-
PDF
- Split View
-
Views
-
Cite
Cite
Jacopo Canzian, Damiano Gentile, Rita De Sanctis, Flavia Jacobs, Chiara Benvenuti, Mariangela Gaudio, Riccardo Gerosa, Giuseppe Saltalamacchia, Rosalba Torrisi, Giovanna Masci, Corrado Tinterri, Alberto Zambelli, Decoding NATALEE and MonarchE eligibility criteria in a real-world cohort of early breast cancer patients, The Oncologist, Volume 30, Issue 5, May 2025, oyaf061, https://doi.org/10.1093/oncolo/oyaf061
Close - Share Icon Share
Abstract
The NATALEE trial expanded the use of adjuvant cyclin-dependent kinase 4/6 inhibitors beyond the MonarchE trial’s criteria for early breast cancer (eBC).
We conducted a retrospective analysis comparing a large real-world (RW) cohort of 762 consecutive eBC patients with those enrolled in the NATALEE and MonarchE randomized controlled trials (RCTs) to evaluate differences in eligibility. Our analysis revealed that 41.7% of RW patients met NATALEE’s eligibility criteria, significantly more than the 21.8% who met MonarchE’s criteria, reflecting NATALEE’s broader indication. Real-world patients were older, had less advanced tumors, and were less likely to be treated with adjuvant chemotherapy compared to the RCT populations. None of the RW patients was deemed eligible for ribociclib based solely on high genomic risk. These findings underscore significant differences in clinical characteristics and potential treatment eligibility, highlighting the need for critical assessment of RCTs results in clinical practice.
Introduction
Among cyclin-dependent kinase 4/6 inhibitors (CDK4/6i), adjuvant abemaciclib and ribociclib have recently demonstrated improved outcomes in patients with hormone receptor-positive (HR+)/human epidermal growth factor receptor 2 negative (HER2−) early breast cancer (eBC) with intermediate to high risk of recurrence, while palbociclib has not.1-4 The NATALEE study broadened CDK4/6i eligibility beyond that of MonarchE and integrated genomic testing into the selection criteria. However, translating the results of these randomized clinical trials (RCTs) to real-world (RW) setting remains challenging due to the variability of patient characteristics, not fully represented in RCTs, together with the lack of predictive factors for treatment response.
We assessed the clinical impact of applying the NATALEE study eligibility criteria compared to the MonarchE study in a RW HR+/HER2− eBC population to determine the proportion of patients potentially eligible for ribociclib vs abemaciclib. Additionally, we examined the relevance of the genomic risk in selecting RW patients eligible for ribociclib.
Patients and methods
We retrospectively analyzed consecutive HR+/HER2− eBC patients treated at Humanitas Research Hospital in Milan (Italy) between January 2021 and December 2022. The inclusion criteria were: histologically confirmed diagnosis of stage I-III HR+/HER2− eBC, and undergoing surgery with curative intent. Exclusion criteria encompassed having only noninvasive breast cancer (carcinoma in situ) or receiving prior neoadjuvant chemotherapy. Baseline clinical-pathological characteristics and treatment modalities were systematically collected using medical records.
Descriptive statistics were used to summarize data. To statistically assess the differences between the RW populations eligible according to NATALEE and MonarchE, the chi-square test (χ²) was applied, and a P-value was generated for each variable. A P-value < 0.05 was considered statistically significant.
Results
Of the 1099 eBC patients evaluated, 762 were eligible for analysis (Figure 1A). Among these, 414/762 (54.3%) were at stage I, 186 (24.4%) were at stage IIA, 99 (13.0%) at stage IIB, 63 (8.0%) at stage III, and 503 (66.0%) had node-negative disease (pN0) (Table S1).
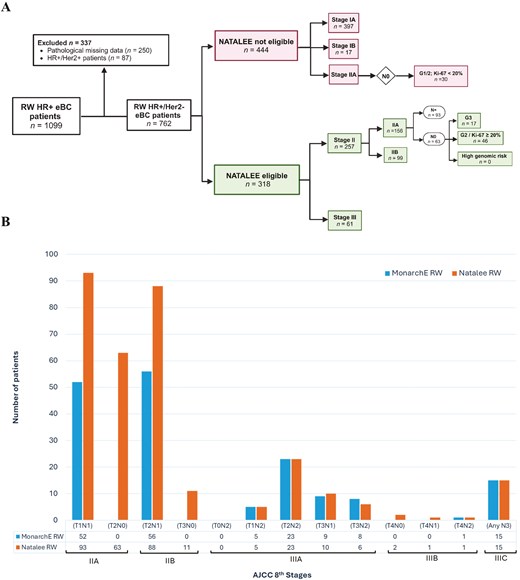
(A) Flow chart of real-world patients potentially eligible for adjuvant Ribociclib using the NATALEE study eligibility criteria. (B) Differences in the number of real-world patients eligible for NATALEE and MonarchE for each cancer stage.
