-
PDF
- Split View
-
Views
-
Cite
Cite
Tomoki Fukui, Katsukiyo Kitabayashi, Nobuyuki Ogasawara, Shinji Hasegawa, Subepicardial aneurysm with free wall rupture and its successful surgical intervention: a case report, European Heart Journal - Case Reports, Volume 5, Issue 2, February 2021, ytab048, https://doi.org/10.1093/ehjcr/ytab048
Close - Share Icon Share
Abstract
Subepicardial aneurysm (SEA) is an uncommon but potentially fatal complication of acute myocardial infarction (MI) associated with an increased risk of free wall rupture (FWR) leading to sudden death. We describe a rare case of a silent myocardial infarction complicated by SEA and subsequent FWR, resulting in cardiac tamponade.
A 68-year-old man with no previous chest symptoms presented with syncope. Cardiac computed tomography incidentally revealed a small aneurysmal cavity at the inferolateral wall of the left ventricle, which was overlooked on initial transthoracic echocardiography. Coronary angiography demonstrated a narrowed first obtuse marginal branch with coronary slow flow, suggesting that spontaneous recanalization of the occluded obtuse marginal branch induced SEA and subsequent FWR. The patient underwent an emergency left ventricular aneurysm repair. The post-operative course was uneventful, and the patient was discharged from the hospital on post-operative day 20.
This case emphasizes the importance of prompt detection and surgical intervention for SEA. Subepicardial aneurysm should be suspected in patients with pericardial effusion and suspected MI. Cardiac computed tomography is not only useful in the detection of such cases but also facilitates the development of a successful surgical strategy.
Subepicardial aneurysm (SEA) is an under-recognized condition despite being associated with an increased risk of free wall rupture leading to sudden death.
Prompt detection and surgical intervention are essential to prevent devastating outcomes in SEA.
Subepicardial aneurysm should be suspected in patients with pericardial effusion and suspected myocardial infarction
Cardiac computed tomography is useful to detect this condition and facilitates the development of a successful surgical strategy.
Introduction
The major mechanical complications of acute myocardial infarction (MI) include free wall rupture (FWR), ventricular septum rupture, and papillary muscle rupture. Now that primary percutaneous coronary intervention is a well-established reperfusion strategy, the incidence of mechanical complications after ST-elevation MI has significantly decreased to <1%, with an FWR rate of 0.52%. However, FWR remains lethal nonetheless, with a mortality rate of 63%, mainly due to cardiac tamponade.1,2 Subepicardial aneurysm (SEA), an under-recognized and unusual cardiac aneurysm occurring post-MI, is highly associated with FWR. Herein, we report the case of an SEA and FWR following a silent MI, incidentally detected by cardiac computed tomography (CCT) and requiring emergency surgery.
Timeline
| Time . | Event . |
|---|---|
| Day 1 | Admission with syncope. Initial transthoracic echocardiography (TTE): Mild pericardial effusion. Cardiac computed tomography: An aneurysmal cavity (18 mm × 13 mm) at the inferolateral wall of the left ventricle with a suspected subepicardial aneurysm (SEA). Repeat TTE: Detection of an aneurysmal cavity with colour Doppler flow. Coronary angiography: A narrowed first obtuse marginal branch with coronary slow flow. Emergency cardiac surgery with suture repair. |
| Day 4 | Extubation. Removal of the intra-aortic balloon pump. |
| Day 7 | Discharge from the intensive care unit. |
| Day 10 | Follow-up TTE confirmed the disappearance of SEA. |
| Day 21 | Discharge from our hospital. |
| Time . | Event . |
|---|---|
| Day 1 | Admission with syncope. Initial transthoracic echocardiography (TTE): Mild pericardial effusion. Cardiac computed tomography: An aneurysmal cavity (18 mm × 13 mm) at the inferolateral wall of the left ventricle with a suspected subepicardial aneurysm (SEA). Repeat TTE: Detection of an aneurysmal cavity with colour Doppler flow. Coronary angiography: A narrowed first obtuse marginal branch with coronary slow flow. Emergency cardiac surgery with suture repair. |
