-
PDF
- Split View
-
Views
-
Cite
Cite
Young Saing Kim, Jun Ho Ji, Sung Yong Oh, Suee Lee, Seok Jae Huh, Ji Hyun Lee, Ki‐Hoon Song, Choon Hee Son, Mee Sook Roh, Gyeong Won Lee, Jeeyun Lee, Seung Tae Kim, Chan Kyu Kim, Joung Soon Jang, In Gyu Hwang, Hee Kyung Ahn, Lee Chun Park, So Yeon Oh, Seong‐Geun Kim, Sang‐Cheol Lee, Do‐Hyoung Lim, Soon Il Lee, Jung Hun Kang, A Randomized Controlled Trial of Epidermal Growth Factor Ointment for Treating Epidermal Growth Factor Receptor Inhibitor‐Induced Skin Toxicities, The Oncologist, Volume 25, Issue 1, January 2020, Pages e186–e193, https://doi.org/10.1634/theoncologist.2019-0221
Close - Share Icon Share
Abstract
The efficacy of epidermal growth factor (EGF) receptor (EGFR) inhibitors in patients with non‐small cell lung cancer (NSCLC), pancreatic cancer (PC), or colorectal cancer (CRC) has been demonstrated. However, dermatological reactions to these inhibitors can cause significant physical and psychosocial discomfort. The objective of the present study was to evaluate the efficacy of EGF ointment for EGFR inhibitor‐related skin adverse events (ERSEs).
This placebo‐controlled, double‐blind, multicenter, pilot phase III trial enrolled patients with NSCLC, PC, or CRC treated with EGFR inhibitors. Patients with grade ≥2 ERSEs were included. Patients were randomized to three treatment arms: arm 1, placebo; arm 2, 1 ppm of EGF ointment; and arm 3, 20 ppm of EGF ointment. Patients applied ointment to their skin lesions twice daily.
Efficacy evaluation was available for 80 patients (9 for PC, 28 for NSCLC, and 43 for CRC). Responses were 44.4% in arm 1, 61.5% in arm 2, and 77.8% in arm 3. There was a linear correlation between EGF concentrations and responses (p = .012). Quality of life (QoL) was assessed for 74 patients. Maximum changes in composite scores by Skindex‐16 after treatment were significantly different among arms (mean ± SD: −5.2 ± 8.6 for arm 1, −11.7 ± 14.2 for arm 2, and − 18.6 ± 17.7 for arm 3; p = .008). EGF arms showed significant improvement in emotions (p = .005) and functioning (p = .044) scores over the placebo arm.
EGF ointment is effective for managing ERSEs. It can also improve patients’ QoL compared with placebo. Clinical trial identification number. NCT02284139
Implications for Practice
Patients with non‐small cell lung cancer, pancreatic cancer, or colorectal cancer who are treated with epidermal growth factor (EGF) receptor (EGFR) inhibitors may experience dermatologic reactions to their treatment. This study investigated the benefit of an EGF ointment in the treatment of these adverse events and observed the ointment to be effective in managing EGFR inhibitor‐related skin adverse events.
Introduction
Epidermal growth factor (EGF) receptor (EGFR) signaling is involved in the pathogenesis and progression of a variety of cancers [1]. Thus, EGFR is an important target for antitumor therapy. Currently, tyrosine kinase inhibitors (TKIs) and monoclonal antibodies that can inhibit EGFR signaling have been approved for cancer treatment either alone or in combination with cytotoxic chemotherapy or radiation therapy. EGFR TKIs (e.g., erlotinib, gefitinib, afatinib) are standard first‐line therapy for patients with advanced non‐small cell lung cancer (NSCLC) harboring EGFR‐activating mutations [2-4]. It has been shown that anti‐EGFR monoclonal antibodies such as cetuximab and panitumumab can improve objective response and survival of patients with RAS wild‐type metastatic colorectal cancer (CRC) [5, 6]. In locoregionally advanced head and neck cancer, addition of cetuximab to radiotherapy can improve locoregional control and survival of patients compared with radiotherapy alone [7].
EGFR is widely expressed in normal skin tissue. It plays an important role in skin homeostasis and wound healing [8, 9]. The most common adverse reactions of EGFR inhibitors are skin toxicities, including acneiform rash, xerosis, paronychial inflammation, pruritus, photosensitivity, and hair and eyelash alteration [10-12]. Because EGFR inhibitor‐associated skin toxicities can induce physical discomfort and sometimes psychological problems, the occurrence of skin toxicity may adversely affect the quality of life (QoL) and social functioning of patients [13, 14]. In addition, skin toxicities can potentially affect treatment duration, leading to treatment interruptions and early discontinuation of therapy. Therefore, in clinical practice, effective management for skin toxicities is important to maximize benefits of EGFR inhibitors.
Epidermal growth factor can stimulate the proliferation and differentiation of epithelial tissue and facilitate skin regeneration and wound healing [15, 16]. In addition, previous clinical studies have suggested that topical application of recombinant human EGF may have a beneficial role in preventing or minimizing radiation‐induced dermatitis and mucositis [17-19]. Given the therapeutic mechanism of EGF, its topical application might be effective for EGFR inhibitor‐induced skin reactions. Our previous phase II study has shown encouraging results that the use of EGF ointment can effectively reduce erlotinib‐induced skin toxicity and improve QoL [20]. The objective of this study was to report results of a subsequent, placebo‐controlled, randomized clinical trial of EGF ointment for the management of EGFR inhibitor‐related skin adverse events (ERSEs).
