-
PDF
- Split View
-
Views
-
Cite
Cite
Jishnu Malgie, Mariëlle I Wilde, Hans-Peter Brunner-La Rocca, Mireille E Emans, Grytsje A De Boer, Charlotte E P Siegers, Antonius M W van Stipdonk, Alexander J Wardeh, Jeroen Schaap, Sandra Sanders-van Wijk, Mieke van den Heuvel, Eric Wierda, Rudolf A De Boer, Stefan Koudstaal, Jasper J Brugts, Newly diagnosed heart failure with reduced ejection fraction: timing, sequencing, and titration of guideline-recommended medical therapy, European Heart Journal, 2025;, ehaf244, https://doi.org/10.1093/eurheartj/ehaf244
Close - Share Icon Share
Abstract
Despite guidelines recommending rapid initiation and up-titration of Guideline-recommended medical therapy (GRMT) for heart failure (HF) with reduced ejection fraction (HFrEF), its feasibility in daily practice remains unclear. TITRATE-HF studies the feasibility of rapid GRMT implementation in de novo HFrEF patients, investigating titration patterns and identifying barriers to effective treatment.
This analysis focuses on the de novo HFrEF patients included in the TITRATE-HF study, an ongoing prospective HF registry conducted in 48 Dutch hospitals. A detailed logbook for each GRMT drug class was recorded, from diagnosis to six months, including initiations, dose adjustments, discontinuations, and reasons for changes.
The study included 1508 de novo HFrEF patients (median age: 70 years [inter-quartile ranges, IQR 62–77]; 31% women; median left ventricular ejection fraction: 30% [IQR 25–35]). At 6 weeks, 46% of patients were using quadruple therapy. Within 6 weeks post-HFrEF diagnosis, 50% of patients were prescribed quadruple therapy at some point, with 84% remaining on it after 180 days. At 6 months, 66.3% of patients were prescribed quadruple therapy, but only 1.3% achieved target doses for all four drug classes. While side effects accounted for 20%–37% of cases where target doses were not reached, a large proportion was attributed to physicians accepting suboptimal doses. Drug titrations occurred frequently in the first 60 days after diagnosis, fading afterwards. Discontinuation rates for angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, angiotensin receptor–neprilysin inhibitors, beta-blocker, mineralocorticoid receptor antagonists, and sodium–glucose cotransporter 2 inhibitors were 13%, 11%, 9%, 9%, 11%, and 9%, respectively, primarily due to side effects or intolerances. Rechallenging these drug classes was successful in over 83% of patients.
The TITRATE-HF study demonstrates that rapid initiation of GRMT for HFrEF is feasible in real-world clinical practice. Nonetheless, our results highlight the urgency for a proactive approach and ongoing dose titration of pharmacological therapy beyond the initial first months to fully optimize treatment.

See the editorial comment for this article ‘Advocating for better care of patients with heart failure’, by K.F. Docherty et al., https://doi.org/10.1093/eurheartj/ehaf224.
Introduction
Guideline-recommended medical therapy (GRMT) is fundamental for the management of heart failure (HF) with reduced ejection fraction (HFrEF). Despite the life-saving benefits of these drugs within weeks to months of their initiation, adequate implementation in the real-world remains challenging. Suboptimal prescription rates of GRMT are generally linked to limiting patient, physician, and healthcare system factors.1–3
Previous studies have mainly focused on chronic HFrEF patients, who should have already attained optimal GRMT. Consequently, there is a gap in our understanding at what stage during the titration process implementation barriers are encountered. It is therefore imperative to study the start of the titration process, especially in de novo HF patients, as well as the sequencing of these drugs. While many patients already use GRMT drugs for other comorbid conditions, about half of patients are GRMT naïve.4
The STRONG-HF trial provided pivotal insights, which demonstrated that rapid up-titration of GRMT combined with high-intensity care can significantly improve clinical outcomes in patients with acute HF.5 In line with the STRONG-HF results, current guidelines now recommend rapid initiation and up-titration within 6 weeks after HF hospitalization if patients are in reasonably stable condition.6,7 However, while STRONG-HF included a few patients with de novo HF admitted in a decompensated state at higher risk, the majority of patients had chronic (worsening) HF. This raises important questions about the feasibility of rapid GRMT implementation in real-world clinical practice, particularly in outpatient settings where de novo HF patients are often managed.
The TITRATE-HF study investigates GRMT sequencing and titration in de novo HFrEF patients starting at the day of diagnosis. The study aims to assess the feasibility of rapid GRMT implementation in real-world clinical practice, and identify side effects and other implementation barriers encountered during the titration process.
Methods
Study design
The design of the TITRATE-HF study has been published previously.8 In summary, TITRATE-HF is an ongoing prospective HF registry in the Netherlands investigating guideline adherence, implementation barriers for GRMT, and long-term clinical outcomes across the different stages of HF. Inclusion criteria include a HF diagnosis with current or previous left ventricular ejection fraction (LVEF) < 50%. Patients were excluded if they had a life expectancy <1 year, a major cardiovascular event (myocardial infarction, open-heart surgery, or stroke) within 2 months before giving informed consent, or advanced HF with anticipated left ventricular assist device or heart transplantation within 6 months post-study enrolment. A total of 48 hospitals throughout the Netherlands participated, as presented in Supplementary data online, Table S1. The study was approved by all local ethics committees (MEC-2022-0252) and complies with the principles outlined in the Declaration of Helsinki.9 TITRATE-HF is registered with ClinicalTrials.gov (NCT06386042). All patients provided written informed consent.
Patient population
From June 2022 to February 2024, the study enrolled 4288 patients with HFrEF, HF with mildly reduced ejection fraction, and HF with improved ejection fraction. Patients were further stratified into de novo HF (n = 1732), chronic HF (n = 2240), and worsening HF (n = 316) groups, in accordance with the definitions of the 2021 ESC HF guidelines,6 as listed in Supplementary data online, Table S2. For this analysis, all patients with de novo HFrEF (n = 1510) were included. Two patients were excluded from prospective analysis due to data quality (n = 1508 available for analysis).
Data collection
Data were collected from the electronic patient record at baseline and at pre-specified 6 month intervals (± 2 months). Baseline data included demographics, medical history, vital parameters, electrocardiogram (ECG), echocardiography, laboratory results, and medication use. At follow-up, all HF and non-HF hospitalizations, urgent visits requiring intravenous diuretics, and deaths were recorded, as well as laboratory results, and ECG data.
Medication logbook of GRMT changes
GRMT for HFrEF consists of four drug classes: renin–angiotensin system inhibitors (RASi), beta-blockers (BB), mineralocorticoid receptor antagonists (MRA), and sodium–glucose cotransporter 2 inhibitors (SGLT2i). RASi encompasses angiotensin-converting enzyme inhibitors (ACEi), angiotensin II receptor blockers (ARB), or angiotensin receptor–neprilysin inhibitors (ARNI). When relevant, the subcategories of RASi are presented separately.
Baseline GRMT use and all changes in GRMT up to 6-month follow-up were recorded in a medication log, including the type of drug, dose, date of change, and the reason for change. This included registrations for starting and stopping a drug, as well as every dose up-titration and every dose down-titration. The list of pre-defined options for discontinuing or down-titrating a drug class has been published in the TITRATE-HF design paper.8 At each follow-up visit, reasons for non-use and not reaching target dose were recorded.
Survey of implementation barriers (patient, physician, and system level)
In April and May 2024, an online survey was distributed to all TITRATE-HF investigators to assess the view of healthcare providers on their preferences for GRMT implementation and to gauge their opinions on the most important implementation barriers. The survey questions relevant to this paper are provided in Supplementary data online, Table S3.
