-
PDF
- Split View
-
Views
-
Cite
Cite
Miyuki Nakahara, Masao Takemoto, Yoshio Arai, Takuya Tsuchihashi, A case report of rescue of a patient with a left ventricular free wall rupture associated with a small subtle ST-elevation myocardial infarction, European Heart Journal - Case Reports, Volume 6, Issue 7, July 2022, ytac270, https://doi.org/10.1093/ehjcr/ytac270
Close - Share Icon Share
Abstract
Left ventricular (LV) free wall ruptures (LVFWRs) of myocardial infarctions (MIs) are still one of the most fatal mechanical complications after an acute MI (AMI). LVFWRs are estimated to occur in 0.01% to 0.52% of patients following an ST-elevation MI (STEMI) and are rarely reported in the setting of a non- or subtle-ST-elevation MI.
We herein present a report of a 92-year-old male rescue case with an LVFWR following a small subtle-STEMI. Contrast cardiac computed tomography was useful to diagnose the LVFWR. An emergent cardiac surgery was performed. Finally, the patient’s life was saved.
This case demonstrates that even without clinical evidence of transmural infarction such as non- or subtle-STEMI, those patients may carry a risk of fatal complications including LVFWR, especially in older age and a first lateral wall AMI without collateral flow, as in this present case. Thus, the physicians should be aware of the possibility of LVFWRs even in the setting of an AMI without or with subtle-ST-elevation. High clinical suspicion and vigilance are the cornerstones of a timely and accurate diagnosis of LVFWR. This is the first report of a rescue case of a patient with an LVFWR associated with a subtle-STEMI.
Left ventricular (LV) free wall ruptures (FWRs) associated with non- or subtle-ST-elevation myocardial infarction (MI) are uncommon but rarely occur.
It is important to evaluate the electrocardiogram in posterior leads V7–V9 which can lead to early detection of occlusion MI, especially posterior MI.
Finally, this case demonstrates that even without clinical evidence of transmural infarction such as non- or subtle-STEMI, those patients may carry a risk of fatal complications including LVFWR.
Thus, the physicians should be aware of the possibility of an LVFWR even in the setting of an acute MI without or with subtle ST-elevation.
High clinical suspicion and vigilance are the cornerstones of a timely and accurate diagnosis of LVFWR.
Introduction
Since the advent of primary percutaneous coronary intervention (PCI), reperfusion therapies have led to a substantial reduction in the frequency of mechanical complications including left ventricular (LV) free wall ruptures (FWRs) associated with myocardial infarctions (MIs).1,2 However, LVFWRs are still one of the most fatal mechanical complications after an acute MI (AMI).1 Although the recent clinical trials have demonstrated that LVFWRs are estimated to occur in 0.01% to 0.52% of patients following an ST-elevation MI (STEMI),1,3 it is rarely reported in the setting of a subtle- or non-ST-elevation MI (NSTEMI).4,5 The aim of this case report was to describe a 92-year-old male rescue case with an LVFWR following a small subtle-STEMI.
Timeline
| On admission to the emergency room (ER) | The patient was in cardiogenic shock. The electrocardiogram exhibited subtle ST-elevation in aVL. The echocardiography yielded a cardiac tamponade. |
| 40 min after admission to the ER | After 490 mL of blood was evacuated by pericardiocentesis, his blood pressure improved to 104/62 mmHg. His creatine kinase was 273 IU/L. |
| 70 min after admission to the ER | The computed tomography revealed the postero-lateral myocardial infarction and left ventricular free wall rupture (LVFWR). |
| 100 min after admission to the ER | An emergent coronary angiography revealed an occlusion of left circumflex-obtuse marginal artery. Then, a diagnosis of cardiac tamponade caused by an LVFWR was made. |
| 160 min after admission to the ER | The patient was transferred to the regional cardiac surgery department to be performed emergent cardiac surgery. |
| 12 days after the event | The patient was transferred to our hospital to undergo cardiac rehabilitation. |
| 36 days after the event | He was discharged from our hospital and transferred to a regional hospital to continue cardiac rehabilitation. |
| 1 year after the event | He has remained well without any symptoms. |
