-
PDF
- Split View
-
Views
-
Cite
Cite
Shreya Patel, Emily Brehm, Liying Gao, Saniya Rattan, Ayelet Ziv-Gal, Jodi A. Flaws, Bisphenol A Exposure, Ovarian Follicle Numbers, and Female Sex Steroid Hormone Levels: Results From a CLARITY-BPA Study, Endocrinology, Volume 158, Issue 6, 1 June 2017, Pages 1727–1738, https://doi.org/10.1210/en.2016-1887
Close - Share Icon Share
Abstract
Bisphenol A (BPA) is an industrial chemical found in thermal receipts and food and beverage containers. Previous studies have shown that BPA can affect the numbers and health of ovarian follicles and the production of sex steroid hormones, but they often did not include a wide range of doses of BPA, used a small sample size, focused on relatively short-term exposures to BPA, and/or did not examine the consequences of chronic BPA exposure on the ovaries or steroid levels. Thus, this study was designed to examine the effects of a wide range of doses of BPA on ovarian morphology and sex steroid hormone production. Specifically, this study tested the hypothesis that prenatal and continuous BPA exposure reduces ovarian follicle numbers and sex steroid hormone levels. To test this hypothesis, rats were dosed with vehicle, ethinyl estradiol (0.05 and 0.5 μg/kg body weight/d), or BPA (2.5, 25, 250, 2500, and 25,000 μg/kg body weight/d) from gestation day 6 until 1 year as part of the Consortium Linking Academic and Regulatory Insights on BPA Toxicity (CLARITY-BPA). Ovaries and sera were collected on postnatal days 1, 21, and 90, and at 6 months and 1 year. The ovaries were subjected to histological evaluation of follicle numbers and the sera were subjected to measurements of estradiol and progesterone. Collectively, these data indicate that BPA exposure at some doses and time points affects ovarian follicle numbers and sex steroid levels, but these effects are different than those observed with ethinyl estradiol exposure and some previous studies on BPA.
Bisphenol A (BPA) is a high-volume industrial chemical used in the production of polycarbonate plastics and epoxy resins, as well as in the manufacturing of food and drink containers, thermal receipt paper, and dental sealants (1–3). BPA can leach out of these products when exposed to high temperatures, UV rays, acidic or basic compounds, or simply from repeated use. Owing to its ubiquitous presence in the environment and its ability to leach out of products, humans are exposed to BPA daily. BPA has been measured in numerous human tissues and fluids, including, but not limited to, urine (4, 5), serum (6–8), plasma (9, 10), amniotic fluid (11, 12), placental tissue (13), and fetal serum (13, 14). Recently, the World Health Organization and the Food and Agriculture Organization of the United Nations estimated the human exposure levels to BPA to be between 0.4 and 1.4 µg/kg body weight (bw)/d (15). According to the US Environmental Protection Agency, the currently accepted lowest observed adverse effect level for BPA is 50 mg/kg bw/d, and the Environmental Protection Agency safe reference dose is 50 µg/kg bw/d (16). However, BPA can potentially have adverse effects at lower doses and induce effects with a nonmonotonic dose-response (17–19).
Several experimental studies have examined the effects of BPA on the ovary (20). These studies focused on the effects of BPA on follicle numbers and sex steroid hormone production because the normal production of ovarian follicles and sex steroid hormones is required for female fertility (21). Some of these animal studies indicate that exposure to BPA alters ovarian follicle numbers, but the types of alterations differ between studies and largely depend on the dose of BPA, the ovarian outcome examined, and/or the window of exposure. Rivera et al. (22) reported that neonatal exposure to BPA (50 µg/kg bw/d) by subcutaneous injection decreases the primordial follicle pool and increases the number of multioocyte and atretic follicles in lambs at postnatal day (PND) 30. Rodriguez et al. (23) reported that neonatal exposure to BPA (20 mg/kg bw/d) by subcutaneous injection reduces the primordial follicle pool in rats at PND 8. Furthermore, in utero exposure to oral BPA (0.5, 20, and 50 µg/kg bw/d) disrupts germ cell nest breakdown in female offspring in mice at PND 4 (24).
Some animal studies also indicate that BPA exposure disrupts steroidogenesis, but the types and degrees of alteration differ between studies, likely due to differences in dose of BPA, route of exposure, and/or timing/length of exposure (20). Specifically, studies indicate that exposure to BPA increases serum estradiol levels in mice and rats (25, 26); however, other studies indicate that exposure to BPA does not affect steroidogenesis except at 100 and 300 mg BPA/kg bw/d in rats (27–29).