Statistical analysis
Continuous variables were reported as means and standard deviations (SD) or medians and inter-quartile ranges (IQR), as appropriate. Categorical variables were reported as frequencies and percentages. Doses were summarized as a mean percentage relative to target doses according to the 2021 ESC HF guidelines.6 The daily dose of loop diuretics was standardized and expressed as the equivalent dose of furosemide in mg. For patients using bumetanide, the furosemide equivalent dose was calculated by multiplying the bumetanide dose by a factor of 40. The sequencing order of the four GRMT drug classes was determined based on the date of their start registration in the medication log. In case of simultaneous initiation, both drug classes were awarded the lowest rank (i.e. second and third were both awarded second). Additionally, at the day of HF diagnosis, we assessed whether patients were naïve to GRMT drug classes or already using at least one of these drugs for other comorbid conditions. A multivariate regression model was used to identify variables associated with the prescription of quadruple GRMT at 6 weeks. The model included at least age, sex, body mass index (BMI), LVEF, New York Heart Association (NYHA) class, and all variables with a P-value <.10 in univariate analyses. Missing data were addressed using multivariate imputation by chained equations. Additionally, a sensitivity analysis was performed using only complete cases to evaluate the impact of missing data on the results. The Kaplan–Meier estimator was used to present survival and hospitalization rates over the 6 months follow-up in all patients. Between-group differences were assessed using the log-rank test. Patients who died or were lost to follow-up were censored in the survival analyses. For prescription-level analyses, a last-observation-carried-forward approach was applied to minimize information loss for these patients. Sensitivity analyses confirmed consistent results independent of the approach. All analyses were performed with SPSS (version 29), R (version 4.3.2.), and RStudio (version 2023.12.0).
Results
Of the 1508 de novo HFrEF patients included in this study, 790 (52.4%) were GRMT naïve and 718 (47.6%) already received at least one GRMT drug class prior to HF diagnosis due to other indications. Baseline characteristics are summarized in Table 1. Overall, the median age was 70 years [IQR 62–77], 31% of patients were female, and the median LVEF was 30% [IQR 25–35]). The use of concomitant medication and device therapy are summarized in Supplementary data online, Tables S4 and S5, respectively.
| . | All de novo HFrEF patients . | GRMT naïve patients . | Patients using ≥1 GRMT drug class prior to HF diagnosis . | P-value . |
|---|---|---|---|---|
| Patients, n | 1508 | 790 | 718 | |
| Age, years, (median [IQR]) | 70 [62, 77] | 69 [59, 76] | 72 [64, 78] | <.001 |
| Female sex, n (%) | 466 (30.9) | 239 (30.3) | 227 (31.6) | .61 |
| Non-ischaemic CMP, n (%) | 952 (64.0) | 541 (69.4) | 411 (58.0) | <.001 |
| NYHA functional class, n (%) | .01 | |||
| I | 142 (9.6) | 91 (11.7) | 51 (7.2) | |
| II | 1020 (68.8) | 523 (67.4) | 497 (70.3) | |
| III | 249 (16.8) | 119 (15.3) | 130 (18.4) | |
| IV | 72 (4.9) | 43 (5.5) | 29 (4.1) | |
| BMI, kg/m2 (median [IQR]) | 25.9 [23.2, 29.5] | 25.7 [22.9, 29.1] | 26.2 [23.6, 29.9] | .03 |
| Systolic BP, mm Hg (mean (SD)) | 126.9 (21.7) | 125.9 (22.5) | 127.9 (20.8) | .07 |
| Diastolic BP, mm Hg (mean (SD)) | 76.1 (13.1) | 76.0 (13.4) | 76.1 (12.9) | .92 |
| Heart rate, b.p.m. (median [IQR]) | 81 [68, 95] | 81 [68, 96] | 80 [68, 93] | .13 |
| Heart rhythm (%) | <.001 | |||
| SR | 1059 (71.4) | 610 (78.5) | 449 (63.6) | |
| AF | 385 (26.0) | 147 (18.9) | 238 (33.7) | |
| PM | 39 (2.6) | 20 (2.6) | 19 (2.7) | |
| LVEF, %, (median [IQR]) | 30 [25, 35] | 29 [23, 35] | 30 [25, 35] | <.001 |
| History of stroke, n (%) | 132 (8.8) | 56 (7.1) | 76 (10.6) | .02 |
| History of hypertension, n (%) | 775 (51.4) | 307 (38.9) | 468 (65.2) | <.001 |
| History of hypercholesterolemia, n (%) | 535 (35.5) | 238 (30.1) | 297 (41.4) | <.001 |
| Obesity, n (%) | 323 (21.4) | 151 (19.1) | 172 (24.0) | .03 |
| History of diabetes, n (%) | 318 (21.1) | 127 (16.1) | 191 (26.6) | <.001 |
| Smoking, n (%) | .01 | |||
| No | 631 (43.3) | 327 (43.0) | 304 (43.7) | |
| Quit | 558 (38.3) | 273 (35.9) | 285 (40.9) | |
| Yes | 268 (18.4) | 161 (21.2) | 107 (15.4) | |
| History of COPD, n (%) | 157 (10.4) | 76 (9.6) | 81 (11.3) | .33 |
| History of OSAS, n (%) | 123 (8.2) | 55 (7.0) | 68 (9.5) | .09 |
| eGFR, mL/min/1.73 m2 (median [IQR]) | 66 [53, 81] | 69 [54, 82] | 64 [51, 80] | .01 |
| eGFR category, n (%) | .01 | |||
| <30 | 52 (3.5) | 24 (3.1) | 28 (3.9) | |
| 30–59 | 509 (34.2) | 241 (30.9) | 268 (37.8) | |
| ≥60 | 929 (62.3) | 516 (66.1) | 413 (58.3) | |
| NTproBNP, pg/mL (median [IQR]) | 1882 [787, 4575] | 1860 [755, 4345] | 1962 [819, 4740] | .25 |
| . | All de novo HFrEF patients . | GRMT naïve patients . | Patients using ≥1 GRMT drug class prior to HF diagnosis . | P-value . |
|---|---|---|---|---|
| Patients, n | 1508 | 790 | 718 | |
| Age, years, (median [IQR]) | 70 [62, 77] | 69 [59, 76] | 72 [64, 78] | <.001 |
| Female sex, n (%) | 466 (30.9) | 239 (30.3) | 227 (31.6) | .61 |
| Non-ischaemic CMP, n (%) | 952 (64.0) | 541 (69.4) | 411 (58.0) | <.001 |
| NYHA functional class, n (%) | .01 | |||
| I | 142 (9.6) | 91 (11.7) | 51 (7.2) | |
| II | 1020 (68.8) | 523 (67.4) | 497 (70.3) | |
| III | 249 (16.8) | 119 (15.3) | 130 (18.4) | |
| IV | 72 (4.9) | 43 (5.5) | 29 (4.1) | |
| BMI, kg/m2 (median [IQR]) | 25.9 [23.2, 29.5] | 25.7 [22.9, 29.1] | 26.2 [23.6, 29.9] | .03 |
| Systolic BP, mm Hg (mean (SD)) | 126.9 (21.7) | 125.9 (22.5) | 127.9 (20.8) | .07 |
| Diastolic BP, mm Hg (mean (SD)) | 76.1 (13.1) | 76.0 (13.4) | 76.1 (12.9) | .92 |
| Heart rate, b.p.m. (median [IQR]) | 81 [68, 95] | 81 [68, 96] | 80 [68, 93] | .13 |
| Heart rhythm (%) | <.001 | |||
| SR | 1059 (71.4) | 610 (78.5) | 449 (63.6) | |
| AF | 385 (26.0) | 147 (18.9) | 238 (33.7) | |
| PM | 39 (2.6) | 20 (2.6) | 19 (2.7) | |
| LVEF, %, (median [IQR]) | 30 [25, 35] | 29 [23, 35] | 30 [25, 35] | <.001 |
| History of stroke, n (%) | 132 (8.8) | 56 (7.1) | 76 (10.6) | .02 |
| History of hypertension, n (%) | 775 (51.4) | 307 (38.9) | 468 (65.2) | <.001 |
| History of hypercholesterolemia, n (%) | 535 (35.5) | 238 (30.1) | 297 (41.4) | <.001 |
| Obesity, n (%) | 323 (21.4) | 151 (19.1) | 172 (24.0) | .03 |
| History of diabetes, n (%) | 318 (21.1) | 127 (16.1) | 191 (26.6) | <.001 |
| Smoking, n (%) | .01 | |||
| No | 631 (43.3) | 327 (43.0) | 304 (43.7) | |
| Quit | 558 (38.3) | 273 (35.9) | 285 (40.9) | |
| Yes | 268 (18.4) | 161 (21.2) | 107 (15.4) | |
| History of COPD, n (%) | 157 (10.4) | 76 (9.6) | 81 (11.3) | .33 |
| History of OSAS, n (%) | 123 (8.2) | 55 (7.0) | 68 (9.5) | .09 |
| eGFR, mL/min/1.73 m2 (median [IQR]) | 66 [53, 81] | 69 [54, 82] | 64 [51, 80] | .01 |
| eGFR category, n (%) | .01 | |||
| <30 | 52 (3.5) | 24 (3.1) | 28 (3.9) | |
| 30–59 | 509 (34.2) | 241 (30.9) | 268 (37.8) | |
| ≥60 | 929 (62.3) | 516 (66.1) | 413 (58.3) | |
| NTproBNP, pg/mL (median [IQR]) | 1882 [787, 4575] | 1860 [755, 4345] | 1962 [819, 4740] | .25 |
HFrEF, heart failure with reduced ejection fraction; GRMT, guideline-recommended medical therapy; HF, heart failure; IQR, inter-quartile range; CMP, cardiomyopathy; NYHA, New York Heart Association; BMI, body mass index; BP, blood pressure; SD, standard deviation; b.p.m.: beats per minute; SR, sinus rhythm; AF, atrial fibrillation/atrial flutter; PM, pacemaker; LVEF, left ventricular ejection fraction; COPD, chronic obstructive pulmonary disease; OSAS, obstructive sleep apnea syndrome; eGFR, estimated glomerular filtration rate.