| Day 4 | Extubation. Removal of the intra-aortic balloon pump. |
| Day 7 | Discharge from the intensive care unit. |
| Day 10 | Follow-up TTE confirmed the disappearance of SEA. |
| Day 21 | Discharge from our hospital. |
| Time . | Event . |
|---|---|
| Day 1 | Admission with syncope. Initial transthoracic echocardiography (TTE): Mild pericardial effusion. Cardiac computed tomography: An aneurysmal cavity (18 mm × 13 mm) at the inferolateral wall of the left ventricle with a suspected subepicardial aneurysm (SEA). Repeat TTE: Detection of an aneurysmal cavity with colour Doppler flow. Coronary angiography: A narrowed first obtuse marginal branch with coronary slow flow. Emergency cardiac surgery with suture repair. |
| Day 4 | Extubation. Removal of the intra-aortic balloon pump. |
| Day 7 | Discharge from the intensive care unit. |
| Day 10 | Follow-up TTE confirmed the disappearance of SEA. |
| Day 21 | Discharge from our hospital. |
| Time . | Event . |
|---|---|
| Day 1 | Admission with syncope. Initial transthoracic echocardiography (TTE): Mild pericardial effusion. Cardiac computed tomography: An aneurysmal cavity (18 mm × 13 mm) at the inferolateral wall of the left ventricle with a suspected subepicardial aneurysm (SEA). Repeat TTE: Detection of an aneurysmal cavity with colour Doppler flow. Coronary angiography: A narrowed first obtuse marginal branch with coronary slow flow. Emergency cardiac surgery with suture repair. |
| Day 4 | Extubation. Removal of the intra-aortic balloon pump. |
| Day 7 | Discharge from the intensive care unit. |
| Day 10 | Follow-up TTE confirmed the disappearance of SEA. |
| Day 21 | Discharge from our hospital. |
Case presentation
A 68-year-old man presented to the emergency department with syncope. He had a history of hypertension and hyperlipidaemia but was not on any treatment. At admission, the patient had regained consciousness, and complained of an intermittent chest discomfort. His blood pressure was 88/64 mmHg, heart rate 94 beats per minute, and oxygen saturation 100%. On physical examination, jugular veins were clearly distended. Chest auscultation revealed no murmurs. Chest X-ray was normal. An electrocardiogram showed abnormal Q waves, inverted T waves in lead aVL, and flattened T waves in lead I (Figure 1). Initial transthoracic echocardiography (TTE) revealed preserved left ventricular ejection fraction, mild pericardial effusion of 6 mm in diastole, mild mitral regurgitation, and dilated inferior vena cava without respiratory variation. Blood tests showed an elevated troponin I level of 1784 pg/mL (reference range: 0–26 pg/mL) and C-reactive protein level of 1.74 mg/dL (reference range: 0.00–0.14 mg/dL). Creatinine kinase, creatinine kinase myocardial band, and white blood cell count were within the normal range. CCT revealed circumferential pericardial effusion, and an unexpected aneurysmal cavity (18 mm × 13 mm) in the inferolateral wall of the left ventricle (LV) with a thin wall and a narrow neck, suggestive of SEA (Figure 2). Repeat TTE confirmed an aneurysmal cavity in the basal-inferolateral LV. Doppler colour flow imaging showed blood flow within the aneurysmal cavity (Figure 3, Videos 1–3). Subsequent coronary angiography demonstrated a narrowed first obtuse marginal branch (OM) with coronary slow flow (Figure 4, Supplementary material online, Video S1). Considering anatomical relationships, the aneurysmal region corresponded to the perfusion territory of the first OM. Therefore, we clinically diagnosed FWR derived from SEA after MI of the first OM.
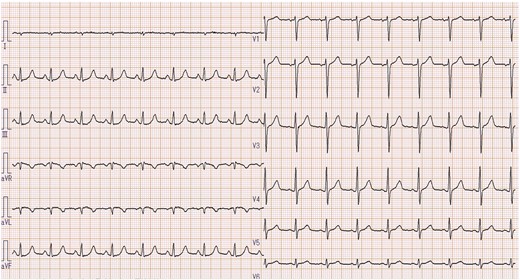
Electrocardiogram on admission showing an abnormal Q wave and an inverted T wave in lead aVL, with a flattened T wave in a lead I.
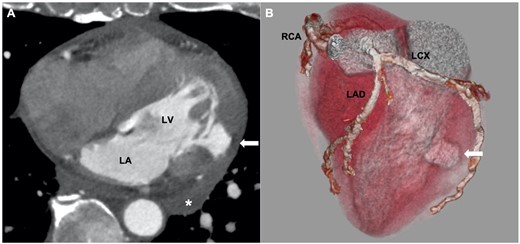
(A) Cardiac computed tomography showing mild pericardial effusion (*), and an aneurysmal cavity at the inferolateral wall of left ventricle (LV) with a thin wall and a narrow neck (white arrow). (B) Three-dimensional reconstruction image showed that the aneurysm corresponded to the perfusion territory of first obtuse marginal branch (white arrow). LA, left atrium; LAD, left anterior descending artery; LCX, left circumflex artery; RCA, right coronary artery.