Materials and Methods
Study Design and Participants
This double‐blind, randomized, placebo‐controlled, multicenter, pilot phase III study was performed at 11 institutions in South Korea. Enrollment criteria were age ≥20 years; histologically proven NSCLC, CRC, or pancreatic cancer (PC) that was locally advanced or metastatic; treatment with EGFR inhibitors gefitinib, erlotinib, afatinib, or cetuximab; grade ≥2 ERSEs according to National Cancer Institute's Common Terminology Criteria for Adverse Events (NCI‐CTCAE) version 4.0; Eastern Cooperative Oncology Group performance status (ECOG PS) ≤2; adequate bone marrow, hepatic, and renal functions; and an estimated life expectancy of at least 3 months. Exclusion criteria were dermatologic treatment for skin lesions within 4 weeks, prior organ transplantation, history of hypersensitivity to EGF ointment or chemotherapeutic agents used in this study, or patients receiving immunosuppressive agents (such as steroids). All patients provided written informed consent before participation. This study was performed in accordance with the Declaration of Helsinki. It was approved by Institutional Review Boards or independent ethics committees of investigational sites. This study was registered at ClinicalTrials.gov (NCT02284139).
Human Investigation Comment
We performed the human investigations after approval by each institution's review board (including that of Dona‐A University Hospital) and in accord with an assurance filed with and approved by the Korean Food and Drug Administration. In addition, such data was required to be anonymized so as to protect the identities of participants involved in the research.
We obtained informed consent from each participant or each participant's guardian.
Treatment and Assessment
Eligible patients were randomly assigned at a 1:1:1 ratio to receive placebo (arm 1), 1 ppm concentration of EGF ointment (arm 2), or 20 ppm of EGF ointment (arm 3). For the purpose of double blinding, all the investigational products were made indistinguishable in appearance between placebo arm and EGF ointment arms and were identified by code numbers only. Randomization was performed using an interactive voice response system with stratification according to cancer types. Masking was achieved by labeling the experimental medication with a unique code number linked to the randomization scheme. EGF and placebo ointments were presented in identical packaging.
Patients applied placebo (arm 1) or EGF ointment (arm 2 or 3) to grade ≥2 skin lesions twice daily. Treatment was continued unless there was deterioration of ERSEs, unacceptable toxicity, withdrawal consent, or investigator decision (such as judgment of no treatment effect).
The primary endpoint was response rate (RR) of EGF ointment. Response was defined as follows: (a) reduction of ERSEs from grade ≥2 to grade ≤ 1 or (b) grade ≥3 ERSEs downgrading to grade 2 and lasting for at least 2 weeks. ERSEs were categorized into palmar‐plantar erythrodysesthesia syndrome, acneiform rash, dry skin, pruritus, or paronychia.
The evaluation of ERSEs was performed by a physician in charge of patients. Because the evaluation of ERSEs could be subjective, investigators trained in the assessment method before and during an interim meeting and registered the patient's photograph in the e‐CRF Web site with the patient's consent.
The effectiveness of EGF ointment was assessed at 2 weeks after the treatment and every 4 weeks thereafter. If a patient used the treatment for less than 1 week, the case was unavailable for evaluating response. If skin lesions showed no improvement after application of the ointment for 8 weeks, the treatment was stopped and classified as “no effect.” If the investigator determined that additional medication was needed to improve skin lesions and symptoms, oral or intravenous administration of antibiotics, antihistamines, and steroids was allowed during the study. However, topical agents were not permitted. Secondary endpoints included QoL and safety. QoL was evaluated with the Korean version of Skindex‐16 questionnaire. Skindex‐16 is a validated, skin‐disease‐specific, brief quality‐of‐life instrument. The Skindex‐16 survey comprises three domains: symptoms (items 1–4), emotions (items 5–11), and functioning (items 12–16). Responses were scored with a 7‐point scale ranging from 0 (never bothered) to 6 (constantly bothered) [21]. Patients responded to 16 items relating to skin condition at baseline and every 4 weeks thereafter. Safety was monitored throughout the study. Adverse events were graded according to NCI‐CTCAE v.4.0.
Statistical Analysis
For sample size calculation, a Cochran‐Armitage test for trend in proportion was used to detect a linear trend using a one‐sided Z test with continuity correction [22]. Assuming that RRs would be 40% for arm 1, 60% for arm 2, and 80% for arm 3 [20], 27 patients were required for each treatment arm to achieve 91% power with a significance level of .05. Considering a dropout rate of 10%, we planned to recruit a total of 90 patients.
Categorical data were summarized as counts and percentages. For continuous variables, summary statistics included number, mean, SD, median, and range. Comparison between treatment arms with respect to categorical variables was performed with a chi‐squared test or Fisher's exact test if the expected number of observations in any cell was less than 5. The Clopper‐Pearson method was used to calculate 95% confidence intervals (CIs) of RRs. For Skindex‐16, response to each item was transferred from a 0–6 scale to a 0–100 scale. Composite scores were calculated as mean scores of all items. Each of the three domain scores was calculated as the mean of related items. A Skindex‐16 change score of 10 points before and after treatment was considered clinically significant [23, 24]. Changes of domain scores and composite score were compared between treatment arms by the Kruskal‐Wallis test or Mann‐Whitney U test. All statistical analyses were performed using SAS statistical software for Windows, version 9.4 (SAS Institute Inc., Cary, NC).