| On admission to the emergency room (ER) | The patient was in cardiogenic shock. The electrocardiogram exhibited subtle ST-elevation in aVL. The echocardiography yielded a cardiac tamponade. |
| 40 min after admission to the ER | After 490 mL of blood was evacuated by pericardiocentesis, his blood pressure improved to 104/62 mmHg. His creatine kinase was 273 IU/L. |
| 70 min after admission to the ER | The computed tomography revealed the postero-lateral myocardial infarction and left ventricular free wall rupture (LVFWR). |
| 100 min after admission to the ER | An emergent coronary angiography revealed an occlusion of left circumflex-obtuse marginal artery. Then, a diagnosis of cardiac tamponade caused by an LVFWR was made. |
| 160 min after admission to the ER | The patient was transferred to the regional cardiac surgery department to be performed emergent cardiac surgery. |
| 12 days after the event | The patient was transferred to our hospital to undergo cardiac rehabilitation. |
| 36 days after the event | He was discharged from our hospital and transferred to a regional hospital to continue cardiac rehabilitation. |
| 1 year after the event | He has remained well without any symptoms. |
| On admission to the emergency room (ER) | The patient was in cardiogenic shock. The electrocardiogram exhibited subtle ST-elevation in aVL. The echocardiography yielded a cardiac tamponade. |
| 40 min after admission to the ER | After 490 mL of blood was evacuated by pericardiocentesis, his blood pressure improved to 104/62 mmHg. His creatine kinase was 273 IU/L. |
| 70 min after admission to the ER | The computed tomography revealed the postero-lateral myocardial infarction and left ventricular free wall rupture (LVFWR). |
| 100 min after admission to the ER | An emergent coronary angiography revealed an occlusion of left circumflex-obtuse marginal artery. Then, a diagnosis of cardiac tamponade caused by an LVFWR was made. |
| 160 min after admission to the ER | The patient was transferred to the regional cardiac surgery department to be performed emergent cardiac surgery. |
| 12 days after the event | The patient was transferred to our hospital to undergo cardiac rehabilitation. |
| 36 days after the event | He was discharged from our hospital and transferred to a regional hospital to continue cardiac rehabilitation. |
| 1 year after the event | He has remained well without any symptoms. |
| On admission to the emergency room (ER) | The patient was in cardiogenic shock. The electrocardiogram exhibited subtle ST-elevation in aVL. The echocardiography yielded a cardiac tamponade. |
| 40 min after admission to the ER | After 490 mL of blood was evacuated by pericardiocentesis, his blood pressure improved to 104/62 mmHg. His creatine kinase was 273 IU/L. |
| 70 min after admission to the ER | The computed tomography revealed the postero-lateral myocardial infarction and left ventricular free wall rupture (LVFWR). |
| 100 min after admission to the ER | An emergent coronary angiography revealed an occlusion of left circumflex-obtuse marginal artery. Then, a diagnosis of cardiac tamponade caused by an LVFWR was made. |
| 160 min after admission to the ER | The patient was transferred to the regional cardiac surgery department to be performed emergent cardiac surgery. |
| 12 days after the event | The patient was transferred to our hospital to undergo cardiac rehabilitation. |
| 36 days after the event | He was discharged from our hospital and transferred to a regional hospital to continue cardiac rehabilitation. |
| 1 year after the event | He has remained well without any symptoms. |
Case presentation
A 92-year-old male was brought to the emergency room (ER) in our hospital because of a depressed level of consciousness and shock. He had a history of diabetes mellitus and hypertension. His baseline medications included telmisartan 20 mg, nateglinide 270 mg, and metformin 750 mg daily. His blood pressure was 55/34 mmHg, pulse rate 91 beats per minute and regular, and respiratory rate 26 per minute. His jugular venous pulse was elevated, and he had quiet heart sounds. Pulsus paradoxus was observed. His lower extremities were cold with no oedema. Because of his hypotension, depressed level of consciousness, and hypoxia, a bolus injection of 100 μg of norepinephrine, intravenous administration of saline, and 20 μg/kg/min of dopamine and mechanical ventilator support after intubation