-
PDF
- Split View
-
Views
-
Cite
Cite
Shirish M Gadgeel, Otto Fajardo, Fabrice Barlesi, Jeong Eun Kim, Razelle Kurzrock, David M Thomas, Ritika Jagtiani, Johannes Noe, Sven Schwemmers, Christos Nikolaidis, Real-world characteristics and survival outcomes of patients with metastatic ALK fusion-positive solid tumors treated with standard-of-care therapies, The Oncologist, Volume 30, Issue 5, May 2025, oyaf005, https://doi.org/10.1093/oncolo/oyaf005
Close - Share Icon Share
Abstract
Anaplastic lymphoma kinase (ALK) fusions can be found in different solid tumors. This study aims to describe the clinical characteristics and investigate survival outcomes of patients with ALK fusion-positive solid tumors (excluding non-small cell lung cancer [NSCLC]) treated with standard-of-care therapies in a real-world setting.
Data for patients with metastatic solid tumors (excluding NSCLC) who had ≥1 Foundation Medicine comprehensive genomic profiling (CGP) test between January 1, 2011 and September 30, 2023, were obtained from a nationwide (US-based) de-identified multi-tumor clinico-genomic database. Patients with ALK wild-type (ALK-WT) tumors were matched with patients with ALK fusion-positive tumors (4:1 ratio) using pre-specified baseline characteristics. Two models were used to analyze survival outcomes: Model 1 used the CGP report date as the index date; Model 2 used the date of metastatic diagnosis as the index date (including adjustment for immortal time bias).
Overall, 22 and 88 patients were included in the ALK fusion-positive and ALK-WT cohorts, respectively. Co-alterations were rare in the ALK fusion-positive cohort. Median overall survival was consistently lower in patients with ALK fusion-positive tumors compared with patients with ALK-WT tumors, across all analyses (hazard ratios between 1.8 and 2.0).
Data from this study suggest that ALK fusions have a negative prognostic effect in metastatic solid tumors and highlight the need for further investigation of ALK inhibitors in the tumor-agnostic setting.
Non-small cell lung cancer (NSCLC) patients with anaplastic lymphoma kinase (ALK) fusions are routinely treated with ALK inhibitors. Preliminary data suggest that ALK inhibitors may also be efficacious across different tumor types. Nevertheless, ALK fusions are rare outside of NSCLC, which hinders the study of ALK inhibitors in randomized clinical trials. Our data show that ALK fusions have a negative prognostic effect in patients with metastatic solid tumors (other than NSCLC) treated with standard-of-care therapies in a real-world setting. These findings advocate for further research on the use of ALK inhibitors in patients with ALK fusion-positive solid tumors beyond NSCLC.
Introduction
Anaplastic lymphoma kinase (ALK) fusions are critical oncogenic drivers of tumor growth and proliferation across a broad number of solid tumors but are considered to be rare. ALK alterations (comprising activating mutations, amplifications, and fusions/rearrangements) occur in ~3.3% of cancers.1-3 In contrast, ALK fusions/rearrangements are detected in ~0.2%-0.8% of cancers overall, with varying frequencies in different malignancies (3–7% of non-small cell lung cancer [NSCLC] vs 0.2% of non‐NSCLC tumors; >50% of inflammatory myofibroblastic tumors [IMTs] and anaplastic large cell lymphomas [ALCLs]).1-4
Crizotinib was the first ALK inhibitor to be approved for the treatment of ALK fusion-positive NSCLC, followed by ceritinib, alectinib, brigatinib, and lorlatinib.3,5 These ALK inhibitors have revolutionized the treatment of patients with ALK fusion-positive NSCLC, and are approved as front-line treatment options for these patients.3,6-15 Recent data have also demonstrated a clinical benefit for ALK inhibition in patients with ALK fusion-positive NSCLC in the adjuvant setting, resulting in the approval of alectinib in this setting.10,16
Outside of NSCLC, patients with ALK fusion-positive solid tumors are poorly served by current standard-of-care therapies, which have limited efficacy and/or significant off-target toxicity. Therefore, there is an unmet need for precision therapies that provide durable clinical benefits by selectively targeting ALK fusions and anticipated off-target resistance mechanisms. Evidence that ALK fusions are strong oncogenic drivers across tumor types suggests that ALK inhibitors may have tumor-agnostic activity; however, due to the rarity of ALK fusions, investigating the efficacy of ALK inhibitors in indications beyond NSCLC is challenging.3 Thus, the characteristics and natural history of patients with tumors harboring ALK fusions other than NSCLC have not been widely studied.
It is important to understand how survival outcomes differ between patients with ALK fusion-positive tumors versus patients with ALK wild-type (ALK-WT) tumors, under standard-of-care treatments, in order to determine whether ALK fusions may be clinically prognostic. This study was designed to broaden current knowledge of clinical characteristics and overall survival (OS) of patients with ALK fusion-positive solid tumors (excluding NSCLC) in a real-world setting, using data from clinical practice, and to characterize the prognostic value of the biomarker in the tumor-agnostic context.
Patients and methods
Study design and data source
This study was a retrospective analysis of clinical characteristics and survival outcomes of patients with ALK fusion-positive or ALK-WT solid tumors, using information from the nationwide (US-based) de-identified Flatiron Health-Foundation Medicine multi-tumor clinico-genomic database (CGDB; version September 2023). Retrospective, longitudinal clinical (patient-level structured and unstructured) data were derived from electronic health records (EHRs) and curated via technology-enabled abstraction. These were then linked to genomic data derived from the Foundation Medicine comprehensive genomic profiling (CGP) tests in the Flatiron Health-Foundation Medicine CGDB, using de-identified, deterministic matching.17 During the study period, de-identified data were obtained from ~280 Flatiron Health cancer clinics in the US (~800 care sites). As this study did not directly enroll patients, no ethics committee approval was required. The de-identified data were subject to obligations to prevent re-identification and protect patient confidentiality.
Patient population
Patients were eligible for inclusion if they had ≥1 documented clinical visit in the Flatiron Health network between January 1, 2011 and September 30, 2023 and underwent CGP testing by Foundation Medicine before October 1, 2023 using one of Foundation Medicine’s solid tumor assays, FoundationOne or FoundationOneCDx.18 Only patients with a diagnosis of de novo Stage IV metastatic disease (Stage IV disease at diagnosis), who had not received an ALK inhibitor in any prior line of therapy, were considered for further analyses.