Although these previous studies provide important information on the effects of BPA on the ovary and steroid production, they often did not include a wide range of doses of BPA or did not control for potential unintended exposure to other compounds with estrogenic activity, such as dietary phytoestrogens. Furthermore, previous studies only focused on relatively short-term exposures to BPA and did not examine the consequences of chronic BPA exposure on the ovaries or steroid levels. Thus, the purpose of this study was to test the hypothesis that prenatal and continuous BPA exposure affects folliculogenesis and the production of sex steroid hormones in female rats. This hypothesis was tested as part of the Consortium Linking Academic and Regulatory Insights on BPA Toxicity (CLARITY-BPA) program. The CLARITY-BPA study is a collaboration between academic and federal government scientists, organized by the National Toxicology Program (NTP), the National Institute of Environmental Health Sciences (NIEHS), and the U.S. Food and Drug Administration (FDA) National Center for Toxicological Research (NCTR) (30, 31). The goal of this research consortium is to combine the strengths of both academic and guideline-compliant studies to obtain better translational research (30, 31). The CLARITY-BPA studies included a wide range of BPA doses ranging from 2.5 µg/kg bw/d to 25,000 µg/kg bw/d, as well as two doses (0.05 and 0.5 µg/kg bw/d) of a reference estrogen control [ethinyl estradiol (EE2)]. Exposure by oral gavage began on gestation day 6 and continued by direct dosing of the pups after birth until weaning at PND 21 (stop dose arm) or continuously for up to 1 year.
Materials and Methods
This study was conducted as part of the CLARITY-BPA Consortium. The methods for this consortium have been published in detail (31), but are briefly described next.
Reagents
BPA [CAS no. 80-05-7; TCI America, Portland, OR, catalog no. B0494, lot no. 111909/AOHOK (air-milled), >99% pure] and EE2 (CAS no. 57-63-6; Sigma-Aldrich, St. Louis, MO, catalog no. E4876, lot no. 071M1492V, >99% pure) were used in these studies (29). The purities of BPA and EE2 were verified at 6-month intervals during the study and again at the end of the study to confirm test particle stability. The vehicle used to deliver BPA and EE2 was 0.3% aqueous carboxymethylcellulose (Sigma-Aldrich, catalog no. C5013, lot no. 041M0105V).
Animals
NCTR Sprague-Dawley rats (strain code 23) from the NCTR rodent breeding colony were used in all experiments. Breeders were housed in polysulfone cages with hard chip bedding and glass water bottles in rooms at 23 ± 3°C with a relative humidity of 50 ± 20%, and provided food (soy- and alfalfa-free verified casein diet 10 IF, 5K96; Purina Mills, Richmond, IN, catalog no. 1810069) and water for ad libitum consumption from weaning (approximately PND 21), and their offspring were housed under the same study conditions from birth. All animal rooms were under 12-hour light/12-hour dark cycles, with lights on at 6:00 am. All animal use and procedures were approved by the NCTR Laboratory Animal Care and Use Committee and conducted in an Association for Assessment and Accreditation of Laboratory Animal Care–accredited facility.
Dosing
Timed pregnant rats were dosed by gavage with vehicle control (0.3% carboxymethylcellulose), five different doses of BPA (2.5, 25, 250, 2500, and 25,000 μg/kg bw/d), and two doses of EE2 (0.05 and 0.5 μg/kg bw/d) from gestation day 6 until the start of labor. Starting on PND 1 (day of birth is PND 0), the pups were directly gavaged with the same dose level of vehicle, BPA, or EE2 as their dams. Each dose group was split into two dosing arms, a continuously dosed group and a stop dose group, with the latter having treatment terminated at weaning on PND 21.
The selected doses of BPA were based on the results from a 90-day BPA study conducted by the NCTR prior to the CLARITY-BPA program (29), estimates of human exposure levels (1, 20), and agreement among NIEHS-funded university-based researchers and NTP and FDA scientists to focus on a dose range of regulatory concern. Furthermore, the NIEHS-funded university-based researchers in the CLARITY-BPA program agreed to include two doses of EE2 (0.05 and 0.5 µg/kg bw/d) to determine the sensitivity of the animal model to EE2.
All doses were administered at the NCTR by daily gavage with a modified Hamilton Microlab ML511C programmable 115V pump (Hamilton Company, Reno, NV). Dosing was always conducted from the lowest to highest dose within a dosing pump, and cleaning and maintenance of the equipment were performed as described in Delclos et al. (29). The accuracy of dose delivery from the pumps was assessed every 3 months and established to be within 10% of the target volume accuracy.
