality and morbidity.614–618
7.5. Post-operative management
Several studies have demonstrated that it is possible to stratify the risk of post-operative complications and mortality with a simple surgical Apgar score. This post-event stratification might enable patients to be redirected to higher-intensity care units. The importance of such risk stratification is underscored by the results of the EuSOS group. In this 7 day cohort study, 46 539 consecutive adult NCS patients in 498 hospitals across 28 European nations were included: 1855 patients (4%) died before hospital discharge and 1358 (73%) of those patients were not admitted to critical care at any stage after surgery.7 This concept of failure-to-rescue has gained a lot of attention in peri-operative medicine in the last few years and strategies have been proposed to address this issue.619–622
Severe post-operative pain occurs in 5–10% of patients, increases sympathetic drive, and delays recovery.623,624 A recent study demonstrated that time-weighted average pain scores within 72 h after surgery were significantly associated with myocardial injury in patients undergoing NCS;625 this finding underscores the importance of effective post-operative analgesia to reduce post-operative CV risk.
The place of non-steroidal anti-inflammatory drugs (NSAIDs) in the treatment of post-operative pain in cardiac patients undergoing NCS is a point of debate. Diclofenac has been shown to carry the highest CV risk of any of the non-selective NSAIDs.626,627 The CV risk of ibuprofen seems to be comparable with that of celecoxib.628 Naproxen has a better CV safety profile than diclofenac and ibuprofen.629,630 One randomized trial found that parecoxib and valdecoxib did not increase thromboembolic events in patients undergoing NCS. A meta-analysis of 32 randomized trials did not find an increased CV risk when comparing parecoxib/valdecoxib with placebo,631 and a single-centre observational study with >10 000 patients undergoing arthroplasty found no association between NSAID use and post-operative MI.632 In contrast, another meta-analysis of three randomized trials including 2604 major surgery patients detected a 2.3-fold increase in MACEs in the group with COX-2 inhibitors. In a position paper, The ESC Working Group on Cardiovascular Pharmacotherapy advises that non-aspirin NSAIDs should generally not be used in patients with established or at high risk of CVD.633
Recommendations for peri-operative monitoring and anaesthesia
 |
 |
Recommendations for peri-operative monitoring and anaesthesia
 |
 |
8. Peri-operative cardiovascular complications
Specific challenges apply to detecting CV complications that occur peri-operatively. First, due to anaesthesia and analgesia, PMI, which is the most common CV complication, is largely asymptomatic in ∼90% of patients and is therefore missed in routine clinical practice in the absence of surveillance for PMI.41,101,111,413,636–641 Second, post-operative pain, nausea, surgical wounds, and drains may interfere with the early identification of acute cardiac disorders, such as PMI, Takotsubo syndrome, tachyarrhythmias, and acute HF. Third, cardiologists are usually not directly involved in post-operative care; therefore, the early detection and early treatment of cardiac complications is performed by non-cardiologists, sometimes with little training in the early detection of acute cardiac disorders. Given the relatively high prevalence of cardiac complications, their high morbidity and mortality, and the availability of effective therapy, high awareness combined with surveillance for PMI in high-risk patients (known CAD, PAD, insulin-dependent DM, or symptoms suggestive of cardiac disorders) undergoing intermediate- or high-risk NCS is recommended to overcome these challenges.41,101,109–111,118,413,636–639,642,643 All measures need to be carefully aligned with the responsible surgeon.
Chronic cardiac disorders, such as CAD, seem to provide a substrate for cardiac complications during and after surgery.41,413,636,637 Several related chronic conditions (e.g. diabetes and renal insufficiency), which are likely to be surrogates for undiagnosed cardiac disease, are also strongly associated with peri-operative cardiac complications (Figure 17).41,413,636,637
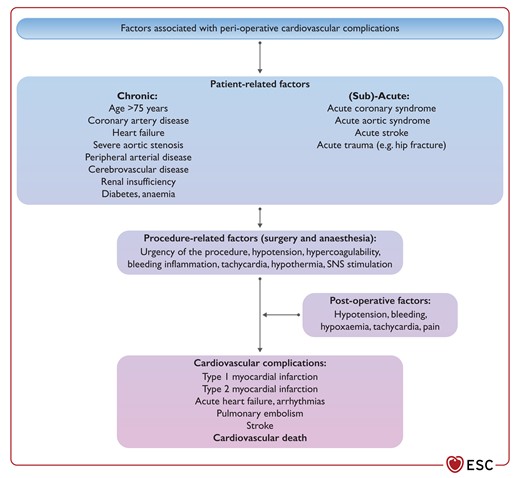
Factors associated with peri-operative cardiovascular complications. SNS, sympathetic nervous system.
Acute conditions such as trauma, surgery, and anaesthesia itself induce activation of the sympathetic nervous system, inflammation, stress, hypercoagulable, and catabolic states, all of which may trigger cardiac complications.41,413,636,637 While the risk of CV complications after NCS is highest in the immediate peri-operative period, it seems to remain increased for a prolonged ‘vulnerable period’ of 3–5 months.8
8.1. Peri-operative myocardial infarction/injury
Peri-operative MI (PMI) is defined as acute cardiomyocyte injury (post-operative hs-cTn T/I release) with or without accompanying symptoms, and with or without ECG or imaging evidence of acute myocardial ischaemia. Peri-operative MI can only be reliably and rapidly detected using PMI surveillance with hs-cTn T/I measurements before and serially after surgery (e.g. 24 and 48 h post-operatively). In the BASEL-PMI study, circa 15% of patients with pre-existing CAD/PAD or aged >65 years undergoing major NCS developed PMI.8 As most PMI occurs during the operation itself or in the immediate post-operative period, during which high doses of anaesthetics and/or analgesics are required, ∼90% of patients with PMI do not report typical symptoms and are therefore missed in routine clinical practice.41,101,111,413,636–639 This is of major concern, as the mortality risk associated with PMI is also high in patients without symptoms.41,101,111,413,636–639 Similarly, the mortality risk associated with PMI is high in patients without additional ECG and/or imaging evidence of myocardial ischaemia.8,41,101,109–111,118,413,564,636–639,641 Overall, 30 day mortality in patients developing PMI is ∼10%.8,41,101,109–111,118,413,564,636–639,641 No single intervention has yet been proven to be unequivocally beneficial in the prevention of PMI.185,644
It is important to highlight that PMI is not a homogenous disease. Several different pathophysiologies and clinical phenotypes may underlie PMI (Figure 18). At least one additional criterion (ischaemic pain; ischaemic ECG changes; imaging evidence of new loss of viable myocardium, or new regional wall motion abnormality in a pattern consistent with an ischaemic aetiology; and thrombus in coronary angiography) is required for patients with PMI to also meet the definition of peri-operative myocardial infarction, according to the fourth universal definition.643 Because is often initially unclear whether the patient will end up fulfilling the criteria for peri-operative myocardial infarction, the broad term PMI is preferred in the initial assessment. In order to properly interpret the aetiology of elevated post-operative hs-cTn T/I concentrations, a baseline pre-operative concentration is necessary to determine whether the increase is acute or chronic (see Section 4).643 To identify the underlying pathophysiology and define causal therapy, systematic work-up and early differentiation of primarily non-cardiac causes (e.g. severe sepsis, PE) vs. the different cardiac causes—including type-1 MI, type-2 MI, tachyarrhythmia, and acute HF—is of major importance (Figures 18 and 19). Transthoracic echocardiography is helpful in the work-up of most patients with PMI.
