-
PDF
- Split View
-
Views
-
Cite
Cite
Arvind Sahadev Singh, Kothandam Sivakumar, Case report: pericardial adhesions from a previous coronary artery bypass surgery contain a left ventricular free wall rupture after an acute myocardial infarction to form a pseudoaneurysm, European Heart Journal - Case Reports, Volume 2, Issue 3, September 2018, yty081, https://doi.org/10.1093/ehjcr/yty081
Close - Share Icon Share
Abstract
Fatal mechanical complications of acute myocardial infarctions include free wall rupture and ventricular septal rupture. If pericardial adhesions wall off a free wall rupture, it may lead to formation of pseudoaneurysms that are characterized by a narrow mouth. Even though pseudoaneurysms are common after myocardial infarctions, they may also occur following surgery, trauma, and infections rarely.
We present a case of a 62-year-old man who developed a left ventricular pseudoaneurysm 2 weeks after thrombolysis for an acute inferolateral myocardial infarction. Multiple non-invasive imaging modalities demonstrated the anatomy, regional and global ventricular function, distortion of mitral annulus by the eccentric large aneurysm. Pericardial scars after a previous coronary bypass surgery contained this left ventricular free wall rupture and helped in providing a safe window period for corrective surgery.
While left ventricular pseudoaneurysms that develop following myocardial infarctions warrant emergency surgery due to the high impending chances of rupture and tamponade, previous surgical pericardial adhesions guarded against an imminent collapse. Multimodality imaging of the aneurysm helped in planning the surgical strategy.
Pseudoaneurysms following acute myocardial infarctions contained by previous surgical adhesions give a window of opportunity to plan surgical correction in a safe period.
Multimodal imaging provides information that is needed in managing these patients.
Introduction
Mechanical complications of myocardial infarction range from ventricular septal rupture, papillary muscle rupture to formation of pseudoaneurysm following ventricular free wall rupture. True myocardial aneurysm is characterized by a broad neck and by the presence of remnants of myocardium and coronary arteries in their walls.1,2 A pseudoaneurysm develops when cardiac rupture is contained by pre-existing adhesions between epicardium and parietal pericardium, has a narrow neck, is devoid of myocardial components and has higher chances (30–45%) of rupture.2–4 It leads to heart failure, thromboembolism, angina, and arrhythmias. A left ventricular free wall rupture following an acute myocardial infarction was serendipitously contained by the pericardial adhesions due to previous coronary bypass surgery. This prevented an acute catastrophic cardiac tamponade but resulted in the formation of a pseudoaneurysm that led to symptoms of heart failure due to reduction in left ventricular contractile efficiency. Surgical strategy was planned when multimodal imaging demonstrated the anatomical, functional consequences of the aneurysm, and graft vessel patency.
Timeline
| Time . | Events . |
|---|---|
| Early December 2013 | Acute coronary syndrome, unstable angina, normal left ventricular ejection fraction, managed with antiplatelets, heparin, beta blockers, statins, and nitrates |
| After 2 days | Coronary angiogram showed ostioproximal left anterior descending coronary artery disease with 99% stenosis and totally occluded distal right coronary artery beyond posterior descending branch |
| Late December 2013 | Coronary artery bypass graft surgery, left internal mammary artery graft to left anterior descending coronary artery, and reversed saphenous venous graft to posterolateral ventricular branch of distal right coronary artery |
| January 2014 | Asymptomatic, continues on statins, aspirin and medications for diabetes mellitus and hypertension, normal ejection fraction on echocardiography |
| Early February 2018 | Acute coronary syndrome, ST-segment elevation lateral wall myocardial infarction, left ventricular ejection fraction 40%, akinetic lateral wall, thrombolysed with reteplase in another centre that is remote from a cardiac catheterization laboratory |
| Late February 2018 | Progressive dyspnoea, hospitalized in our centre, echocardiogram shows large posterior left ventricular pseudoaneurysm, left ventricle contracts ineffectively due to ‘to-and-fro’ blood flows into the pseudoaneurysm, mild Grade II mitral regurgitation. Pulmonary artery systolic pressures were 45 mmHg by tricuspid regurgitation Doppler |