Tissue collection
Pregnant dams were allowed to give birth naturally. Their pups then were euthanized on PNDs 1, 21, and 90, as well as at 6 months and 1 year of age. The present study used samples from female offspring (n = 8 to 10 per treatment group per time point); only one female per litter was used per treatment group, so no adjustment for litter was needed in the statistical analysis. Cycling females were euthanized when predicted to be in estrus based on a vaginal smear from the previous day. This method was not always successful in predicting estrous cyclicity on the day of collections. Because it is important to measure hormone levels from animals on the same day of the estrous cycle, n < 10 in some treatment groups used for hormone analysis. The ovaries were collected, and one ovary per animal was fixed in Dietrich’s solution and the other was snap frozen in liquid nitrogen. The fixed and frozen ovaries were shipped from the NCTR to the University of Illinois. The fixed ovaries were used to assess ovarian follicle numbers, as described later, and the frozen ovaries were stored at −80°C for future studies. Furthermore, sera were collected from all female offspring, frozen, and shipped from the NCTR to the University of Illinois for analysis of estradiol and progesterone levels, as described later. Samples were received from both the stop-dose and continuous dosing arms of the study. All samples were received without knowledge of the treatment group, and data were not decoded until data collection of follicle numbers and hormone levels was complete and the raw data were locked in the NTP Chemical Effects in Biological Systems database.
Assessment of ovarian follicle numbers
The fixed ovaries were transferred to 70% ethanol, embedded in paraffin wax, and serial sectioned (8 µm) using a microtome. The serial sections were mounted on glass slides and stained with hematoxylin and eosin. Follicle numbers were counted in every 10th section using previously defined criteria (32–35). Briefly, primordial follicles contained an oocyte surrounded by a single layer of squamous granulosa cells, primary follicles contained an oocyte surrounded by a single layer of cuboidal granulosa cells, preantral follicles contained an oocyte surrounded by at least two layers of cuboidal granulosa cells and theca cells, and antral follicles contained an oocyte surrounded by multiple layers of cuboidal granulosa cells with a fluid-filled antral space and theca cells. All primordial and primary follicles were counted in each section regardless of nuclear material in the oocyte, whereas only preantral and antral follicles with nuclear material in the oocyte were counted to avoid double counting the larger follicle types that can span multiple sections. Follicles transitioning between stages were counted as follicles within the more immature stage of the two stages. Follicles were considered unhealthy when they showed numerous apoptotic granulosa cells or oocyte abnormalities. All other follicles were considered healthy.
Measurement of estradiol and progesterone
The serum estradiol and progesterone levels were quantified by enzyme-linked immunosorbent assays at the University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core (estradiol kits were from Calbiotech, Spring Valley CA; progesterone kits were from IBL, Minneapolis, MN). The Core used assays that were validated according to the Endocrine Society’s Sex Steroid Assay Reporting Task Force (https://med.virginia.edu/research-in-reproduction/wp-content/uploads/sites/311/2015/12/Steroid-Method-Valid-Proc.pdf). The assays were read on a Bio-Tek Instruments EL800 universal microplate reader. The Core laboratory performed all analyses without knowledge of treatment group. The intra-assay and interassay coefficients of variation for estradiol were 6.1% and 8.9%, respectively. The intra-assay and interassay coefficients of variation for progesterone were 3.6% and 9.0%, respectively.
Statistical analysis
Data analysis was conducted using SPSS statistical software (SPSS, Chicago, IL). In the case of data collected from cycling females (PND 90 and at 6 months and 1 year), only the data collected from females in estrus were used in hormone analysis. Therefore, n < 10 for some endpoints. Data were expressed as means and standard errors of the mean (SEM). Multiple comparisons between normally distributed experimental groups were made using one-way analysis of variance. In cases when the analysis of variance was significant at P ≤ 0.05, Dunnett two-sided t tests were used to determine which treatment groups were significantly different from the control. Nonnormally distributed data were analyzed using a Kruskall–Wallis test. BPA and EE2 were compared with the vehicle in separate analyses. Statistical significance was assigned at P ≤ 0.05.
Results
Effects of EE2 and BPA on ovarian morphology on PND 1
On PND 1, EE2, or BPA exposure did not significantly affect the number of healthy or apoptotic germ cells compared with control (Fig. 1, n = 9 to 10). It also did not affect the number of primordial follicles present in the ovaries compared with control (Fig. 1, n = 9 to 10).
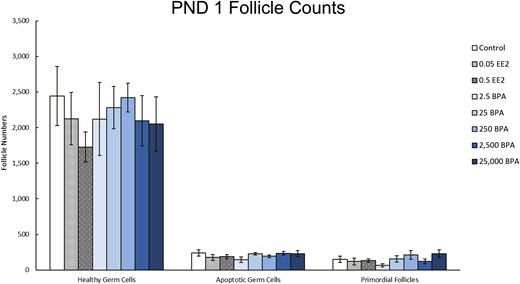
Effects of EE2 and BPA on ovarian morphology on PND 1. On PND 1, pups from each group were euthanized and one ovary from each animal was fixed for histological evaluation of germ cell and ovarian follicle numbers. The graph represents the means ± SEM of the number of healthy germ cells, apoptotic germ cells, and primordial follicles present in 9 to 10 ovaries per treatment group.