Patients with ALK fusion-positive NSCLC were excluded from this analysis since this is a well-studied population with several approved targeted therapies.6-15 Other exclusion criteria included treatment with an unlabeled study drug as part of a clinical trial, >1 CGP test, a visit gap of >90 days after initial diagnosis, multiple cancer diagnoses, no initial diagnosis date, a CGP report date before initial diagnosis, initial diagnosis within 3 months before data cut-off, and death before 2012 (year of Foundation Medicine CGP start).
Determination of ALK status
Patients were considered ALK fusion-positive if their tumors had a 3’ ALK fusion with a protein-coding 5’ gene fusion partner, predicted to be in frame with an intact kinase domain. These fusions had a predicted known/likely functional status as defined by Foundation Medicine. Patients were deemed ALK-WT when no qualifying ALK alterations were observed by CGP.
Covariate matching and statistical analyses
Patients with ALK-WT tumors were matched with patients with ALK fusion-positive tumors (4:1 ratio to minimize bias)19 using pre-selected baseline characteristics. Matching of patients within histological subtypes was conducted using the Mahalanobis distance method.20 Covariates used for matching included age, gender, race, tumor type, practice type (academic vs community), Eastern Cooperative Oncology Group performance status (ECOG PS) from 30 days before to 7 days after the index date, year of CGP, time from initial diagnosis to CGP report date, and number of lines of treatment prior to CGP report date. An absolute mean difference of <0.1 was used to indicate negligible differences between groups.21
Two models were used for the analysis: Model 1 used the CGP report date as the index date, whereas Model 2 used the date of metastatic diagnosis as the index date (including adjustment for immortal time bias using left truncation).22,23 Descriptive analyses were used to assess patient characteristics; assessments of frequencies were used for categorical variables, and means and standard deviations (SDs) for continuous variables. Overall survival was analyzed using the Kaplan-Meier method and Cox regression; medians and 95% confidence intervals (CIs) were calculated, along with hazard ratios (HRs) and associated 95% CIs. Sensitivity analyses were performed to assess the potential impact of tumor protein p53 (TP53) gene alterations (common in Stage IV cancers) on prognosis.
Objectives
The primary objective of this study was to compare real-world OS in patients with ALK-WT versus ALK fusion-positive tumors, excluding NSCLC. OS was defined as the length of time in months from the index date until death from any cause or the censoring date (ie, last visit or encounter date). Secondary objectives included: description of patient characteristics and treatment patterns; characterization of ALK fusion partners and genetic alterations such as tumor mutational burden (TMB), microsatellite instability (MSI), and functional co-occurring alterations in actionable oncogenes (RET, BRAF, ERBB2, EGFR, NTRK, ROS1, MET, and KRAS), as well as other common alterations that manifest in the metastatic setting, such as TP53 mutations.24
Results
Patient characteristics
Of the 525 patients with ALK fusion-positive solid tumors selected from the CGDB, 22 met the eligibility criteria and were included in the analysis (Figure 1). Of the 503 patients who did not meet the eligibility criteria, 152 were excluded for not having a de novo Stage IV diagnosis, and a further 328 were excluded because they had NSCLC. In the ALK fusion-positive cohort, the mean age was 62.6 years compared to 62.2 years in the matched ALK-WT cohort. Three patients (13.6%) had received ≥2 prior lines of therapy (Table 1).
| ALK fusion-positive (N = 22) . | ALK-WT (N = 8869) . | ||
|---|---|---|---|
| Matched (N = 88) . | Non-matched (N = 8,781) . | ||
| Sex, n (%) | |||
| Female | 13 (59.1) | 45 (51.1) | 4163 (47.4) |
| Male | 9 (40.9) | 43 (48.9) | 4618 (52.6) |
| Mean age, years (SD)a | 62.6 (15.7) | 62.2 (11.9) | 62.7 (12.2) |
| Race, n (%) | |||
| Asian | 0 | 0 | 203 (2.3) |
| Black/African American | 2 (9.1) | 4 (4.5) | 816 (9.3) |
| Hispanic/Latino | 0 | 0 | 16 (0.2) |
| White | 18 (81.8) | 80 (90.9) | 5623 (64.0) |
| Other/missing | 2 (9.1) | 4 (4.5) | 2123 (24.2) |
| ECOG PS, n (%) | |||