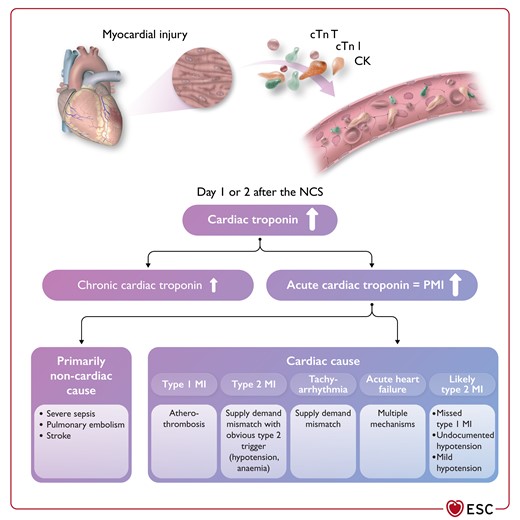
Differential diagnosis of elevated post-operative cardiac troponin concentrations.
CK, creatinine kinase; cTn l, cardiac troponin I; cTn T, cardiac troponin T; MI, myocardial infarction; PMI, peri-operative myocardial infarction/injury. Please be aware that the accuracy of physicians’ judgement in the classification of type-1 vs. type-2 MI in the peri-operative setting may be lower vs. the non-operative setting.647
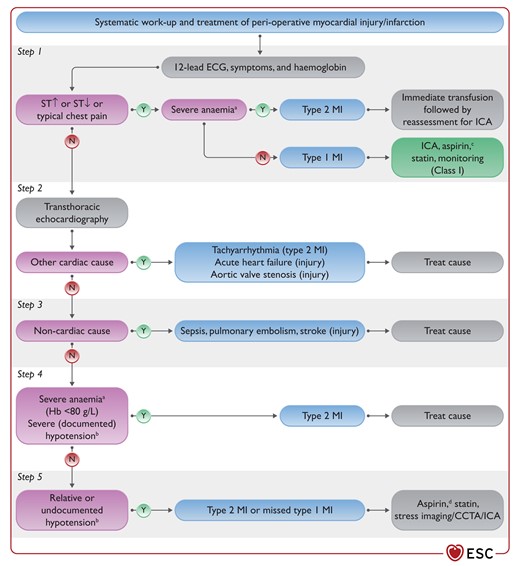
Systematic work-up (aetiology) and therapy of peri-operative myocardial infarction/injury.
CCTA, coronary computed tomography angiography; ECG, electrocardiogram; Hb, haemoglobin; ICA, invasive coronary angiography; MI, myocardial infarction; N, no; ST, ST-segment. Y, yes; Most patients with type-2 MI and silent type-1 MI should be scheduled for stress imaging or CCTA/ICA as outpatients after discharge, depending on symptoms prior to or after surgery and known CAD. aOr active bleeding. bOr other type-2 MI trigger such as hypoxaemia, tachycardia, hypertension. cDual antiplatelet therapy after coronary stenting. dPossibly in combination with dabigatran 110 mg b.i.d.
The term myocardial injury following NCS (MINS) has been used for a selected subset of patients with cardiac PMI, in whom cardiomyocyte injury was deemed most likely due to CAD with myocardial ischaemia (i.e. secondary to supply–demand mismatch or thrombosis), in the absence of an atypical surgical physiological stress, and no evidence of a cardiac non-CAD aetiology, e.g. rapid AF, acute HF).101,109,638,641,642,645,646 In approximately half of all patients with PMI, the underlying pathophysiology cannot be reliably ascertained based on the commonly available documentation and is assumed to be likely type-2 MI due to undocumented or relative hypotension, but may also include missed type-1 MI, or missed PE in cases in which CT angiography has not yet been performed post-operatively.110,647 It is therefore important to highlight that PMI surveillance also facilitates the detection of primarily non-cardiac disorders with immediate therapeutic consequences, such as PE, that would otherwise have been missed.
The prevalence of PMI depends on patient-related, procedural-related, and post-operative factors, and the required minimum extent of acute myocardial injury, quantified by absolute increase in hs-cTn T/I (e.g. the 99th percentile ULN) above the pre-operative hs-cTn T/I concentration.8,41,101,109–111,118,413,564,636–639,641,648 An absolute increase in more than the ULN above pre-operative concentrations has consistently been shown to be associated with a relevant increase in 30 day and long-term mortality, and other non-fatal post-operative cardiac complications, and can easily be determined for each hs-cTn T/I assay. This threshold is therefore recommended for clinical use.8,41,101,109–111,118,413,564,636–639,641,648 However, further studies are warranted regarding optimal thresholds. Emerging data suggest that surveillance for PMI is cost-effective.117,119
Identification of the most likely pathophysiology is critical for selection of the most appropriate therapy. Evidence from a large single-centre retrospective cohort suggests that involvement of a cardiologist in the work-up and therapy of these patients was associated with lower mortality.649 Mortality substantially differs among the different phenotypes: 30 day all-cause mortality and the composite of 30 day CV death, life-threatening arrhythmia, acute MI occurring after day 3, and cardiac decompensation are highest in patients with acute HF and primarily extra-cardiac PMI, such as severe sepsis or PE, intermediate for type-1 MI and tachyarrhythmias, and modestly elevated in likely type-2 MI.110 Type-2 MI patients are usually treated as for type-1 MI, although the evidence for this is limited.