| March 2018 | Surgical correction with closure of pseudoaneurysm using a bovine pericardial buttressed patch, normal ejection fraction on post-operative echocardiography |
| April 2018 | One-month follow-up after ventricular reconstructive surgery, normal ejection fraction, no mitral regurgitation. Continues on aspirin, statin, betablockers, angiotensin converting enzyme inhibitors and antidiabetics. New York Heart Association (NYHA) Class I, diuretics stopped |
| Time . | Events . |
|---|---|
| Early December 2013 | Acute coronary syndrome, unstable angina, normal left ventricular ejection fraction, managed with antiplatelets, heparin, beta blockers, statins, and nitrates |
| After 2 days | Coronary angiogram showed ostioproximal left anterior descending coronary artery disease with 99% stenosis and totally occluded distal right coronary artery beyond posterior descending branch |
| Late December 2013 | Coronary artery bypass graft surgery, left internal mammary artery graft to left anterior descending coronary artery, and reversed saphenous venous graft to posterolateral ventricular branch of distal right coronary artery |
| January 2014 | Asymptomatic, continues on statins, aspirin and medications for diabetes mellitus and hypertension, normal ejection fraction on echocardiography |
| Early February 2018 | Acute coronary syndrome, ST-segment elevation lateral wall myocardial infarction, left ventricular ejection fraction 40%, akinetic lateral wall, thrombolysed with reteplase in another centre that is remote from a cardiac catheterization laboratory |
| Late February 2018 | Progressive dyspnoea, hospitalized in our centre, echocardiogram shows large posterior left ventricular pseudoaneurysm, left ventricle contracts ineffectively due to ‘to-and-fro’ blood flows into the pseudoaneurysm, mild Grade II mitral regurgitation. Pulmonary artery systolic pressures were 45 mmHg by tricuspid regurgitation Doppler |
| March 2018 | Surgical correction with closure of pseudoaneurysm using a bovine pericardial buttressed patch, normal ejection fraction on post-operative echocardiography |
| April 2018 | One-month follow-up after ventricular reconstructive surgery, normal ejection fraction, no mitral regurgitation. Continues on aspirin, statin, betablockers, angiotensin converting enzyme inhibitors and antidiabetics. New York Heart Association (NYHA) Class I, diuretics stopped |
| Time . | Events . |
|---|---|
| Early December 2013 | Acute coronary syndrome, unstable angina, normal left ventricular ejection fraction, managed with antiplatelets, heparin, beta blockers, statins, and nitrates |
| After 2 days | Coronary angiogram showed ostioproximal left anterior descending coronary artery disease with 99% stenosis and totally occluded distal right coronary artery beyond posterior descending branch |
| Late December 2013 | Coronary artery bypass graft surgery, left internal mammary artery graft to left anterior descending coronary artery, and reversed saphenous venous graft to posterolateral ventricular branch of distal right coronary artery |
| January 2014 | Asymptomatic, continues on statins, aspirin and medications for diabetes mellitus and hypertension, normal ejection fraction on echocardiography |
| Early February 2018 | Acute coronary syndrome, ST-segment elevation lateral wall myocardial infarction, left ventricular ejection fraction 40%, akinetic lateral wall, thrombolysed with reteplase in another centre that is remote from a cardiac catheterization laboratory |
| Late February 2018 | Progressive dyspnoea, hospitalized in our centre, echocardiogram shows large posterior left ventricular pseudoaneurysm, left ventricle contracts ineffectively due to ‘to-and-fro’ blood flows into the pseudoaneurysm, mild Grade II mitral regurgitation. Pulmonary artery systolic pressures were 45 mmHg by tricuspid regurgitation Doppler |
| March 2018 | Surgical correction with closure of pseudoaneurysm using a bovine pericardial buttressed patch, normal ejection fraction on post-operative echocardiography |
| April 2018 | One-month follow-up after ventricular reconstructive surgery, normal ejection fraction, no mitral regurgitation. Continues on aspirin, statin, betablockers, angiotensin converting enzyme inhibitors and antidiabetics. New York Heart Association (NYHA) Class I, diuretics stopped |
| Time . | Events . |
|---|---|