Effects of prenatal and preweaning exposure to EE2 and BPA on ovarian morphology and hormone levels on PND 21
On PND 21, EE2 exposure did not affect the numbers of primordial, primary, and preantral follicles, but it decreased antral follicles when compared with control [Fig. 2(a), n = 8 to 10, P < 0.05]. Exposure to BPA significantly decreased primordial (250 µg/kg bw/d), primary (2.5 and 250 µg/kg bw/d), and preantral (2.5 µg/kg bw/d) follicle numbers compared with control [Fig. 2(a), n = 8 to 10, P ≤ 0.05]. Additionally, exposure to BPA (2.5 and 250 µg/kg bw/d) significantly decreased the number of total healthy follicles compared with control [Fig. 2(a), n = 8 to 10, P ≤ 0.05].
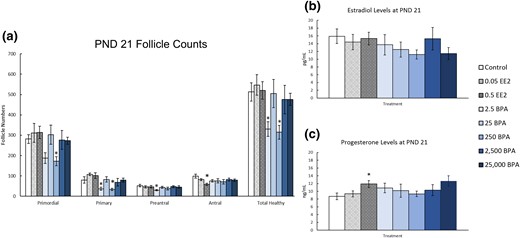
Effects of EE2 and BPA on ovarian morphology and sex steroid hormones on PND 21. On PND 21, rats from each group were euthanized and one ovary from each animal was fixed for histological evaluation of different ovarian follicle types. Additionally, serum was collected from the blood of each of the animals and used in the measurement of sex steroid hormones. (a) Graph represents the means ± SEM of the number of the different follicle types and total healthy follicles present in 8 to 10 ovaries per treatment group. (b) Graph represents the means ± SEM of the amount of estradiol present in the serum of 8 to 10 animals per group. (c) Graph represents the means ± SEM of the amount of progesterone present in the serum of 8 to 10 animals per group. *P ≤ 0.05, difference between the control group and doses of BPA or EE2 (n = 8 to 10).
On PND 21, exposure to EE2 or BPA did not affect serum estradiol levels when compared with control [Fig. 2(b), n = 8 to 10]. In contrast, exposure to EE2 (0.5 µg/kg bw/d) significantly increased serum progesterone levels by ∼2 ng/mL when compared with control [Fig. 2(c), n = 8 to 10, P ≤ 0.05]. Exposure to BPA did not affect serum progesterone levels when compared with control [Fig. 2(c), n = 8 to 10].
Effects of prenatal and preweaning exposure to EE2 and BPA on ovarian morphology and hormone levels on PND 90
On PND 90, exposure to EE2 or BPA did not affect the numbers of different follicle types when compared with control [Fig. 3(a), n = 8 to 10]. Additionally, exposure to EE2 or BPA did not significantly affect serum estradiol or progesterone levels compared with control [Fig. 2(b) and 2(c), n = 4 to 9]. EE2 exposure (0.5 µg/kg bw/d) increased serum progesterone levels, but this change was not statistically significant when compared with control [Fig. 2(c), n = 7, P = 0.058].
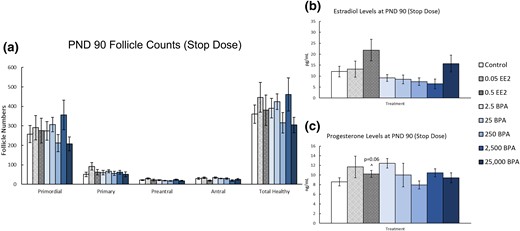
Effects of prenatal and preweaning exposure to EE2 and BPA on ovarian morphology and sex steroid hormones on PND 90. On PND 90, rats from each group were euthanized and one ovary from each animal was fixed for histological evaluation of different ovarian follicle types. Additionally, serum was collected from the blood of each of the animals and used in the measurement of sex steroid hormones. (a) Graph represents the means ± SEM of the number of the different follicle types and total healthy follicles present in 8 to 10 ovaries per treatment group. (b) Graph represents the means ± SEM of the amount of estradiol present in the serum of four to nine animals per group. (c) Graph represents the means ± SEM of the amount of progesterone present in the serum of four to nine animals per group. ^P > 0.05.
Effects of continuous exposure to EE2 and BPA on ovarian morphology and hormone levels on PND 90
On PND 90, continuous exposure to EE2 (0.5 µg/kg bw/d) significantly decreased the number of preantral follicles when compared with control [Fig. 4(a), n = 8 to 10, P ≤ 0.05]. However, BPA did not significantly affect the number of ovarian follicles when compared with control [Fig. 4(a), n = 8 to 10]. Additionally, continuous EE2 or BPA exposure did not affect serum estradiol or progesterone levels when compared with control [Fig. 4(b) and 4(c), n = 6 to 9].