Results
Patients
Between June 2015 and October 2017, 90 patients were enrolled in this study, and 89 patients were randomly assigned (supplemental online Fig. 1). Ten enrolled patients were excluded from response analysis because of the following reasons: consent withdrawal (n = 5), follow‐up loss (n = 3), not meeting eligibility criteria (n = 1), and treatment violation (n = 1). Of the remaining 80 patients, 43 had CRC, 28 had NSCLC, and 9 had PC. The most commonly used EGFR inhibitor was cetuximab (53.8%), followed by erlotinib (37.5%) and afatinib (8.8%). Characteristics of patients evaluated were similar among the three treatment arms (Table 1).
| Characteristics . | Arm 1 (n = 27), n (%) . | Arm 2 (n = 26), n (%) . | Arm 3 (n = 27), n (%) . | p value . |
|---|---|---|---|---|
| Sex | .078 | |||
| Male | 13 (48.1) | 19 (73.1) | 20 (74.1) | |
| Female | 14 (51.9) | 7 (26.9) | 7 (25.9) | |
| Age | .081 | |||
| Median (range) | 57 (43–83) | 64.(30–79) | 56 (42–83) | |
| >60 years | 11 (40.7) | 17 (65.4) | 10 (37.0) | |
| ECOG PS | .920 | |||
| 0 | 8 (29.6) | 8 (30.8) | 7 (15.9) | |
| 1–2 | 19 (70.4) | 18 (69) | 20 (74.1) | |
| Operation history | .902 | |||
| No | 17 (63.0) | 15 (57.7) | 17 (63.0) | |
| Yes | 10 (37.0) | 11 (42.3) | 10 (37.0) | |
| Concomitant medication (P.O.) | ||||
| Yes | 6 (22.2) | 3 (11.5) | 5 (18.5) | .662 |
| Antibiotics | 5 (18.5) | 3 (11.5) | 3 (11.1) | |
| Antihistamine | 5 (11.1) | 0 | 3 (11.1) | |
| Steroid | 2 (7.4) | 0 | 1 (3.7) | |
| Cancer type | .944 | |||
| Colorectal | 15 (55.6) | 15 (57.7) | 13 (48.1) | |
| NSCLC | 9 (33.3) | 9 (34.6) | 10 (37.0) | |
| Pancreas | 3 (11.1) | 2 (7.7) | 4 (14.8) | |
| EGFR inhibitor | .965 | |||
| Cetuximab + FOLFIRI | 15 (55.6) | 15 (57.7) | 13 (48.1) | |
| Erlotiniba | 7 (25.9) | 6 (23.1) | 8 (14.8) | |
| Afatiniba | 2 (7.4) | 3 (11.5) | 2 (7.4) | |
| Erlotinibb + gemcitabine | 3 (11.1) | 2 (7.7) | 4 (14.8) |
| Characteristics . | Arm 1 (n = 27), n (%) . | Arm 2 (n = 26), n (%) . | Arm 3 (n = 27), n (%) . | p value . |
|---|---|---|---|---|
| Sex | .078 | |||
| Male | 13 (48.1) | 19 (73.1) | 20 (74.1) | |
| Female | 14 (51.9) | 7 (26.9) | 7 (25.9) | |
| Age | .081 | |||
| Median (range) | 57 (43–83) | 64.(30–79) | 56 (42–83) | |
| >60 years | 11 (40.7) | 17 (65.4) | 10 (37.0) | |
| ECOG PS | .920 | |||
| 0 | 8 (29.6) | 8 (30.8) | 7 (15.9) | |
| 1–2 | 19 (70.4) | 18 (69) | 20 (74.1) | |
| Operation history | .902 | |||
| No | 17 (63.0) | 15 (57.7) | 17 (63.0) | |
| Yes | 10 (37.0) | 11 (42.3) | 10 (37.0) | |
| Concomitant medication (P.O.) | ||||
| Yes | 6 (22.2) | 3 (11.5) | 5 (18.5) | .662 |
| Antibiotics | 5 (18.5) | 3 (11.5) | 3 (11.1) | |
| Antihistamine | 5 (11.1) | 0 | 3 (11.1) | |
| Steroid | 2 (7.4) | 0 | 1 (3.7) | |
| Cancer type | .944 | |||
| Colorectal | 15 (55.6) | 15 (57.7) | 13 (48.1) | |
| NSCLC | 9 (33.3) | 9 (34.6) | 10 (37.0) | |
| Pancreas | 3 (11.1) | 2 (7.7) | 4 (14.8) | |
| EGFR inhibitor | .965 | |||
| Cetuximab + FOLFIRI | 15 (55.6) | 15 (57.7) | 13 (48.1) | |
| Erlotiniba | 7 (25.9) | 6 (23.1) | 8 (14.8) | |
| Afatiniba | 2 (7.4) | 3 (11.5) | 2 (7.4) | |
| Erlotinibb + gemcitabine | 3 (11.1) | 2 (7.7) | 4 (14.8) |
aTreatment for non‐small cell lung cancer.
bErlotinib 100 mg was used for the treatment of pancreas cancer.