| 0 | 5 (22.7) | 26 (29.5) | 2146 (24.4) |
| 1 | 9 (40.9) | 28 (31.8) | 2816 (32.1) |
| 2 | 0 | 0 | 822 (9.4) |
| ≥3 | 1 (4.5) | 4 (4.5) | 237 (2.7) |
| Missing | 7 (31.8) | 30 (34.1) | 2760 (31.4) |
| Number of prior lines of treatment, n (%) | |||
| 0 | 3 (13.6) | 12 (13.6) | 1247 (14.2) |
| 1 | 7 (31.8) | 32 (36.4) | 3363 (38.3) |
| 2 | 1 (4.5) | 4 (4.5) | 1120 (12.8) |
| ≥3 | 2 (9.1) | 8 (9.1) | 1208 (13.7) |
| Missing | 9 (40.9) | 32 (36.4) | 1843 (21.0) |
| Year of CGP report, n (%) | |||
| <2019 | 4 (18.2) | 16 (18.2) | 3213 (36.6) |
| 2019 | 2 (9.1) | 8 (9.1) | 1441 (16.4) |
| 2020 | 6 (27.3) | 25 (28.4) | 1381 (15.7) |
| 2021 | 6 (27.3) | 25 (28.4) | 1303 (14.8) |
| 2022 | 3 (13.6) | 10 (11.4) | 1190 (13.6) |
| 2023 | 1 (4.5) | 4 (4.5) | 253 (2.9) |
| Mean follow-up time from CGP report, months (IQR) | 5.0 (16.8) | 10.6 (16.9) | 9.0 (16.1) |
| Mean time from initial diagnosisb to CGP report date, months (IQR) | 2.1 (5.6) | 2.7 (9.8) | 3.9(17.8) |
| ALK fusion-positive (N = 22) . | ALK-WT (N = 8869) . | ||
|---|---|---|---|
| Matched (N = 88) . | Non-matched (N = 8,781) . | ||
| Sex, n (%) | |||
| Female | 13 (59.1) | 45 (51.1) | 4163 (47.4) |
| Male | 9 (40.9) | 43 (48.9) | 4618 (52.6) |
| Mean age, years (SD)a | 62.6 (15.7) | 62.2 (11.9) | 62.7 (12.2) |
| Race, n (%) | |||
| Asian | 0 | 0 | 203 (2.3) |
| Black/African American | 2 (9.1) | 4 (4.5) | 816 (9.3) |
| Hispanic/Latino | 0 | 0 | 16 (0.2) |
| White | 18 (81.8) | 80 (90.9) | 5623 (64.0) |
| Other/missing | 2 (9.1) | 4 (4.5) | 2123 (24.2) |
| ECOG PS, n (%) | |||
| 0 | 5 (22.7) | 26 (29.5) | 2146 (24.4) |
| 1 | 9 (40.9) | 28 (31.8) | 2816 (32.1) |
| 2 | 0 | 0 | 822 (9.4) |
| ≥3 | 1 (4.5) | 4 (4.5) | 237 (2.7) |
| Missing | 7 (31.8) | 30 (34.1) | 2760 (31.4) |
| Number of prior lines of treatment, n (%) | |||
| 0 | 3 (13.6) | 12 (13.6) | 1247 (14.2) |
| 1 | 7 (31.8) | 32 (36.4) | 3363 (38.3) |
| 2 | 1 (4.5) | 4 (4.5) | 1120 (12.8) |
| ≥3 | 2 (9.1) | 8 (9.1) | 1208 (13.7) |
| Missing | 9 (40.9) | 32 (36.4) | 1843 (21.0) |
| Year of CGP report, n (%) | |||
| <2019 | 4 (18.2) | 16 (18.2) | 3213 (36.6) |
| 2019 | 2 (9.1) | 8 (9.1) | 1441 (16.4) |
| 2020 | 6 (27.3) | 25 (28.4) | 1381 (15.7) |
| 2021 | 6 (27.3) | 25 (28.4) | 1303 (14.8) |
| 2022 | 3 (13.6) | 10 (11.4) | 1190 (13.6) |
| 2023 | 1 (4.5) | 4 (4.5) | 253 (2.9) |
| Mean follow-up time from CGP report, months (IQR) | 5.0 (16.8) | 10.6 (16.9) | 9.0 (16.1) |
| Mean time from initial diagnosisb to CGP report date, months (IQR) | 2.1 (5.6) | 2.7 (9.8) | 3.9(17.8) |
aPatients with a birth year of 1938 or earlier may have an adjusted birth year in Flatiron datasets due to patient de-identification requirements.
bOf de novo metastatic disease.
ALK, anaplastic lymphoma kinase; CGP, comprehensive genomic profiling; ECOG PS, Eastern Cooperative Oncology Group performance status; IQR, interquartile range; SD, standard deviation; WT, wild type.
| ALK fusion-positive (N = 22) . | ALK-WT (N = 8869) . | ||
|---|---|---|---|
| Matched (N = 88) . | Non-matched (N = 8,781) . | ||
| Sex, n (%) | |||
| Female | 13 (59.1) | 45 (51.1) | 4163 (47.4) |
| Male | 9 (40.9) | 43 (48.9) | 4618 (52.6) |
| Mean age, years (SD)a | 62.6 (15.7) | 62.2 (11.9) | 62.7 (12.2) |
| Race, n (%) | |||
| Asian | 0 | 0 | 203 (2.3) |
| Black/African American | 2 (9.1) | 4 (4.5) | 816 (9.3) |
| Hispanic/Latino | 0 | 0 | 16 (0.2) |
| White | 18 (81.8) | 80 (90.9) | 5623 (64.0) |
| Other/missing | 2 (9.1) | 4 (4.5) | 2123 (24.2) |
| ECOG PS, n (%) | |||
| 0 | 5 (22.7) | 26 (29.5) | 2146 (24.4) |
| 1 | 9 (40.9) | 28 (31.8) | 2816 (32.1) |
| 2 | 0 | 0 | 822 (9.4) |
| ≥3 | 1 (4.5) | 4 (4.5) | 237 (2.7) |
| Missing | 7 (31.8) | 30 (34.1) | 2760 (31.4) |
| Number of prior lines of treatment, n (%) | |||
| 0 | 3 (13.6) | 12 (13.6) | 1247 (14.2) |
| 1 | 7 (31.8) | 32 (36.4) | 3363 (38.3) |
| 2 | 1 (4.5) | 4 (4.5) | 1120 (12.8) |
| ≥3 | 2 (9.1) | 8 (9.1) | 1208 (13.7) |
| Missing | 9 (40.9) | 32 (36.4) | 1843 (21.0) |
| Year of CGP report, n (%) | |||
| <2019 | 4 (18.2) | 16 (18.2) | 3213 (36.6) |
| 2019 | 2 (9.1) | 8 (9.1) | 1441 (16.4) |
| 2020 | 6 (27.3) | 25 (28.4) | 1381 (15.7) |
| 2021 | 6 (27.3) | 25 (28.4) | 1303 (14.8) |
| 2022 | 3 (13.6) | 10 (11.4) | 1190 (13.6) |
| 2023 | 1 (4.5) | 4 (4.5) | 253 (2.9) |
| Mean follow-up time from CGP report, months (IQR) | 5.0 (16.8) | 10.6 (16.9) | 9.0 (16.1) |
