-
PDF
- Split View
-
Views
-
Cite
Cite
Paul M Haller, Patrick Sulzgruber, Christoph Kaufmann, Bastiaan Geelhoed, Juan Tamargo, Sven Wassmann, Renate B Schnabel, Dirk Westermann, Kurt Huber, Alexander Niessner, Thomas Gremmel, Bleeding and ischaemic outcomes in patients treated with dual or triple antithrombotic therapy: systematic review and meta-analysis, European Heart Journal - Cardiovascular Pharmacotherapy, Volume 5, Issue 4, October 2019, Pages 226–236, https://doi.org/10.1093/ehjcvp/pvz021
Close - Share Icon Share
Abstract
The combination of oral anticoagulation with a P2Y12 inhibitor and aspirin in patients with atrial fibrillation (AF) undergoing percutaneous coronary intervention (PCI) is associated with a high bleeding risk. Dual antithrombotic therapy (DAT) with omission of aspirin is a promising option to reduce bleedings, but carries a yet unknown risk of ischaemic events. We therefore sought to systematically review and analyse randomized controlled trials investigating DAT vs. triple antithrombotic therapy (TAT) in patients with AF following PCI and/or acute coronary syndrome (ACS).
We included four trials with overall 9317 patients (5039 DAT, 4278 TAT) in our analysis. Dual antithrombotic therapy was associated with a significant reduction in thrombolysis in myocardial infarction major bleeding [hazard ratio (HR) 0.52, 95% confidence interval (CI) 0.42–0.65; P = 0.0001], while the composite trial-defined ischaemic endpoint did not differ significantly between DAT and TAT (HR 0.98, 95% CI 0.79–1.22; P = 0.88). There was also no difference regarding the occurrence of myocardial infarction (MI; HR 1.16, 95% CI 0.92–1.46; P = 0.21) or stent thrombosis (HR 1.25, 95% CI 0.69–2.26; P = 0.46). Absolute numbers for MI were 131/4278 (3.1%) with TAT and 182/5039 (3.6%) with DAT, and for stent thrombosis 32/4278 (0.75%) and 52/5039 (1%), respectively. A post hoc power calculation based on the size and event rate of this meta-analysis revealed 80% power to detect a 37% and 100% increase in MI and stent thrombosis, respectively.
Dual antithrombotic therapy significantly reduces bleedings compared with TAT and seems to have a similar effect in preventing ischaemic endpoints in AF patients post-PCI or ACS. Future investigations are needed to determine its applicability specifically in patients at high risk of ischaemic outcomes.
Introduction
The antithrombotic management of patients with atrial fibrillation (AF) who have undergone percutaneous coronary intervention (PCI) with stent implantation or experience acute coronary syndrome (ACS) remains a major challenge in clinical practice. Oral anticoagulation (OAC) is indicated in AF patients presenting with an increased risk for the development of thromboembolic events. In contrast, dual antiplatelet therapy (DAPT) with a P2Y12 inhibitor plus aspirin is usually prescribed for 6–12 months after stent implantation to prevent adverse cardiovascular events, including stent thrombosis.1–3
Facing the dilemma that DAPT significantly reduces the risk of stent thrombosis compared with OAC but is inferior to OAC in the prevention of stroke and systemic embolism, it is common guideline-supported practice to combine all three agents as triple antithrombotic therapy (TAT).4,5 Although the use of TAT is associated with a lower burden of both stent thrombosis and thromboembolic events, this pharmacotherapeutic approach carries an almost four-fold increased major bleeding risk and doubles the incidence of intracranial haemorrhage when compared with OAC alone.6–8 Therefore, the treatment strategy in AF patients after stent implantation or ACS needs to balance the risk of stent thrombosis and thromboembolic events against the risk of major bleeding, to reach an optimal net clinical benefit in treated individuals.9–11 Consequently, efforts to seek novel therapeutic strategies in this high-risk patient population were prompted.
In this regard, dual antithrombotic therapy (DAT), i.e. the combination of OAC with only one antiplatelet agent, represents a promising approach to reduce the risk of bleeding. Meanwhile, different randomized controlled clinical trials compared the efficacy and safety of DAT vs. TAT in AF patients with ACS and/or undergoing PCI and stenting.12–16 In these trials, bleeding events were significantly lower with DAT compared with TAT. However, as a major drawback, these studies were not sufficiently powered to rule out an increased risk for ischaemic events with DAT vs. TAT.
In order to analyse the benefits and potential risks of DAT in AF patients following ACS and/or PCI with stent implantation, we performed a systematic review and meta-analysis of randomized clinical trials.
Methods
Reference search and study selection
The present analysis followed the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement (Supplementary material online, Table S1). We performed a systematic search on the main databases PubMed, Web of Science, and Embase until 23 March 2019. The following subject headings and keywords were used in different combinations: ‘pci’, ‘percutaneous coronary intervention’, ‘coronary intervention’, ‘coronary stenting’, ‘dual antithrombotic therapy’, ‘dual therapy’, ‘triple therapy’, ‘triple antithrombotic therapy’, ‘apixaban’, ‘rivaroxaban’, ‘edoxaban’, ‘dabigatran’, ‘vitamin k antagonist’, ‘warfarin’, ‘phenprocoumon’, ‘oral anticoagulation’, ‘oral anticoagulants’, ‘dual anti platelet therapy’, ‘dual anti-platelet therapy’, ‘clopidogrel’, and ‘aspirin’. All randomized controlled trials comparing DAT with TAT for patients having AF and an indication for DAPT were included. Dual antithrombotic therapy was defined by a study protocol mandated regime including any OAC with any P2Y12 inhibitor. TAT was defined by adding low-dose aspirin to any DAT regimen. For the present analysis, we included only trials where randomization was performed within 2 weeks after ACS/PCI. We also restricted our search to studies reporting on clinical outcomes, including bleedings by any definition, ischaemic outcomes [i.e. myocardial infarction (MI), stroke, stent thrombosis], and death. In addition, we only considered studies published in English and performed in humans. Two investigators independently screened all retrieved references by title and abstract (P.M.H. and C.K.), and additionally, reviewed references from full-text articles for other relevant studies. Extraction of data from full-text articles was performed independently by two physicians (P.M.H. and T.G.) using a predefined data recording form. Recorded data were further evaluated for potential inconsistencies with disagreements resolved by consensus. We extracted data on author, year of publication, number of participants, data on study drugs (timing, dose, duration), primary and secondary endpoints [absolute numbers, hazard ratios (HRs) with corresponding confidence intervals (CIs)], and patient characteristics. The primary safety endpoint of the present meta-analysis was major bleeding defined by the thrombolysis in myocardial infarction (TIMI) bleeding scale and the primary efficacy endpoint was the trial-defined ischaemic endpoint of major adverse cardiovascular events and death. Secondary endpoints included the composite of TIMI major and minor bleeding, as well as the individual extracted ischaemic endpoints, including death, MI, stroke, and stent thrombosis.