| . | All de novo HFrEF patients . | GRMT naïve patients . | Patients using ≥1 GRMT drug class prior to HF diagnosis . | P-value . |
|---|---|---|---|---|
| Patients, n | 1508 | 790 | 718 | |
| Age, years, (median [IQR]) | 70 [62, 77] | 69 [59, 76] | 72 [64, 78] | <.001 |
| Female sex, n (%) | 466 (30.9) | 239 (30.3) | 227 (31.6) | .61 |
| Non-ischaemic CMP, n (%) | 952 (64.0) | 541 (69.4) | 411 (58.0) | <.001 |
| NYHA functional class, n (%) | .01 | |||
| I | 142 (9.6) | 91 (11.7) | 51 (7.2) | |
| II | 1020 (68.8) | 523 (67.4) | 497 (70.3) | |
| III | 249 (16.8) | 119 (15.3) | 130 (18.4) | |
| IV | 72 (4.9) | 43 (5.5) | 29 (4.1) | |
| BMI, kg/m2 (median [IQR]) | 25.9 [23.2, 29.5] | 25.7 [22.9, 29.1] | 26.2 [23.6, 29.9] | .03 |
| Systolic BP, mm Hg (mean (SD)) | 126.9 (21.7) | 125.9 (22.5) | 127.9 (20.8) | .07 |
| Diastolic BP, mm Hg (mean (SD)) | 76.1 (13.1) | 76.0 (13.4) | 76.1 (12.9) | .92 |
| Heart rate, b.p.m. (median [IQR]) | 81 [68, 95] | 81 [68, 96] | 80 [68, 93] | .13 |
| Heart rhythm (%) | <.001 | |||
| SR | 1059 (71.4) | 610 (78.5) | 449 (63.6) | |
| AF | 385 (26.0) | 147 (18.9) | 238 (33.7) | |
| PM | 39 (2.6) | 20 (2.6) | 19 (2.7) | |
| LVEF, %, (median [IQR]) | 30 [25, 35] | 29 [23, 35] | 30 [25, 35] | <.001 |
| History of stroke, n (%) | 132 (8.8) | 56 (7.1) | 76 (10.6) | .02 |
| History of hypertension, n (%) | 775 (51.4) | 307 (38.9) | 468 (65.2) | <.001 |
| History of hypercholesterolemia, n (%) | 535 (35.5) | 238 (30.1) | 297 (41.4) | <.001 |
| Obesity, n (%) | 323 (21.4) | 151 (19.1) | 172 (24.0) | .03 |
| History of diabetes, n (%) | 318 (21.1) | 127 (16.1) | 191 (26.6) | <.001 |
| Smoking, n (%) | .01 | |||
| No | 631 (43.3) | 327 (43.0) | 304 (43.7) | |
| Quit | 558 (38.3) | 273 (35.9) | 285 (40.9) | |
| Yes | 268 (18.4) | 161 (21.2) | 107 (15.4) | |
| History of COPD, n (%) | 157 (10.4) | 76 (9.6) | 81 (11.3) | .33 |
| History of OSAS, n (%) | 123 (8.2) | 55 (7.0) | 68 (9.5) | .09 |
| eGFR, mL/min/1.73 m2 (median [IQR]) | 66 [53, 81] | 69 [54, 82] | 64 [51, 80] | .01 |
| eGFR category, n (%) | .01 | |||
| <30 | 52 (3.5) | 24 (3.1) | 28 (3.9) | |
| 30–59 | 509 (34.2) | 241 (30.9) | 268 (37.8) | |
| ≥60 | 929 (62.3) | 516 (66.1) | 413 (58.3) | |
| NTproBNP, pg/mL (median [IQR]) | 1882 [787, 4575] | 1860 [755, 4345] | 1962 [819, 4740] | .25 |
| . | All de novo HFrEF patients . | GRMT naïve patients . | Patients using ≥1 GRMT drug class prior to HF diagnosis . | P-value . |
|---|---|---|---|---|
| Patients, n | 1508 | 790 | 718 | |
| Age, years, (median [IQR]) | 70 [62, 77] | 69 [59, 76] | 72 [64, 78] | <.001 |
| Female sex, n (%) | 466 (30.9) | 239 (30.3) | 227 (31.6) | .61 |
| Non-ischaemic CMP, n (%) | 952 (64.0) | 541 (69.4) | 411 (58.0) | <.001 |
| NYHA functional class, n (%) | .01 | |||
| I | 142 (9.6) | 91 (11.7) | 51 (7.2) | |
| II | 1020 (68.8) | 523 (67.4) | 497 (70.3) | |
| III | 249 (16.8) | 119 (15.3) | 130 (18.4) | |
| IV | 72 (4.9) | 43 (5.5) | 29 (4.1) | |
| BMI, kg/m2 (median [IQR]) | 25.9 [23.2, 29.5] | 25.7 [22.9, 29.1] | 26.2 [23.6, 29.9] | .03 |
| Systolic BP, mm Hg (mean (SD)) | 126.9 (21.7) | 125.9 (22.5) | 127.9 (20.8) | .07 |
| Diastolic BP, mm Hg (mean (SD)) | 76.1 (13.1) | 76.0 (13.4) | 76.1 (12.9) | .92 |
| Heart rate, b.p.m. (median [IQR]) | 81 [68, 95] | 81 [68, 96] | 80 [68, 93] | .13 |
| Heart rhythm (%) | <.001 | |||
| SR | 1059 (71.4) | 610 (78.5) | 449 (63.6) | |
| AF | 385 (26.0) | 147 (18.9) | 238 (33.7) | |
| PM | 39 (2.6) | 20 (2.6) | 19 (2.7) | |
| LVEF, %, (median [IQR]) | 30 [25, 35] | 29 [23, 35] | 30 [25, 35] | <.001 |
| History of stroke, n (%) | 132 (8.8) | 56 (7.1) | 76 (10.6) | .02 |
| History of hypertension, n (%) | 775 (51.4) | 307 (38.9) | 468 (65.2) | <.001 |
| History of hypercholesterolemia, n (%) | 535 (35.5) | 238 (30.1) | 297 (41.4) | <.001 |
| Obesity, n (%) | 323 (21.4) | 151 (19.1) | 172 (24.0) | .03 |
| History of diabetes, n (%) | 318 (21.1) | 127 (16.1) | 191 (26.6) | <.001 |
| Smoking, n (%) | .01 | |||
| No | 631 (43.3) | 327 (43.0) | 304 (43.7) | |
| Quit | 558 (38.3) | 273 (35.9) | 285 (40.9) | |
| Yes | 268 (18.4) | 161 (21.2) | 107 (15.4) | |
| History of COPD, n (%) | 157 (10.4) | 76 (9.6) | 81 (11.3) | .33 |
| History of OSAS, n (%) | 123 (8.2) | 55 (7.0) | 68 (9.5) | .09 |
| eGFR, mL/min/1.73 m2 (median [IQR]) | 66 [53, 81] | 69 [54, 82] | 64 [51, 80] | .01 |
| eGFR category, n (%) | .01 | |||
| <30 | 52 (3.5) | 24 (3.1) | 28 (3.9) | |
| 30–59 | 509 (34.2) | 241 (30.9) | 268 (37.8) | |
| ≥60 | 929 (62.3) | 516 (66.1) | 413 (58.3) | |
| NTproBNP, pg/mL (median [IQR]) | 1882 [787, 4575] | 1860 [755, 4345] | 1962 [819, 4740] | .25 |
HFrEF, heart failure with reduced ejection fraction; GRMT, guideline-recommended medical therapy; HF, heart failure; IQR, inter-quartile range; CMP, cardiomyopathy; NYHA, New York Heart Association; BMI, body mass index; BP, blood pressure; SD, standard deviation; b.p.m.: beats per minute; SR, sinus rhythm; AF, atrial fibrillation/atrial flutter; PM, pacemaker; LVEF, left ventricular ejection fraction; COPD, chronic obstructive pulmonary disease; OSAS, obstructive sleep apnea syndrome; eGFR, estimated glomerular filtration rate.