| Mean time from initial diagnosisb to CGP report date, months (IQR) | 2.1 (5.6) | 2.7 (9.8) | 3.9(17.8) |
| ALK fusion-positive (N = 22) . | ALK-WT (N = 8869) . | ||
|---|---|---|---|
| Matched (N = 88) . | Non-matched (N = 8,781) . | ||
| Sex, n (%) | |||
| Female | 13 (59.1) | 45 (51.1) | 4163 (47.4) |
| Male | 9 (40.9) | 43 (48.9) | 4618 (52.6) |
| Mean age, years (SD)a | 62.6 (15.7) | 62.2 (11.9) | 62.7 (12.2) |
| Race, n (%) | |||
| Asian | 0 | 0 | 203 (2.3) |
| Black/African American | 2 (9.1) | 4 (4.5) | 816 (9.3) |
| Hispanic/Latino | 0 | 0 | 16 (0.2) |
| White | 18 (81.8) | 80 (90.9) | 5623 (64.0) |
| Other/missing | 2 (9.1) | 4 (4.5) | 2123 (24.2) |
| ECOG PS, n (%) | |||
| 0 | 5 (22.7) | 26 (29.5) | 2146 (24.4) |
| 1 | 9 (40.9) | 28 (31.8) | 2816 (32.1) |
| 2 | 0 | 0 | 822 (9.4) |
| ≥3 | 1 (4.5) | 4 (4.5) | 237 (2.7) |
| Missing | 7 (31.8) | 30 (34.1) | 2760 (31.4) |
| Number of prior lines of treatment, n (%) | |||
| 0 | 3 (13.6) | 12 (13.6) | 1247 (14.2) |
| 1 | 7 (31.8) | 32 (36.4) | 3363 (38.3) |
| 2 | 1 (4.5) | 4 (4.5) | 1120 (12.8) |
| ≥3 | 2 (9.1) | 8 (9.1) | 1208 (13.7) |
| Missing | 9 (40.9) | 32 (36.4) | 1843 (21.0) |
| Year of CGP report, n (%) | |||
| <2019 | 4 (18.2) | 16 (18.2) | 3213 (36.6) |
| 2019 | 2 (9.1) | 8 (9.1) | 1441 (16.4) |
| 2020 | 6 (27.3) | 25 (28.4) | 1381 (15.7) |
| 2021 | 6 (27.3) | 25 (28.4) | 1303 (14.8) |
| 2022 | 3 (13.6) | 10 (11.4) | 1190 (13.6) |
| 2023 | 1 (4.5) | 4 (4.5) | 253 (2.9) |
| Mean follow-up time from CGP report, months (IQR) | 5.0 (16.8) | 10.6 (16.9) | 9.0 (16.1) |
| Mean time from initial diagnosisb to CGP report date, months (IQR) | 2.1 (5.6) | 2.7 (9.8) | 3.9(17.8) |
aPatients with a birth year of 1938 or earlier may have an adjusted birth year in Flatiron datasets due to patient de-identification requirements.
bOf de novo metastatic disease.
ALK, anaplastic lymphoma kinase; CGP, comprehensive genomic profiling; ECOG PS, Eastern Cooperative Oncology Group performance status; IQR, interquartile range; SD, standard deviation; WT, wild type.
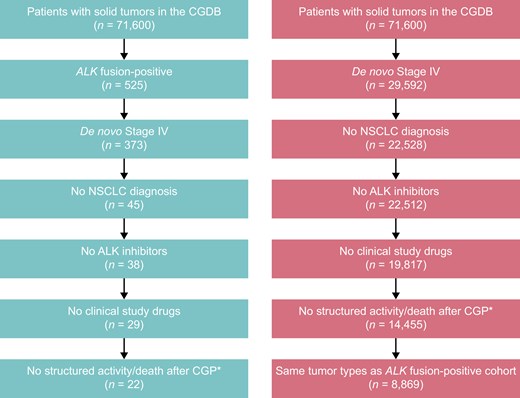
ALK fusion-positive and ALK-WT cohort attrition. *Also excluded patients with no initial metastatic disease diagnosis date or a diagnosis within 3 months before the data cut-off, patients who died before 2012, patients with multiple cancer diagnoses, patients with more than 1 CGP report, and patients with a CGP report date before the initial diagnosis. ALK, anaplastic lymphoma kinase; CGDB, clinico-genomic database; CGP, comprehensive genomic profiling; NSCLC, non-small cell lung cancer; WT, wild type.
The ALK fusion-positive cohort included 8 distinct tumor/histological subtypes, the most common of which were colorectal cancer (n = 9; 40.9%), sarcoma (n = 3; 13.6%), and prostate cancer (n = 3; 13.6%) (Figure 2). A total of 17 different ALK fusion partners were detected, of which the most common were EML4 (n = 3; 13.6%), STRN (n = 3; 13.6%), and SPTBN1 (n = 2; 9.1%); ARHGAP15, ACTG2, CLIP4, CLTC, EPAS1, ERC1, FCHSD2, FN1, KIF5C, SLC8A1, SLMAP, SPINK5, TNS1, and ZNF143 were detected in 1 patient each.

Tumor types in the ALK fusion-positive cohort (N = 22). Patients with ALK fusion-positive NSCLC were excluded. ALK, anaplastic lymphoma kinase; RCC, renal cell carcinoma.
A total of 8869 patients with ALK-WT solid tumors met the eligibility criteria. After covariate matching 4:1 with the ALK fusion-positive cohort (by patient characteristics, tumor type, ECOG PS, number of prior therapies, practice type, and timing of CGP), the matched ALK-WT cohort included 88 patients. The mean time from initial diagnosis to CGP report date was comparable between the ALK-positive and matched ALK-WT cohorts (2.1 [interquartile range (IQR) 5.6] and 2.7 [IQR 9.8] months, respectively; Table 1).
Genomic alterations
Co-alterations were very rare in both the ALK fusion-positive and matched ALK-WT cohorts (Table 2), except for KRAS alterations in the matched ALK-WT cohort (n = 22; 25.0%). TP53 alterations were equally common in both cohorts (50.0% in the ALK fusion-positive cohort and 44.3% in the matched ALK-WT cohort). TMB and MSI were also assessed, with few patients found to be TMB-high or MSI-high, and with a similar frequency in the 2 matched cohorts. However, ~30% of patients in the ALK fusion-positive cohort had unknown/missing TMB or MSI status (vs only 1%-2% of patients in the ALK-WT cohort).