were immediately started. However, the hypotension did not satisfactorily improve and his blood pressure was 68/46 mmHg. The 12-lead electrocardiogram exhibited subtle-ST-elevation in lead aVL and flat T waves in leads I and aVL and precordial leads V4∼6 (Figure 1A). The chest X-ray was unremarkable. Echocardiography yielded a preserved LV systolic function without any overt wall motion abnormalities but revealed findings of an entire circumferential effusion of the pericardial space of 10 mm (yellow arrows in Figure 1B) and right atrial collapse. Thus, with a diagnosis of cardiac tamponade, a pericardiocentesis and insertion of a pericardial drain was immediately performed. After 490 mL of fresh blood was evacuated by the pericardial drain, haemodynamic stability was obtained thereafter, and the blood pressure improved to 104/62 mmHg. The white cell count was 10.9 × 103/μl, creatine kinase 273 IU/L, aspartate aminotransferase 47 IU/L, lactate dehydrogenase 192 U/L, and creatinine 1.07 mg/dl (Table 1). The emergency room doctor did not evaluate a troponin test. The head computed tomography was normal, however, contrast computed tomography (CT) also revealed an entire circumferential pericardial effusion (yellow arrows in Figure 2A and B), but no evidence of an aortic dissection. Interestingly, findings of poor contrasting of the postero-lateral wall of the LV (white arrows in Figure 2A and B) and leakage of contrast into the pericardial space were observed (red arrows in Figure 2A and B). An emergent coronary angiography revealed an occlusion of the distal site of the left circumflex (LCX)-obtuse marginal (OM) artery without any collateral vessels (white arrow in Figure 3A), 75% stenosis of the left anterior descending (LAD) artery (green arrow in Figure 3A), and no significant stenosis of the right coronary artery (Figure 3B). Then, a diagnosis of cardiac tamponade caused by an LVFWR associated with a subtle-STEMI developing into cardiogenic shock was made. Thus, the patient was urgently transferred to the regional cardiac surgery department to undergo an emergent cardiac surgery. Anti-platelet therapy was withheld as the patient was being considered for the emergent cardiac surgery for the LVFWR. Then, because the troponin test was positive, we made a definite diagnosis of a subtle-STEMI. The findings during the operation demonstrated an area with a haemorrhage on the pericardial surface of the LV postero-lateral wall surrounding a 3 cm LVFWR and transmural MI (Figure 4). A direct suture of a patch to cover the rupture and infarcted myocardium was performed. No coronary artery bypass graft was performed, because the LAD and LCX-OM arteries were very narrow and the perfused area of the LCX-OM artery was very small. Twelve days after the event, the patient was transferred to our hospital to undergo cardiac rehabilitation. Because of his frailty after the cardiac surgery, he needed a long hospital stay for his rehabilitation. Thirty-six days after the event, he was discharged from our hospital and transferred to a regional hospital to continue his cardiac rehabilitation. The echocardiography before his discharge revealed a normal LV systolic function without any overt wall motion abnormalities. The pharmacological therapy at the time of discharge was aspirin 100 mg, bisoprolol 1.25 mg, pitavastatin 1 mg, telmisartan 20 mg, and metformin 750 mg daily. He has remained well without any symptoms for 1 year after the event.
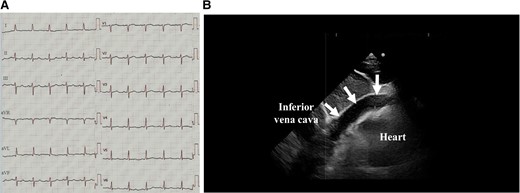
The 12-lead electrocardiograms (A) and echocardiography (B) on admission. The white arrows in Figure 1B delineate a pericardial effusion.
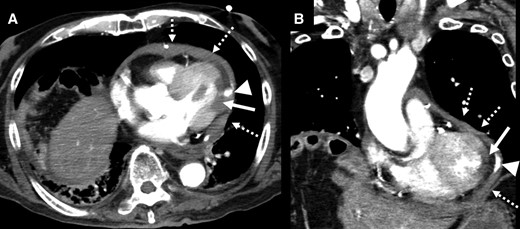
Horizontal (A) and vertical (B) images of the contrast cardiac computed tomography revealed a pericardial effusion (dotted white arrows), poor contrasting of the postero-lateral wall of the left ventricle (white arrows), and leakage of contrast into the pericardial space (triangle arrows).
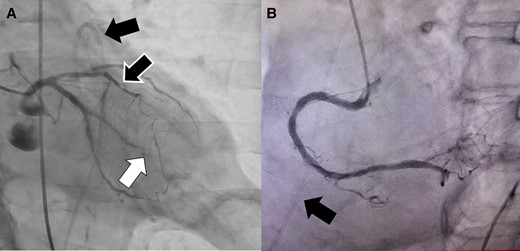
An emergent coronary angiography revealed an occlusion of the distal site of the left circumflex (LCX)-obtuse marginal (OM) artery without any collateral vessels (white arrow) (A), 75% stenosis of the left anterior descending artery (black and white arrow) (A), and no significant stenosis of the right coronary artery (B). The black arrow shows a pericardial drain.