Statistical analysis
The effect of DAT vs. TAT was analysed using models with generic inverse variance testing. HR along with 95% CI was extracted and transformed using natural logarithm before analysis. In case HRs were not reported, we retrieved the log-HR and the corresponding variance based on a previously described formula17,18: log-HR = 2 * [(number of observed events in group 1) − (number of observed events in group 2)]/(number of observed events group 1) + (number of observed events observed events in group 2)] and
variance (log-HR) = 4/[(number of observed events in group 1) + (number of observed events in group 2)].
Due to the expected heterogeneity across studies with respect to study drugs, endpoint definition and duration of follow-up, we meta-analysed all results using random effects models (DerSimonian and Laird). Statistical heterogeneity of the undertaken comparisons was assessed by χ2 statistics and was further stratified according to the Higgins I2 statistics. In general, we report heterogeneity as either being low (I2 <25%), moderate (I2 25–50%), or high (I2 >50%). Sensitivity analysis was performed to evaluate the robustness of our findings by testing whether any study in particular had large influence on the overall results. Additionally, we did undertake subgroup analyses based on the two different doses of dabigatran that have been investigated in one trial.
Assessment of publication bias was not possible by funnel plots based on the low number (<10) of studies included. Finally, all studies were evaluated regarding their methodological quality (low, intermediate, and high risk, respectively) according to the Cochrane’s Collaboration risk of bias assessment tool. For statistical analyses and their presentation, we used Review Manager [RevMan, (Computer program) Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014] and the ‘meta’ package19 in ‘R’ Version 3.4.3.20 Additionally, a post hoc power calculation for endpoints was performed using a two-sided alpha of 0.05 and the ‘sampsi’ command in STATA IC 12.0. A post hoc power calculation based on the size and event rate of this meta-analysis revealed 80% power to detect a 37% and 100% increase in MI or stent thrombosis, respectively.
Results
Of 571 initially retrieved references, data from seven references13,–16,21–23 reporting on four randomized clinical trials were included (Figure 1); WOEST (use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: an open-label, randomized, controlled trial), PIONEER AF-PCI (Open-Label, Randomized, Controlled, Multicenter Study Exploring Two Treatment Strategies of Rivaroxaban, and a Dose-Adjusted Oral Vitamin K Antagonist Treatment Strategy in Subjects with Atrial Fibrillation who Undergo Percutaneous Coronary Intervention), RE-DUAL PCI (Randomized Evaluation of Dual Antithrombotic Therapy With Dabigatran vs. Triple Therapy With Warfarin in Patients With Nonvalvular Atrial Fibrillation Undergoing Percutaneous Coronary Intervention), and AUGUSTUS (An open-Label, 2×2 factorial, randomized controlled trial to evaluate the safety of apixaban vs. vitamin K antagonist and aspirin vs. placebo in patients with atrial fibrillation and acute coronary syndrome and/or percutaneous coronary intervention).13–16 In total, these trials included 9317 patients (5039 DAT, 4278 TAT). A summary of study characteristics is provided in Table 1. All four studies were judged to be of overall high quality (Supplementary material online, Table S2). The AUGUSTUS trial was the only one using a double-blinded randomization between DAT and TAT, whereas the comparison of the respective OAC regimens was open label.16 Accordingly, it is the only trial to be judged with low risk of performance bias, as all other studies used an open label design. All studies had an independent committee for event adjudication unaware of the group allocation (detection bias) and used appropriate randomization techniques.
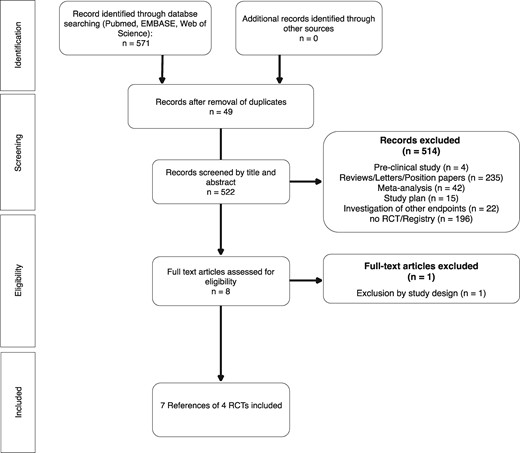
The PRISMA flowchart. The PRIMSA flowchart presenting the study identification and selection process.