Description of RW cohorts: MonarchE vs NATALEE eligibility
In our RW cohort, 167/762 (21.8%) and 318/762 (41.7%) met eligibility criteria for the MonarchE and NATALEE trials, respectively. A higher proportion of RW patients eligible for NATALEE (RW-N) were at stage II compared to those eligible for MonarchE (RW-M) (80.1% vs 64.6%). Additionally, 23.9% of RW patients without lymph node involvement (pN0) were eligible only for NATALEE (Figure 1B).
Oncotype DX testing was performed on 157 RW-N patients, mostly classified as stage IIA (59.2%), though none were eligible based solely on their Recurrence Score. More RW-N patients did not receive adjuvant chemotherapy compared to RW-M patients (64.5% vs 56.4%).
RW cohort vs RCTs: population characteristics
Compared to RCTs, RW patients were older (median age 53 vs 51 for MonarchE, 54 vs 52 for NATALEE) and had less advanced tumor stages. RW patients had higher stage IIa (31.1% vs 11.5% for MonarchE, 49.0% vs 19.0% for NATALEE) and stage IIb (33.5% vs 14.0% for MonarchE, 31.0% vs 21.0% for NATALEE), while RCT patients had more stage III (74.1% for MonarchE, 60.0% for NATALEE; P < .001). RCT patients also had greater lymph node involvement, with a significantly higher proportion of ≥ 4 positive nodes (P < .001). Fewer RW patients received adjuvant chemotherapy compared to NATALEE (35.5% vs 48.0%) and MonarchE (43.7% vs 58.5%) (Table 1).
Differences between the clinical characteristics of the MonarchE and NATALEE randomized trial (RCT) populations and the real-world (RW) population.
| MonarchE . | χ² . | NATALEE . | χ² . | |||
|---|---|---|---|---|---|---|
| RCT . | RW . | RCT . | RW . | |||
| Number of patients | 2808 | 167/762 (21.8%) | 2549 | 318/762 (41.8%) | ||
| Median age | 51 y | 53 y | P-value = .0015 | 52 y | 54 y | |
| Stage | P-value < .001 | P < .001 | ||||
| I | 2 (0.1%) | / | / | / | ||
| IIA | 323 (11.5%) | 52 (31.1%) | 479 (19%) | 156 (49%) | ||
| IIB | 389 (13.9%) | 56 (33.5%) | 532 (21%) | 99 (31.1%) | ||
| III | 2081 (74.1%) | 59 (35.3%) | 1528 (60%) | 63 (19.9%) | ||
| Tumor size | P-value = .0025 | |||||
| T0, T1, Tx, Tis | 780 (27.8%) | 60 (35.9%) | / | 101 (31.7%) | ||
| T2 | 1372 (48.9%) | 88 (52.6%) | / | 183 (57.5%) | ||
| T3, T4 | 607 (21.6%) | 19 (11.3%) | / | 34 (10.7%) | ||
| Nodal status surgery | ||||||
| N0 | 7 (0.2%) | / | 378 (14.8%) | 76 (23.9%) | ||
| N1 | 1118 (39.8%) | 117 (70%) | 1062 (41.7%) | 192 (60.3%) | ||