Sequencing order of GRMT in de novo HFrEF patients
In patients who were naïve to GRMT (n = 790), RASi and BB were most commonly initiated as the first drug class. Additionally, MRA were most often started as the third, and SGLT2i as the fourth drug class (Figure 1).

GRMT sequencing order in de novo HFrEF patients who were naïve to GRMT prior to HF diagnosis. GRMT, guideline-recommended medical therapy; HFrEF, heart failure with reduced ejection fraction; HF, heart failure; RAS, renin–angiotensin system; MRA, mineralocorticoid receptor antagonist; SGLT2, sodium–glucose cotransporter −2
In the GRMT naïve subgroup, simultaneous initiation of multiple drug classes was observed in 73.9%, 28.2%, and 7.8% of patients for at least two, at least three, and all four drug classes, respectively. On the day of HF diagnosis, 41.9% of these patients started RASi, 33.0% started BB, 29.4% started MRA, and 15.7% started SGLT2i.
GRMT prescription levels after 6 weeks and 180 days in de novo HFrEF
The initiation speed for each drug class is illustrated in Figure 2, with specific details for ACEi, ARB, and ARNI shown in Supplementary data online, Figure S1. At 6 weeks post-HF diagnosis, 46.1% (n = 695) of patients were prescribed all four drug classes (Structured Graphical Abstract). At this point, 84.8% (n = 1279) of all patients were using RASi, 38.4% (n = 579) were using ARNI, 83.7% (n = 1262) were on BB, 73.7% (n = 1111) were taking MRA, and 67.7% (n = 1021) were using SGLT2i. By 180 days post-diagnosis, the percentage of patients on quadruple therapy increased to 66.3%, with 90.7% using RASi, 65.8% using ARNI, 88.0% on BB, 87.9% on MRA, and 85.5% on SGLT2i. In the first 6 months following diagnosis, the median number of days on which GRMT titration steps occurred was 5 days [IQR 4–7]. During the initial 6 weeks, the median number of days on which titration steps occurred was 3 days [IQR 2–4], while between 6 weeks and 6 months, the median was 2 days [IQR 1–4]. Patients enrolled during their index hospitalization (n = 305, 20.2%) were significantly more likely to achieve quadruple therapy at 6 weeks compared with those enrolled in an outpatient setting (58.4% vs 43.0%, P < .001). There was no significant difference in prescription of quadruple GRMT at 6 weeks between GRMT naïve and GRMT non-naïve patients (44.8% vs 47.2%, P = .357). Among the 755 patients (50.1%) who were prescribed quadruple therapy at some point within the first 6 weeks after diagnosis, 633 patients (83.8%) were still on quadruple therapy at 180 days.
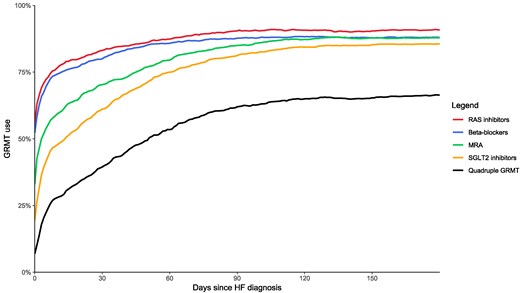
GRMT prescription rates from diagnosis until 6-month follow-up. GRMT, guideline-recommended medical therapy; RAS, renin–angiotensin system; MRA, mineralocorticoid receptor antagonist; SGLT2, sodium–glucose cotransporter 2; HF, heart failure
Within the first 180 days after diagnosis, 3.2% (n = 48), 6.2% (n = 93), 5.9% (n = 89), and 9.4% (n = 142) of patients were never prescribed RASi, BB, MRA, and SGLT2i, respectively. In total, 22.6% (n = 341) of patients did not use quadruple therapy at any point during the first 180 days after diagnosis. Among patients never prescribed RASi, 31% (n = 15) had a systolic blood pressure below 100 mmHg, a history of dialysis, a serum potassium level of 5.5 mmol/L or higher, or other contraindications or intolerances. The remaining 69% (n = 33) had no documented reasons for the non-use of RASi. For patients never prescribed BB, 26% (n = 24) had a heart rate below 60 b.p.m. or other contraindications or intolerances, while in 74% (n = 69) of cases, the reasons for BB non-use were not known. Among patients never using MRA, 28% (n = 25) had an estimated glomerular filtration rate (eGFR) of 30 mL/min/1.73 m² or less, a history of dialysis, a serum potassium level exceeding 5 mmol/L, or other contraindications or intolerances. In addition, 18% (n = 26) of patients never prescribed SGLT2i had an eGFR of 20 mL/min/1.73 m² or less, a history of dialysis, or other contraindications or intolerances, while 82% (n = 116) had no documented reasons for non-use.
Differences between patients who were prescribed quadruple therapy at 6 weeks after diagnosis (n = 695) vs those who were not (n = 813) are summarized in Supplementary data online, Table S6. Patients receiving quadruple therapy at 6 weeks were slightly younger (68 [IQR 59, 76] vs 72 [IQR 64, 78] years, P < .001), had a lower LVEF (28% [IQR 22, 35] vs 30% [IQR 25, 35], P < .001), and had less co-morbidities such as hypertension, diabetes and renal dysfunction (all P ≤ .03). Multivariate predictors for the use of quadruple therapy 6 weeks after diagnosis are summarized in Supplementary data online, Table S7. In summary, lower age, lower LVEF, higher NYHA class, higher eGFR, and shorter QRS duration on ECG were significantly and independently associated with increased rates of quadruple GRMT prescription at 6 weeks after diagnosis. Similar results were found in sensitivity analysis.