| . | WOEST . | PIONEER AF-PCI . | RE-DUAL PCI . | AUGUSTUS . | Meta-analysis . |
|---|---|---|---|---|---|
| Author | Dewilde et al. | Gibson et al. | Cannon et al. | Lopes et al. | |
| Year of publication | 2013 | 2016 | 2017 | 2019 | |
| Follow-up (months) | 12 | 12 | 14 | 6 | |
| Randomized to DAT, n | 279 | 709 | 1744 | 2307 | 5039 |
| % of meta-analysis | 2.99 | 7.61 | 18.72 | 24.76 | 54 |
| Randomized to TAT, n | 284 | 706 | 981 | 2307 | 4278 |
| % of meta-analysis | 3.05 | 7.58 | 10.53 | 24.76 | 46 |
| Total study population included, n | 563 | 1415 | 2725 | 4614 | 9317 |
| % of meta-analysis | 6 | 15.2 | 29.2 | 49.5 | 100 |
| Women, n (% within trial) | 115/563 (20.4) | 369/1415 (26.1) | 655/2725 (24.0) | 1337 (29.0) | 2476 |
| % of meta-analysis | — | — | — | — | 26.6 |
| Acute coronary syndrome, n (% within trial) | 155/563 (27.5) | 722/1415 (51.0) | 1375/2725 (50.5) | 2811/4595 (61.2) | 5063 |
| % of meta-analysis | 1.7 | 7.7 | 14.8 | 30.2 | 54.3 |
| Atrial fibrillation, n (% within trial) | 326/470 (69.4) | 1415/1415 (100) | 2725/2725 (100) | 4614/4614 (100) | 9080 |
| % of meta-analysis | — | — | — | — | 97.5 |
| Patients undergoing PCI with stent placement, n (% within trial) | 553/563 (98.2) | 1413/1415 (99.9) | 2725/2725 (100) | 3498/4595 (76.1) | 8189 |
| % of meta-analysis | — | — | — | — | 87.9 |
| Stent type, n (% within trial) | |||||
| Drug eluting | 364/563 (64.7) | 932/1415 (65.9) | 2251/2725 (82.6) | NR | 3547a |
| % of analysis | — | — | — | — | 75.4a |
| Bare metal | 175/563 (31.1) | 455/1415 (32.2) | 404/2725 (14.8) | NR | 1034a |
| % of analysis | — | — | — | — | 22a |
| Both | 14/563 (2.5) | 26/1415 (1.8) | 41/2725 (1.5) | NR | 81a |
| % of analysis | — | — | — | — | 1.7 |
| P2Y12 inhibitor use, n (% within trial) | |||||
| Clopidogrel | 563/563 (100) | 1340/1415 (94.7) | 2398/2725 (88) | 4165/4496 (92.6) | 8466 |
| % of meta-analysis | — | — | — | — | 90.9 |
| Prasugrel | 0/563 | 17/1415 (1.2) | 0/2725 (0) | 51/4496 (1.1) | 68 |
| % of meta-analysis | — | — | — | — | 0.7 |
| Ticagrelor | 0/563 | 58/1415 (4.1) | 327/2725 (12) | 280/4496 (6.2) | 665 |
| % of meta-analysis | — | — | — | — | 7.1 |
| . | WOEST . | PIONEER AF-PCI . | RE-DUAL PCI . | AUGUSTUS . | Meta-analysis . |
|---|---|---|---|---|---|
| Author | Dewilde et al. | Gibson et al. | Cannon et al. | Lopes et al. | |
| Year of publication | 2013 | 2016 | 2017 | 2019 | |
| Follow-up (months) | 12 | 12 | 14 | 6 | |
| Randomized to DAT, n | 279 | 709 | 1744 | 2307 | 5039 |
| % of meta-analysis | 2.99 | 7.61 | 18.72 | 24.76 | 54 |
| Randomized to TAT, n | 284 | 706 | 981 | 2307 | 4278 |
| % of meta-analysis | 3.05 | 7.58 | 10.53 | 24.76 | 46 |
| Total study population included, n | 563 | 1415 | 2725 | 4614 | 9317 |
| % of meta-analysis | 6 | 15.2 | 29.2 | 49.5 | 100 |
| Women, n (% within trial) | 115/563 (20.4) | 369/1415 (26.1) | 655/2725 (24.0) | 1337 (29.0) | 2476 |
| % of meta-analysis | — | — | — | — | 26.6 |
| Acute coronary syndrome, n (% within trial) | 155/563 (27.5) | 722/1415 (51.0) | 1375/2725 (50.5) | 2811/4595 (61.2) | 5063 |
| % of meta-analysis | 1.7 | 7.7 | 14.8 | 30.2 | 54.3 |
| Atrial fibrillation, n (% within trial) | 326/470 (69.4) | 1415/1415 (100) | 2725/2725 (100) | 4614/4614 (100) | 9080 |
| % of meta-analysis | — | — | — | — | 97.5 |
| Patients undergoing PCI with stent placement, n (% within trial) | 553/563 (98.2) | 1413/1415 (99.9) | 2725/2725 (100) | 3498/4595 (76.1) | 8189 |
| % of meta-analysis | — | — | — | — | 87.9 |
| Stent type, n (% within trial) | |||||
| Drug eluting | 364/563 (64.7) | 932/1415 (65.9) | 2251/2725 (82.6) | NR | 3547a |
| % of analysis | — | — | — | — | 75.4a |
| Bare metal | 175/563 (31.1) | 455/1415 (32.2) | 404/2725 (14.8) | NR | 1034a |
| % of analysis | — | — | — | — | 22a |
| Both | 14/563 (2.5) | 26/1415 (1.8) | 41/2725 (1.5) | NR | 81a |
| % of analysis | — | — | — | — | 1.7 |
| P2Y12 inhibitor use, n (% within trial) | |||||
| Clopidogrel | 563/563 (100) | 1340/1415 (94.7) | 2398/2725 (88) | 4165/4496 (92.6) | 8466 |
| % of meta-analysis | — | — | — | — | 90.9 |
| Prasugrel | 0/563 | 17/1415 (1.2) | 0/2725 (0) | 51/4496 (1.1) | 68 |
| % of meta-analysis | — | — | — | — | 0.7 |
| Ticagrelor | 0/563 | 58/1415 (4.1) | 327/2725 (12) | 280/4496 (6.2) | 665 |
| % of meta-analysis | — | — | — | — | 7.1 |
Characteristics of the included trials are presented as reported in the respective publications. The 2.5 mg b.i.d. rivaroxaban group of the PIONEER AF-PCI trial was excluded. Also, the AUGUSTUS trial did not report data on all randomized individuals.
DAT, dual antithrombotic therapy; NR, not reported; PCI, percutaneous coronary intervention; TAT, triple antithrombotic therapy.