| Early December 2013 | Acute coronary syndrome, unstable angina, normal left ventricular ejection fraction, managed with antiplatelets, heparin, beta blockers, statins, and nitrates |
| After 2 days | Coronary angiogram showed ostioproximal left anterior descending coronary artery disease with 99% stenosis and totally occluded distal right coronary artery beyond posterior descending branch |
| Late December 2013 | Coronary artery bypass graft surgery, left internal mammary artery graft to left anterior descending coronary artery, and reversed saphenous venous graft to posterolateral ventricular branch of distal right coronary artery |
| January 2014 | Asymptomatic, continues on statins, aspirin and medications for diabetes mellitus and hypertension, normal ejection fraction on echocardiography |
| Early February 2018 | Acute coronary syndrome, ST-segment elevation lateral wall myocardial infarction, left ventricular ejection fraction 40%, akinetic lateral wall, thrombolysed with reteplase in another centre that is remote from a cardiac catheterization laboratory |
| Late February 2018 | Progressive dyspnoea, hospitalized in our centre, echocardiogram shows large posterior left ventricular pseudoaneurysm, left ventricle contracts ineffectively due to ‘to-and-fro’ blood flows into the pseudoaneurysm, mild Grade II mitral regurgitation. Pulmonary artery systolic pressures were 45 mmHg by tricuspid regurgitation Doppler |
| March 2018 | Surgical correction with closure of pseudoaneurysm using a bovine pericardial buttressed patch, normal ejection fraction on post-operative echocardiography |
| April 2018 | One-month follow-up after ventricular reconstructive surgery, normal ejection fraction, no mitral regurgitation. Continues on aspirin, statin, betablockers, angiotensin converting enzyme inhibitors and antidiabetics. New York Heart Association (NYHA) Class I, diuretics stopped |
Case presentation
Following a coronary bypass surgery with mammary graft to left anterior descending coronary artery and venous graft to posterolateral ventricular branch four years previously, a 61-year-old diabetic hypertensive man presented with acute lateral wall myocardial infarction. Due to remote access to a cardiac catheterization laboratory for a primary angioplasty, he was managed with thrombolysis initially. After 2 weeks, he presented with New York Heart Association (NYHA) Class II dyspnoea to our centre. Clinical examination showed sinus tachycardia at 106 per minute, normal blood pressures, left ventricular third sound, absence of murmurs, and basal lung crepitations. His electrocardiogram revealed an old inferior wall infarct and chest radiograph showed cardiomegaly with pulmonary venous congestion. There was a left ventricular free wall rupture forming a pseudoaneurysm measuring 8 × 15 cm with a 16 mm mouth of entry from the left ventricle between the two heads of the papillary muscles, identified on transthoracic echocardiography (Figure 1, Supplementary material online, Videos S1 and S2). The systolic colour Doppler flows from the ventricle into the large pseudoaneurysm and diastolic flow back into the left ventricle created a to-and-fro spectral Doppler pattern and reduced ventricular efficiency. Regional strain analysis identified the scarred akinetic inferoposterior and lateral segments (Figure 2).
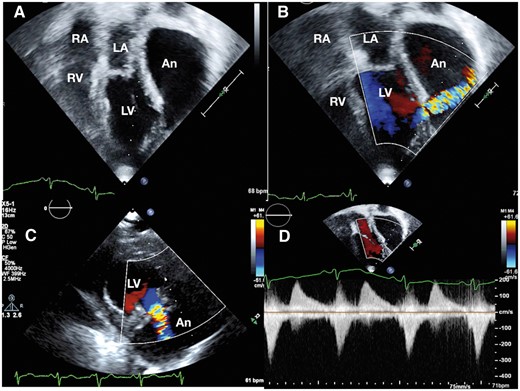
Transthoracic echocardiogram on Epiq 7 platform (Philips N.V, Netherlands) from apical view (A) shows a very large pseudoaneurysm with a narrow neck from the posterolateral wall of the left ventricle extending superiorly to the left of left atrium. The right atrium and right ventricle are normal in size. Colour Doppler imaging demonstrates systolic flows into the aneurysm in apical view (B). The mouth of the aneurysm is located between the two heads of papillary muscles of the left ventricle (C). To-and-fro flows on spectral Doppler (D) demonstrates ineffective left ventricular contractility. An, pseudoaneurysm; LA, left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle.
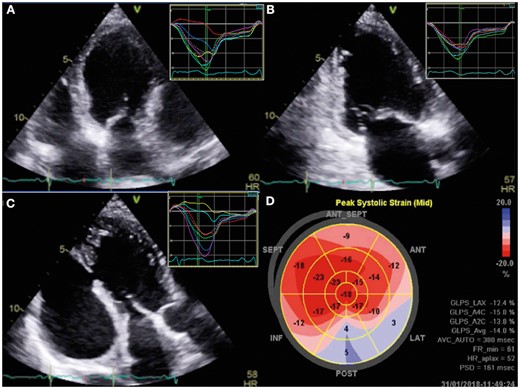
Speckle imaging on Vivid E95 platform (GE Health care, Solingen, Germany) from four chamber (A), two chamber (B), and three chamber (C) views demonstrated normal segmental strain in anterior, anteroseptal and septal walls and post-systolic shortening with positive strain in posterior and lateral walls. The polar bull’s eye map shows the segmental differences in strain.