In a randomized, placebo-controlled trial, 1754 patients (mean age 70 years) who had developed MINS after NCS (mainly orthopaedic, general, and vascular surgery) were randomly assigned (1:1) to receive dabigatran 110 mg orally b.i.d. or matched placebo within 35 days of MINS;650 60% of patients were already on aspirin or a P2Y12 inhibitor. The median peak measured hs-cTn concentration associated with the diagnosis of MINS was 82 ng/L. More than 90% of MINS events occurred without a clinical symptom or sign of cardiac ischaemia. Dabigatran/placebo was initiated a median of 6 days after the operation and the average time on study drug was ∼9 months. Among patients with MINS randomly assigned to receive dabigatran (n = 877) or placebo (n = 877), the composite primary efficacy outcome of a major vascular complication—including vascular mortality, MI, non-haemorrhagic stroke, peripheral arterial thrombosis, amputation, and symptomatic VTE—occurred in fewer patients randomized to dabigatran than placebo (97 [11%] of 877 patients assigned to dabigatran vs. 133 [15%] of 877 patients assigned to placebo; HR, 0.72; 95% CI, 0.55–0.93; P = 0.0115). There was no increase in major bleeding. Based on these data, in patients with MINS and at low risk of bleeding, initiation of dabigatran 110 mg orally b.i.d. may be considered about 1 week after NCS.
8.2. Spontaneous myocardial infarction (after day 2)
The incidence of post-operative spontaneous MI after day 2 seems to be about 0.5% within 30 days, and 1–2% within 365 days for patients undergoing major NCS with established CAD, PAD, or aged >65 years.8 In the immediate post-operative period (<5 days), bleeding is a major concern and limits the use of antiplatelet and anticoagulant therapy, depending on the site and extent of surgery. Otherwise, the same principles as for MI therapy should generally be applied following the recommendations of speciality guidelines.98,171
8.3. Takotsubo syndrome
The incidence of peri-operative Takotsubo syndrome remains unknown, as none of the studies with PMI screening used echocardiography in all patients during PMI work-up. Increased awareness in the non-operative setting has led to a substantial increase in the detection of Takotsubo syndrome, and the use of TTE in the work-up of PMI is strongly encouraged. Anecdotal evidence suggests that it is likely that the incidence is also higher than expected in the peri-operative setting.8,41,101,109–111,118,413,564,636–639,641,644
8.4. Acute heart failure
The incidence of post-operative acute HF seems to be 1–2% within 30 days and 4–6% within 365 days in patients with established CAD, PAD, or aged >65 years undergoing major NCS.8 Pre-existing diagnosed or undiagnosed chronic HF and volume loading in the peri-operative and post-operative periods are important contributors. In the absence of studies specifically investigating acute HF post-operatively, the general principles of acute HF diagnostic work-up and therapy should be applied.651
8.5. Venous thromboembolism
The incidence of VTE in the peri-operative phase is currently unknown and likely underreported due to the lack of systematic screening methods and the limited validity of diagnostic tools (e.g. D-dimer, typical pain symptoms) in this setting. It is associated with high peri-operative mortality (∼17%).322 Risk factors for post-operative VTE/PE include type of surgery (e.g. high-risk hip arthroplasty, open prostatectomy, open surgery for malignancy), acute renal insufficiency, MI, and post-operative infection.322 Stratification of the extent of embolism (e.g. massive, submassive, and subsegmental; high risk, intermediate high/low risk, and low risk) is important to predict mortality and guide therapeutic strategy.652 Pulmonary embolism should be suspected in patients with PMI without a clear cause. Close haemodynamic monitoring and monitoring of RV function (echocardiography, CT) is essential to determine which PE patients require aggressive therapy. There is currently a lack of evidence regarding adequate antithrombotic therapy in patients with post-operative PE, since recent major surgery or trauma was a contraindication in previous trials of thrombolytic or anticoagulant therapy.653 Small case series support the use of systemic thrombolysis, surgical thrombectomy,654 or catheter-directed therapies in massive PE. In general, anticoagulation, preferably LMWH or fondaparinux, should be initiated as early as possible.652 Oral anticoalgulant therapy, preferably NOAC due to the lower bleeding risk, should be initiated, depending on post-operative renal function and bleeding risk, as early as possible for at least 3 months.652
Rescue thrombolytic therapy is recommended for patients with PE and haemodynamic deterioration on anticoagulation treatment in the post-operative phase, if possible, according to bleeding risk.652 As an alternative to thrombolytic therapy for massive PE, surgical embolectomy or percutaneous catheter-directed treatment should be considered for patients with haemodynamic deterioration on anticoagulation treatment, particularly in patients with high bleeding risk.
8.6. Atrial fibrillation and other relevant arrhythmias
Post-operative AF is defined as new-onset AF in the immediate post-operative period; its incidence ranges between 2–30%, with peak incidence 2–4 days post-operatively.655,656
Although many post-operative AF episodes are self–terminating and some are asymptomatic, post-operative AF has been associated with a four- to five-fold risk of AF recurrence in the 5 years following cardiac surgery, while the risk of recurrence after NCS is less well described.656–660 Importantly, post-operative AF is a risk factor for stroke, MI, and death compared with non-post-operative AF patients.656,658,661 Post-operative AF may also lead to haemodynamic instability, prolonged hospital stay, infections, renal complications, bleeding, increased in-hospital death, and greater healthcare costs.662–664 The essential principles of the prevention and management of post-operative AF are outlined in Figure 20.
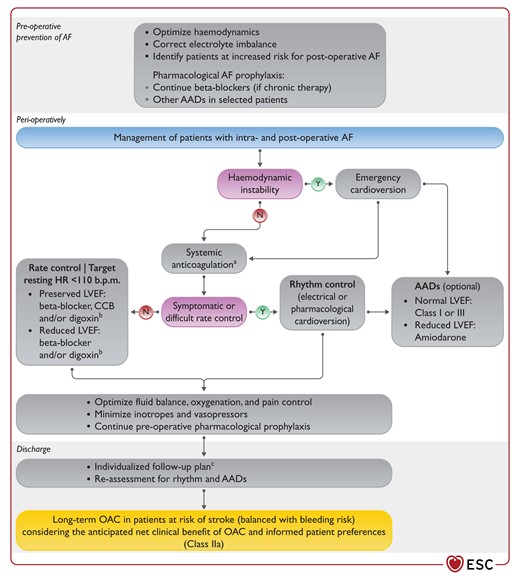
Prevention and management of post-operative atrial fibrillation.