Calculations on stent types are based on data without the AUGUSTUS trial as they have not been reported.
| . | WOEST . | PIONEER AF-PCI . | RE-DUAL PCI . | AUGUSTUS . | Meta-analysis . |
|---|---|---|---|---|---|
| Author | Dewilde et al. | Gibson et al. | Cannon et al. | Lopes et al. | |
| Year of publication | 2013 | 2016 | 2017 | 2019 | |
| Follow-up (months) | 12 | 12 | 14 | 6 | |
| Randomized to DAT, n | 279 | 709 | 1744 | 2307 | 5039 |
| % of meta-analysis | 2.99 | 7.61 | 18.72 | 24.76 | 54 |
| Randomized to TAT, n | 284 | 706 | 981 | 2307 | 4278 |
| % of meta-analysis | 3.05 | 7.58 | 10.53 | 24.76 | 46 |
| Total study population included, n | 563 | 1415 | 2725 | 4614 | 9317 |
| % of meta-analysis | 6 | 15.2 | 29.2 | 49.5 | 100 |
| Women, n (% within trial) | 115/563 (20.4) | 369/1415 (26.1) | 655/2725 (24.0) | 1337 (29.0) | 2476 |
| % of meta-analysis | — | — | — | — | 26.6 |
| Acute coronary syndrome, n (% within trial) | 155/563 (27.5) | 722/1415 (51.0) | 1375/2725 (50.5) | 2811/4595 (61.2) | 5063 |
| % of meta-analysis | 1.7 | 7.7 | 14.8 | 30.2 | 54.3 |
| Atrial fibrillation, n (% within trial) | 326/470 (69.4) | 1415/1415 (100) | 2725/2725 (100) | 4614/4614 (100) | 9080 |
| % of meta-analysis | — | — | — | — | 97.5 |
| Patients undergoing PCI with stent placement, n (% within trial) | 553/563 (98.2) | 1413/1415 (99.9) | 2725/2725 (100) | 3498/4595 (76.1) | 8189 |
| % of meta-analysis | — | — | — | — | 87.9 |
| Stent type, n (% within trial) | |||||
| Drug eluting | 364/563 (64.7) | 932/1415 (65.9) | 2251/2725 (82.6) | NR | 3547a |
| % of analysis | — | — | — | — | 75.4a |
| Bare metal | 175/563 (31.1) | 455/1415 (32.2) | 404/2725 (14.8) | NR | 1034a |
| % of analysis | — | — | — | — | 22a |
| Both | 14/563 (2.5) | 26/1415 (1.8) | 41/2725 (1.5) | NR | 81a |
| % of analysis | — | — | — | — | 1.7 |
| P2Y12 inhibitor use, n (% within trial) | |||||
| Clopidogrel | 563/563 (100) | 1340/1415 (94.7) | 2398/2725 (88) | 4165/4496 (92.6) | 8466 |
| % of meta-analysis | — | — | — | — | 90.9 |
| Prasugrel | 0/563 | 17/1415 (1.2) | 0/2725 (0) | 51/4496 (1.1) | 68 |
| % of meta-analysis | — | — | — | — | 0.7 |
| Ticagrelor | 0/563 | 58/1415 (4.1) | 327/2725 (12) | 280/4496 (6.2) | 665 |
| % of meta-analysis | — | — | — | — | 7.1 |
| . | WOEST . | PIONEER AF-PCI . | RE-DUAL PCI . | AUGUSTUS . | Meta-analysis . |
|---|---|---|---|---|---|
| Author | Dewilde et al. | Gibson et al. | Cannon et al. | Lopes et al. | |
| Year of publication | 2013 | 2016 | 2017 | 2019 | |
| Follow-up (months) | 12 | 12 | 14 | 6 | |
| Randomized to DAT, n | 279 | 709 | 1744 | 2307 | 5039 |
| % of meta-analysis | 2.99 | 7.61 | 18.72 | 24.76 | 54 |
| Randomized to TAT, n | 284 | 706 | 981 | 2307 | 4278 |
| % of meta-analysis | 3.05 | 7.58 | 10.53 | 24.76 | 46 |
| Total study population included, n | 563 | 1415 | 2725 | 4614 | 9317 |
| % of meta-analysis | 6 | 15.2 | 29.2 | 49.5 | 100 |
| Women, n (% within trial) | 115/563 (20.4) | 369/1415 (26.1) | 655/2725 (24.0) | 1337 (29.0) | 2476 |
| % of meta-analysis | — | — | — | — | 26.6 |
| Acute coronary syndrome, n (% within trial) | 155/563 (27.5) | 722/1415 (51.0) | 1375/2725 (50.5) | 2811/4595 (61.2) | 5063 |
| % of meta-analysis | 1.7 | 7.7 | 14.8 | 30.2 | 54.3 |
| Atrial fibrillation, n (% within trial) | 326/470 (69.4) | 1415/1415 (100) | 2725/2725 (100) | 4614/4614 (100) | 9080 |
| % of meta-analysis | — | — | — | — | 97.5 |
| Patients undergoing PCI with stent placement, n (% within trial) | 553/563 (98.2) | 1413/1415 (99.9) | 2725/2725 (100) | 3498/4595 (76.1) | 8189 |
| % of meta-analysis | — | — | — | — | 87.9 |
| Stent type, n (% within trial) | |||||
| Drug eluting | 364/563 (64.7) | 932/1415 (65.9) | 2251/2725 (82.6) | NR | 3547a |
| % of analysis | — | — | — | — | 75.4a |
| Bare metal | 175/563 (31.1) | 455/1415 (32.2) | 404/2725 (14.8) | NR | 1034a |
| % of analysis | — | — | — | — | 22a |
| Both | 14/563 (2.5) | 26/1415 (1.8) | 41/2725 (1.5) | NR | 81a |
| % of analysis | — | — | — | — | 1.7 |
| P2Y12 inhibitor use, n (% within trial) | |||||
| Clopidogrel | 563/563 (100) | 1340/1415 (94.7) | 2398/2725 (88) | 4165/4496 (92.6) | 8466 |
| % of meta-analysis | — | — | — | — | 90.9 |
| Prasugrel | 0/563 | 17/1415 (1.2) | 0/2725 (0) | 51/4496 (1.1) | 68 |
| % of meta-analysis | — | — | — | — | 0.7 |
| Ticagrelor | 0/563 | 58/1415 (4.1) | 327/2725 (12) | 280/4496 (6.2) | 665 |
| % of meta-analysis | — | — | — | — | 7.1 |
Characteristics of the included trials are presented as reported in the respective publications. The 2.5 mg b.i.d. rivaroxaban group of the PIONEER AF-PCI trial was excluded. Also, the AUGUSTUS trial did not report data on all randomized individuals.
DAT, dual antithrombotic therapy; NR, not reported; PCI, percutaneous coronary intervention; TAT, triple antithrombotic therapy.
Calculations on stent types are based on data without the AUGUSTUS trial as they have not been reported.