Dose titration at 6 weeks and 180 days
Table 2 provides an overview of the percentage of patients achieving at least 50% or 100% of target doses at 6 weeks and 6 months post-HF diagnosis. Among all patients, 0.3% were using all four drug classes at target doses at 6 weeks post-diagnosis, rising to 1.3% after 6 months. The number of up-titrations and down-titrations per drug class is depicted in Supplementary data online, Figure S2. Dose titration per drug class, for patients who started the drug class within 6 weeks after HF diagnosis, is presented in Figure 3. The percentage of patients prescribed loop diuretics decreased from 57.1% (n = 861) at baseline to 46.8% (n = 706) at 6 months (P < .001). Among patients who used loop diuretics both at baseline and 6-month follow-up (n = 560), the mean daily dose decreased from 49.1 mg (SD 33.1) to 42.1 mg (SD 27.0) (P < .001).
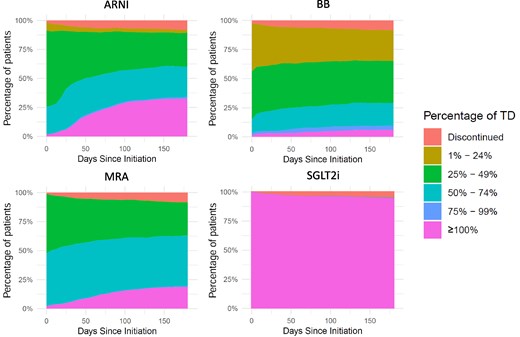
Dose titration across GRMT drug classes in patients initiating therapy within 6 weeks after HF diagnosis. GRMT, guideline-recommended medical therapy; HF, heart failure; ARNI, angiotensin receptor–neprilysin inhibitor; BB, beta-blocker; MRA, mineralocorticoid receptor antagonist; SGLT2i, sodium–glucose cotransporter 2 inhibitor; TD, target dose
| . | Six weeks after diagnosis . | Six months after diagnosis . | ||||
|---|---|---|---|---|---|---|
| . | Any dose . | ≥50% TD . | ≥100% TD . | Any dose . | ≥50% TD . | ≥100% TD . |
| RASi | 84.8% | 39.7%a | 10.5%a | 90.7% | 55.5%a | 25.5%a |
| ARNI | 38.4% | 42.6%a | 9.7%a | 65.8% | 61.7%a | 29.6%a |
| BB | 83.7% | 33.4%a | 6.0%a | 88.0% | 36.0%a | 8.4%a |
| MRA | 73.7% | 56.8%a | 6.8%a | 87.9% | 66.4%a | 18.5%a |
| SGLT2i | 67.7% | 100.0%a | 99.8%a | 85.5% | 100.0%a | 99.5%a |
| Quadruple therapy | 46.1% | 5.3% | 0.3% | 66.3% | 14.0% | 1.3% |
| . | Six weeks after diagnosis . | Six months after diagnosis . | ||||
|---|---|---|---|---|---|---|
| . | Any dose . | ≥50% TD . | ≥100% TD . | Any dose . | ≥50% TD . | ≥100% TD . |
| RASi | 84.8% | 39.7%a | 10.5%a | 90.7% | 55.5%a | 25.5%a |
| ARNI | 38.4% | 42.6%a | 9.7%a | 65.8% | 61.7%a | 29.6%a |
| BB | 83.7% | 33.4%a | 6.0%a | 88.0% | 36.0%a | 8.4%a |
| MRA | 73.7% | 56.8%a | 6.8%a | 87.9% | 66.4%a | 18.5%a |
| SGLT2i | 67.7% | 100.0%a | 99.8%a | 85.5% | 100.0%a | 99.5%a |
| Quadruple therapy | 46.1% | 5.3% | 0.3% | 66.3% | 14.0% | 1.3% |
GRMT, guideline-recommended medical therapy; TD, target dose; RASi, renin–angiotensin system inhibitors; ARNI, angiotensin receptor-neprilysin inhibitors; BB, beta-blockers; MRA, mineralocorticoid receptor antagonists; SGLT2i, sodium–glucose cotransporter-2 inhibitors.
aOf patients using the drug class.
| . | Six weeks after diagnosis . | Six months after diagnosis . | ||||
|---|---|---|---|---|---|---|
| . | Any dose . | ≥50% TD . | ≥100% TD . | Any dose . | ≥50% TD . | ≥100% TD . |
| RASi | 84.8% | 39.7%a | 10.5%a | 90.7% | 55.5%a | 25.5%a |
| ARNI | 38.4% | 42.6%a | 9.7%a | 65.8% | 61.7%a | 29.6%a |
| BB | 83.7% | 33.4%a | 6.0%a | 88.0% | 36.0%a | 8.4%a |
| MRA | 73.7% | 56.8%a | 6.8%a | 87.9% | 66.4%a | 18.5%a |
| SGLT2i | 67.7% | 100.0%a | 99.8%a | 85.5% | 100.0%a | 99.5%a |
| Quadruple therapy | 46.1% | 5.3% | 0.3% | 66.3% | 14.0% | 1.3% |
| . | Six weeks after diagnosis . | Six months after diagnosis . | ||||
|---|---|---|---|---|---|---|
| . | Any dose . | ≥50% TD . | ≥100% TD . | Any dose . | ≥50% TD . | ≥100% TD . |
| RASi | 84.8% | 39.7%a | 10.5%a | 90.7% | 55.5%a | 25.5%a |
| ARNI | 38.4% | 42.6%a | 9.7%a | 65.8% | 61.7%a | 29.6%a |
| BB | 83.7% | 33.4%a | 6.0%a | 88.0% | 36.0%a | 8.4%a |
| MRA | 73.7% | 56.8%a | 6.8%a | 87.9% | 66.4%a | 18.5%a |
| SGLT2i | 67.7% | 100.0%a | 99.8%a | 85.5% | 100.0%a | 99.5%a |
| Quadruple therapy | 46.1% | 5.3% | 0.3% | 66.3% | 14.0% | 1.3% |
GRMT, guideline-recommended medical therapy; TD, target dose; RASi, renin–angiotensin system inhibitors; ARNI, angiotensin receptor-neprilysin inhibitors; BB, beta-blockers; MRA, mineralocorticoid receptor antagonists; SGLT2i, sodium–glucose cotransporter-2 inhibitors.
aOf patients using the drug class.
Side effects and intolerance in GRMT
During the first 180 days, in 5.0%, 4.1%, 8.0%, 15.2%, 8.7%, and 0.1% of patients down-titration occurred for ACEi, ARB, ARNI, BB, MRA, and SGLT2i, respectively. A full overview of the reasons for down-titration is presented in Figure 4. The most common reason for down-titration was hypotension for RASi, bradycardia for BB, and hyperkalemia for MRA. For SGLT2i, only one down-titration occurred, which was at the request of the patient. During the first 180 days, 13.4%, 10.9%, 8.6%, 9.4%, 11.4%, and 8.6% of patients discontinued ACEi, ARB, ARNI, BB, MRA, and SGLT2i, respectively. Stopping ACEi or ARB in order to initiate ARNI was not regarded as a discontinuation. An overview of the reasons for discontinuation is presented in Figure 5. Common causes for discontinuing GRMT were cough and hypotension for ACEi, hypotension for RASi and BB, bradycardia for BB, worsening renal function for MRA and urogenital infection for SGLT2i. However, ‘other side effects’ were generally the most common reason for discontinuation, accounting for 13% to 38% of cases, depending on the drug class. Supplementary data online, Table S8 provides an overview of the three most common reasons for discontinuation for each GRMT drug class, along with the percentage of patients experiencing these side effects. Of the patients who discontinued ARNI, BB, MRA, and SGLT2i, 27.5%, 40.6%, 40.1%, and 35.3%, respectively, were rechallenged with the same medication class. The average number of days between discontinuation and reintroduction was 27.5 days for ARNI, 18.5 days for BB, 25.0 days for MRA, and 27.6 days for SGLT2i. Discontinuation after rechallenge occurred in 16.7% for ARNI, 7.5% for BB, 10.9% for MRA, and 7.7% for SGLT2i.