| ALK fusion-positive (N = 22) . | ALK-WT (N = 8869) . | ||
|---|---|---|---|
| Matched (N = 88) . | Non-matched (N = 8781) . | ||
| TMB status, n (%) | |||
| High (≥20 mut/Mb) | 1 (4.5) | 3 (3.4) | 198 (2.3) |
| Medium (<20, ≥5.7 mut/Mb) | 2 (9.1) | 16 (18.2) | 1286 (14.6) |
| Low (<5.7 mut/Mb) | 13 (59.1) | 68 (77.3) | 7284 (83.0) |
| Missing | 6 (27.3) | 1 (1.1) | 13 (0.1) |
| MSI-high, n (%) | |||
| Yes | 1 (4.5) | 4 (4.5) | 117 (1.3) |
| No | 14 (63.6) | 82 (93.2) | 7700 (87.7) |
| Unknown/missing | 7 (31.8) | 2 (2.2) | 964 (11.0) |
| Oncogenic alterations, n (%) | |||
| NTRK rearrangement | 0 | 0 | 16 (0.2) |
| ROS1 rearrangement | 0 | 0 | 12 (0.1) |
| RET rearrangement | 0 | 0 | 11 (0.1) |
| BRAF alteration | 0 | 5 (5.7) | 424 (4.8) |
| EGFR alteration | 1 (4.5) | 1 (1.1) | 45 (0.5) |
| KRAS alteration | 1 (4.5) | 22 (25.0) | 3078 (35.1) |
| ERBB2 amplification | 1 (4.5) | 1 (1.1) | 353 (4.0) |
| MET alteration | 0 | 0 | 17 (0.2) |
| TP53 alteration | 11 (50.0) | 39 (44.3) | 5359 (61.0) |
| ALK fusion-positive (N = 22) . | ALK-WT (N = 8869) . | ||
|---|---|---|---|
| Matched (N = 88) . | Non-matched (N = 8781) . | ||
| TMB status, n (%) | |||
| High (≥20 mut/Mb) | 1 (4.5) | 3 (3.4) | 198 (2.3) |
| Medium (<20, ≥5.7 mut/Mb) | 2 (9.1) | 16 (18.2) | 1286 (14.6) |
| Low (<5.7 mut/Mb) | 13 (59.1) | 68 (77.3) | 7284 (83.0) |
| Missing | 6 (27.3) | 1 (1.1) | 13 (0.1) |
| MSI-high, n (%) | |||
| Yes | 1 (4.5) | 4 (4.5) | 117 (1.3) |
| No | 14 (63.6) | 82 (93.2) | 7700 (87.7) |
| Unknown/missing | 7 (31.8) | 2 (2.2) | 964 (11.0) |
| Oncogenic alterations, n (%) | |||
| NTRK rearrangement | 0 | 0 | 16 (0.2) |
| ROS1 rearrangement | 0 | 0 | 12 (0.1) |
| RET rearrangement | 0 | 0 | 11 (0.1) |
| BRAF alteration | 0 | 5 (5.7) | 424 (4.8) |
| EGFR alteration | 1 (4.5) | 1 (1.1) | 45 (0.5) |
| KRAS alteration | 1 (4.5) | 22 (25.0) | 3078 (35.1) |
| ERBB2 amplification | 1 (4.5) | 1 (1.1) | 353 (4.0) |
| MET alteration | 0 | 0 | 17 (0.2) |
| TP53 alteration | 11 (50.0) | 39 (44.3) | 5359 (61.0) |
aOnly variants of “known” or “likely” functional status were included.
ALK, anaplastic lymphoma kinase; BRAF; v-raf murine sarcoma viral oncogene homolog B1; ERBB2, Erb-B2 receptor tyrosine kinase 2; EGFR, epidermal growth factor receptor; KRAS, kirsten rat sarcoma viral oncogene homologue; MET; mesenchymal epithelial transition factor; MSI, microsatellite instability; NTRK, neurotrophic tyrosine receptor kinase; RET, rearranged during transfection; ROS1, ROS proto-oncogene 1; TMB, tumor mutational burden; TP53, tumor protein p53; WT, wild type.
| ALK fusion-positive (N = 22) . | ALK-WT (N = 8869) . | ||
|---|---|---|---|
| Matched (N = 88) . | Non-matched (N = 8781) . | ||
| TMB status, n (%) | |||
| High (≥20 mut/Mb) | 1 (4.5) | 3 (3.4) | 198 (2.3) |
| Medium (<20, ≥5.7 mut/Mb) | 2 (9.1) | 16 (18.2) | 1286 (14.6) |
| Low (<5.7 mut/Mb) | 13 (59.1) | 68 (77.3) | 7284 (83.0) |
| Missing | 6 (27.3) | 1 (1.1) | 13 (0.1) |
| MSI-high, n (%) | |||
| Yes | 1 (4.5) | 4 (4.5) | 117 (1.3) |
| No | 14 (63.6) | 82 (93.2) | 7700 (87.7) |
| Unknown/missing | 7 (31.8) | 2 (2.2) | 964 (11.0) |
| Oncogenic alterations, n (%) | |||
| NTRK rearrangement | 0 | 0 | 16 (0.2) |
| ROS1 rearrangement | 0 | 0 | 12 (0.1) |
| RET rearrangement | 0 | 0 | 11 (0.1) |
| BRAF alteration | 0 | 5 (5.7) | 424 (4.8) |
| EGFR alteration | 1 (4.5) | 1 (1.1) | 45 (0.5) |
| KRAS alteration | 1 (4.5) | 22 (25.0) | 3078 (35.1) |
| ERBB2 amplification | 1 (4.5) | 1 (1.1) | 353 (4.0) |
| MET alteration | 0 | 0 | 17 (0.2) |
| TP53 alteration | 11 (50.0) | 39 (44.3) | 5359 (61.0) |
| ALK fusion-positive (N = 22) . | ALK-WT (N = 8869) . | ||
|---|---|---|---|
| Matched (N = 88) . | Non-matched (N = 8781) . | ||
| TMB status, n (%) | |||
| High (≥20 mut/Mb) | 1 (4.5) | 3 (3.4) | 198 (2.3) |
| Medium (<20, ≥5.7 mut/Mb) | 2 (9.1) | 16 (18.2) | 1286 (14.6) |
| Low (<5.7 mut/Mb) | 13 (59.1) | 68 (77.3) | 7284 (83.0) |
| Missing | 6 (27.3) | 1 (1.1) | 13 (0.1) |
| MSI-high, n (%) | |||
| Yes | 1 (4.5) | 4 (4.5) | 117 (1.3) |
| No | 14 (63.6) | 82 (93.2) | 7700 (87.7) |
| Unknown/missing | 7 (31.8) | 2 (2.2) | 964 (11.0) |
| Oncogenic alterations, n (%) | |||
| NTRK rearrangement | 0 | 0 | 16 (0.2) |
| ROS1 rearrangement | 0 | 0 | 12 (0.1) |
| RET rearrangement | 0 | 0 | 11 (0.1) |
| BRAF alteration | 0 | 5 (5.7) | 424 (4.8) |
| EGFR alteration | 1 (4.5) | 1 (1.1) | 45 (0.5) |
| KRAS alteration | 1 (4.5) | 22 (25.0) | 3078 (35.1) |
| ERBB2 amplification | 1 (4.5) | 1 (1.1) | 353 (4.0) |
| MET alteration | 0 | 0 | 17 (0.2) |
| TP53 alteration | 11 (50.0) | 39 (44.3) | 5359 (61.0) |
aOnly variants of “known” or “likely” functional status were included.