Three trials tested a vitamin K antagonist in combination with a P2Y12 inhibitor and aspirin as TAT13–15 and one trial also randomized the oral anticoagulant irrespective of DAT or TAT allocation.16 In general, each of the four trials tested a different oral anticoagulant in the DAT group (warfarin, rivaroxaban, dabigatran, or apixaban). The primary indication for OAC was AF in all trials. However, the WOEST trial also included patients with other indications than AF (31% of the WOEST study population and 1.5% of the meta-analysed population). Furthermore, clopidogrel was used in most patients (8466/9317, 90.9%), with only 665 and 68 patients treated with ticagrelor and prasugrel, respectively (7.1% and 0.7% of the meta-analysed patient cohort, respectively). The duration of follow-up ranged from 6 to 14 months. In total, 8189 patients (87.9%) were treated with PCI and 1097 patients (11.8%) had a medically managed ACS and did not undergo PCI. Of note, only the AUGUSTUS trial also enrolled patients without PCI. In the PIONEER AF-PCI trial, three different treatment regimens were tested, of which only two entered the present meta-analysis. The omitted group of the PIONEER AF-PCI trial received a very low dose of rivaroxaban (2.5 mg b.i.d.) in combination with aspirin and clopidogrel. Since this dosage of rivaroxaban is not approved for the prevention of ischaemic stroke and systemic embolism in AF, the respective patient group was not considered.15 Furthermore, the RE-DUAL PCI trial tested two different dosages of dabigatran. We primarily used the combined group for the analysis, but provide data on the individual doses (110 mg or 150 mg b.i.d., respectively) wherever possible.13 In general, all four studies reported the analysed outcomes.
Primary and secondary safety endpoint
Dual antithrombotic therapy (n = 82/5039) compared with TAT (n = 128/4278) was associated with a significant reduction in TIMI major bleeding (Figure 2A; HR 0.52, 95% CI 0.42–0.65; P = 0.0001) with moderate heterogeneity (I2 0%). Furthermore, the composite endpoint of TIMI major and minor bleeding occurred less frequently with DAT (n = 196/5039) compared with TAT (n = 337/4278), with low heterogeneity (Figure 2B; HR 0.52, 95% CI 0.44–0.6; P < 0.00001; I2 7%). Findings were consistent for the subgroup meta-analysis investigating the individual doses of dabigatran (Supplementary material online, Figure S1A and B). Of note, the PIONEER AF-PCI trial also included study-defined clinically relevant bleedings in this outcome.15
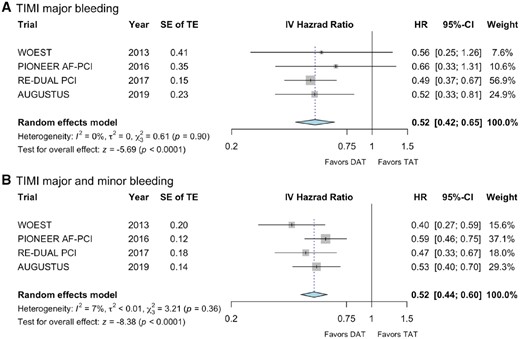
Forest plots for (A) thrombolysis in myocardial infarction major bleeding and (B) thrombolysis in myocardial infarction major in combination with thrombolysis in myocardial infarction minor bleeding are shown with standard errors of the treatment effect and calculated hazard ratio with corresponding 95% confidence interval. In (B), the PIONEER AF-PCI trial also included trial-defined clinically significant bleeding in the analysis. CI, confidence interval; DAT, dual antithrombotic therapy; HR, hazard ratio; IV, inverse variance; SE, standard error; TAT, triple antithrombotic therapy; TE, treatment effect; TIMI, thrombolysis in myocardial infarction.
Primary and secondary efficacy endpoints
With respect to the meta-analysis of the trial-defined secondary efficacy endpoints, a composite of ischaemic endpoints and death (Table 2), we observed no significant difference between DAT (n = 479/5039) and TAT (n = 366/4278) with high heterogeneity (HR 0.98, 95% CI 0.79–1.22; P = 0.88; I2 51%; Figure 3A).
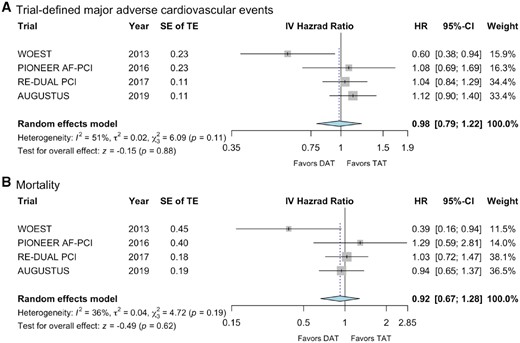
Forest plots for (A) trial-defined MAE and (B) mortality. The individual endpoints building the composite endpoint major adverse cardiovascular events are provided in Table 2. For the analysis of death, all-cause mortality was used of all trials, except the PIONEER AF-PCI trial, which only reported data on cardiovascular mortality. CI, confidence interval; DAT, dual antithrombotic therapy; HR, hazard ratio; IV, inverse variance; SE, standard error; TAT, triple antithrombotic therapy; TE, treatment effect.
| . | WOEST . | PIONEER AF-PCI . | RE-DUAL PCI . | AUGUSTUS . |
|---|---|---|---|---|
| Drug compound for anticoagulation | ||||
| In TAT group | Warfarin | Warfarin | Warfarin | Apixaban/warfarin |
| In DAT group | Warfarin | Rivaroxaban | Dabigatran | Apixaban/warfarin |
| ASA before randomization/latest time for randomization | Up to 4 h after PCI | Up to 72 h after PCI | Up to 120 h after PCI | Up to 14 days after PCI, mean duration 6.6 ± 4.2 days |
| Intended duration of TAT at randomizationa | 1–12 months |
|
| 6 months |
| Permanent cessation of protocol mandated therapy before scheduled termination date |
|
|
|
|
| Composite ischaemic endpoint included | ||||
| Mortality | x | xb | x | x |
| Myocardial infarction | x | x | x | x |
| Stroke | x | x | x | x |
| Stent thrombosis | x | x | ||
| Target vessel revascularization | x | |||
| Systemic embolism | x | |||
| Revascularization | x | x | ||
| . | WOEST . | PIONEER AF-PCI . | RE-DUAL PCI . | AUGUSTUS . |
|---|---|---|---|---|
| Drug compound for anticoagulation | ||||
| In TAT group | Warfarin | Warfarin | Warfarin | Apixaban/warfarin |
| In DAT group | Warfarin | Rivaroxaban | Dabigatran | Apixaban/warfarin |
| ASA before randomization/latest time for randomization | Up to 4 h after PCI | Up to 72 h after PCI | Up to 120 h after PCI | Up to 14 days after PCI, mean duration 6.6 ± 4.2 days |
| Intended duration of TAT at randomizationa | 1–12 months |
|
| 6 months |
| Permanent cessation of protocol mandated therapy before scheduled termination date |
|
|
|
|
| Composite ischaemic endpoint included | ||||
| Mortality | x | xb | x | x |
| Myocardial infarction | x | x | x | x |
| Stroke | x | x | x | x |
| Stent thrombosis | x | x | ||
| Target vessel revascularization | x | |||
| Systemic embolism | x | |||
| Revascularization | x | x | ||
The duration of TAT was at the discretion of the treating physician in WOEST, had to be pre-specified before randomization by the treating physician in PIONEER AF-PCI and was guided by the type of stent (BMS vs. DES) in RE-DUAL PCI.