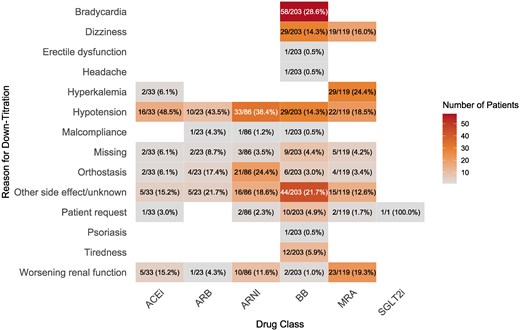
Number of down-titrations per reason and drug class. The percentages indicate the proportion of the reported down-titrations caused by each side-effect within each drug class. ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor–neprilysin inhibitors; BB, beta-blocker; MRA, mineralocorticoid receptor antagonist; SGLT2i, sodium–glucose cotransporter 2 inhibitor
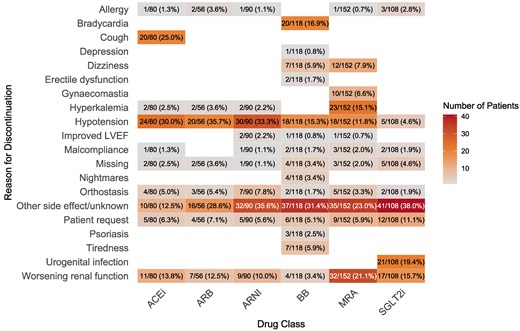
Number of discontinuations per reason and drug class. The percentages indicate the proportion of the reported discontinuations caused by each side-effect within each drug class. LVEF, left ventricular ejection fraction; ACEi, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor–neprilysin inhibitor; BB, beta-blocker; MRA, mineralocorticoid receptor antagonist; SGLT2i, sodium–glucose cotransporter 2 inhibitor
The reasons for not reaching target doses at the 6-month follow-up are summarized in Table 3. Across all drug classes, except for SGLT2i, the most frequently reported reasons for not achieving target dose were side effects/intolerances and the healthcare provider’s acceptance of a suboptimal dose.
| . | ACEi . | ARB . | ARNI . | BB . | MRA . | SGLT2i . | |
|---|---|---|---|---|---|---|---|
| Reasons for not reaching target dose | Still in titration process | 31 (19%) | 35 (26%) | 143 (22%) | 165 (15%) | 148 (14%) | 0 (0%) |
| Side effects/intolerances | 61 (37%) | 27 (20%) | 229 (35%) | 347 (31%) | 311 (30%) | 1 (20%) | |
| Request of patient/malcompliance | 1 (1%) | 4 (3%) | 10 (2%) | 20 (2%) | 17 (2%) | 1 (20%) | |
| Acceptance of suboptimal dose by health care provider | 61 (37%) | 62 (46%) | 225 (35%) | 532 (47%) | 480 (47%) | 0 (0%) | |
| Unknown reasons | 11 (7%) | 7 (5%) | 40 (6%) | 73 (6%) | 68 (7%) | 3 (60%) | |
| . | ACEi . | ARB . | ARNI . | BB . | MRA . | SGLT2i . | |
|---|---|---|---|---|---|---|---|
| Reasons for not reaching target dose | Still in titration process | 31 (19%) | 35 (26%) | 143 (22%) | 165 (15%) | 148 (14%) | 0 (0%) |
| Side effects/intolerances | 61 (37%) | 27 (20%) | 229 (35%) | 347 (31%) | 311 (30%) | 1 (20%) | |
| Request of patient/malcompliance | 1 (1%) | 4 (3%) | 10 (2%) | 20 (2%) | 17 (2%) | 1 (20%) | |
| Acceptance of suboptimal dose by health care provider | 61 (37%) | 62 (46%) | 225 (35%) | 532 (47%) | 480 (47%) | 0 (0%) | |
| Unknown reasons | 11 (7%) | 7 (5%) | 40 (6%) | 73 (6%) | 68 (7%) | 3 (60%) | |
ACEi, angiotensin converting enzyme inhibitors; ARB, angiotensin receptor blockers; ARNI, angiotensin receptor-neprilysin inhibitors; BB, beta-blockers; MRA, mineralocorticoid receptor antagonists; SGLT2i, sodium–glucose cotransporter-2 inhibitors.
| . | ACEi . | ARB . | ARNI . | BB . | MRA . | SGLT2i . | |
|---|---|---|---|---|---|---|---|
| Reasons for not reaching target dose | Still in titration process | 31 (19%) | 35 (26%) | 143 (22%) | 165 (15%) | 148 (14%) | 0 (0%) |
| Side effects/intolerances | 61 (37%) | 27 (20%) | 229 (35%) | 347 (31%) | 311 (30%) | 1 (20%) | |
| Request of patient/malcompliance | 1 (1%) | 4 (3%) | 10 (2%) | 20 (2%) | 17 (2%) | 1 (20%) | |
| Acceptance of suboptimal dose by health care provider | 61 (37%) | 62 (46%) | 225 (35%) | 532 (47%) | 480 (47%) | 0 (0%) | |
| Unknown reasons | 11 (7%) | 7 (5%) | 40 (6%) | 73 (6%) | 68 (7%) | 3 (60%) | |
| . | ACEi . | ARB . | ARNI . | BB . | MRA . | SGLT2i . | |
|---|---|---|---|---|---|---|---|
| Reasons for not reaching target dose | Still in titration process | 31 (19%) | 35 (26%) | 143 (22%) | 165 (15%) | 148 (14%) | 0 (0%) |
| Side effects/intolerances | 61 (37%) | 27 (20%) | 229 (35%) | 347 (31%) | 311 (30%) | 1 (20%) | |
| Request of patient/malcompliance | 1 (1%) | 4 (3%) | 10 (2%) | 20 (2%) | 17 (2%) | 1 (20%) | |
| Acceptance of suboptimal dose by health care provider | 61 (37%) | 62 (46%) | 225 (35%) | 532 (47%) | 480 (47%) | 0 (0%) | |
| Unknown reasons | 11 (7%) | 7 (5%) | 40 (6%) | 73 (6%) | 68 (7%) | 3 (60%) | |
ACEi, angiotensin converting enzyme inhibitors; ARB, angiotensin receptor blockers; ARNI, angiotensin receptor-neprilysin inhibitors; BB, beta-blockers; MRA, mineralocorticoid receptor antagonists; SGLT2i, sodium–glucose cotransporter-2 inhibitors.
Clinical outcomes in de novo HFrEF
A Kaplan–Meier curve of cardiovascular mortality, all-cause mortality, HF hospitalization, and a composite endpoint of all-cause mortality and HF hospitalization is provided in Supplementary data online, Figure S3. Analysis of the composite endpoint indicated no significant difference between patients who were prescribed quadruple therapy at 6 weeks after diagnosis compared with those who were not (log-rank P = .96) (see Supplementary data online, Figure S4). At 180 days, event-free survival rates were 92.7% in the quadruple therapy group compared with 92.8% in the non-quadruple therapy group. Similarly, when comparing patients receiving three or more GRMT drug classes at ≥50% of target doses at 6 weeks to those using fewer classes or lower doses, no significant differences were observed in the composite endpoint (log-rank P = .68) (see Supplementary data online, Figure S5).
TITRATE-HF survey: implementation barriers
The survey of 117 healthcare providers (see Supplementary data online, Table S9) from 38 TITRATE-HF participating hospitals showed that the majority, 82.9%, viewed patient factors as the primary barrier to GRMT implementation. Specific patient-related barriers included challenges with managing blood pressure and worsening renal function. In contrast, 9.4% of respondents cited physician-related barriers, such as failure to up-titrate medication for patients deemed ‘stable’ and a lack of expertise in GRMT. Finally, system barriers were mentioned by 7.7% of respondents and primarily included waiting lists for outpatient clinics. Overall, 48.7% of participants felt that HF clinics were inadequately equipped to handle the (time) demands of optimal GRMT implementation and titration.