ALK, anaplastic lymphoma kinase; BRAF; v-raf murine sarcoma viral oncogene homolog B1; ERBB2, Erb-B2 receptor tyrosine kinase 2; EGFR, epidermal growth factor receptor; KRAS, kirsten rat sarcoma viral oncogene homologue; MET; mesenchymal epithelial transition factor; MSI, microsatellite instability; NTRK, neurotrophic tyrosine receptor kinase; RET, rearranged during transfection; ROS1, ROS proto-oncogene 1; TMB, tumor mutational burden; TP53, tumor protein p53; WT, wild type.
Overall survival
Overall survival was analyzed using 2 models. Model 1 used the CGP report date as the index whereas Model 2 used the date of initial diagnosis as the index, and included adjustment for immortal time bias. Results were consistent across the analyses (Table 3; Figure 3). When the CGP report date was used as the index (Model 1), median OS was 6.1 months (95% CI, 2.0-19.4) in the ALK fusion-positive cohort and 15.6 months (95% CI, 11.3-21.5) in the matched ALK-WT cohort; the HR was 1.8 (95% CI, 1.0-3.1). When the date of initial diagnosis was used as the index (Model 2), median OS was 13.4 months (95% CI, 6.1-25.4) in the ALK fusion-positive cohort and 25.2 months (95% CI, 21.3-35.2) in the matched ALK-WT cohort, with an HR of 2.0 (95% CI, 1.1-3.4). Lastly, when the date of initial diagnosis was used as the index (Model 2) and after adjusting for immortal time bias, median OS was 9.7 months (95% CI, 1.2-18.4) in the ALK fusion-positive cohort and 20.0 months (95% CI, 13.4-25.2) in the matched ALK-WT cohort, and the HR was 1.8 (95% CI, 1.0-3.2).
| No. of deaths, n (%) . | Median OS, months (95% CI) . | HR, 95% CI . | ||
|---|---|---|---|---|
| Model 1a | ALK fusion-positive cohort (N = 22) | 17 (77.3%) | 6.1 (2.0-19.4) | 1.8 (1.0-3.1) |
| Matched ALK-WT cohort (N = 88) | 48 (54.5%) | 15.6 (11.3-21.5) | ||
| Model 2b | ALK fusion-positive cohort (N = 22) | 17 (77.3%) | 13.4 (6.1-25.4) | 2.0 (1.1-3.4) |
| Matched ALK-WT cohort (N = 88) | 49 (55.7%) | 25.2 (21.3-35.2) | ||
| Model 2b (corrected for immortal time bias) | ALK fusion-positive cohort (N = 22) | 17 (77.3%) | 9.7 (1.2-18.4) | 1.8 (1.0-3.2) |
| Matched ALK-WT cohort (N = 88) | 49 (55.7%) | 20.0 (13.4-25.2) | ||
| No. of deaths, n (%) . | Median OS, months (95% CI) . | HR, 95% CI . | ||
|---|---|---|---|---|
| Model 1a | ALK fusion-positive cohort (N = 22) | 17 (77.3%) | 6.1 (2.0-19.4) | 1.8 (1.0-3.1) |
| Matched ALK-WT cohort (N = 88) | 48 (54.5%) | 15.6 (11.3-21.5) | ||
| Model 2b | ALK fusion-positive cohort (N = 22) | 17 (77.3%) | 13.4 (6.1-25.4) | 2.0 (1.1-3.4) |
| Matched ALK-WT cohort (N = 88) | 49 (55.7%) | 25.2 (21.3-35.2) | ||
| Model 2b (corrected for immortal time bias) | ALK fusion-positive cohort (N = 22) | 17 (77.3%) | 9.7 (1.2-18.4) | 1.8 (1.0-3.2) |
| Matched ALK-WT cohort (N = 88) | 49 (55.7%) | 20.0 (13.4-25.2) | ||
aModel 1: using CGP report date as the index;
bModel 2: using date of initial diagnosis as the index.
ALK, anaplastic lymphoma kinase; CI, confidence interval; CGP, comprehensive genomic profiling; HR, hazard ratio; OS, overall survival; WT, wild type.
| No. of deaths, n (%) . | Median OS, months (95% CI) . | HR, 95% CI . | ||
|---|---|---|---|---|
| Model 1a | ALK fusion-positive cohort (N = 22) | 17 (77.3%) | 6.1 (2.0-19.4) | 1.8 (1.0-3.1) |
| Matched ALK-WT cohort (N = 88) | 48 (54.5%) | 15.6 (11.3-21.5) | ||
| Model 2b | ALK fusion-positive cohort (N = 22) | 17 (77.3%) | 13.4 (6.1-25.4) | 2.0 (1.1-3.4) |
| Matched ALK-WT cohort (N = 88) | 49 (55.7%) | 25.2 (21.3-35.2) | ||
| Model 2b (corrected for immortal time bias) | ALK fusion-positive cohort (N = 22) | 17 (77.3%) | 9.7 (1.2-18.4) | 1.8 (1.0-3.2) |
| Matched ALK-WT cohort (N = 88) | 49 (55.7%) | 20.0 (13.4-25.2) | ||
| No. of deaths, n (%) . | Median OS, months (95% CI) . | HR, 95% CI . | ||
|---|---|---|---|---|
| Model 1a | ALK fusion-positive cohort (N = 22) | 17 (77.3%) | 6.1 (2.0-19.4) | 1.8 (1.0-3.1) |
| Matched ALK-WT cohort (N = 88) | 48 (54.5%) | 15.6 (11.3-21.5) | ||
| Model 2b | ALK fusion-positive cohort (N = 22) | 17 (77.3%) | 13.4 (6.1-25.4) | 2.0 (1.1-3.4) |
| Matched ALK-WT cohort (N = 88) | 49 (55.7%) | 25.2 (21.3-35.2) | ||
| Model 2b (corrected for immortal time bias) | ALK fusion-positive cohort (N = 22) | 17 (77.3%) | 9.7 (1.2-18.4) | 1.8 (1.0-3.2) |
| Matched ALK-WT cohort (N = 88) | 49 (55.7%) | 20.0 (13.4-25.2) | ||
aModel 1: using CGP report date as the index;
bModel 2: using date of initial diagnosis as the index.