The PIONEER AF-PCI trial reported only on cardiovascular mortality. All other trials reported all-cause mortality.
| . | WOEST . | PIONEER AF-PCI . | RE-DUAL PCI . | AUGUSTUS . |
|---|---|---|---|---|
| Drug compound for anticoagulation | ||||
| In TAT group | Warfarin | Warfarin | Warfarin | Apixaban/warfarin |
| In DAT group | Warfarin | Rivaroxaban | Dabigatran | Apixaban/warfarin |
| ASA before randomization/latest time for randomization | Up to 4 h after PCI | Up to 72 h after PCI | Up to 120 h after PCI | Up to 14 days after PCI, mean duration 6.6 ± 4.2 days |
| Intended duration of TAT at randomizationa | 1–12 months |
|
| 6 months |
| Permanent cessation of protocol mandated therapy before scheduled termination date |
|
|
|
|
| Composite ischaemic endpoint included | ||||
| Mortality | x | xb | x | x |
| Myocardial infarction | x | x | x | x |
| Stroke | x | x | x | x |
| Stent thrombosis | x | x | ||
| Target vessel revascularization | x | |||
| Systemic embolism | x | |||
| Revascularization | x | x | ||
| . | WOEST . | PIONEER AF-PCI . | RE-DUAL PCI . | AUGUSTUS . |
|---|---|---|---|---|
| Drug compound for anticoagulation | ||||
| In TAT group | Warfarin | Warfarin | Warfarin | Apixaban/warfarin |
| In DAT group | Warfarin | Rivaroxaban | Dabigatran | Apixaban/warfarin |
| ASA before randomization/latest time for randomization | Up to 4 h after PCI | Up to 72 h after PCI | Up to 120 h after PCI | Up to 14 days after PCI, mean duration 6.6 ± 4.2 days |
| Intended duration of TAT at randomizationa | 1–12 months |
|
| 6 months |
| Permanent cessation of protocol mandated therapy before scheduled termination date |
|
|
|
|
| Composite ischaemic endpoint included | ||||
| Mortality | x | xb | x | x |
| Myocardial infarction | x | x | x | x |
| Stroke | x | x | x | x |
| Stent thrombosis | x | x | ||
| Target vessel revascularization | x | |||
| Systemic embolism | x | |||
| Revascularization | x | x | ||
The duration of TAT was at the discretion of the treating physician in WOEST, had to be pre-specified before randomization by the treating physician in PIONEER AF-PCI and was guided by the type of stent (BMS vs. DES) in RE-DUAL PCI.
The PIONEER AF-PCI trial reported only on cardiovascular mortality. All other trials reported all-cause mortality.
Furthermore, we meta-analysed the individual endpoints of death, MI, stroke, and stent thrombosis. For this comparison, only data on cardiovascular mortality were considered for the PIONEER AF-PCI trial, as all-cause mortality was not reported in the primary publication.15 We did not observe any difference regarding death between DAT (n = 186/5039) and TAT (n = 149/4278) with moderate heterogeneity (HR 0.92, 95% CI 0.67–1.28; P = 0.62; I2 36%, Figure 3B). There was also no significant difference with respect to the incidence of MI with low heterogeneity (HR 1.16, 95% CI 0.92–1.46; P = 0.21; I2 0%, Figure 4A), or stent thrombosis with moderate heterogeneity (HR 1.25, 95% CI 0.69–2.26; P = 0.46; I2 34%, Figure 4B) or stroke with low heterogeneity (HR 0.96, 95% CI 0.64–1.44; P = 0.85; I2 0%, Figure 4C). Absolute event rates for MI, stent thrombosis, and stroke were 182, 52, and 56 out of 5039 patients with DAT and 131, 32, and 48 out of 4278 patients with TAT, respectively. Corresponding subgroup analysis with respect to the two investigated doses of dabigatran (110 mg and 150 mg b.i.d., respectively), did not reveal any different findings (Supplementary material online, Figures S1–S5).
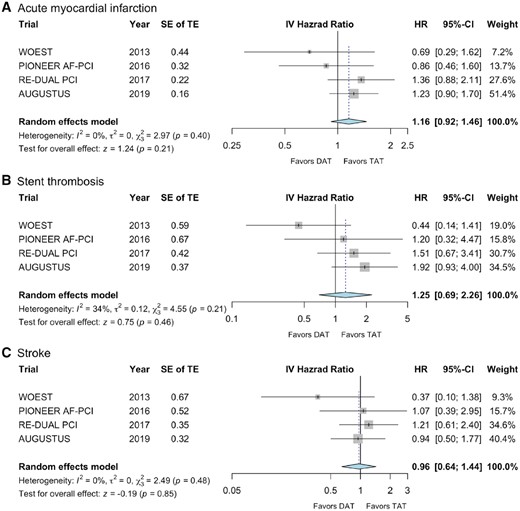
Forest plots for (A) acute myocardial infarction, (B) stent thrombosis, and (C) stroke. CI, confidence interval; DAT, dual antithrombotic therapy; HR, hazard ratio; IV, inverse variance; SE, standard error; TAT, triple antithrombotic therapy; TE, treatment effect.