Proposed solutions from the healthcare providers included increasing the capacity of dedicated HF outpatient clinics, educating patients and health care providers about HF and the importance of GRMT, and allocating more time for outpatient consultations. In case of side effects limiting further up-titration, more than 20% of respondents suggested accepting suboptimal treatment or up-titrating more slowly. Detailed responses and proposed solutions can be found in Supplementary data online, Table S10.
Discussion
The TITRATE-HF study provides novel insights into sequencing and titration of GRMT for patients with de novo HF, starting right at the moment of diagnosis. Supported by the results of the STRONG-HF trial, which focused mainly on not yet optimally treated worsening HF patients, the 2023 ESC HF guideline update recommends rapid initiation of GRMT within 6 weeks after hospitalization.5,7 Therefore, questions of feasibility in de novo HF patients and in a real-world clinical setting arose, where TITRATE-HF provides important complimentary data to STRONG-HF. In addition, based mainly upon the findings of STRONG-HF, there is a general consensus in the 2023 ESC HF guideline update to initiate multiple drug classes at low doses with the goal of quickly achieving quadruple therapy.7 Afterwards, the focus shifts to up-titrating the dose to target dose, with the aim of reaching optimal GRMT within 6 weeks after HF diagnosis. The current analysis demonstrates that about half of de novo HFrEF patients in our population received quadruple GRMT during the first 6 weeks after diagnosis, and that 84% of those patients were able to maintain quadruple GRMT prescription after 180 days. This study found that most GRMT initiation occurred within the first 60 days following HF diagnosis, after which new drug classes were rarely introduced. Taken together, these findings underscore the importance of the first weeks post-HF diagnosis to initiate and up-titrate patients on quadruple therapy. However, these findings also highlight the need to critically evaluate and optimize the achieved therapy after the first 60 days as an ongoing process and effort. We advocate for fast up-titration of GRMT in the initial weeks following HF diagnosis, when tolerated. However, a personalized approach remains an option, allowing slower optimization with ongoing evaluation for patients who do not tolerate the initial rapid approach. Nonetheless, the central aim remains to prescribe quadruple therapy, preferably at the optimal dose, within six weeks after HF diagnosis. Physicians should therefore be encouraged to implement GRMT with a sense of urgency and timing as specified in the 2023 HF guideline update.7 Every patient interaction should be viewed as an opportunity to optimize treatment and advance patient care.
The 2021 ESC HF guidelines do not recommend a specific sequencing order, but instead recommend rapid initiation of all four GRMT drug classes.6 In light of this latest guideline, multiple sequencing strategies have been proposed in the literature. For example, Packer and McMurray suggest starting with an SGLT2i in combination with a BB, while Greene et al. suggest starting all four drug classes simultaneously at low doses before up-titrating to higher doses.10,11 The TITRATE-HF study reveals that the traditional sequencing method of starting with RASi, followed by BB, then MRA, and finally an SGLT2i is still the most common approach. However, especially in light of STRONG-HF, the order of sequencing might be less important compared with ensuring an ‘as fast-as-possible’ approach to initiating all four drug classes in a patient.5 The TITRATE-HF study also shows that simultaneous initiation of multiple drug classes was common with almost three quarters of patients being initiated on at least two drug classes at the same time. Notably, almost 8% of patients were started on all four drug classes simultaneously. We feel that prospective trials that compare different sequencing strategies are warranted, as e.g. immediate start of SGLT2i and MRA likely is better tolerated than ARNI and BB, and may allow faster and more complete up-titration.
Six months after HFrEF diagnosis, only 1.3% of patients were prescribed all four drug classes at the full target doses recommended by the ESC HF guidelines.6 This prompts a crucial question: is the set bar for attaining target dose too high? Even in the landmark randomized controlled trials (RCTs) of the GRMT drugs, with carefully selected typical trial populations, target doses of the study drugs or background therapies were often not achieved.3 While RCTs have shown a benefit of higher doses compared with lower doses, especially for BB and ACEi, ensuring quadruple therapy is likely more important than up-titrating the dose.12–15 The TITRATE-HF study shows that while new drug classes were initiated often just after HF diagnosis, dose up-titration occurred infrequently. The multinational study EVOLUTION-HF demonstrated a higher frequency of dose up-titrations but also exhibited elevated rates of discontinuation compared with the relatively stable prescription rates observed during the initial 6 months of follow-up in TITRATE-HF.16 This raises the question whether the lower doses administered in TITRATE-HF may have contributed to a lower discontinuation rate. Although studies have consistently demonstrated the superiority of higher doses over lower doses, real-world clinical practice is a delicate balance between dose titration and tolerability. This balance is crucial to ensure patients not only initiate but also persistently continue using all GRMT drug classes. However, a secondary analysis from STRONG-HF demonstrated higher doses of GRMT to be associated with better outcomes, underscoring the need to titrate patients as close to target doses as possible.17 These insights stress the importance of continuing to up-titrate the dose as close to target dose as possible, also after the first initiation phase. While a proportion of patients in TITRATE-HF did not reach target dose due to side effects or intolerances, in most cases, not reaching target dose was attributed to other factors, such as physician factors or system barriers. These cases, where there are no clear contraindications to up-titration, present a major opportunity to improve treatment (and consequently) outcomes for patients.
In this current analysis, 66% of de novo HFrEF patients were using quadruple therapy at 6 months post-HF diagnosis. In contrast, baseline data from TITRATE-HF showed that 44% of chronic and worsening HFrEF patients were using quadruple therapy at inclusion.4 Several factors might explain this difference. First, the de novo HF patients in TITRATE-HF were diagnosed after the implementation of the 2021 ESC HF guidelines. These guidelines recommend the rapid initiation of all four drug classes without a specific sequencing order, emphasizing the timely achievement of quadruple GRMT.6 This differs from previous guidelines that recommended adding drug classes sequentially and titrating to the maximally tolerated dose before introducing the next drug class.18,19 Second, as shown by an analysis from the SwedeHF registry, participation in a study could motivate healthcare providers to pursue quicker initiation of quadruple therapy.20 However, it is important to note that there were no study specific visits between zero and 6 months, as TITRATE-HF studied care as provided in clinical routine. Last, physicians might adopt a more aggressive treatment strategy for de novo HF patients, taking advantage of the opportunity to control the disease from the onset. This approach aligns with the urgency of translating guideline recommendations into routine clinical practice. In contrast, chronic HF is a population where GRMT is up-titrated less often and where the focus shifts more towards symptom management. In this population, periods of perceived disease stability can, incorrectly, decrease the urgency to act.
Although prior data on side effects as reasons for discontinuation or down-titration were limited, some patterns have been observed. Previous studies have identified hyperkalemia and worsening renal function as common reasons for discontinuation of ACEi, ARBs, and MRAs, as well as bradycardia for BB.21,22 The detailed and mandatory medication logbook in TITRATE-HF found similar main reasons for dose down-titrations. However, despite an extensive pre-defined option list, the most common reason for discontinuing a drug class was an ‘other side-effect’. This suggests that real-world tolerability of GRMT may extend beyond commonly established parameters such as blood pressure, heart rate, and serum potassium levels. Instead, reasons for discontinuation may rather lie in general ‘unwell-being’, ‘fatigue’, or ‘weakness’, which could also be related to HF symptoms.23 It should however be noted that when discontinued drugs were reintroduced, over four out of five patients were able to tolerate the drug class. These findings suggest that discontinuation due to side effects might often be temporary or manageable. Clinicians should consider rechallenging patients under careful monitoring, particularly for non-severe side effects, if clinical judgement allows.