ALK, anaplastic lymphoma kinase; CI, confidence interval; CGP, comprehensive genomic profiling; HR, hazard ratio; OS, overall survival; WT, wild type.
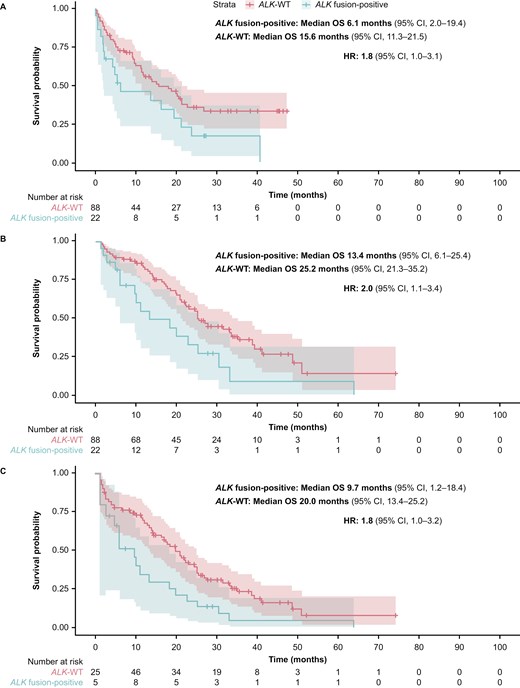
Kaplan-Meier plot estimates of OS comparing the ALK fusion-positive cohort (N = 22) with the matched ALK-WT cohort (N = 88) using (A) the CGP report date as the index (Model 1); (B) the initial diagnosis date as the index (Model 2); and (C) the initial diagnosis date as the index (Model 2*), and corrected for immortal time bias. *The number of patients is the same in all panels (ie, N = 22 for the ALK fusion-positive cohort and N = 88 for the ALK-WT cohort). Panel C shows the analysis using Model 2 and corrected for immortal time bias, which used left truncation to estimate survival. Only 5 patients with ALK fusion-positive tumors and 25 patients with ALK-WT tumors had both a metastatic diagnosis and a CGP date at time zero. For the remaining patients, who satisfied cohort entry criteria at later times, this immortal time was taken into account when calculating OS. ALK, anaplastic lymphoma kinase; CGP, comprehensive genomic profiling; CI, confidence interval; HR, hazard ratio; OS, overall survival; WT, wild type.
Sensitivity analyses
Sensitivity analyses were performed to determine whether the presence of TP53 co-alterations had an impact on the prognosis for these patients. After adjusting for the presence of TP53 alterations, the median OS with Model 1 was 6.1 months (95% CI, 2.0-19.4) in the ALK fusion-positive cohort and 11.6 months (95% CI, 9.7-14.4) in the matched ALK-WT cohort; the HR was 1.4 (95% CI, 0.8-2.3) (Figure 4). With Model 2 after adjusting for the presence of TP53 alterations, median OS was 13.4 months (95% CI, 6.1-25.4) in the ALK fusion-positive cohort and 21.3 months (95% CI, 17.5-26.4) in the matched ALK-WT cohort, with an HR of 1.7 (95% CI, 1.0-2.9). Lastly, with Model 2 after adjusting for immortal time bias and the presence of TP53 alterations, median OS was 9.7 months (95% CI, 1.2-18.4) in the ALK fusion-positive cohort and 14.4 months (95% CI, 9.2-19.3) in the matched ALK-WT cohort, and the HR was 1.5 (95% CI, 0.8-2.5).
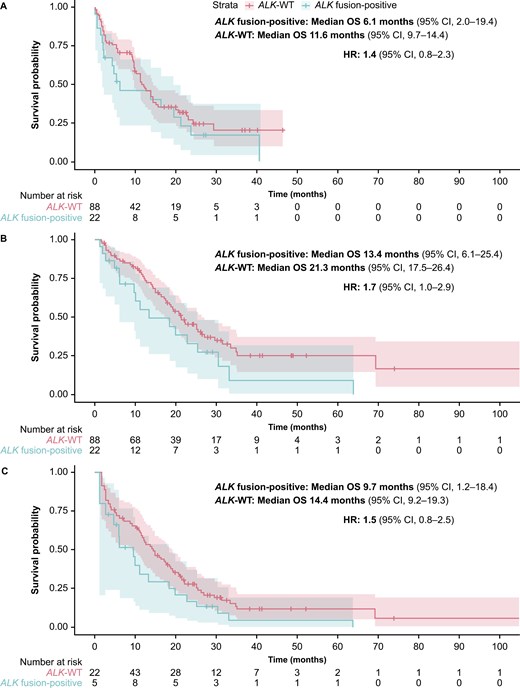
Sensitivity analyses: Kaplan-Meier estimates of OS analysis comparing the ALK fusion-positive cohort (N = 22) with the matched ALK-WT cohort (N = 88) and correcting for TP53 alterations, using (A) the CGP report date as the index (Model 1); (B) the initial diagnosis date as the index (Model 2); and (C) the initial diagnosis date as the index (Model 2*), and corrected for immortal time bias. *The number of patients is the same in all panels (ie, N = 22 for the ALK fusion-positive cohort and N = 88 for the ALK-WT cohort). Panel C shows the analysis using Model 2 and corrected for immortal time bias, which used left truncation to estimate survival. Only 5 patients with ALK fusion-positive tumors and 22 patients with ALK-WT tumors had both a metastatic diagnosis and a CGP date at time 0. For the remaining patients, who satisfied cohort entry criteria at later times, this immortal time was taken into account when calculating OS. ALK, anaplastic lymphoma kinase; CGP, comprehensive genomic profiling; CI, confidence interval; HR, hazard ratio; OS, overall survival; TP53, tumor protein p53; WT, wild type.