AAD, antiarrhythmic drug; AF, atrial fibrillation; b.p.m., beats per minute; CCB, calcium channel blocker; HR, heart rate; LVEF, left ventricular ejection fraction. N, no; Y, yes. aDepending on the CHA2DS2VASC-score, and post-operative bleeding risk. bIn the acute post-operative phase, unless blood pressure is high, combination of low-dose beta-blocker and loading with digoxin is preferred to avoid hypotension. cShould include a cardiology visit before month 3. Adapted from the 2020 ESC Guidelines on the Diagnosis and Management of Atrial Fibrillation.99
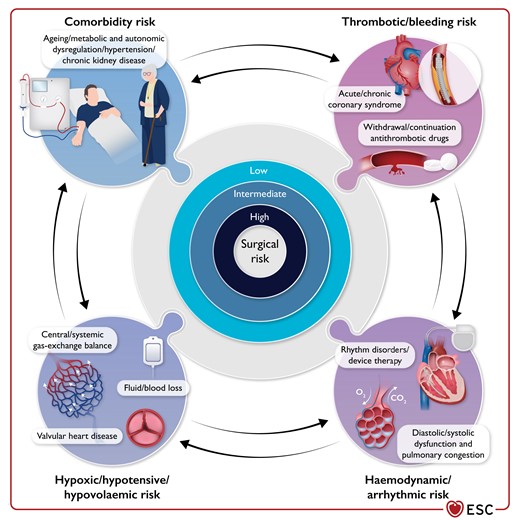
Central illustration: the complex interplay between the intrinsic risk of surgery and the patient risk of peri-operative cardiovascular complications.
8.6.1. Prevention of post-operative atrial fibrillation
Pre-operative use of beta-blockers is associated with reduced incidence of post-operative AF,204,665–667 but not major adverse events such as death, stroke, or AKI.668 Importantly, in a large RCT, peri-operative metoprolol was associated with increased mortality risk.185 In a meta-analysis, amiodarone (oral or i.v.) was equally effective in reducing post-operative AF as beta-blockers,207 whereas their combination was better than beta-blockers alone.208 Lower cumulative doses of amiodarone (<3000 mg) may be effective, with fewer adverse events.669–671 Data for other interventions—such as statins,672,673 magnesium,674 sotalol,666 colchicine,675 or corticosteroids676—are not robust.677,678
8.6.2. Management of post-operative atrial fibrillation
8.6.2.1. Rate and/or rhythm control
In haemodynamically unstable patients with post-operative AF, an emergent electrical or possibly pharmacological (i.e. i.v. administration of amiodarone666 or vernakalant,679 if consistent with the clinical situation) cardioversion is indicated.99 In haemodynamically stable patients with post-operative AF, ensuring optimal ventricular rate control during the arrhythmia is mandatory (using, for example beta-blockers or verapamil, as needed). As post-operative AF is often self-terminating, non–emergent cardioversion may not be needed. If performed in patients with AF lasting ≥48 h, non-emergency cardioversion of post-operative AF should follow the principles of pericardioversion thromboprophylaxis (that is, can be performed only after the left atrial thrombosis is excluded by TEE or postponed for 3 weeks of therapeutic OAC therapy). Of note, a RCT of patients with post-operative AF after cardiac surgery showed no net clinical advantage of rhythm (i.e. achieving and maintenance of sinus rhythm) vs. rate control strategy.680 Therefore, rate or rhythm control treatment decisions in patients with haemodynamically stable post-operative AF should be based on patient’s symptoms and shared informed treatment decision-making.99
8.6.2.2. Prevention of atrial fibrillation-related thromboembolic complications
In all patients with post-operative AF, it should be considered to initiate therapeutic anticoagulation as soon as possible during in-hospital treatment, depending on the individual stroke risk (CHA2DS2-VASc) and the bleeding risk after surgery. In a large meta-analysis, patients with post-operative AF had 62% higher risk of early stroke and 37% higher risk of long-term stroke compared with patients without post-operative AF (long-term stroke rates were 2.4% in post-operative AF vs. 0.4% in patients without AF), and a 44% and 37% higher risk of early and long-term mortality, respectively.661 Importantly, post-operative AF was more strongly associated with the long-term stroke risk in patients undergoing NCS (HR, 2.00; 95% CI, 1.70–2.35) than in patients undergoing cardiac surgery (HR, 1.20; 95% CI, 1.07–1.34; P < 0.0001).661
Evidence on the effects of long-term OAC therapy for the prevention of stroke or systemic embolism in patients with post-operative AF is from observational studies.664,681–685 In a recent study, post-operative AF following NCS was associated with similar long-term thromboembolic risk as common, non-surgical AF, and OAC use was associated with comparable lower risk of thromboembolism and all–cause mortality in both groups (mean CHA2DS2-VASc-score 3.0 ± 1.7).686
Based on the available evidence, long-term OAC should be considered in all patients with post-operative AF at risk of stroke. If anticoagulation is parenterally initiated, LMWH or fondaparinux is recommended (over UFH) for most patients. Non-vitamin K antagonist oral anticoagulants should be preferred over VKA for long-term treatment. Re-evaluation of the continuation of OAC may be performed after a period of 3 months. A small RCT (ASPIRE-AF; NCT03968393) on the optimal long-term OAC use among NCS patients developing post-operative AF is ongoing.
8.7. Peri-operative stroke
With respect to NCS, peri-operative stroke has been reported in 0.08–0.70% of patients undergoing general surgery, 0.2–0.9% of patients requiring orthopaedic surgery, 0.6–0.9% of lung operations, and 0.8–3.0% of surgeries involving the peripheral vasculature.687,688 The associated mortality ranges from 18–26%.687,688 A more recent analysis of 523 059 patients undergoing NCS reported a lower incidence of peri-operative stroke (0.1%), although the occurrence of this adverse event was associated with an eight-fold increase in peri-operative mortality within 30 days, corresponding to an absolute risk increase of >20%.689 Peri-operative stroke is mainly ischaemic or cardioembolic, and AF is often the leading underlying condition. Triggers include withdrawal of anticoagulation and the hypercoagulable state related to surgery. Additional aetiologies include atheroembolism, originating from the aorta or the supra-aortic vessels, and local atherothrombosis in the presence of intracranial small vessel disease. Hypoperfusion—related to peri-operative arterial hypotension and/or severe stenosis of the cervicocranial vessels—is an unusual cause of peri-operative stroke.690 Rarely, peri-operative stroke may be due to air, fat, or paradoxical embolisms.