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group performance status; EGFR, epidermal growth factor receptor; FOLFIRI, folinic acid, fluorouracil, and irinotecan; NSCLC, non‐small cell lung cancer; P.O., per os.
| Characteristics . | Arm 1 (n = 27), n (%) . | Arm 2 (n = 26), n (%) . | Arm 3 (n = 27), n (%) . | p value . |
|---|---|---|---|---|
| Sex | .078 | |||
| Male | 13 (48.1) | 19 (73.1) | 20 (74.1) | |
| Female | 14 (51.9) | 7 (26.9) | 7 (25.9) | |
| Age | .081 | |||
| Median (range) | 57 (43–83) | 64.(30–79) | 56 (42–83) | |
| >60 years | 11 (40.7) | 17 (65.4) | 10 (37.0) | |
| ECOG PS | .920 | |||
| 0 | 8 (29.6) | 8 (30.8) | 7 (15.9) | |
| 1–2 | 19 (70.4) | 18 (69) | 20 (74.1) | |
| Operation history | .902 | |||
| No | 17 (63.0) | 15 (57.7) | 17 (63.0) | |
| Yes | 10 (37.0) | 11 (42.3) | 10 (37.0) | |
| Concomitant medication (P.O.) | ||||
| Yes | 6 (22.2) | 3 (11.5) | 5 (18.5) | .662 |
| Antibiotics | 5 (18.5) | 3 (11.5) | 3 (11.1) | |
| Antihistamine | 5 (11.1) | 0 | 3 (11.1) | |
| Steroid | 2 (7.4) | 0 | 1 (3.7) | |
| Cancer type | .944 | |||
| Colorectal | 15 (55.6) | 15 (57.7) | 13 (48.1) | |
| NSCLC | 9 (33.3) | 9 (34.6) | 10 (37.0) | |
| Pancreas | 3 (11.1) | 2 (7.7) | 4 (14.8) | |
| EGFR inhibitor | .965 | |||
| Cetuximab + FOLFIRI | 15 (55.6) | 15 (57.7) | 13 (48.1) | |
| Erlotiniba | 7 (25.9) | 6 (23.1) | 8 (14.8) | |
| Afatiniba | 2 (7.4) | 3 (11.5) | 2 (7.4) | |
| Erlotinibb + gemcitabine | 3 (11.1) | 2 (7.7) | 4 (14.8) |
| Characteristics . | Arm 1 (n = 27), n (%) . | Arm 2 (n = 26), n (%) . | Arm 3 (n = 27), n (%) . | p value . |
|---|---|---|---|---|
| Sex | .078 | |||
| Male | 13 (48.1) | 19 (73.1) | 20 (74.1) | |
| Female | 14 (51.9) | 7 (26.9) | 7 (25.9) | |
| Age | .081 | |||
| Median (range) | 57 (43–83) | 64.(30–79) | 56 (42–83) | |
| >60 years | 11 (40.7) | 17 (65.4) | 10 (37.0) | |
| ECOG PS | .920 | |||
| 0 | 8 (29.6) | 8 (30.8) | 7 (15.9) | |
| 1–2 | 19 (70.4) | 18 (69) | 20 (74.1) | |
| Operation history | .902 | |||
| No | 17 (63.0) | 15 (57.7) | 17 (63.0) | |
| Yes | 10 (37.0) | 11 (42.3) | 10 (37.0) | |
| Concomitant medication (P.O.) | ||||
| Yes | 6 (22.2) | 3 (11.5) | 5 (18.5) | .662 |
| Antibiotics | 5 (18.5) | 3 (11.5) | 3 (11.1) | |
| Antihistamine | 5 (11.1) | 0 | 3 (11.1) | |
| Steroid | 2 (7.4) | 0 | 1 (3.7) | |
| Cancer type | .944 | |||
| Colorectal | 15 (55.6) | 15 (57.7) | 13 (48.1) | |
| NSCLC | 9 (33.3) | 9 (34.6) | 10 (37.0) | |
| Pancreas | 3 (11.1) | 2 (7.7) | 4 (14.8) | |
| EGFR inhibitor | .965 | |||
| Cetuximab + FOLFIRI | 15 (55.6) | 15 (57.7) | 13 (48.1) | |
| Erlotiniba | 7 (25.9) | 6 (23.1) | 8 (14.8) | |
| Afatiniba | 2 (7.4) | 3 (11.5) | 2 (7.4) | |
| Erlotinibb + gemcitabine | 3 (11.1) | 2 (7.7) | 4 (14.8) |
aTreatment for non‐small cell lung cancer.
bErlotinib 100 mg was used for the treatment of pancreas cancer.
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group performance status; EGFR, epidermal growth factor receptor; FOLFIRI, folinic acid, fluorouracil, and irinotecan; NSCLC, non‐small cell lung cancer; P.O., per os.