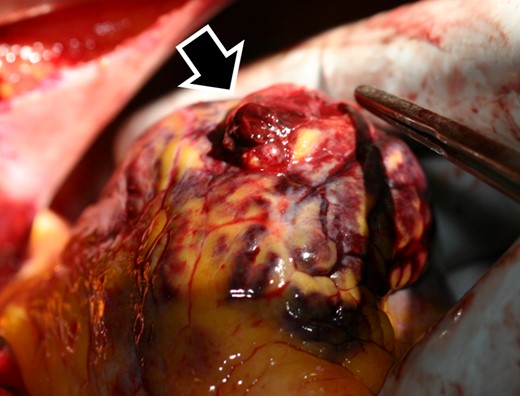
The findings during the operation demonstrated a 3 cm left ventricular free wall rupture (black and white arrow).
| Blood cell count . | value . | Normal range . | Serum chemistry . | value . | Normal range . |
|---|---|---|---|---|---|
| White blood cell (×1000/µL) | 10.9* | 3.3∼8.6 | Total protein (g/dL) | 5.8* | 6.6∼8.1 |
| Haemoglobin (g/dL) | 11.8 | 11.6∼14.8 | Albumin (g/dL) | 2.6* | 4.1∼5.1 |
| Haematocrit (%) | 35.3 | 35.1∼44.4 | Aspartate aminotransferase (U/L) | 47* | 13∼30 |
| Platelets (×104/µL) | 17.6 | 158∼348 | Alanine aminotransferase (U/L) | 22 | 7∼23 |
| Lactate dehydrogenase (U/L) | 192 | 124∼222 | |||
| Blood gas (10 L/min O2 on mask) | Blood urea nitrogen (mg/dL) | 21.9* | 8∼20 | ||
| FiO2 (%) | 80% | NA | Creatinine (mg/dL) | 1.07* | 0.46∼0.79 |
| pH | 7.286* | 7.35∼7.45 | Natrium (mmol/L) | 138 | 138∼145 |
| pO2 (mmHg) | 197.4 | 80 < | Potassium (mmol/L) | 4.5 | 3.6∼4.8 |
| pCO2 (mmHg) | 32.1* | 35∼45 | Chlorine (mmol/L) | 106 | 99∼109 |
| HCO3− (mmol/L) | 14.8* | 22∼26 | Creatinine kinase (U/L) | 273* | 41∼153 |
| BE (mEq/L) | −10.4* | −3∼3 | C-reactive protein (mg/dL) | 3.56* | 0.00∼0.25 |
| Lactate (mg/dL) | 76* | 4∼16 | Ammonia (µg/dL) | 46 | 12∼66 |
| Blood cell count . | value . | Normal range . | Serum chemistry . | value . | Normal range . |
|---|---|---|---|---|---|
| White blood cell (×1000/µL) | 10.9* | 3.3∼8.6 | Total protein (g/dL) | 5.8* | 6.6∼8.1 |
| Haemoglobin (g/dL) | 11.8 | 11.6∼14.8 | Albumin (g/dL) | 2.6* | 4.1∼5.1 |
| Haematocrit (%) | 35.3 | 35.1∼44.4 | Aspartate aminotransferase (U/L) | 47* | 13∼30 |
| Platelets (×104/µL) | 17.6 | 158∼348 | Alanine aminotransferase (U/L) | 22 | 7∼23 |
| Lactate dehydrogenase (U/L) | 192 | 124∼222 | |||
| Blood gas (10 L/min O2 on mask) | Blood urea nitrogen (mg/dL) | 21.9* | 8∼20 | ||
| FiO2 (%) | 80% | NA | Creatinine (mg/dL) | 1.07* | 0.46∼0.79 |
| pH | 7.286* | 7.35∼7.45 | Natrium (mmol/L) | 138 | 138∼145 |
| pO2 (mmHg) | 197.4 | 80 < | Potassium (mmol/L) | 4.5 | 3.6∼4.8 |
| pCO2 (mmHg) | 32.1* | 35∼45 | Chlorine (mmol/L) | 106 | 99∼109 |
| HCO3− (mmol/L) | 14.8* | 22∼26 | Creatinine kinase (U/L) | 273* | 41∼153 |
| BE (mEq/L) | −10.4* | −3∼3 | C-reactive protein (mg/dL) | 3.56* | 0.00∼0.25 |
| Lactate (mg/dL) | 76* | 4∼16 | Ammonia (µg/dL) | 46 | 12∼66 |
*abnormal value. NA, not available.