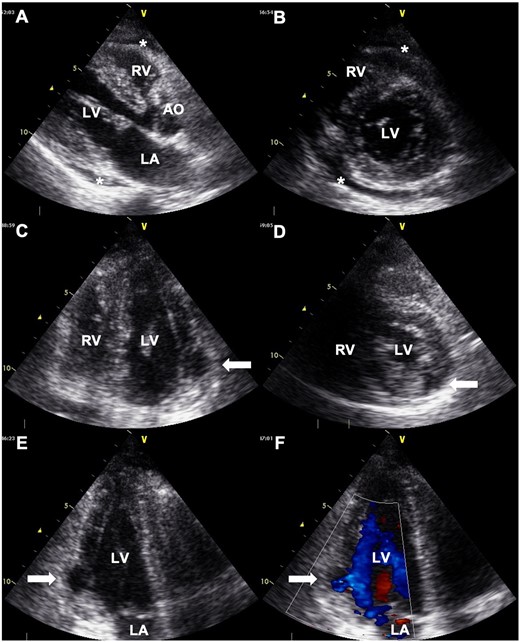
Transthoracic echocardiography showing mild pericardial effusion (*), and an aneurysmal cavity located at the basal-inferolateral left ventricle (LV) (white arrows). (A) Long-axis view. (B) Short-axis view at the chordae level. (C) Apical four-chamber view. (D) Short-axis view at the papillary muscle level. (E) Apical three-chamber view. (F) Doppler colour flow imaging of apical three-chamber view showing blood flow within an aneurysmal cavity. AO, aorta; LA, left atrium; RV, right ventricle.
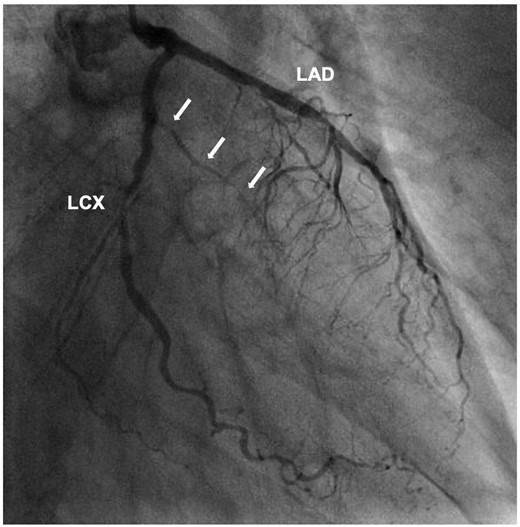
Coronary angiography of left coronary artery showing a narrowed first obtuse marginal branch with coronary slow flow (white arrows). LAD, left anterior descending artery; LCX, left circumflex artery.
The patient underwent an emergency cardiac surgery immediately. Intraoperatively, the pericardial space was found to have a small blood clot. The aneurysmal cavity and pericardial space were separated by the thinning myocardium layer with a small liner wound, where blood oozing was identified. After incising the wound from the outside, the connection between the aneurysm and cardiac lumen through a narrow orifice was confirmed. The first OM branch was ligated. Left ventricle around the aneurysm was sutured and fixed by surgical glue (Figure 5). The surgery was completed successfully without any intraoperative complications. Follow-up TTE confirmed the disappearance of SEA. The post-operative course was uneventful, and the patient was discharged on post-operative Day 20. Currently, no cardiac events have been recorded in the 6-month follow-up period.
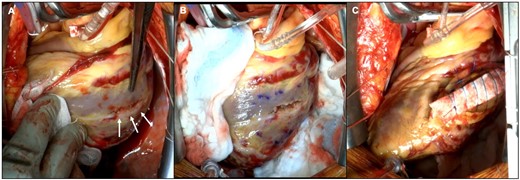
Intraoperative images. (A) The aneurysmal cavity and pericardial space were separated by the layer of a thinning myocardium with a small line wound (white arrows). (B) After incising the wound from the outside, the connection between the aneurysm and the cardiac lumen through a narrow orifice was confirmed. (C) The left ventricle was sutured and fixed by surgical glue.
Transthoracic echocardiography showing an aneurysmal cavity located in the basal-inferolateral left ventricle. (S1) Apical four-chamber view. (S2) Short-axis view (S3) Apical three-chamber view.