Efficacy of EGF Ointment
Baseline ERSEs of patients were evaluated. Acneiform rash and pruritus were the main ERSEs in participants of this study. Grade 3 ERSEs were observed in 8 (10%) patients. There were no significant differences in baseline NCI‐CTCAE ratings of ERSEs among the three arms. According to predefined response criteria, RR was 44.4% (95% CI, 25.5%–64.7%) in arm 1, 61.5% (95% CI, 40.6%–79.8%) in arm 2, and 77.8% (95% CI, 57.7%–91.4%) in arm 3 (p = .042). Representative photographs of patients are shown in Figures 1–2, and 3. RRs were significantly different between arm 1 and the combination of arms 2 and 3 (44.4% vs. 69.8%; p = .028). There was a significant linear correlation between EGF concentration and response (p = .012; Table 2). However, response did not show significant association with clinical characteristics such as sex, age, ECOG PS, surgery, concomitant medication, cancer type, or type of EGFR inhibitor (Table 3). Among patients with colon cancer who were treated with cetuximab (n = 43), RR was significantly higher in arm 3 than that in arm 1 (76.9% vs. 40.0%; p = .049). In patients treated with EGFR TKIs (n = 37), RR was 50.0% in arm 1, 72.7% in arm 2, and 78.6% in arm 3 (p = .209; supplemental online Fig. 2). A total of 14 (17.5%) patients received concomitant oral medication for the management of ERSEs. There were no significant concomitant medication differences among study arms (p = .662; Table 1). There was no influence on response of EGF ointment by concomitant medication (p = .797; Table 3). In patients not receiving concomitant oral medication for the management of ERSEs (n = 66), RR in arm 2 (60.9%) or arm 3 (77.3%) was higher than that in arm 1 (42.9%, p = .070; test for trend, p = .066), although it was not significantly higher. Adverse events related to study treatment were observed in two patients in arm 2 (skin fissure and pyogenic granuloma) and one patient in arm 3 (periungual skin overgrowth; supplemental online Fig. 3).
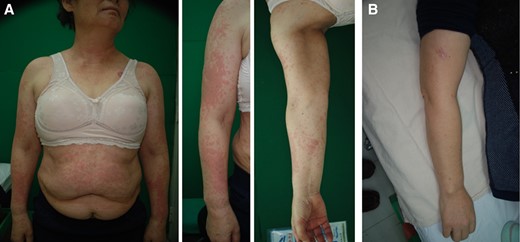
A 63‐year‐old female patient with non‐small cell lung cancer treated with erlotinib (150 mg). (A): Erythematous multiple macules and patches were observed on face, upper extremities, chest, and trunk. (B): Improved skin lesions following 4 weeks of treatment (arm 2; EGF 1 ppm).
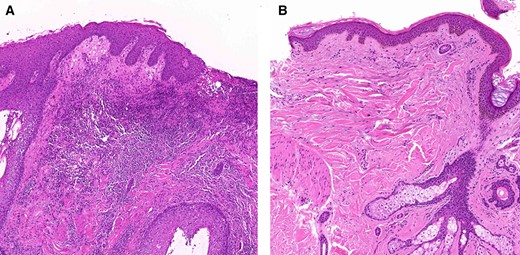
Histopathological findings of Figure 1. (A): Dense dermal infiltration of neutrophils and lymphohistiocytes and spongiosis of epidermis with focal pustular scab formation. (B): Markedly reduced inflammatory cell infiltration after 4 weeks of treatment (arm 2; EGF 1 ppm).
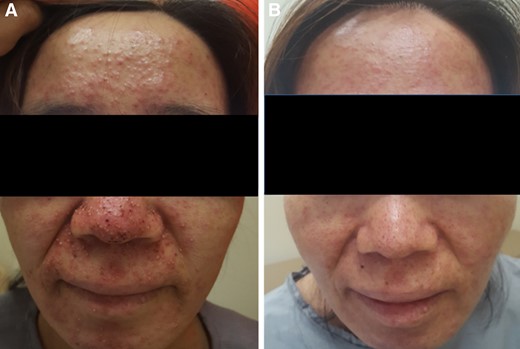
A 54‐year‐old female patient with colon cancer treated with cetuximab‐based regimen. (A): Acneiform papules and pustules, and crusts on the face. (B): Improved skin lesions following 4 weeks of treatment (arm 3; EGF 20 ppm).
Calculated by Pearson's chi‐square test.
Calculated by Cochran Armitage trend test.
Calculated by Pearson's chi‐square test.
Calculated by Cochran Armitage trend test.
| Characteristics . | Total, n . | Response, n (%) . | p valuea . |
|---|---|---|---|
| Sex | .130 | ||
| Male | 52 | 35 (67.3) | |
| Female | 28 | 14 (50.0) | |
| Age | .087 | ||
| ≤60 years | 42 | 22 (52.4) | |
| >60 years | 38 | 27 (71.1) | |
| ECOG PS | .965 | ||
| 0 | 23 | 14 (60.9) | |
| 1–2 | 57 | 35 (61.4) | |
| Surgery history | .642 | ||
| No | 49 | 31 (63.3 | |
| Yes | 31 | 18 (58.1) | |
| Concomitant medication | .797 | ||
| No | 66 | 40 (60.6) | |
| Yes | 14 | 9 (64.3) | |
| Cancer type | .432 | ||
| Colorectal | 43 | 24 (55.8) | |
| NSCLC | 28 | 18 (64.3) | |
| Pancreas | 9 | 7 (77.8) | |
| EGFR inhibitor | .282 | ||
| Cetuximab | 43 | 25 (58.1) | |
| Erlotinib or afatinib | 37 | 25 (67.6) |
| Characteristics . | Total, n . | Response, n (%) . | p valuea . |
|---|---|---|---|
| Sex | .130 | ||
| Male | 52 | 35 (67.3) | |
| Female | 28 | 14 (50.0) | |
| Age | .087 | ||
| ≤60 years | 42 | 22 (52.4) | |
| >60 years | 38 | 27 (71.1) | |
| ECOG PS | .965 | ||
| 0 | 23 | 14 (60.9) | |
| 1–2 | 57 | 35 (61.4) | |
| Surgery history | .642 | ||
| No | 49 | 31 (63.3 | |
| Yes | 31 | 18 (58.1) | |
| Concomitant medication | .797 | ||
| No | 66 | 40 (60.6) | |
| Yes | 14 | 9 (64.3) | |
| Cancer type | .432 | ||
| Colorectal | 43 | 24 (55.8) | |
| NSCLC | 28 | 18 (64.3) | |
| Pancreas | 9 | 7 (77.8) | |
| EGFR inhibitor | .282 | ||
| Cetuximab | 43 | 25 (58.1) | |
| Erlotinib or afatinib | 37 | 25 (67.6) |
aChi‐squared test.