Effects of continuous exposure to EE2 and BPA on ovarian morphology and sex steroid hormones on PND 90. On PND 90, rats from each group were euthanized and one ovary from each animal was fixed for histological evaluation of different ovarian follicle types. Additionally, serum was collected from the blood of each of the animals and used in the measurement of sex steroid hormones. (a) Graph represents the means ± SEM of the number of the different follicle types and total healthy follicles present in 8 to 10 ovaries per treatment group. (b) Graph represents the means ± SEM of the amount of estradiol present in the serum of six to nine animals per group. (c) Graph represents the means ± SEM of the amount of progesterone present in the serum of six to nine animals per group. *Significant difference between the control group and doses of BPA or EE2 (n = 8 to 10; P ≤ 0.05).
Effects of prenatal and preweaning exposure to EE2 and BPA on ovarian morphology and hormone levels at 6 months
At 6 months, prenatal and preweaning exposure to EE2 (0.5 µg/kg bw/d) significantly decreased the number of preantral and antral follicles when compared with control [Fig. 5(a), n = 9 to 10, P ≤ 0.05]. Exposure to BPA did not significantly affect the numbers of any ovarian follicle type when compared with control [Fig. 5(a), n = 9 to 10]. Similarly, exposure to EE2 or BPA did not affect serum estradiol or progesterone levels at 6 months when compared with control [Fig. 5(b) and 5(c), n = 5 to 9].
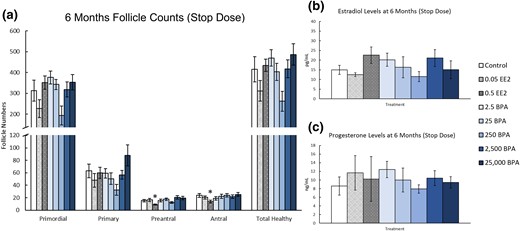
Effects of prenatal and preweaning exposure to EE2 and BPA on ovarian morphology and sex steroid hormones at 6 months. At 6 months, rats from each group were euthanized and one ovary from each animal was fixed for histological evaluation of different ovarian follicle types. Additionally, serum was collected from the blood of each of the animals and used in the measurement of sex steroid hormones. (a) Graph represents the means ± SEM of the number of the different follicle types and total healthy follicles present in 9 to 10 ovaries per treatment group. (b) Graph represents the means ± SEM of the amount of estradiol present in the serum of five to nine animals per group. (c) Graph represents the means ± SEM of the amount of progesterone present in the serum of five to nine animals per group. *P ≤ 0.05, difference between the control group and doses of BPA or EE2 (n = 9 to 10).
Effects of continuous exposure to EE2 and BPA on ovarian morphology and hormone levels at 6 months
Continuous exposure to EE2 and BPA did not significantly affect the numbers of any ovarian follicle type at 6 months when compared with control [Fig. 6(a), n = 10]. Furthermore, EE2 or BPA exposure did not significantly affect most serum estradiol or progesterone levels when compared with control [Fig. 6(b) and 6(c), n = 3 to 8]. However, EE2 exposure (0.5 µg/kg bw/d) decreased serum progesterone levels at 6 months [Fig. 6(c), n = 7, P ≤ 0.05].

Effects of continuous exposure to EE2 and BPA on ovarian morphology and sex steroid hormones at 6 months. At 6 months, rats from each group were euthanized and one ovary from each animal was fixed for histological evaluation of different ovarian follicle types. Additionally, serum was collected from the blood of each of the animals and used in the measurement of sex steroid hormones. (a) Graph represents the means ± SEM of the number of the different follicle types and total healthy follicles present in 10 ovaries per treatment group. (b) Graph represents the means ± SEM of the amount of estradiol present in the serum of three to eight animals per group. (c) Graph represents the means ± SEM of the amount of progesterone present in the serum of three to eight animals per group. *Significant difference between the control group and EE2 (n = 9 to 10; P < 0.05).
Effects of prenatal and preweaning exposure to EE2 and BPA on ovarian morphology and hormone levels at 1 year
At 1 year, prenatal and preweaning exposure to EE2 or BPA did not significantly affect the numbers of most ovarian follicle types, but EE 0.5 μg/kg bw/d) increased primary follicles when compared with control [Fig. 7(a), n = 9 to 10]. Additionally, exposure to EE2 or BPA did not significantly affect serum estradiol or progesterone levels when compared with control [Fig. 7(b) and 7(c), n = 5 to 10].
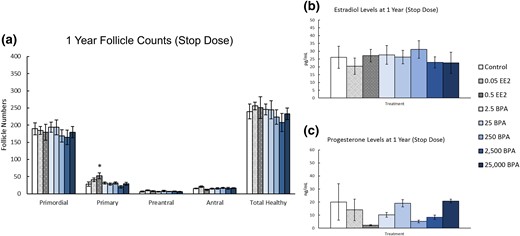
Effects of prenatal and preweaning exposure to EE2 and BPA on ovarian morphology and sex steroid hormones at 1 year. At 1 year, rats from each group were euthanized and one ovary from each animal was fixed for histological evaluation of different ovarian follicle types. Additionally, serum was collected from the blood of each of the animals and used in the measurement of sex steroid hormones. (a) Graph represents the means ± SEM of the number of the different follicle types and total healthy follicles present in 9 to 10 ovaries per treatment group. (b) Graph represents the means ± SEM of the amount of estradiol present in the serum of 5 to 10 animals per group. (c) Graph represents the means ± SEM of the amount of progesterone present in the serum of 5 to 10 animals per group. *Significant difference between the control group and EE2 (n = 9 to 10; P ≤ 0.05).