Discussion
Subepicardial aneurysm was first defined pathologically in 1983. Although cardiac aneurysms following MI were classically divided into true- and pseudoaneurysms, Epstein and Hutchins proposed SEA as a new addition because of its peculiar features, i.e., abrupt interruption of the myocardium with an intact epicardium, narrow neck, and propensity to rupture spontaneously regardless of the components of the aneurysmal wall.3 A previous report indicated that SEA was detected in 0.2% of the 1,140 cases of MI on autopsy; however, the accurate incidence remains uncertain because of the limited literature and difficulties involved in diagnosis.3,4 Many SEAs may imperceptibly develop into pseudoaneurysms or end in frank rupture, resulting in sudden death prior to diagnosis.
True- and pseudoaneurysms are generally managed conservatively unless haemodynamic instability or life-threatening arrhythmias occur.5 However, SEA has a high risk of FWR, leading to sudden death.4 Although successful conservative management for asymptomatic SEAs have been described, the standard therapy is an emergency surgical intervention.6,7 Thus, prompt detection of SEA is essential to prevent devastating outcomes. Previously, SEA was detected by TTE or left ventriculography.4 While left ventriculography can visualize the extravasation of contrast medium into the aneurysmal cavity, it is invasive and can induce myocardial rupture. Transthoracic echocardiography is a widespread, non-invasive diagnostic tool that helps evaluate cardiac structure and function even in the setting of haemodynamic instability. Additionally, contrast-enhanced echocardiography can assist visualization of SEA.8,9 Thanks to advances in imaging technologies, CCT and cardiac magnetic resonance (CMR) are widely applied in the diagnosis of SEAs because of their ability to evaluate the aneurysmal morphology multi-directionally. Cardiac magnetic resonance can reveal not only aneurysms but also infarcted myocardium and left ventricular dynamics.10,11 Cardiac computed tomography can also evaluate aneurysmal morphology—the three-dimensional reconstruction provides the anatomical relationship between the coronary arteries and LV, as seen in this case.12 Furthermore, these two modalities are suitable for detecting and classifying the cardiac aneurysms. Subepicardial aneurysms and pseudoaneurysms have similarities in having narrow necks and a propensity to cause myocardial tearing; however, SEAs do not involve the regional bulge of LV contour, which is observed in true- and pseudoaneurysms. Careful observation with multimodality imaging is required when seeking the cause of pericardial effusion, and in suspected MI patients. In regard to our case, detecting an SEA on initial TTE would be difficult as SEA could only be observed in very limited slices because of its small size.
Free wall rupture usually occurs within 1 week, with majority occurring within 24 h of MI onset.13 Female sex, advanced age, presence of Q waves in the initial ECG, elevated troponin levels, and late reperfusion are reported as risk factors for FWR.1,14 Although FWR is an important clue to SEA, SEA has different characteristics. Giltner et al. reviewed 20 SEA cases including 5 females. Time from MI ranged from 1 day to 1 year, with a median of 1 month. The aneurysmal site was the inferior or inferolateral wall in 80% and the anterior wall in 15%.4 In our case, SEA was typically located at the inferolateral wall; however, the onset of MI was uncertain because of the absence of chest syndrome before admission and the relatively minor abnormalities seen on blood tests and electrocardiogram. SEAs are generally found in ST-elevation MI patients, and this case is unique in its occurrence post a silent MI.10 It is presumable that spontaneous recanalization of an occluded first OM following a silent MI caused SEA, leading to FWR. The syncope could have occurred due to the sudden drop in blood pressure due to cardiac tamponade.
Surgical techniques for SEA include patch repair, suture, aneurysmectomy, and infarctectomy.4,15 Additionally, the surgical procedure depends on the location of the SEA, sometimes requiring coronary artery bypass grafting and valvular surgery.11 Therefore, preoperative anatomical assessment, as done by CCT, is essential for a safe and precise surgical strategy. Cardiovascular surgeons could recognize the aneurysmal site preoperatively with the assistance of imaging, and perform the operation without the need for mitral valve surgery.
Conclusion
We report a case of successful surgery for SEA with FWR. Subepicardial aneurysm should be suspected when seeking the cause of pericardial effusion and in patients with suspected MI; CCT is useful in its detection and in devising a successful surgical strategy.
Lead author biography
Dr Tomoki Fukui graduated from the Chiba University, Japan in 2014. He works as a clinical fellow at the Department of Cardiology, Japan Community Healthcare Organization Osaka Hospital, Japan. He is interested in cardiovascular interventions and echocardiography.
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Slide sets: A fully edited slide set detailing these cases and suitable for local presentation is available online as Supplementary data.
Consent: The authors confirm that written consent for submission and publication of this case report including images and associated text has been obtained from the patient in line with COPE guidance.
Conflict of interest: none declared.
Funding: None declared





Comments