Sensitivity analyses revealed, that the omission of any trial did not result in a change of direction or significance level of treatment effect of any investigated outcome, with one exception: upon omission of the WOEST trial (Figure 5A–G), we observed a borderline significance for an increased risk of stent thrombosis with DAT when both dabigatran groups from RE-DUAL PCI were considered (Figure 5F; HR 1.64, 95% CI 0.99–2.71; P = 0.05, I2 0%) and a significant increase in stent thrombosis with DAT compared with TAT if only the low-dose dabigatran group from RE-DUAL PCI was considered (HR 1.77, 95% CI 1.06–2.95; P = 0.03; I2 0%).
Endpoints with omission of the WOEST trial. Forest plots for (A) TIMI major bleeding, (B) TIMI major and minor bleeding, (C) trial-defined MACE, (D) mortality, (E) acute myocardial infarction, (F) stent thrombosis and (G) stroke with omission of the WOEST trial. CI, confidence interval; DAT, dual antithrombotic therapy; HR, hazard ratio; IV, inverse variance; MACE, major adverse cardiovascular events; SE, standard error; TAT, triple antithrombotic therapy; TE, treatment effect; TIMI, thrombolysis in myocardial infarction.
Based on the sample size of this meta-analysis it would have been possible to detect a relative increase of 37% or more for MI and a relative increase of 100% or more for stent thrombosis between DAT and TAT (alpha = 0.05, Power 80%, respectively).
Discussion
The present meta-analysis systematically compares DAT with TAT as assessed in four landmark clinical trials.13–16 As expected, DAT was associated with a markedly lower bleeding risk than TAT. Furthermore, DAT carried a similar risk of thrombotic and thromboembolic events like TAT, and may therefore represent a viable treatment option in most AF patients in need of combined antithrombotic therapy following PCI or ACS.
Our results are in line with previous meta-analyses comparing DAT with TAT regarding the occurrence of adverse bleeding and ischaemic events in anticoagulated patients after PCI.17,24 Since many major bleedings occur in the first few weeks after the initiation of TAT,25 DAT may be particularly beneficial if started early following PCI or ACS. In detail, previous studies revealed a major bleeding rate of 2.2% during the first month of TAT when compared with 4–12% major bleedings over 1 year suggesting a particularly increased bleeding risk in the early phase of TAT.25,26 This applies primarily to patients receiving TAT with warfarin since data on the exact timing of major bleeding episodes in patients receiving TAT with a non-vitamin K antagonist oral anticoagulant (NOAC) have not been published, so far. However, the currently available analyses of the AUGUSTUS trial also suggest a more pronounced bleeding risk in the first 30 days after initiation of TAT with apixaban.16 Thus, we decided to confine our literature search to clinical trials where the randomization to DAT vs. TAT took place within 14 days after an ACS or PCI at the latest. As a consequence, the ISAR-TRIPLE trial, which compared 6-week TAT followed by DAT with 6-month TAT in AF patients undergoing PCI, was not included into our meta-analysis.12 However, as patients in the respective studies were randomized to DAT vs. TAT within several days after the index event (up to 1 week) a minimal duration of aspirin therapy of 1 week seems justified in all patients. The omission of clopidogrel instead of aspirin in the studied DAT regimen represents another major difference of ISAR-TRIPLE compared with other included trials.12–16 Therefore, and in contrast to Golwala et al.,17 we chose not to incorporate the event data from the landmark analysis of the ISAR-TRIPLE trial.
In all four included trials, DAT consisted of OAC in combination with a P2Y12 inhibitor alone, while patients on TAT also received low-dose aspirin therapy. The omission of aspirin may be beneficial in two ways: first, by avoiding aspirin, thromboxane synthesis as one of the most important pathways of physiological platelet activation remains intact. As evidenced by all randomized trials comparing DAT with TAT and the current meta-analysis,12–16 the omission of one antiplatelet agent decreases the risk of bleeding in patients with impaired plasmatic coagulation due to OAC. In PIONEER AF-PCI and RE-DUAL PCI it is unclear to which extent the omission of aspirin or the use of a NOAC instead of warfarin contributed to the reduction of bleeding events in patients receiving DAT.13,15 However, WOEST and AUGUSTUS clearly show that skipping aspirin alone significantly lowers the risk of bleeding compared with TAT, an effect that occurs most likely due to improved cellular haemostasis.14,16 Second, without aspirin, mucosal prostaglandin generation is not impaired and patients are less prone to upper gastrointestinal bleedings.27–31 Since P2Y12 antagonists, at least in part, exert their antiplatelet effects by fostering the antiplatelet action of prostacyclin,32 it has been speculated that by inhibiting prostacyclin synthesis aspirin may attenuate the antiplatelet effects of P2Y12 blockers.33 Consequently, the omission of aspirin may allow P2Y12 inhibitors to fully unleash their antiplatelet potential, and monotherapy with a P2Y12 antagonist may provide more than just 50% of the antiplatelet effect of DAPT. However, there are currently no data supporting the speculation of an improved antiplatelet potential of P2Y12 inhibitors in the absence of aspirin in patients on OAC. Consequently, randomized clinical trials evaluating which antiplatelet agent to omit when shifting from TAT to DAT would be of great interest.
While clopidogrel was the only P2Y12 blocker prescribed in the WOEST trial, patients enrolled in the other trials could also be treated with ticagrelor (or prasugrel). However, these newer and more potent P2Y12 inhibitors were only used in a small proportion of patients (7.1% and 0.7% of the meta-analysed patient population, respectively). With 12% of patients on ticagrelor, RE-DUAL PCI had the highest number treated with a new generation P2Y12 blocker among these three trials. The particular subanalysis of RE-DUAL PCI revealed a similar safety and efficacy profile of DAT with ticagrelor and clopidogrel, respectively.13,23 Further clinical trials are warranted to investigate the net benefit of DAT with ticagrelor or prasugrel, especially in AF patients at high risk of stent thrombosis.