In an attempt to attenuate the risk of peri-operative stroke, antiplatelet/anticoagulant treatments should be continued whenever possible throughout the peri-operative period. Alternatively, the period of drug withdrawal should be kept as short as possible, while weighting thromboembolic and haemorrhagic risks (see Section 5.2). Adequate selection of the anaesthetic technique (regional vs. neuraxial vs. general anaesthesia), prevention and treatment of AF, euglycaemic control (avoiding both hyperglycaemia and hypoglycaemia), and meticulous peri-operative control of BP may all contribute to reducing the risk of peri-operative stroke.
If post-operative stroke occurs, it must trigger immediate action: angio-CT and neurology/neurosurgical consultation with the goal to restore flow in the case of acute thrombotic occlusion.
Recommendations for peri-operative cardiovascular complications
 |
 |
 |
 |
Recommendations for peri-operative cardiovascular complications
 |
 |
 |
 |
9. Key messages
The occurrence of CV complications in the peri-operative phase of NCS has a dramatic impact on prognosis.
The risk of CV complications in patients undergoing NCS is determined by patient-related factors, type of surgery or procedure, and the circumstances under which surgery takes place (elective vs. emergency procedure; local or tertiary hospital).
Specific patient-related risk factors may be reduced by adequate pre-operative risk assessment and initiation of effective risk-reduction strategies.
The quantification of surgical risk as low, intermediate, and high is helpful in identifying the group of patients who should most benefit from preventive, diagnostic, and therapeutic approaches to concomitant CV conditions.
Proper selection of type and timing of the surgical procedure may reduce the risk of complications.
It is important that patients’ values, quality of life, and preferences regarding the benefits and risks of surgery are taken into consideration, and that well-informed patients are involved in the decisions. Risk should be communicated to the patient in absolute terms (e.g. 1 out of 100).
Clinical examination, patient-reported functional capacity, and non-invasive tests represent the cornerstone of pre-operative cardiac assessment.
Instrumental and functional cardiac examination tools should be selected in view of the surgical risk, relative diagnostic proficiency, and healthcare resource utilization and costs.
The peri-operative evaluation of elderly patients who require elective major NCS should include frailty screening, which has proven to be an excellent predictor of unfavourable health outcomes in the older surgical population.
Treatment of pre-existing or newly diagnosed CV conditions (e.g. coronary and peripheral vascular disease, rhythm disorders, and HF) should be individualized according to the pre-operative risk of NCS, and considering the recommendations of speciality guidelines.
A multidisciplinary approach to evaluate whether the treatment of concomitant cardiac conditions before scheduled NCS improves peri-operative safety without unnecessary delay is encouraged.
Efficient peri-operative management of antithrombotic therapies in patients scheduled for NCS aims to offer the potential benefit of preventing thrombotic events without excessive bleeding complications.
It is important to clearly and concisely communicate with patients, with simple verbal and written instructions, about changes in medication in the pre- and post-operative phases.
Management in the peri-operative phase of NCS aims to avoid haemodynamic imbalance, while ensuring sufficient cardioprotective action.
Healthcare providers are recommended to have high awareness of peri-operative CV complications combined with surveillance for PMI in high-risk patients undergoing intermediate- or high-risk NCS.
Routine assessment of treatment quality through specific indicators is important to document and measure the success of preventive and therapeutic strategies in patients undergoing NCS.
10. Gaps in evidence
The age cut-off for individuals (considered to be cardiovascularly healthy) benefiting from risk stratification work-out before NCS needs to be evaluated.
Further studies are needed to characterize outcome differences in NCS between men and women, and between different countries, in order to individualize peri-operative management and improve patient safety.
Evidence on the additive value of cardiac biomarkers, hand-held ultrasound, problem FOCUS, and stress echocardiography for cardiac risk stratification of patients scheduled for NCS presenting with previously unknown cardiac murmur, dyspnoea, oedema, and chest pain is still lacking. The impact of FOCUS on outcomes of urgent or time-sensitive surgery needs further investigation.
The impact of stress imaging (echocardiography or MRI) before NCS on reduction of peri-operative CV complications in non-ischaemic heart diseases needs further research.
The role of right heart catheterization in patients with advanced HF or patients with severe pulmonary hypertension undergoing NCS is unknown.
It is unknown whether artificial intelligence-based systems facilitate prompt detection and response to imminent adverse events in high-risk cardiac patients undergoing high-risk NCS.
Systematic and structured research to investigate pathophysiology, causes, and time distribution of serious peri-operative arrhythmic events among patients undergoing NCS is still needed.
Strategies for timing of pre-operative CIED control dependent on device type, urgency, and type of NCS, and risk of EMI during NCS need to be developed to ensure maximal patient safety.
Benefit of routine myocardial revascularization of high-risk CCS patients (except left main or three-vessel CAD, reduced LV function) before elective intermediate- and high-risk NCS is not well-established.
More evidence regarding the need for bridging of anticoagulation in patients with MHVs is needed.
There is a lack of evidence regarding the optimal strategies before emergent or time-sensitive NCS for patients on antithrombotic treatment at high risk of thromboembolic events, including the: (i) use of extracorporeal haemoperfusion or NOAC antidotes (ongoing trial NCT04233073); (ii) use of albumin, extracorporeal haemoperfusion, or PB2452-specific antidote to antagonized ticagrelor (ongoing trial NCT04286438 for PB2452); and (iii) premature cessation or bridging during interruption of oral P2Y12-receptor inhibitors (glycoprotein IIb/IIIa receptor inhibitors or cangrelor).
There is lack of well-powered studies to evaluate the role of platelet function testing to guide the strategy for treatment of NCS patients on antiplatelet therapy.
Evidence regarding the need for and benefit of anticoagulation in NCS patients with post-operative AF is still lacking (ongoing ASPIRE-AF trial: NCT03968393).
Prophylactic strategies to reduce the incidence of post-operative AF in NCS patients additional to beta-blocker maintenance in patients already on this treatment need to be evaluated.
The optimal cardiac work-up and therapy for patients with PMI within and outside hospital settings need to be evaluated.
Studies are needed to investigate the impact of the treatment of peri-operative hypotension on post-operative outcomes, the use of new HF drug classes (SGLT2 inhibitors and vericiguat), and the use of NSAIDs as a temporary treatment for acute post-operative pain.
Prospective studies are needed to investigate the incremental value of anaemia algorithms and blood-sparing strategies (use of blood-sparing blood tubes) to reduce the risk of anaemia-associated adverse outcomes among CV patients undergoing NCS.