The TITRATE-HF study has several strengths to consider. To our knowledge, it is the first registry to study de novo HF and meticulously document every titration step across all GRMT drug classes starting at the day of diagnosis, including reasons for changes, and for not reaching target doses at the 6 month follow-up. Initiated after the release of the 2021 ESC HF guideline, the study reflects the adoption and implementation of this new guideline, encompassing the latest drug classes such as ARNI and SGLT2i as class I indications for HFrEF patients.6 The study also has several noteworthy limitations. The large number of participating hospitals paired with a relatively low number of inclusions per site could introduce selection bias. However, with consecutive patient enrolment and minimal inclusion and exclusion criteria, we believe the studied population accurately reflects real-world clinical practice, aligning with the central aim of this quality of care project on GRMT prescription. While we did not find significant differences in clinical outcomes between patients initiated on quadruple therapy within 6 weeks and those who were not, these secondary analyses should be interpreted in light of the lack of power, co-morbidities, and the non-randomized nature of our study. Another limitation is that the prognosis of this cohort appears more favourable than that of typical epidemiological cohorts. This is likely attributable to several factors, including the relatively younger patient population and the enrolment of most patients in the outpatient setting rather than following acute HF hospitalization. Furthermore, the relatively larger proportion of patients with non-ischaemic cardiomyopathy, which is associated with reversibility and a better prognosis compared with ischaemic cardiomyopathy, may have contributed to the cohort’s more favourable outcomes.24,25 In contemporary HF cohorts, including STRONG-HF and HF-OPT, we might be observing a shift that ischaemic aetiology is no longer the predominant aetiology.5,26 Lastly, the relatively high rates of GRMT prescription in this cohort may have further improved prognosis compared with other cohorts. While these factors may limit the generalizability of the findings, they offer valuable insights into the risk profile of de novo HF patients treated with contemporary GRMT in the current era. Furthermore, the study reflects hospital care in the Netherlands, which may limit its generalizability to other healthcare systems. Nevertheless, similar challenges and implementation barriers are likely present in other settings. However, given the country’s status as a high-income nation with a well-structured and accessible healthcare system, it serves as an excellent testing ground for assessing the feasibility of recent guideline recommendations such as rapid initiation and up-titration of quadruple therapy within 6 weeks.
Clinical implications
First, the TITRATE-HF study not only demonstrates the feasibility and achievement of rapid GRMT sequencing within a modern health care system, but also highlights the importance of the first weeks after HF diagnosis as the key window for GRMT implementation. Additionally, it emphasizes the necessity for ongoing attention to pharmacological treatment beyond these initial weeks following HF diagnosis, with a focus on continued titration towards optimal dosing. This is especially relevant considering that optimizing GRMT beyond 90 days post-diagnosis further improves LVEF and remodelling in de novo HFrEF patients.26 Second, discontinuation due to non-severe intolerances or side effects may not always need to be permanent. When clinically appropriate, our findings suggest that physicians should be encouraged to consider rechallenging GRMT drug classes to patients, as we have observed relatively high success rates with these rechallenges. Third, previous TITRATE-HF findings showed that dedicated HF clinics may be a potential solution for better GRMT implementation by reducing system barriers.4 However, a large number of healthcare providers also reported inadequate capacity or time availability for providing optimal HF care and fast up-titration, warranting a call for action. Fourth, our results highlight the challenge of achieving target doses for GRMT in real-world settings, influenced not only by physician and system factors, but also by side effects and other patient-specific factors. Balancing drug intolerance with the maximally achievable dose is key to optimizing treatment. This balance should be thoroughly discussed with the patient in the outpatient clinic, with a clear emphasis on the life-saving benefits of these medications.
Conclusion
The TITRATE-HF study highlights both the opportunities and challenges for GRMT implementation in the first weeks after HFrEF diagnosis. Our findings underscore the feasibility of rapidly initiating all GRMT drug classes during the early treatment phase, demonstrating the potential for improving patient outcomes through early intervention and optimization. However, the study also emphasizes the importance and urgency of continuous dose up-titration to ensure further optimization of medical therapy as an ongoing target, also beyond the first months. Here lies an important task for both the healthcare provider and the healthcare system: providing an ongoing proactive effort in the optimization of GRMT.
Acknowledgements
The TITRATE-HF investigators thank consenting patients for enabling this study. This study is supported by the Dutch Network for Cardiovascular Research (WCN), the Netherlands Heart Institute (NLHI), the Dutch Cardiac Society (NVVC), and the National Heart Failure Working Group as partners of Dutch Cardiovascular Alliance (DCVA). This study collaborates with the Netherlands Heart Registration (NHR) and is aligned with the quality of care dataset, and also collaborates within the Heart4Data Consortium (funded by the Dutch Heart Foundation and ZonMw (2021-B015)).
Supplementary data
Supplementary data are available at European Heart Journal online.
Declarations
Disclosure of Interest
J.M.: received a speaker fee from Boehringer Ingelheim; M.I.W.: none declared; H.P.B.R.: received grants from Roche Diagnostics and Innovative Health Initiative Joint Undertaking under grant agreement No. 101112022, consulting fees from Novartis, Boehringer Ingelheim, Vifor Pharma, Roche Diagnostics, and AstraZeneca, speaker fees and other services from Roche Diagnostics, payment for export testimony from Novartis, and participation on board of CeleCor Therapeutics; M.E.E.: received speaker fees from AstraZeneca, Vifor, Boehringer Ingelheim, and Novartis; G.A.de.B.: reports no conflict of interest; C.e.P.S.: received speaker fees for education on geriatric cardiology, participation on national committee ‘guideline for ‘treatment choice aid’ for frail older patient with multimorbidity’; A.M.W.S.: received speaker fees from Abbott, Novartis, AstraZeneca; A.J.W.: none declared; J.S.: received an educational grant and speaker/consultancy fees from Boehringer Ingelheim, Daiichi Sankyo, AstraZeneca, General Electric and Novo Nordisk, all not related to this work; S.S.W.: received grants and/or speaker fees from Boehringer Ingelheim, Novartis, Artazenica, Roche, Pfizer. M.H.: none declared; E.W.: none declared; R.A.B.: received research grants to institution from Alnylam, AstraZeneca, Abbott, Bristol-Myers Squibb, Cardior Pharmaceuticals GmbH, NovoNordisk, and Roche, reports speaker engagements with and/or received fees from and/or served on an advisory board; payments to self from Abbott, AstraZeneca, Bristol Myers Squibb, Cardior Pharmaceuticals GmbH, NovoNordisk, and Roche, received travel support from Abbott, Cardior Pharmaceuticals GmbH, and NovoNordisk, and is President of Dutch Cardiac Society; S.K.: has had speaker engagements with Novartis, Boehringer Ingelheim, and Astra Zeneca; J.J.B: reports an independent research grant from Abbott paid to the Institute and speaker engagements for invited lectures or advisory board fees from Astra Zeneca, Abbott, Boehringer Ingelheim, Bayer, Daiichi Sankyo, Novartis, and Vifor in the past five years.
Data Availability
The data underlying this article cannot be shared publicly due to internal regulations, patient consent, and data regulations for outside Erasmus Medical Center. Researchers interested in collaboration are invited to contact the corresponding author.
Funding
TITRATE-HF is made possible in part by funding to ICIN Netherlands Heart Institute (NLHI) as CRO by an independent research grant from Novartis, Boehringer Ingelheim, AstraZeneca, Bayer, Abbott, and Vifor Pharma. This study was initiated by the authors and is designed, conducted, interpreted, and reported independently of the sponsors. The board and steering committee received no funding for this project. All authors have approved the manuscript before submission.
Pre-registered Clinical Trial Number
TITRATE-HF is registered with ClinicalTrials.gov (NCT06386042).
References
Author notes
Jishnu Malgie and Mariëlle I Wilde contributed equally to the study.