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group performance status; EGFR, epidermal growth factor receptor; NSCLC, non‐small cell lung cancer.
| Characteristics . | Total, n . | Response, n (%) . | p valuea . |
|---|---|---|---|
| Sex | .130 | ||
| Male | 52 | 35 (67.3) | |
| Female | 28 | 14 (50.0) | |
| Age | .087 | ||
| ≤60 years | 42 | 22 (52.4) | |
| >60 years | 38 | 27 (71.1) | |
| ECOG PS | .965 | ||
| 0 | 23 | 14 (60.9) | |
| 1–2 | 57 | 35 (61.4) | |
| Surgery history | .642 | ||
| No | 49 | 31 (63.3 | |
| Yes | 31 | 18 (58.1) | |
| Concomitant medication | .797 | ||
| No | 66 | 40 (60.6) | |
| Yes | 14 | 9 (64.3) | |
| Cancer type | .432 | ||
| Colorectal | 43 | 24 (55.8) | |
| NSCLC | 28 | 18 (64.3) | |
| Pancreas | 9 | 7 (77.8) | |
| EGFR inhibitor | .282 | ||
| Cetuximab | 43 | 25 (58.1) | |
| Erlotinib or afatinib | 37 | 25 (67.6) |
| Characteristics . | Total, n . | Response, n (%) . | p valuea . |
|---|---|---|---|
| Sex | .130 | ||
| Male | 52 | 35 (67.3) | |
| Female | 28 | 14 (50.0) | |
| Age | .087 | ||
| ≤60 years | 42 | 22 (52.4) | |
| >60 years | 38 | 27 (71.1) | |
| ECOG PS | .965 | ||
| 0 | 23 | 14 (60.9) | |
| 1–2 | 57 | 35 (61.4) | |
| Surgery history | .642 | ||
| No | 49 | 31 (63.3 | |
| Yes | 31 | 18 (58.1) | |
| Concomitant medication | .797 | ||
| No | 66 | 40 (60.6) | |
| Yes | 14 | 9 (64.3) | |
| Cancer type | .432 | ||
| Colorectal | 43 | 24 (55.8) | |
| NSCLC | 28 | 18 (64.3) | |
| Pancreas | 9 | 7 (77.8) | |
| EGFR inhibitor | .282 | ||
| Cetuximab | 43 | 25 (58.1) | |
| Erlotinib or afatinib | 37 | 25 (67.6) |
aChi‐squared test.
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group performance status; EGFR, epidermal growth factor receptor; NSCLC, non‐small cell lung cancer.
QoL Outcomes
QoL analysis was available for 74 patients. Results are summarized in Table 4. Improved mean score was indicated by a negative mean difference. In the placebo arm, mean changes from baseline were not significant for any domain of Skindex‐16. On the other hand, there were significant improvements in symptoms, emotions, and composite scores for both arm 2 and arm 3. There were significant differences in mean changes of emotions (p = .008) and composite scores (p = .008) among the three arms. Compared with placebo treatment, EGF ointment (arms 2 and 3 combined) was associated with significantly better improvements in emotions (p = .005), functioning (p = .044), and composite scores (p = .005).
| Treatment arm . | Skindex‐16 domain and composite scoresa . | |||
|---|---|---|---|---|
| Symptoms . | Emotions . | Functioning . | Composite . | |
| Arm 1, placebo | ||||
| Mean ± SD | −6.9 ± 21.4 | −6.3 ± 12.4 | −2.2 ± 12.1 | −5.2 ± 8.6 |
| Median (range) | −8.3 (−50.0., 50.0) | −3.6 (−31.0, 11.9) | 0 (−30.0, 30.0) | −2.6 (−27.1, 7.3) |
| Arm 2, EGF 1 ppm | ||||
| Mean ± SD | −11.2 ± 16.3 | −14.3 ± 17.2 | −8.6 ± 16.9 | −11.7 ± 14.2 |
| Median (range) | −8.3 (−45.8, 20.8) | −10.7 (−50.0, 21.4) | 0 (−60.0, 6.7) | −7.3 (−40.6, 16.7) |
| Arm 3, EGF 20 ppm | ||||
| Mean ± SD | −14.8 ± 19.1 | −24.8 ± 25.1 | −12.9 ± 18.4 | −18.6 ± 17.7 |
| Median (range) | −10.4 (−83.3, 4.2) | −17.9 (−83.3, 7.1) | −6.7 (−70.0, 6.7) | −12.5 (−62.5, 5.2) |
| p value | ||||
| Arm 1 vs. Arm 2 vs. Arm 3b | .750 | .008 | .058 | .008 |
| Arm 1 vs. Arms 2 + 3c | .466 | .005 | .044 | .005 |
| Treatment arm . | Skindex‐16 domain and composite scoresa . | |||
|---|---|---|---|---|
| Symptoms . | Emotions . | Functioning . | Composite . | |
| Arm 1, placebo | ||||
| Mean ± SD | −6.9 ± 21.4 | −6.3 ± 12.4 | −2.2 ± 12.1 | −5.2 ± 8.6 |
| Median (range) | −8.3 (−50.0., 50.0) | −3.6 (−31.0, 11.9) | 0 (−30.0, 30.0) | −2.6 (−27.1, 7.3) |
| Arm 2, EGF 1 ppm | ||||
| Mean ± SD | −11.2 ± 16.3 | −14.3 ± 17.2 | −8.6 ± 16.9 | −11.7 ± 14.2 |
| Median (range) | −8.3 (−45.8, 20.8) | −10.7 (−50.0, 21.4) | 0 (−60.0, 6.7) | −7.3 (−40.6, 16.7) |
| Arm 3, EGF 20 ppm | ||||
| Mean ± SD | −14.8 ± 19.1 | −24.8 ± 25.1 | −12.9 ± 18.4 | −18.6 ± 17.7 |
| Median (range) | −10.4 (−83.3, 4.2) | −17.9 (−83.3, 7.1) | −6.7 (−70.0, 6.7) | −12.5 (−62.5, 5.2) |
| p value | ||||
| Arm 1 vs. Arm 2 vs. Arm 3b | .750 | .008 | .058 | .008 |
| Arm 1 vs. Arms 2 + 3c | .466 | .005 | .044 | .005 |
aNegative score indicates an improved Skindex‐16 score.