11. Sex differences
Sex and gender may significantly affect the management and outcomes of patients with specific diseases undergoing NCS. There are sex- and gender-dependent clinical phenotypes of comorbidities and risk factors, which may have an impact on peri-operative morbidity and mortality. However, there is a paucity of data specifically addressing the interplay between sex, age, and comorbidities in patients scheduled for NCS.
The pre-operative assessment before NCS might take sex into consideration, since the age-adjusted incidence of CVD is lower in women than men, and the risk of undetected disease could therefore be lower in women. However, no data exist on sex-specific assessment strategies.
The in-hospital mortality during surgery was recently reported to be lower in women than men.41,691 In contrast, among 609 735 patients who underwent elective NCS between 2009 and 2016, the odds of post-operative 90 day mortality were higher among women with HF than men with HF.692 Further studies are needed to provide more information about outcome differences between men and women in NCS.
Some studies have reported a higher risk of bleeding in women than men, but other studies could not confirm this. No trials have systematically investigated the impact of sex differences regarding efficacy and safety of continuation vs. interruption of antithrombotic therapy in patients undergoing NCS.
The prevalence of anaemia in women of reproductive age is as high as 30% (WHO Global Anaemia estimates),693 resulting in millions of women undergoing surgery every year despite pre-operative anaemia. Furthermore, since women have lower blood volumes and lower haemoglobin values than men, but face the same surgical blood loss as men, they are exposed to far higher risk of post-operative complications. Also, higher transfusion rates and volumes have been reported in women compared with men in elective surgery.694 It is therefore of particular importance that clinicians follow the Patient Blood Management program in women undergoing NCS. Other sex-related differences in physiology, and pharmacokinetics and pharmacodynamics of anaesthetic drugs may influence the anaesthesia plan, pain management, post-operative recovery, and patient satisfaction.
Sex differences regarding presentation, electrophysiological substrate, complications, or long-term outcomes have been reported in patients undergoing CIED implantation,695,696 and female sex is a well-known risk factor for stroke in patients with AF.697 However, no specific data exist that suggest sex differences in risk profile or outcomes of patients with CIEDs or arrhythmias who undergo NCS.
12. ‘What to do’ and ‘what not to do’ messages from the Guidelines
13. Quality indicators
Quality indicators (QIs) are tools that may be used to evaluate care quality, including structural, process, and outcomes of care.698 They may also serve as a mechanism for enhancing adherence to guideline recommendations, through associated quality improvement initiatives and the benchmarking of care providers.699,700 As such, the role of QIs in improving care and outcomes for CV disease is increasingly being recognized by healthcare authorities, professional organizations, payers, and the public.698
The ESC understands the need for measuring and reporting quality and outcomes of CV care and has established methods for the development of the ESC QIs for the quantification of care and outcomes for CV diseases.698 To date, the ESC has developed QI suites for a number of CV diseases701–703 and embedded these in respective ESC Clinical Practice Guidelines (2020 ESC Guidelines for the diagnosis and management of atrial fibrillation; 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure; and 2021 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy).99,412,481,704 Furthermore, the ESC aims to integrate its QIs with clinical registries such as the EURObservational Research Programme (EORP) and the European Unified Registries On Heart Care Evaluation and Randomized Trials (EuroHeart) project705 to provide ‘real-world’ data about the patterns and outcomes of care for CV disease across Europe.
In parallel with the writing of this Clinical Practice Guideline document, a process has been initiated to develop QIs for patients undergoing NCS using the ESC methodology and through collaboration with patient representatives and domain experts. Such QIs may be used for evaluation of the quality of care for this group of patients and enable capture of important aspects of care delivery. The QIs, alongside their measurement specifications and development process, will be published in a separate paper.
14. Central illustration
There is a complex interplay between the intrinsic risk of surgery and the patient-related risk of peri-operative CV complications. This latter risk depends on the baseline general and CV status of patients scheduled for NCS. For each patient, the proper quantification and communication of the surgical risk require close cooperation between cardiologists, surgeons, anaesthesiologists, general practitioners, and other healthcare providers (Figure 21).
15. Supplementary data
Supplementary data is available at European Heart Journal online.
16. Data availability statement
No new data were generated or analysed in support of this research.
17. Author information
Author/Task Force Member Affiliations: Salvatore Cassese, Klinik für Herz- und Kreislauferkrankungen, Deutsches Herzzentrum München, Munich, Germany; Trygve S. Hall, Department of Cardiology, Oslo University Hospital, Ullevål, Oslo, Norway; Magdy Abdelhamid, Cardiology Department, Faculty of Medicine, Kase Al Ainy, Cairo University, Cairo, Egypt; Emanuele Barbato, Advanced Biomedical Sciences, University Federico II, Naples, Italy, Cardiovascular Center Aalst, OLV Hospital, Aalst, Belgium; Stefan De Hert, Department of Anesthesiology and Perioperative Medicine, Ghent University Hospital/Ghent University, Ghent, Belgium; Tobias Geisler, Department of Cardiology and Angiology, University Hospital Tübingen, Tübingen, Germany; Lynne Hinterbuchner, Department of Cardiology, Clinic of Internal Medicine II, Paracelsus Medical University of Salzburg, Salzburg, Austria; Borja Ibanez, Clinical Research Department, Centro Nacional de Investigaciones Cardiovasculares (CNIC), Madrid, Spain, Cardiology Department, Fundación Jiménez Díaz Hospital, Madrid, Spain, CIBERCV Centro Investigacion Biomedica en Red, Madrid, Spain; Ingrid de Laval (Sweden), ESC Patient Forum, Sophia Antipolis, France; Radosław Lenarczyk, Department of Cardiology, Congenital Heart Diseases and Electrotherapy, The Medical University of Silesia, Division of Medical Sciences in Zabrze, Zabrze, Poland, Silesian Center for Heart Disease, Zabrze, Poland; Ulrich R. Mansmann, Institute for Medical Data Processing, Biometry, and Epidemiology, Ludwig-Maximilian's University, Munich, Germany; Paul McGreavy (United Kingdom), ESC Patient Forum, Sophia Antipolis, France; Christian Mueller, Cardiovascular Research Institute Basel, University Heart Center, University Hospital Basel, Basel, Switzerland, University of