Effect of continuous exposure to EE2 and BPA on ovarian morphology and hormone levels at 1 year
At 1 year, continuous exposure to EE2 (0.5 µg/kg bw/d) significantly increased the number of primary follicles present when compared with control [Fig. 8(a), n = 8 to 10, P ≤ 0.05]. Exposure to BPA did not significantly affect the numbers of any ovarian follicle types compared with control [Fig. 8(a), n = 8 to 10]. Exposure to EE2 (0.05 and 0.5 µg/kg bw/d) significantly decreased serum estradiol levels when compared with control [Fig. 8(b), n = 3 to 9, P ≤ 0.05]. Additionally, BPA exposure at 2500 and 25,000 µg/kg bw/d significantly decreased serum estradiol levels when compared with control [Fig. 8(b), n = 3 to 9, P ≤ 0.05]. BPA exposure at 2.5, 25, and 250 µg/kg bw/d also decreased estradiol levels compared with control, but this change was not statistically significant [Fig. 8(b), n = 3 to 9]. Conversely, EE2 or BPA exposure did not significantly affect serum progesterone levels when compared with control [Fig. 8(c), n = 3 to 9].
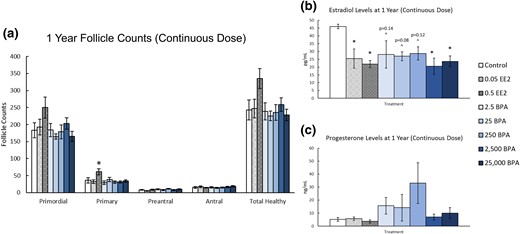
Effects of continuous exposure to EE2 and BPA on ovarian morphology and sex steroid hormones at 1 year. At 1 year, rats from each group were euthanized and one ovary from each animal was fixed for histological evaluation of different ovarian follicle types. Additionally, serum was collected from the blood of each of the animals and used in the measurement of sex steroid hormones. (a) Graph represents the means ± SEM of the number of the different follicle types and total healthy follicles present in 8 to 10 ovaries per treatment group. (b) Graph represents the means ± SEM of the amount of estradiol present in the serum of three to nine animals per group. (c) Graph represents the means ± SEM of the amount of progesterone present in the serum of three to nine animals per group. *P ≤ 0.05, difference between the control group and doses of BPA or EE2 (n = 3 to 9); ^P > 0.05.
Discussion
The CLARITY-BPA consortium allowed us to examine the effects of short-term and long-term exposure to EE2 and BPA on ovarian morphology and sex steroid hormone production. Our results show that exposure to BPA (2.5 and 250 µg/kg bw/d) decreased the numbers of primordial, primary, preantral, and total healthy follicle numbers at PND 21. Exposure to EE2 (0.5 µg/kg bw/d) decreased preantral follicles (PND 90, 6 months), decreased antral follicles (PND 21 and 6 months), and increased the number of primary follicles (1 year) when compared with control. Additionally, both BPA (2500 and 25,000 µg/kg bw/d) and EE2 (0.05 and 0.5 µg/kg bw/d) exposure decreased estradiol levels in animals dosed for 1 year. Collectively, these results indicate that EE2 and BPA exposures at some doses and time points affect ovarian follicle numbers and sex steroid levels in the rat. The most consistent effects were observed with 0.5 µg/kg bw/d EE2 (Fig. 9). This particular treatment significantly affected follicle numbers at PND 21, PND 90, 6 months, and 1 year. Furthermore, it altered hormone levels at PND 21, PND 90, 6 months, and 1 year. Consistent effects were also observed with all treatment groups on estradiol levels after 1 year of continuous dosing (Fig. 9). Both EE2 treatments (0.05 and 0.5 µg/kg bw/d) and two BPA treatments (2500 and 25,000 µg/kg bw/d) significantly reduced estradiol levels, and several BPA treatments (2.5, 25, and 250 µg/kg bw/d) caused a borderline significant reduction in estradiol levels, with P values ranging from 0.08 to 0.14 (Fig. 9).