| N2, N3 | 1682 (59.9%) | 50 (30%) | P-value < .001 | 1105 (43%) | 50 (15,7%) | P < .001 |
| NX | / | / | 272 (11%) | / | ||
| Grade | P-value < .001 | P < .001 | ||||
| G1 | 209 (7.4%) | 2 (1.2%) | 213 (9%) | 3 (0.9%) | ||
| G2 | 1373 (48.9%) | 109 (65.2%) | 1460 (61.9%) | 235 (73.9%) | ||
| G3 | 1090 (38.8%) | 56 (33.5%) | 684 (29%) | 78 (24.5%) | ||
| Ki-67a | P-value < .001 | P < .001 | ||||
| < 20% | 953 (33.9%) | 23 (13.7%) | 1199 (47%) | 146 (46%) | ||
| ≥ 20% | 1262 (44.9%) | 144 (86.2%) | 920 (36.1%) | 172 (67%) | ||
| Chemotherapy | ||||||
| Adjuvant | 1642 (58.5%) | 73 (43.7%) | 1223 (48%) | 113 (35.5%) | ||
| MonarchE . | χ² . | NATALEE . | χ² . | |||
|---|---|---|---|---|---|---|
| RCT . | RW . | RCT . | RW . | |||
| Number of patients | 2808 | 167/762 (21.8%) | 2549 | 318/762 (41.8%) | ||
| Median age | 51 y | 53 y | P-value = .0015 | 52 y | 54 y | |
| Stage | P-value < .001 | P < .001 | ||||
| I | 2 (0.1%) | / | / | / | ||
| IIA | 323 (11.5%) | 52 (31.1%) | 479 (19%) | 156 (49%) | ||
| IIB | 389 (13.9%) | 56 (33.5%) | 532 (21%) | 99 (31.1%) | ||
| III | 2081 (74.1%) | 59 (35.3%) | 1528 (60%) | 63 (19.9%) | ||
| Tumor size | P-value = .0025 | |||||
| T0, T1, Tx, Tis | 780 (27.8%) | 60 (35.9%) | / | 101 (31.7%) | ||
| T2 | 1372 (48.9%) | 88 (52.6%) | / | 183 (57.5%) | ||
| T3, T4 | 607 (21.6%) | 19 (11.3%) | / | 34 (10.7%) | ||
| Nodal status surgery | ||||||
| N0 | 7 (0.2%) | / | 378 (14.8%) | 76 (23.9%) | ||
| N1 | 1118 (39.8%) | 117 (70%) | 1062 (41.7%) | 192 (60.3%) | ||
| N2, N3 | 1682 (59.9%) | 50 (30%) | P-value < .001 | 1105 (43%) | 50 (15,7%) | P < .001 |
| NX | / | / | 272 (11%) | / | ||
| Grade | P-value < .001 | P < .001 | ||||
| G1 | 209 (7.4%) | 2 (1.2%) | 213 (9%) | 3 (0.9%) | ||
| G2 | 1373 (48.9%) | 109 (65.2%) | 1460 (61.9%) | 235 (73.9%) | ||
| G3 | 1090 (38.8%) | 56 (33.5%) | 684 (29%) | 78 (24.5%) | ||
| Ki-67a | P-value < .001 | P < .001 | ||||
| < 20% | 953 (33.9%) | 23 (13.7%) | 1199 (47%) | 146 (46%) | ||
| ≥ 20% | 1262 (44.9%) | 144 (86.2%) | 920 (36.1%) | 172 (67%) | ||
| Chemotherapy | ||||||
| Adjuvant | 1642 (58.5%) | 73 (43.7%) | 1223 (48%) | 113 (35.5%) | ||
aKi-67 cutoff differs between the 2 studies; NATALEE trial split the cut off into ≤ 20% and > 20%.
Differences between the clinical characteristics of the MonarchE and NATALEE randomized trial (RCT) populations and the real-world (RW) population.