Basel, Basel, Switzerland; Claudio Muneretto, Cardiothoracic, University of Brescia Italy, Brescia, Italy; Alexander Niessner, Department of Internal Medicine II, Division of Cardiology, Medical University of Vienna, Vienna, Austria; Tatjana S. Potpara, School of Medicine, Belgrade University, Belgrade, Serbia, Cardiology Clinic, University Clinical Center of Serbia, Belgrade, Serbia; Arsen D. Ristić, Faculty of Medicine, Belgrade University, Belgrade, Serbia, Department of Cardiology, University Clinical Center of Serbia, Belgrade, Serbia; L. Elif Sade, Heart and Vascular Institute, University of Pittsburgh Medical Center, Pittsburgh, PA, United States of America, Cardiology, University of Baskent, Ankara, Turkey; Henrik Schirmer, Department of Cardiology, Akershus University Hospital, Lørenskog, Norway, Institute of Clinical Medicine, Campus Ahus, University of Oslo, Lørenskog, Norway; Stefanie Schüpke, ISAResearch Center, Deutsches Herzzentrum München, Munich, Germany, Heart Alliance, DZHK (German Center for Cardiovascular Research), Munich, Germany; Henrik Sillesen, Vascular Surgery, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark, Clinical Medicine, University of Copenhagen, Copenhagen, Denmark; Helge Skulstad, Department of Cardiology, Clinic of heart-, lung- and vessel disease, Oslo University Hospital, Oslo, Norway, Institute of Clinical Medicine, University of Oslo, Oslo, Norway; Lucia Torracca, Cardiac Surgery, IRCCS Humanitas Research Hospital, Milano, Italy; Oktay Tutarel, Department of Congenital Heart Disease and Paediatric Cardiology, German Heart Centre Munich, TUM School of Medicine, Technical University of Munich, Munich, Germany, DZHK (German Centre for Cardiovascular Research), partner site Munich Heart Alliance, Munich, Germany; Peter Van der Meer, Cardiology, University Medical Center Groningen, Groningen, Netherlands; Wojtek Wojakowski, Division of Cardiology and Structural Heart Diseases, Medical University of Silesia, Katowice, Poland; and Kai Zacharowski, Anaesthesiology, Intensive Care Medicine & Pain Therapy, University Hospital Frankfurt, Goethe University, Frankfurt, Germany.
18. Appendix
ESC Scientific Document Group: includes Document Reviewers and ESC National Cardiac Societies.
Document Reviewers: Juhani Knuuti (CPG Review Coordinator) (Finland), Steen Dalby Kristensen (CPG Review Coordinator) (Denmark), Victor Aboyans (France), Ingo Ahrens (Germany), Sotiris Antoniou (United Kingdom), Riccardo Asteggiano (Italy), Dan Atar (Norway), Andreas Baumbach (United Kingdom), Helmut Baumgartner (Germany), Michael Böhm (Germany), Michael A. Borger (Germany), Hector Bueno (Spain), Jelena Čelutkienė (Lithuania), Alaide Chieffo (Italy), Maya Cikes (Croatia), Harald Darius (Germany), Victoria Delgado (Spain), Philip J. Devereaux (Canada), David Duncker (Germany), Volkmar Falk (Germany), Laurent Fauchier (France), Gilbert Habib (France), David Hasdai (Israel), Kurt Huber (Austria), Bernard Iung (France), Tiny Jaarsma (Sweden), Aleksandra Konradi (Russian Federation), Konstantinos C. Koskinas (Switzerland), Dipak Kotecha (United Kingdom), Ulf Landmesser (Germany), Basil S. Lewis (Israel), Ales Linhart (Czech Republic), Maja-Lisa Løchen (Norway), Michael Maeng (Denmark), Stéphane Manzo-Silberman (France), Richard Mindham (United Kingdom), Lis Neubeck (United Kingdom), Jens Cosedis Nielsen (Denmark), Steffen E. Petersen (United Kingdom), Eva Prescott (Denmark), Amina Rakisheva (Kazakhstan), Antti Saraste (Finland), Dirk Sibbing (Germany), Jolanta M. Siller-Matula (Austria), Marta Sitges (Spain), Ivan Stankovic (Serbia), Rob F. Storey (United Kingdom), Jurrien ten Berg (Netherlands), Matthias Thielmann (Germany), and Rhian M. Touyz (Canada/United Kingdom).
ESC National Cardiac Societies actively involved in the review process of the 2022 ESC Guidelines on assessment and management of patients undergoing non-cardiac surgery
Algeria: Algerian Society of Cardiology, Mohammed Amine Bouzid; Armenia: Armenian Cardiologists Association, Hamayak Sisakian; Austria: Austrian Society of Cardiology, Bernhard Metzler; Belarus: Belorussian Scientific Society of Cardiologists, Vadim Shumavets; Belgium: Belgian Society of Cardiology, Agnès Pasquet; Bosnia and Herzegovina: Association of Cardiologists of Bosnia and Herzegovina, Elnur Smajic; Bulgaria: Bulgarian Society of Cardiology, Maria Milanova; Croatia: Croatian Cardiac Society, Boško Skorić; Cyprus: Cyprus Society of Cardiology, Maria Karakyriou; Czechia: Czech Society of Cardiology, Hana Skalicka; Denmark: Danish Society of Cardiology, Michael Maeng; Egypt: Egyptian Society of Cardiology, Bassem Abd Elhamid; Estonia: Estonian Society of Cardiology, Arno Ruusalepp; Finland: Finnish Cardiac Society, Kati Valtola; France: French Society of Cardiology, Ariel Cohen; Georgia: Georgian Society of Cardiology, Archil Chukhrukidze; Germany: German Cardiac Society, Ilka Ott; Greece: Hellenic Society of Cardiology, Nikos Kafkas; Hungary: Hungarian Society of Cardiology, Zoltán Járai; Iceland: Icelandic Society of Cardiology, Thórdís Jóna Hrafnkelsdóttir; Ireland: Irish Cardiac Society, Patricia Campbell; Israel: Israel Heart Society, Alon Eisen; Italy: Italian Federation of Cardiology, Stefano Urbinati; Kazakhstan: Association of Cardiologists of Kazakhstan, Nazipa Aidargaliyeva; Kosovo (Republic of): Kosovo Society of Cardiology, Arlind Batalli; Kyrgyzstan: Kyrgyz Society of Cardiology, Olga Lunegova; Latvia: Latvian Society of Cardiology, Andrejs Erglis; Lebanon: Lebanese Society of Cardiology, Georges Saade; Lithuania: Lithuanian Society of Cardiology, Andrius Macas; Luxembourg: Luxembourg Society of Cardiology, Cristiana Banu; Malta: Maltese Cardiac Society, Tiziana Felice; Moldova: Moldavian Society of Cardiology, Aurel Grosu; Montenegro: Montenegro Society of Cardiology, Mihailo Vukmirovic; Morocco: Moroccan Society of Cardiology, Aida Soufiani; Netherlands: Netherlands Society of Cardiology, Eric Dubois; North Macedonia: North Macedonian Society of Cardiology, Hristo Pejkov; Norway: Norwegian Society of Cardiology, Erlend Aune; Poland: Polish Cardiac Society, Stanisław Bartuś; Portugal: Portuguese Society of Cardiology, Mário Santos; Romania: Romanian Society of Cardiology, Elisabeta Badila; Russian Federation: Russian Society of Cardiology, Olga Irtyuga; San Marino: San Marino Society of Cardiology, Luca Bertelli; Serbia: Cardiology Society of Serbia, Branko Beleslin; Slovakia: Slovak Society of Cardiology, Martin Dúbrava; Slovenia: Slovenian Society of Cardiology, Zlatko Fras; Spain: Spanish Society of Cardiology, José Luis Ferreiro; Sweden: Swedish Society of Cardiology, Claes Held; Switzerland: Swiss Society of Cardiology, Philippe Meyer; Syrian Arab Republic: Syrian Cardiovascular Association, Walid Bsata; Tunisia: Tunisian Society of Cardiology and Cardio-Vascular Surgery, Afef Ben Halima; Turkey: Turkish Society of Cardiology, Murat Biteker, United Kingdom of Great Britain and Northern Ireland: British Cardiovascular Society, Andrew Archbold; Ukraine: Ukrainian Association of Cardiology, Maksym Sokolov; and Uzbekistan: Association of Cardiologists of Uzbekistan, Nodir Zakirov.