Three-dimensional transoesophageal echocardiogram delineated the morphology and extent of the large pseudoaneurysm, which extended posterolaterally from the left ventricle and distorted the mitral annulus (Figure 3). A reconstructed three chamber long-axis view showed the exact location and extent of the leak (see Supplementary material online, Videos S3 and S4). Multislice gated computed tomogram showed patent bypass grafts, non-dominant left circumflex artery with very small obtuse marginal branches, lack of need for further revascularization, and delineated the aneurysm well (Figure 4). After femoral venous and arterial cannulation that assisted to establish cardiopulmonary bypass, a left posterolateral thoracotomy was performed to gain access to the pseudoaneurysm. Under hypothermic circulatory arrest, the aneurysm was opened from outside and the mouth was closed using bovine pericardial buttressed patch successfully. The symptoms of dyspnoea improved significantly and tachycardia subsided on 1-month follow-up with normal clinical findings. Echocardiography confirmed normal ejection fraction and complete exclusion of the pseudoaneurysm.
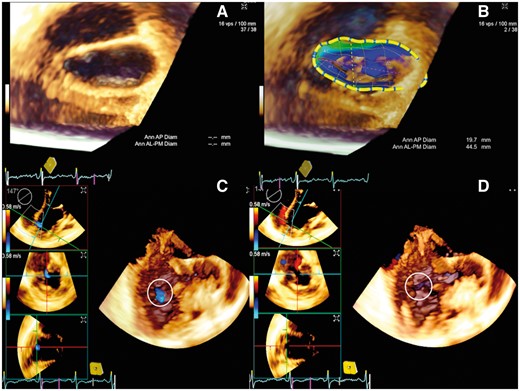
Three-dimensional transoesophageal echocardiogram in Acuson SC2000 prime platform (Siemens, Muenchen, Germany) shows left atrial view of the distorted mitral annulus, which is flattened by the posterior large pseudoanerysm (A) clearly demonstrated in eSie mitral analysis (B). Volume rendered three chamber view in diastole (C) and systole (D) shows the to-and-fro flows into and out of the mouth of the pseudoaneurysm.
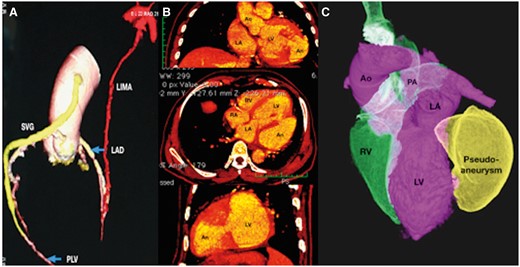
Multislice gated computed tomography (A) demonstrates patent internal mammary graft to left anterior descending coronary artery shown in red colour and venous graft from ascending aorta to posterolateral ventricular branch of right coronary artery shown in yellow colour. Multiplanar reformatted images in coronal, axial, and parasagittal planes (B) shows the mouth of the pseudoaneurysm from the left ventricle. A volume rendered image shows the large pseudoaneurysm shown in yellow arising from the posterolateral wall of the left ventricle.
Discussion
Left ventricular pseudoaneurysms are rare with a prevalence of 0.05%, occur following acute myocardial infarctions contained by adhering pericardium.5 They warrant emergency surgery as they may rupture leading to cardiac tamponade, shock and death contrary to a more benign natural history for true aneurysms.6 The most common cause of left ventricular pseudoaneurysms are secondary to contained rupture following myocardial infarctions and the other causes are post-surgical, trauma, and infections.7 Inferior wall infarctions are more likely to cause pseudoaneurysms than anterior infarctions.5 The most dreaded complication is tamponade, other presentations are ventricular arrhythmias, thromboembolism, dyspnoea from heart failure and persistent angina, even though one early report suggested a benign course.7,8 Electrocardiogram in true and pseudoaneurysm may show persistent ST-segment elevations in the corresponding leads.9
Echocardiography plays a major role in understanding the global and regional left ventricular systolic function and delineating the extent and location of the pseudoaneurysms.9 The ratio of the diameter of the entry orifice to the diameter of the aneurysm differentiates a true aneurysm from a pseudoaneurysm.6 Transoesophageal echocardiography is useful for assessing location of the aneurysm, presence of thrombus, involvement of mitral apparatus, interpapillary distance, global and regional ventricular function.9 Our report shows the utility of three-dimensional echocardiography to show the distortion of the mitral annular shape by the large posterior pseudoaneurysm. This gave us the confidence that a mitral valve surgery will not be needed if the pseudoaneurysm is eliminated surgically. Even though left ventriculography has been historically recommended as the imaging modality of choice, cardiac computed tomography provides equally similar information, as illustrated in our patient.8 Extensive use of these non-invasive imaging helps in diagnosis and management of these pseudoaneurysms.9
Conclusions
Pseudoaneurysms following acute myocardial infarctions contained by previous surgical adhesions give a window of opportunity to plan surgical correction in a safe period. Multimodal imaging provides information that is needed in managing these patients.
Consent: The author/s confirm that written consent for submission and publication of this case report including image(s) and associated text has been obtained from the patient in line with COPE guidance.
Conflict of interest: none declared.




Comments