| MonarchE . | χ² . | NATALEE . | χ² . | |||
|---|---|---|---|---|---|---|
| RCT . | RW . | RCT . | RW . | |||
| Number of patients | 2808 | 167/762 (21.8%) | 2549 | 318/762 (41.8%) | ||
| Median age | 51 y | 53 y | P-value = .0015 | 52 y | 54 y | |
| Stage | P-value < .001 | P < .001 | ||||
| I | 2 (0.1%) | / | / | / | ||
| IIA | 323 (11.5%) | 52 (31.1%) | 479 (19%) | 156 (49%) | ||
| IIB | 389 (13.9%) | 56 (33.5%) | 532 (21%) | 99 (31.1%) | ||
| III | 2081 (74.1%) | 59 (35.3%) | 1528 (60%) | 63 (19.9%) | ||
| Tumor size | P-value = .0025 | |||||
| T0, T1, Tx, Tis | 780 (27.8%) | 60 (35.9%) | / | 101 (31.7%) | ||
| T2 | 1372 (48.9%) | 88 (52.6%) | / | 183 (57.5%) | ||
| T3, T4 | 607 (21.6%) | 19 (11.3%) | / | 34 (10.7%) | ||
| Nodal status surgery | ||||||
| N0 | 7 (0.2%) | / | 378 (14.8%) | 76 (23.9%) | ||
| N1 | 1118 (39.8%) | 117 (70%) | 1062 (41.7%) | 192 (60.3%) | ||
| N2, N3 | 1682 (59.9%) | 50 (30%) | P-value < .001 | 1105 (43%) | 50 (15,7%) | P < .001 |
| NX | / | / | 272 (11%) | / | ||
| Grade | P-value < .001 | P < .001 | ||||
| G1 | 209 (7.4%) | 2 (1.2%) | 213 (9%) | 3 (0.9%) | ||
| G2 | 1373 (48.9%) | 109 (65.2%) | 1460 (61.9%) | 235 (73.9%) | ||
| G3 | 1090 (38.8%) | 56 (33.5%) | 684 (29%) | 78 (24.5%) | ||
| Ki-67a | P-value < .001 | P < .001 | ||||
| < 20% | 953 (33.9%) | 23 (13.7%) | 1199 (47%) | 146 (46%) | ||
| ≥ 20% | 1262 (44.9%) | 144 (86.2%) | 920 (36.1%) | 172 (67%) | ||
| Chemotherapy | ||||||
| Adjuvant | 1642 (58.5%) | 73 (43.7%) | 1223 (48%) | 113 (35.5%) | ||
| MonarchE . | χ² . | NATALEE . | χ² . | |||
|---|---|---|---|---|---|---|
| RCT . | RW . | RCT . | RW . | |||
| Number of patients | 2808 | 167/762 (21.8%) | 2549 | 318/762 (41.8%) | ||
| Median age | 51 y | 53 y | P-value = .0015 | 52 y | 54 y | |
| Stage | P-value < .001 | P < .001 | ||||
| I | 2 (0.1%) | / | / | / | ||
| IIA | 323 (11.5%) | 52 (31.1%) | 479 (19%) | 156 (49%) | ||
| IIB | 389 (13.9%) | 56 (33.5%) | 532 (21%) | 99 (31.1%) | ||
| III | 2081 (74.1%) | 59 (35.3%) | 1528 (60%) | 63 (19.9%) | ||
| Tumor size | P-value = .0025 | |||||
| T0, T1, Tx, Tis | 780 (27.8%) | 60 (35.9%) | / | 101 (31.7%) | ||
| T2 | 1372 (48.9%) | 88 (52.6%) | / | 183 (57.5%) | ||
| T3, T4 | 607 (21.6%) | 19 (11.3%) | / | 34 (10.7%) | ||
| Nodal status surgery | ||||||
| N0 | 7 (0.2%) | / | 378 (14.8%) | 76 (23.9%) | ||
| N1 | 1118 (39.8%) | 117 (70%) | 1062 (41.7%) | 192 (60.3%) | ||
| N2, N3 | 1682 (59.9%) | 50 (30%) | P-value < .001 | 1105 (43%) | 50 (15,7%) | P < .001 |
| NX | / | / | 272 (11%) | / | ||
| Grade | P-value < .001 | P < .001 | ||||
| G1 | 209 (7.4%) | 2 (1.2%) | 213 (9%) | 3 (0.9%) | ||
| G2 | 1373 (48.9%) | 109 (65.2%) | 1460 (61.9%) | 235 (73.9%) | ||
| G3 | 1090 (38.8%) | 56 (33.5%) | 684 (29%) | 78 (24.5%) | ||
| Ki-67a | P-value < .001 | P < .001 | ||||
| < 20% | 953 (33.9%) | 23 (13.7%) | 1199 (47%) | 146 (46%) | ||
| ≥ 20% | 1262 (44.9%) | 144 (86.2%) | 920 (36.1%) | 172 (67%) | ||
| Chemotherapy | ||||||
| Adjuvant | 1642 (58.5%) | 73 (43.7%) | 1223 (48%) | 113 (35.5%) | ||
aKi-67 cutoff differs between the 2 studies; NATALEE trial split the cut off into ≤ 20% and > 20%.