ESC Clinical Practice Guidelines (CPG) Committee: Colin Baigent (Chairperson) (United Kingdom), Magdy Abdelhamid (Egypt), Victor Aboyans (France), Sotiris Antoniou (United Kingdom), Elena Arbelo (Spain), Riccardo Asteggiano (Italy), Andreas Baumbach (United Kingdom), Michael A. Borger (Germany), Jelena Čelutkienė (Lithuania), Maja Cikes (Croatia), Jean-Philippe Collet (France), Volkmar Falk (Germany), Laurent Fauchier (France), Chris P. Gale (United Kingdom), Sigrun Halvorsen (Norway), Bernard Iung (France), Tiny Jaarsma (Sweden), Aleksandra Konradi (Russian Federation), Konstantinos C. Koskinas (Switzerland), Dipak Kotecha (United Kingdom), Ulf Landmesser (Germany), Basil S. Lewis (Israel), Ales Linhart (Czech Republic), Maja-Lisa Løchen (Norway), Richard Mindham (United Kingdom), Jens Cosedis Nielsen (Denmark), Steffen E. Petersen (United Kingdom), Eva Prescott (Denmark), Amina Rakisheva (Kazakhstan), Marta Sitges (Spain), and Rhian M. Touyz (Canada /United Kingdom).
References
Author notes
The two chairpersons contributed equally to the document and are joint corresponding authors.
The two Task Force Coordinators contributed equally to the document
Author/Task Force Member affiliations: listed in author information.
Representing the European Society of Anaesthesiology and Intensive Care (ESAIC)
ESC Clinical Practice Guidelines (CPG) Committee listed in the Appendix.
ESC subspecialty communities having participated in the development of this document:
Associations: Association for Acute CardioVascular Care (ACVC), Association of Cardiovascular Nursing & Allied Professions (ACNAP), European Association of Cardiovascular Imaging (EACVI), European Association of Percutaneous Cardiovascular Interventions (EAPCI), European Heart Rhythm Association (EHRA), and Heart Failure Association (HFA).
Councils: Council of Cardio-Oncology and Council on Valvular Heart Disease.
Working Groups: Adult Congenital Heart Disease, Aorta and Peripheral Vascular Diseases, Cardiovascular Pharmacotherapy, Cardiovascular Surgery, and Thrombosis.
Patient Forum The content of these European Society of Cardiology (ESC) Guidelines has been published for personal and educational use only. No commercial use is authorized. No part of the ESC Guidelines may be translated or reproduced in any form without written permission from the ESC. Permission can be obtained upon submission of a written request to Oxford University Press, the publisher of the European Heart Journal, and the party authorized to handle such permissions on behalf of the ESC (journals.permissions@oup.com).
Disclaimer: The ESC Guidelines represent the views of the ESC and were produced after careful consideration of the scientific and medical knowledge and the evidence available at the time of their publication. The ESC is not responsible in the event of any contradiction, discrepancy, and/or ambiguity between the ESC Guidelines and any other official recommendations or guidelines issued by the relevant public health authorities, particularly in relation to good use of health care or therapeutic strategies. Health professionals are encouraged to take the ESC Guidelines fully into account when exercising their clinical judgment, and in the determination and the implementation of preventive, diagnostic, or therapeutic medical strategies; however, the ESC Guidelines do not override, in any way whatsoever, the individual responsibility of health professionals to make appropriate and accurate decisions in consideration of each patient’s health condition and in consultation with that patient and, where appropriate and/or necessary, the patient’s caregiver. The ESC Guidelines do not exempt health professionals from taking into full and careful consideration the relevant official updated recommendations or guidelines issued by the competent public health authorities, in order to manage each patient’s case in light of the scientifically accepted data pursuant to their respective ethical and professional obligations. It is also the health professional’s responsibility to verify the applicable rules and regulations relating to drugs and medical devices at the time of prescription.
© The European Society of Cardiology 2022. All rights reserved. For permissions please e-mail: journals.permissions@oup.com
All experts involved in the development of these guidelines have submitted declarations of interest. These have been compiled in a report and simultaneously published in a supplementary document to the guidelines. The report is also available on the ESC website www.escardio.org/Guidelines
 See the European Heart Journal online for supplementary data that include background information and detailed discussion of the data that have provided the basis of the guidelines.
See the European Heart Journal online for supplementary data that include background information and detailed discussion of the data that have provided the basis of the guidelines.
 Click here to access the corresponding ESC CardioMed chapters.
Click here to access the corresponding ESC CardioMed chapters.



 See the European Heart Journal online for supplementary data that include background information and detailed discussion of the data that have provided the basis of the guidelines.
See the European Heart Journal online for supplementary data that include background information and detailed discussion of the data that have provided the basis of the guidelines. Click here to access the corresponding ESC CardioMed chapters.
Click here to access the corresponding ESC CardioMed chapters.
