Due to the growing evidence supporting the omission of aspirin in AF patients with PCI or ACS and the introduction of this concept to the respective guidelines of the European Society of Cardiology (ESC),4 European interventional cardiologists feel comfortable prescribing DAT especially in AF patients undergoing elective low-risk PCI, while TAT for at least 1 month is recommended if the ischaemic risk is prevailing.4,34,35 In contrast, North American experts recommended DAT as default strategy in the majority of patients.36 By showing similar rates of ischaemic outcomes with TAT and DAT, respectively, the current meta-analysis encourages the application of the latter strategy. However, the risk of stent thrombosis is particularly high in the first weeks after PCI when the implanted stent is not covered with endothelium and was numerically increased in patients on DAT in AUGUSTUS and in the low-dose dabigatran group of RE-DUAL PCI.13,16 Further, upon omission of the WOEST trial, we observed a borderline significance for an increased risk of stent thrombosis with DAT when both dabigatran groups from RE-DUAL PCI were included and a significant increase in stent thrombosis with DAT compared with TAT when only the low-dose dabigatran group from RE-DUAL PCI was considered. Accordingly, as suggested in the current guidelines of the ESC,4 it is of utmost importance to carefully select patients eligible for DAT instead of TAT by weighing their bleeding risk against their individual risk of stent thrombosis. In particular, severe consequences of stent thrombosis, e.g. due to left main stenting, and an increased risk of stent thrombosis, e.g. due to bifurcation intervention, stent malapposition, multiple stent implantation, or small diameter stents need to be taken into account. Similarly, patients with clinical characteristics associated with a high ischaemic risk, such as ACS and diabetes mellitus need specific attention. Even though a prolonged duration of TAT seems reasonable in individuals presenting with an increased ischaemic risk, as also recommended by current ESC guidelines,4 there is no evidence-based approach how to identify these patients and how to select the optimal duration of TAT, so far.
Among the four included trials, only the WOEST trial reported a significantly reduced overall mortality with DAT compared with TAT.14 We therefore performed a subanalysis on this secondary endpoint with omission of the WOEST trial. As expected, this subanalysis showed a similar overall mortality in patients on DAT and TAT, respectively. Since the number of thrombotic events and in particular the number of stent thrombosis was low even in this meta-analysis, the trend for an increased risk of stent thrombosis with DAT compared with TAT when the WOEST trial was omitted, may represent a chance finding. AUGUSTUS was by far the largest trial comparing DAT with TAT in AF patients following ACS and/or PCI. Overall, AUGUSTUS accounts for approximately 50% of the meta-analysed population. Moreover, compared with previous trials, AUGUSTUS adds important data on DAT and TAT with another NOAC and warfarin, respectively. Unfortunately, individual patient data of the included trials are not publicly available at the moment and a patient-level meta-analysis is therefore currently not feasible. Since trial-level data were used for the assessment of outcomes, differences in baseline characteristics across the trials and their potential influence on the investigated outcomes could not be evaluated in detail. Moreover, our meta-analysis comprises the overall results of the WOEST trial, even though only 69% of the included patients had AF. The decision to pool the results of all patients enrolled was based on subanalyses of the WOEST trial showing no significant differences in the primary endpoints based on the indication of OAC (AF, venous thromboembolism, mechanical valves, and others).14 Although there is insufficient evidence that a dose of 15 mg rivaroxaban prevents stroke in AF, we decided to include patients randomized to DAT with 15 mg rivaroxaban in the PIONEER AF-PCI trial because this regimen has already been introduced in the guidelines of the ESC as well as in the European Heart Rhythm Association (EHRA) Practical Guide 20184,34 and is therefore part of current clinical practice in the treatment of AF patients undergoing PCI. In contrast, TAT with very low-dose rivaroxaban is not a therapeutic option in these patients according to current guidelines. Another limitation of this meta-analysis is the substantial heterogeneity between the four included trials regarding primary ischaemic endpoint definition, duration of follow-up, as well as type, dose and duration of antithrombotic therapy. In particular the duration of concomitant aspirin therapy in the TAT groups varies considerably between WOEST, PIONEER AF-PCI, RE-DUAL PCI, and AUGUSTUS. Of note, we predominantly observed low to moderate statistical heterogeneity in the performed analyses, thereby strengthening our findings. Moreover, the information derived from post hoc power calculation needs to be interpreted with great caution and only provides a rough estimation of the potential effect size to be detected with this sample size and event rate. Finally, DAT with the fourth NOAC edoxaban in combination with a P2Y12 inhibitor is currently being investigated in AF patients undergoing PCI in the ENTRUST-AF PCI trial.37 However, ENTRUST-AF PCI will only include 1500 patients and these patients were only randomized to TAT with warfarin vs. DAT with edoxaban. Accordingly, it is unlikely that ENTRUST-AF PCI would affect the conclusions of the current meta-analysis.
Conclusion
In conclusion, DAT is associated with a significantly lower bleeding risk and a similar risk of ischaemic outcomes compared with TAT and may therefore be the preferred treatment strategy in most AF patients undergoing PCI. Future investigations might reveal the ideal antithrombotic regimen in AF patients with PCI at high risk of stent thrombosis, and provide data on DAT regimens with the more potent P2Y12 inhibitors.
Conflict of interest: P.M.H.: reports travel fees from Bayer, Daiichi Sankyo, Boehringer Ingelheim, Menarini, and lecture fees from Beckman Coulter. P.S.: reports grants from Boehringer Ingelheim, grants from Daiichi Sankyo, personal fees from Daiichi Sankyo, outside the submitted work.
C.K., B.G., and J.T. have nothing to disclose. S.W.: reports lecture and consulting fees from Astra Zeneca, Boehringer Ingelheim, Bistrol-Myers Squibb, Daiichi Sankyo, and Pfizer outside the submitted work. R.B.S.: reports lecture and consulting fees from Bristol-Myers Squibb/Pfizer. D.W.: reports personal fees from Bayer, Astra Zeneca, Boehringer Ingelheim, Berlin-Chemie, and Novartis outside of this work. K.H.: Lecture and consulting fees from Astra Zeneca, Bayer, Boehringer-Ingelheim, Bristol-Myers Squibb, Daiichi-Sankyo, and Pfizer. A.N.: Lecture and consulting fees from Astra Zeneca, Bayer, Boehringer-Ingelheim, Bristol-Myers Squibb, Daiichi-Sankyo, and Pfizer. T.G.: Lecture and consulting fees from Bayer, Boehringer-Ingelheim, Bristol-Myers Squibb, Daiichi-Sankyo, and Pfizer.
References
R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing;
Steering Committee of the Physicians' Health Study Research Group.
Author notes
Paul M. Haller and Patrick Sulzgruber authors share first authorship.
Alexander Niessner and Thomas Gremmel authors share senior authorship.