bKruskal‐Wallis test.
cMann‐Whitney U test.
Abbreviation: EGF, epidermal growth factor.
| Treatment arm . | Skindex‐16 domain and composite scoresa . | |||
|---|---|---|---|---|
| Symptoms . | Emotions . | Functioning . | Composite . | |
| Arm 1, placebo | ||||
| Mean ± SD | −6.9 ± 21.4 | −6.3 ± 12.4 | −2.2 ± 12.1 | −5.2 ± 8.6 |
| Median (range) | −8.3 (−50.0., 50.0) | −3.6 (−31.0, 11.9) | 0 (−30.0, 30.0) | −2.6 (−27.1, 7.3) |
| Arm 2, EGF 1 ppm | ||||
| Mean ± SD | −11.2 ± 16.3 | −14.3 ± 17.2 | −8.6 ± 16.9 | −11.7 ± 14.2 |
| Median (range) | −8.3 (−45.8, 20.8) | −10.7 (−50.0, 21.4) | 0 (−60.0, 6.7) | −7.3 (−40.6, 16.7) |
| Arm 3, EGF 20 ppm | ||||
| Mean ± SD | −14.8 ± 19.1 | −24.8 ± 25.1 | −12.9 ± 18.4 | −18.6 ± 17.7 |
| Median (range) | −10.4 (−83.3, 4.2) | −17.9 (−83.3, 7.1) | −6.7 (−70.0, 6.7) | −12.5 (−62.5, 5.2) |
| p value | ||||
| Arm 1 vs. Arm 2 vs. Arm 3b | .750 | .008 | .058 | .008 |
| Arm 1 vs. Arms 2 + 3c | .466 | .005 | .044 | .005 |
| Treatment arm . | Skindex‐16 domain and composite scoresa . | |||
|---|---|---|---|---|
| Symptoms . | Emotions . | Functioning . | Composite . | |
| Arm 1, placebo | ||||
| Mean ± SD | −6.9 ± 21.4 | −6.3 ± 12.4 | −2.2 ± 12.1 | −5.2 ± 8.6 |
| Median (range) | −8.3 (−50.0., 50.0) | −3.6 (−31.0, 11.9) | 0 (−30.0, 30.0) | −2.6 (−27.1, 7.3) |
| Arm 2, EGF 1 ppm | ||||
| Mean ± SD | −11.2 ± 16.3 | −14.3 ± 17.2 | −8.6 ± 16.9 | −11.7 ± 14.2 |
| Median (range) | −8.3 (−45.8, 20.8) | −10.7 (−50.0, 21.4) | 0 (−60.0, 6.7) | −7.3 (−40.6, 16.7) |
| Arm 3, EGF 20 ppm | ||||
| Mean ± SD | −14.8 ± 19.1 | −24.8 ± 25.1 | −12.9 ± 18.4 | −18.6 ± 17.7 |
| Median (range) | −10.4 (−83.3, 4.2) | −17.9 (−83.3, 7.1) | −6.7 (−70.0, 6.7) | −12.5 (−62.5, 5.2) |
| p value | ||||
| Arm 1 vs. Arm 2 vs. Arm 3b | .750 | .008 | .058 | .008 |
| Arm 1 vs. Arms 2 + 3c | .466 | .005 | .044 | .005 |
aNegative score indicates an improved Skindex‐16 score.
bKruskal‐Wallis test.
cMann‐Whitney U test.
Abbreviation: EGF, epidermal growth factor.
Discussion
Although the pathophysiologic mechanism of ERSEs has not yet been fully elucidated, much work has been done to understand these processes and identify mechanism‐based treatment strategies. The current study is the first placebo‐controlled randomized trial to evaluate the efficacy of reactive treatment with topical EGF for ERSEs. EGF ointment significantly improved ERSEs compared with placebo. It was effective for both patients treated with EGFR TKIs (erlotinib and afatinib) and those treated with cetuximab.