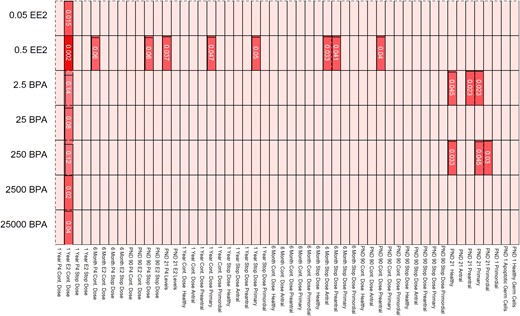
Heat map depicting the effects of EE2 and BPA exposure on ovarian morphology and sex steroid hormones. Data from Figs. 1–8 were used to generate a heat map. The light shading shows nonsignificant findings between controls and treatment groups. Darker shading corresponds to significant differences between control and treatment group (with the lowest P values being the darkest colors).
The observed effects of EE2 on follicle numbers are somewhat similar to previous studies on EE2. Specifically, our study as well as studies by Talsness et al. (36) and Delclos et al. (29) indicate that prenatal exposure to EE2 reduces the number of preantral and/or antral follicles in the rat ovary. However, both Talsness et al. (36) and Delclos et al. (29) reported that EE2 causes cyst formation in the ovary, whereas we did not observe that EE2 causes cyst formation. It is likely that the differences in our results compared with Talsness et al. (36) stem from differences in the doses of EE2. We used 0.05 and 0.5 μg/kg bw, whereas Talsness et al. (36) used 2 μg/kg bw of EE2. It is also likely that the differences in our results compared with Talsness et al. (36) stem from differences in the source of rats. We used NCTR Sprague-Dawley rats (strain code 23) from the NCTR rodent breeding colony, whereas Talsness et al. (36) used Sprague-Dawley rats from Winkelmann (Borchen, Germany). However, we cannot explain differences between the results of our study and Delclos et al. (29). Both studies used the same dose of EE2, the same dosing window, the same route of exposure, and the same strain of rats.
The observed effects of BPA on follicle numbers in rats are similar to previous studies on the effects of BPA in other species such as lambs and mice. In our study, BPA exposure at 2.5 and 250 µg/kg bw/d decreased follicle numbers on PND 21. In a study conducted on lambs, Rivera et al. (22) showed that subcutaneous exposure to 50 µg/kg bw/d BPA from PND 1 to 14 decreased the primordial follicle pool and increased the number of atretic follicles and multioocyte follicles in PND 30 ovaries. In a study conducted on mice, prenatal exposure to 0.5 or 50 µg/kg bw/d BPA decreased the number of primordial follicles present in PND 4 ovaries (24). These studies indicate that several species are susceptible to BPA-induced reductions in follicle numbers. Contrary to the mouse and lamb studies, we did not examine follicle populations at PND 4 or 30. Thus, we may have missed effects of BPA on the PND 4 and PND 30 ovary. It is also possible that the effects of BPA exposure on the rat ovary may occur at later time points than in the mouse or at earlier time points than in the lamb.
Similar to our study, other studies conducted on rats showed that exposure to BPA affects follicle numbers. One particular study found that exposure to BPA (0.5 and 50 µg/kg bw/d) from gestational day 9 to PND 21 decreased the number of primary follicles in female Wistar rats (37). Another study found that exposure to BPA (3 µg/kg bw/d) from gestation day 0 to PND 21 increased the number of primary, secondary, antral, and total follicle numbers in the ovary when compared with control in Wistar rats (38). Although the effects of BPA on specific follicle populations differ between our study and other rat studies, they collectively indicate that prenatal and prepubertal exposure to BPA can affect follicle numbers in the ovary. Any differences in the effects of BPA on specific follicle populations likely stem from the different doses and timing of exposure used in the various rat studies.
Previous studies conducted on rats have also examined the effects of BPA on sex steroid hormone production. Both Fernández et al. (26) and Gámez et al. (38) showed that BPA exposure (2.5 to 6.2 and 25 to 62 mg/kg, and 3 µg/kg bw/d) increased estradiol levels compared with control. In contrast, our results indicate that continuous exposure to BPA decreased estradiol levels compared with control, but only at 1 year. Although both Fernández et al. (26) and Gámez et al. (38) used doses similar to those in our study, it is possible that differences in the observed effects of BPA on hormone levels are the result of different routes of exposure. Fernández et al. (26) administered BPA by subcutaneous injection. Subcutaneous injection of BPA could result in higher systemic BPA levels than oral gavage (39). Thus, the rats from the study by Fernández et al. (26) could have been exposed to higher levels of BPA than the rats in our study, resulting in different effects of BPA on estradiol levels. Gámez et al. (38) administered BPA in the drinking water. Although this is a natural route of exposure to BPA, it is difficult to control the exact amount of BPA that each rat receives through the drinking water and likely that internal doses of BPA differed from those in our study.