Discussion
Our study shows that the clinical characteristics of a RW HR+/HER2− eBC population significantly differ from those in the NATALEE and MonarchE RCTs. Additionally, when applying the eligibility criteria of these RCTs to a RW setting, the number of patients eligible for ribociclib is twice that of those eligible for abemaciclib. Approximately 80% of the RW-N cohort were at stage II. In contrast to the MonarchE criteria, some of these patients could safely forgo lymph node dissection and still be eligible for ribociclib treatment.5 Furthermore, in the NATALEE trial, patients with pN0 disease achieved similar benefits from adjuvant ribociclib as those with pN1-3 disease. Our study suggests that these benefits could extend to an even larger RW population (23.9% vs 14.8%). The NATALEE study, with its higher proportion of patients with lymph node involvement and receiving neoadjuvant/adjuvant chemotherapy, contrasts significantly with the RW setting as highlighted by our findings. The lower proportion of RW patients receiving chemotherapy may be attributed to both their lower-risk profile and the presence of contraindications to this treatment in clinical practice. Given the limited representation of patients who had not received chemotherapy in the NATALEE study, longitudinal RW data will be crucial to evaluate the relative impact of adjuvant ribociclib in this population. Another critical issue in translating the results of RCTs into clinical practice concerns the limited data available on patients with gBRCA1/2 mutations. Despite their low prevalence, a proportion of these patients may be eligible for both adjuvant olaparib and CDK 4/6i, and the optimal treatment strategy for this group has yet to be defined.6,7 Furthermore, the population eligible for adjuvant CDK 4/6i is expected to increase over time, with regional and country-specific variations. Therefore, it will be essential to carefully identify those who will derive the greatest benefit from adjuvant ribociclib, taking into account both clinical efficacy and financial toxicity.
As CDK 4/6i efficacy has been shown to be independent of genomic risk and access to genomic testing remains challenging worldwide, our study highlights its limited utility in patient selection and emphasizes the need for novel predictors of treatment efficacy.8
Given the lack of a standard definition for “high risk’ HR+/HER2− eBC, the response to short-term preoperative endocrine therapy could be a useful predictor of which patients are most likely to benefit from adjuvant CDK4/6i. This hypothesis is currently being investigated in ongoing studies such as POETIC-A (NCT04584853) and ADAPT-Cycle (NCT04055493) studies.
Finally, the discrepancy between statistical significance and clinical relevance in large RCTs underscores the need to balance the treatment benefits with RW impact on patient outcomes and quality of life. For instance, early dropouts in the control arm of the NATALEE study might have amplified the magnitude of benefit of a composite endpoint like invasive disease-free survival (iDFS). Since ribociclib has recently demonstrated increasing benefits in iDFS beyond the completion of 3 years of treatment, a more mature follow-up will be crucial to confirm its potential advantages in terms of overall survival.9 In conclusion, although our study is limited by its retrospective design and the lack of certain demographic data, it clearly highlights the anticipated increase in the population eligible for CDK4/6i treatment. Therefore, the rising patient volume and the extended treatment duration (3 years for ribociclib vs 2 years for abemaciclib) necessitate addressing clinical needs before translating these results into clinical practice.
Future research is crucial to identify subgroups that derive the greatest benefit, maximizing the treatment value.
Author contributions
Jacopo Canzian (Conceptualization, Data curation, Investigation, Formal analysis, Writing—original draft, Writing—review & editing), Damiano Gentile (Data curation, Investigation, Formal analysis, Writing—original draft, Writing—review & editing), Rita De Sanctis (Conceptualization, Data curation, Investigation, Formal analysis, Writing—original draft, Writing—review & editing), Flavia Jacobs (Data curation, Investigation, Formal analysis, Writing—original draft, Writing—review & editing), Chiara Benvenuti (Data curation, Investigation, Formal analysis, Writing—original draft, Writing—review & editing), Mariangela Gaudio (Data curation, Investigation, Formal analysis, Writing—original draft, Writing—review & editing), Riccardo Gerosa (Data curation, Investigation, Formal analysis, Writing—original draft, Writing—review & editing), Giuseppe Saltalamacchia (Data curation, Investigation, Formal analysis, Writing—original draft, Writing—review & editing), Rosalba Torrisi (Data curation, Investigation, Formal analysis, Writing—original draft, Writing—review & editing), Giovanna Masci (Data curation, Investigation, Formal analysis, Writing—original draft, Writing—review & editing), Corrado Tinterri (Data curation, Investigation, Formal analysis, Writing—original draft, Writing—review & editing), and Alberto Zambelli (Conceptualization, Data curation, Investigation, Formal analysis, Writing—original draft, Writing—review & editing)
Funding
None declared.
Conflicts of interest
None declared.
Data availability
The data presented in this study are available on reasonable request from the corresponding author.