The use of ointment containing higher EGF concentration seemed to be more effective in improving skin lesions in the present study. Our results are in accordance with those of preclinical studies demonstrating a dose‐dependent effect of topical application of EGF on wound healing [25, 26]. Interestingly, RR in the placebo arm was 44.4%. A possible explanation for this effect was that the placebo agent contained petrolatum, a skin moisturizing agent. It is known that EGFR inhibitor‐mediated skin rash often improves spontaneously during therapy [27]. A dapsone lotion study for preventing cetuximab related ERSEs has reported that moisturizer (Vanicream Lite Lotion) in the control group could decrease 36.7% of lesions [28]. In clinical practice, physicians usually educate patients to use moisturizing creams to reduce ERSEs. Thus, we considered it appropriate to use moisturizing component as a placebo agent.
Topical corticosteroids are generally used for treating ERSEs, especially skin rash. Unexpectedly, the efficacy of topical corticosteroids has not been evaluated in randomized trials. Their use is based on clinical experience and expert opinion [11,29,30]. Other topical agents such as retinoids and vitamin K1 cream have potential roles for the management of ERSEs [31, 32]. However, their efficacies have not been fully investigated through prospective studies. A recent phase III trial has shown that prophylactic use of vitamin K1 cream in combination with doxycycline cannot decrease the incidence of grade ≥2 skin rash in patients initiating cetuximab therapy compared with doxycycline or vehicle [33].
Given that ERSEs occur in the majority of patients and that treatment of ERSEs sometimes starts late, prophylactic management could be more effective than reactive management. A number of randomized controlled studies have evaluated prophylactic strategies using tetracycline‐class antibiotics with or without topical agents [34-41]. A recent meta‐analysis has revealed that prophylactic treatment with antibiotics is significantly associated with a reduced risk of developing a skin rash of any grade (odds ratio [OR], 0.53; 95% CI, 0.39–0.72) and grade 2–4 rash (OR, 0.36; 95% CI, 0.22–0.60) [42]. Therefore, future studies are needed to determine whether EGF ointment is effective in prophylactic setting and whether it provides additional benefits when it is combined with antibiotics.
Despite improvements in survival because of the use of EGFR inhibitors, metastatic cancers remain incurable. In addition, EGFR inhibitors can cause ERSEs and decrease QoL. Therefore, QoL is an important outcome when assessing the efficacy of treatment strategies for ERSEs. Previous studies [14,43] using Skindex‐16 have noted that the negative effect of ERSEs is the highest in the emotional domain. In our study, the emotional domain was also affected the most (baseline median score: 31.0 vs. 25.0 in symptoms and 16.7 in function). Its improvement after EGF treatment was also the highest.
Nevertheless, there are some limitations in generalizing and applying this finding directly to real practice. This study was a pilot concept trial with a small number of patients. In addition, evaluation of response and patient's QoL was performed using our own criteria because there was no consensus about response evaluation methods. Permitted concomitant medication influence was also a limitation of this trial. Also, basic research is needed to clarify the mechanisms by which ERSE occurs and by which EGF is not absorbed into the circulation to impair activity of the EGFR inhibitors.
Conclusion
This randomized, prospective study showed that EGF ointment was effective in treating ERSEs. EGF ointment had a better effect at a higher dose. Topical EGF was also associated with significant improvement of QoL. Further research is needed to evaluate the efficacy of prophylactic use and the effect of combination therapy to obtain further evidence about the use of EGF ointment for treating ERSEs.
Acknowledgments
This study was supported by Daewoong Pharmaceutical Company and Dong‐A university research fund.
Author Contributions
Conception/design: Young Saing Kim, Jun Ho Ji, Sung Yong Oh, Jung Hun Kang
Provision of study material or patients: Young Saing Kim, Jun Ho Ji, Sung Yong Oh, Suee Lee, Seok Jae Huh, Ji Hyun Lee, Choon Hee Son, Gyeong Won Lee, Jeeyun Lee, Seung Tae Kim, Chan Kyu Kim, Joung Soon Jang, In Gyu Hwang, Hee Kyung Ahn, Lee Chun Park, So Yeon Oh, Seong‐Geun Kim, Sang‐Cheol Lee, Do‐Hyoung Lim, Soon Il Lee, Jung Hun Kang
Collection and/or assembly of data: Young Saing Kim, Jun Ho Ji, Sung Yong Oh, Jung Hun Kang
Data analysis and interpretation: Young Saing Kim, Jun Ho Ji, Sung Yong Oh,Ki‐Hoon Song, Mee Sook Roh, Jung Hun Kang
Manuscript writing: Young Saing Kim, Sung Yong Oh
Final approval of manuscript: Young Saing Kim, Jun Ho Ji, Sung Yong Oh, Suee Lee, Seok Jae Huh, Ji Hyun Lee, Ki‐Hoon Song, Choon Hee Son, Mee Sook Roh, Gyeong Won Lee, Jeeyun Lee, Seung Tae Kim, Chan Kyu Kim, Joung Soon Jang, In Gyu Hwang, Hee Kyung Ahn, Lee Chun Park, So Yeon Oh, Seong‐Geun Kim, Sang‐Cheol Lee, Do‐Hyoung Lim, Soon Il Lee, Jung Hun Kang
Disclosures
The authors indicated no financial relationships.
References
Author notes
Contributed equally.
Disclosures of potential conflicts of interest may be found at the end of this article.