| Blood cell count . | value . | Normal range . | Serum chemistry . | value . | Normal range . |
|---|---|---|---|---|---|
| White blood cell (×1000/µL) | 10.9* | 3.3∼8.6 | Total protein (g/dL) | 5.8* | 6.6∼8.1 |
| Haemoglobin (g/dL) | 11.8 | 11.6∼14.8 | Albumin (g/dL) | 2.6* | 4.1∼5.1 |
| Haematocrit (%) | 35.3 | 35.1∼44.4 | Aspartate aminotransferase (U/L) | 47* | 13∼30 |
| Platelets (×104/µL) | 17.6 | 158∼348 | Alanine aminotransferase (U/L) | 22 | 7∼23 |
| Lactate dehydrogenase (U/L) | 192 | 124∼222 | |||
| Blood gas (10 L/min O2 on mask) | Blood urea nitrogen (mg/dL) | 21.9* | 8∼20 | ||
| FiO2 (%) | 80% | NA | Creatinine (mg/dL) | 1.07* | 0.46∼0.79 |
| pH | 7.286* | 7.35∼7.45 | Natrium (mmol/L) | 138 | 138∼145 |
| pO2 (mmHg) | 197.4 | 80 < | Potassium (mmol/L) | 4.5 | 3.6∼4.8 |
| pCO2 (mmHg) | 32.1* | 35∼45 | Chlorine (mmol/L) | 106 | 99∼109 |
| HCO3− (mmol/L) | 14.8* | 22∼26 | Creatinine kinase (U/L) | 273* | 41∼153 |
| BE (mEq/L) | −10.4* | −3∼3 | C-reactive protein (mg/dL) | 3.56* | 0.00∼0.25 |
| Lactate (mg/dL) | 76* | 4∼16 | Ammonia (µg/dL) | 46 | 12∼66 |
| Blood cell count . | value . | Normal range . | Serum chemistry . | value . | Normal range . |
|---|---|---|---|---|---|
| White blood cell (×1000/µL) | 10.9* | 3.3∼8.6 | Total protein (g/dL) | 5.8* | 6.6∼8.1 |
| Haemoglobin (g/dL) | 11.8 | 11.6∼14.8 | Albumin (g/dL) | 2.6* | 4.1∼5.1 |
| Haematocrit (%) | 35.3 | 35.1∼44.4 | Aspartate aminotransferase (U/L) | 47* | 13∼30 |
| Platelets (×104/µL) | 17.6 | 158∼348 | Alanine aminotransferase (U/L) | 22 | 7∼23 |
| Lactate dehydrogenase (U/L) | 192 | 124∼222 | |||
| Blood gas (10 L/min O2 on mask) | Blood urea nitrogen (mg/dL) | 21.9* | 8∼20 | ||
| FiO2 (%) | 80% | NA | Creatinine (mg/dL) | 1.07* | 0.46∼0.79 |
| pH | 7.286* | 7.35∼7.45 | Natrium (mmol/L) | 138 | 138∼145 |
| pO2 (mmHg) | 197.4 | 80 < | Potassium (mmol/L) | 4.5 | 3.6∼4.8 |
| pCO2 (mmHg) | 32.1* | 35∼45 | Chlorine (mmol/L) | 106 | 99∼109 |
| HCO3− (mmol/L) | 14.8* | 22∼26 | Creatinine kinase (U/L) | 273* | 41∼153 |
| BE (mEq/L) | −10.4* | −3∼3 | C-reactive protein (mg/dL) | 3.56* | 0.00∼0.25 |
| Lactate (mg/dL) | 76* | 4∼16 | Ammonia (µg/dL) | 46 | 12∼66 |
*abnormal value. NA, not available.