It is also important to note that in our study, the continuous dosed control group had higher estradiol levels (∼45 ng/mL, Fig. 8B) than the stop-dose control group (∼25 ng/mL, Fig. 7B). Thus, it is possible that the statistical differences in the decreases in estradiol levels in the BPA- and EE2-treated groups at 1 year are due to an unusual control group. However, it is also possible that the higher estradiol levels are simply due to normal biological or seasonal variance between the stop-dosed animals and continuous-dosed animals. All hormone analyses were conducted at the same time and without knowledge of treatment group. Importantly, note that we may not have had statistical power to observe significant effects of BPA exposure on hormone levels at some doses and time points. We initially planned to collect and analyze 10 serum samples from each treatment group and time point. However, cycling females were euthanized when predicted to be in estrus based on a vaginal smear from the previous day. This method was not always successful in predicting estrous cyclicity on the day of collections. Because it is important to measure hormone levels from animals on the same day of the estrous cycle, the sample size was <10 in some treatment groups used for hormone analysis. This could have reduced our statistical power to observe differences between treatment groups. In fact, we noticed that several BPA treatments reduced estradiol levels compared with controls at 1 year, but this reduction was not always statistically significant at the P < 0.05 level.
Interestingly, the observed effects of BPA and EE2 differ from each other, raising questions about whether BPA and EE2 work via different mechanisms of action. It is well known that EE2 works through estrogen receptors, but it is possible that BPA exerts its effects via different receptors or that it stimulates/inhibits different estrogen-responsive pathways than EE2 in the ovarian follicle. Studies on the effects of BPA on isolated antral follicles from mice indicate that BPA inhibits follicle growth and induces atresia independently of the genomic estrogenic pathway (40). Furthermore, they also indicate that BPA may inhibit follicle growth partially through the aryl hydrocarbon receptor pathway (41). Thus, it is possible that the effects of BPA observed in this study occur through nongenomic estrogen pathways or other receptor pathways such as the aryl hydrocarbon pathway.
When comparing our findings to those of published studies from the CLARITY-BPA consortium, we observed that many of the effects observed in our study occur at the same doses as those shown to cause effects in other CLARITY-BPA studies. Our results show that continuous exposure to BPA at 2.5 and 250 µg/kg bw/d decreases the number of primordial, primary, preantral, and total healthy follicle numbers present in the ovary at PND 21. Similarly, Arambula et al. (42) observed that BPA at 2.5 and 250 µg/kg bw/d increases the expression of Esr1 and Esr2 in the hypothalamus at PND 1. In another published CLARITY-BPA study, Johnson et al. (43) examined the effects of BPA and EE2 doses on the spatial navigational learning and memory in rats. They found that female rats exposed to BPA at 2500 µg/kg bw/d were less likely to locate the correct escape box than controls at PND 90. After exposure to the same dose (BPA 2500 µg/kg bw/d), we observed decreased serum estradiol levels compared with vehicle control at 1 year. Although the effects seen in previously published CLARITY-BPA studies occurred at the same doses of BPA as used in our studies, the effects varied by the age of the rats. Therefore, it is difficult to state whether the effects observed in different studies are related to each other. However, these results indicate that the same doses of BPA have a variety of physiological and behavioral effects on the NCTR Sprague-Dawley rats.
Overall, our results indicate that BPA exposure at some doses and time points alters follicle numbers and hormone production in female rats. Furthermore, because we observed different effects of BPA exposure than EE2 exposure on follicle numbers and hormone levels, it is highly suggested that BPA is acting on the rat ovaries in a manner different from EE2. Future studies should examine why these two chemicals produce different responses at different doses and time points.
Abbreviations:
- BPA
bisphenol A
- bw
body weight
- CLARITY-BPA
Consortium Linking Academic and Regulatory Insights on BPA Toxicity
- EE2
ethinyl estradiol
- FDA
US Food and Drug Administration
- NCTR
National Center for Toxicological Research
- NIEHS
National Institute of Environmental Health Sciences
- NTP
National Toxicology Program
- PND
postnatal day
- SEM
standard error of the mean.
Acknowledgments
The authors thank Luísa Camacho, Barry Delclos, Sherry M. Lewis, and Michelle M. Vanlandingham from the FDA-NCTR for their contribution to the study and insight on the manuscript, and the NCTR animal care, pathology, veterinary services, diet preparation, information technology, microbiology, chemistry, and mass spectroscopy support groups for their critical contribution to the planning and conduct of the study. The authors also thank Dr. Rebecca Smith (Department of Pathobiology, University of Illinois at Urbana–Champaign) for help in generating the heat map.
This work was supported by National Institutes of Health Grant U01ES020835 (to J.A.F.), the Billie A. Field Fellowship (to S.P.), and by National Institutes of Health Grant T32ES007326 (to S.R.). The University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core is supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development /National Institutes of Health (National Centers for Translational Research in Reproduction and Infertility) Grant P50-HD28934.
Disclosure Summary: The authors have nothing to disclose.
References
Author notes
Address all correspondence and requests for reprints to: Jodi A. Flaws, PhD, Department of Comparative Biosciences, University of Illinois at Urbana–Champaign, 2001 South Lincoln Avenue, Room 3223, Urbana, Illinois 61802. E-mail: [email protected].