Discussion
In spite of the introduction of early reperfusion therapies using PCI, mechanical complications, including LVFWRs, now occur in fewer than about 0.1% of patients following an AMI.1,3 Moreover, unfortunately, the mortality rates in patients with those complications have not decreased over the past 2 decades, and patients with those complications have a more than 4-fold in-hospital mortality than those without.1,3 Thus, those complications remain an important determinant of the outcomes after an AMI. LVFWRs are recognized as one of the most fatal mechanical complications and cause of a high mortality in patients with an AMI,1 mainly with STEMIs. However, LVFWRs associated with subtle-STEMIs, as in this present case, or NSTEMIs, are uncommon.4,5 The risk factors of an LVFWR following an AMI have been reported including an old age (>70 years old), female sex, large MI size, ongoing ischaemic symptoms such as chest pain or ST-elevation, persistent hypertension, first AMI without collateral flow, and anterior or lateral AMI.6–8 LVFWRs most commonly occur on the lateral wall (43%), inferior wall (29%), and less commonly the anterior wall (17%).9 If patients with LVFWRs are stable, the use of advanced imaging including cardiac CT and/or magnetic resonance imaging may be useful to confirm the presence and site of the LVFWR,1 as in this present case. The risk factors including an old age of 92 years old and a first lateral wall AMI without collateral flow (Figure 3) were relevant in this case.
The diagnosis of isolated posterior wall MIs is often difficult and sometimes missed. Further, because the infarcted area of the myocardium in this present case was so small, it might not have generated significant ST-changes in other leads other than the posterior leads. Thus, if we evaluated the electrocardiogram in posterior leads V7–V9, we might have been able to pick up the ST elevation.
The underlying mechanism(s) of LVFWRs have still not been completely elucidated, and a transmural infarction is thought to be one of the important prerequisite factors.10 It has been reported that transmural MIs have traditionally existed in 27% to 34% of NSTEMI patients, but NSTEMIs are usually thought to accompany subendocardial MIs.10 A post mortem case report of a patient with an LVFWR associated with an NSTEMI also demonstrated a transmural MI at the site of the LVFWR.4,5 The dissociation and abnormal geometry of the LV wall motion between the non-contractile transmural infarcted region and surrounding contractile healthy and non-infarcted region may play an important role in the occurrence of an LVFWR.8 The size of the MI, in this present case, was small from the findings during the cardiac surgery (Figure 4). Thus, the dissociation and abnormal geometry of the LV wall motion between the infarcted region and non-infarcted region may have delivered a large and excessive pressure that worked on the small infarcted region, and finally the LVFWR occurred and developed into a cardiac tamponade and cardiogenic shock.
Since more than 80% of patients with LVFWRs present with a cardiac tamponade, the initial treatment of the LVFWR is the same as that for an acute cardiac tamponade.9 However, the in-hospital mortality rates of medically treated patients with LVFWRs are extremely high at up to about 90% as compared to about 34% to 60% in those that undergo surgical treatment.7,9,11 Further, the current guidelines of the American College of Cardiology Foundation/American Heart Association12 and European Society of Cardiology13 recommend early surgical intervention for haemodynamically unstable patients with LVFWRs following an AMI. Thus, the early diagnosis and management of an LVFWR are important to improve the outcomes and reduce the mortality, and they require an understanding of the clinical findings that should raise suspicion of an LVFWR and require an early surgical treatment.
Finally, this case demonstrated that even without clinical evidence of a transmural infarction such as non- or subtle-STEMI, those patients may carry a risk of fatal complications including an LVFWR, especially in those with an older age and a first lateral wall AMI without collateral flow, as in this present case. Thus, the physicians should be aware of the possibility of LVFWRs even in the setting of an AMI without or with subtle-ST-elevation. A high clinical suspicion and vigilance are the cornerstones of a timely and accurate diagnosis of an LVFWR. Only two cases have been reported with an LVFWR associated with a NSTEMI. However, both reports were post-mortem cases. To the best of our knowledge, this is the first report of a rescue case with an LVFWR associated with a subtle-STEMI.
Lead author biography
 Dr. Miyuki Nakahara received the MD from Kyushu University, Fukuoka, Japan, in 2018. Since 2018, she began to work as a resident, and since 2020, she has been working as a cardiologist at the Steel Memorial Yawata Hospital, Kitakyushu, Japan. Her medical interests are heart failure and arrhythmias.
Dr. Miyuki Nakahara received the MD from Kyushu University, Fukuoka, Japan, in 2018. Since 2018, she began to work as a resident, and since 2020, she has been working as a cardiologist at the Steel Memorial Yawata Hospital, Kitakyushu, Japan. Her medical interests are heart failure and arrhythmias.
Supplementary material
Supplementary material is available at European Heart Journal – Case Reports online.
Acknowledgements
We thank Mr. John Martin for his linguistic assistance with this paper.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The authors confirm that written consent for the submission and publication of this case report including the images and associated text has been obtained from the patient in line with COPE guidance.
Conflict of interest: None declared.
Funding: None declared.




Comments