Discussion
In this study, we investigated the clinical and demographic characteristics of patients with solid tumors harboring ALK fusions, and the prognostic value of ALK fusions. Co-alterations of common oncogenic drivers were rare in the ALK fusion-positive cohort (0%-4.5%), suggesting that ALK fusions were the main oncogenic drivers in this population. A shorter median OS was observed in patients with ALK fusion-positive versus ALK-WT tumors, with an HR of 1.8, suggesting that the risk of death for patients whose tumors harbor ALK fusions is almost double that of their wild-type counterparts (ie, ALK fusions have a negative prognostic effect). While patients with ALK fusion-positive NSCLC were excluded from this analysis, since this population is well characterized and there are already several approved targeted therapies in this setting, prior studies have also suggested that ALK rearrangements may be associated with worse disease outcomes in NSCLC.25 Genomic alterations in TP53 were quite common in both the ALK fusion-positive and ALK-WT cohorts (50% and 44%, respectively) and data from sensitivity analyses suggest that TP53 alterations, which typically manifest in the metastatic setting, may in general negatively affect the prognosis of these patients. Our data highlight the unmet need for patients with ALK fusion-positive solid tumors other than NSCLC, and warrant further investigation into the use of ALK inhibitors to improve their outcomes.
Several ALK TKIs are approved for patients with ALK fusion-positive NSCLC and crizotinib is also approved in patients with ALK fusion-positive relapsed or refractory, systemic ALCL and patients with ALK fusion-positive unresectable, recurrent or refractory IMTs.6 Investigating the efficacy of ALK inhibitors outside of NSCLC is challenging due to the rarity of ALK fusions,3 and this is supported by our findings; in our study, out of 22 528 patients with metastatic solid tumors (excluding NSCLC) in the CGDB, only 22 patients met the eligibility criteria for inclusion in the ALK fusion-positive cohort. These small numbers of patients means that randomized head-to-head trials would not be feasible, nor would it be possible to stratify patients (eg, by histology or other important patient characteristics, such as lines of prior treatments, tumor stage, etc.) in such studies. In addition, different tumor types might require different standards of care to be used as a comparator. Therefore, modified study designs (eg, single-arm basket or umbrella studies) are needed to generate meaningful data for tumor types with rare molecular alterations.26,27 Evidence suggests that ALK inhibitors may be efficacious across different indications3,28 and there is a need for further investigations of the clinical value of ALK TKIs in a tumor-agnostic way. The signal-seeking MyPathway trial (NCT02091141)28 completed in 2023 and additional studies to investigate ALK TKIs in adult and pediatric patients in a tumor-agnostic setting are currently ongoing or completed (eg, TAPISTRY platform study [NCT04589845]29; iMATRIX study [NCT04774718]30). In our study, patients with ALK fusion-positive tumors treated with standard-of-care therapies had poor survival outcomes, suggesting that this genomic alteration is not a favorable prognostic factor. Therefore, any potential benefit seen with ALK inhibitors in these tumor-agnostic studies would most likely be due to the treatment itself, and not confounded by the presence of the ALK alteration.
Similar analyses using real-world data have recently been published for patients with NTRK fusion-positive or RET fusion-positive solid tumors, which concluded that both NTRK and RET fusions are also negative prognostic factors.22,31,32 Real-world data are used to evaluate patient outcomes based on the presence of certain oncogenic drivers and could therefore be considered as complementary source of evidence (eg, in patients with rare molecular alterations).
Our study has several strengths. We used real-world data from a large database and drawn from EHRs, thus reflecting the nuances of routine clinical practice, and spanning more than a decade of observations. This longitudinal follow-up provides insights into the durability of treatment responses, late effects of therapy, and real-world survival outcomes, beyond the limited follow-up periods typical of clinical trials. Moreover, it allows the exploration of heterogeneity in treatment effects across different patient populations, thus enhancing the generalizability of findings to real-world clinical practice. The use of 2 models for analyzing OS also supports the robustness of our findings. Lastly, there is an established track record of studies investigating the prognostic effect of precision oncology biomarkers, including NTRK fusions31,32 and RET fusions22 in solid tumors, using a similar methodology.
This study also has some limitations. This was a retrospective analysis with a small number of patients with ALK fusion-positive tumors and, although this is reflective of the rare nature of ALK fusions,4 it still limits our ability to draw definitive conclusions. Additionally, since the study used a heterogeneous population, it is unclear if tumor types with relatively long survival/large censoring may have impacted the data, despite the fact that only de novo metastatic tumors (with equally poor prognosis) were included in the analyses, and that underlying patient and clinical characteristics were successfully matched. In addition, clinical characteristics were not always available, with data regarding the number of prior lines of therapy missing for more than a third of patients. Furthermore, since CGP is not yet routinely used across all tumor types, it is not known how representative the patient population is of the wider population of patients with ALK fusion-positive solid tumors. There may also be potential bias if genetic testing was preferentially performed on some patients for reasons that were not captured in this analysis.
In conclusion, our data suggest a negative prognostic value for ALK fusions in metastatic solid tumors, highlighting an unmet medical need for precision therapies that provide durable clinical benefit by selectively targeting ALK fusions. Additional analyses with larger patient populations must be conducted to assess the validity of these findings in the future.
Acknowledgments
Medical writing support for the development of this manuscript, under the direction of the authors, was provided by Rebecca Benatan, BSc, of Ashfield MedComms, an Inizio company, and funded by F. Hoffmann-La Roche Ltd.
Author contributions
Shirish M. Gadgeel (Investigation, Writing—review and editing), Otto Fajardo (Conceptualization, Resources, Data curation, Formal analysis, Writing—review and editing), Fabrice Barlesi (Investigation, Writing—review and editing), Jeong Eun Kim (Investigation, Writing—review and editing), Razelle Kurzrock (Investigation, Writing—review and editing), David M. Thomas (Investigation, Writing—review and editing), Ritika Jagtiani (Writing—review and editing), Johannes Noe (Writing—review and editing), Sven Schwemmers (Writing—review and editing), and Christos Nikolaidis (Conceptualization, Resources, Data curation, Formal analysis, Writing—review and editing)
Funding
This study was funded by F. Hoffmann-La Roche Ltd.
Conflicts of interest
S.M.G.: Consulting or advisory role: AstraZeneca, Pfizer, Gilead, Boehringer-Ingelheim, Arcus, Blueprint, BMS, Mirati, Genentech, Inc./ F. Hoffmann-La Roche Ltd, Merck, Esai, Eli Lilly, Takeda, GSK; Travel expenses: Mirati, Merck. O.F.: Employee of and holds stocks/shares in F. Hoffmann-La Roche Ltd. F.B.: Institutional funding: AbbVie, ACEA, Amgen, AstraZeneca, Bayer, Bristol-Myers Squibb, Boehringer-Ingelheim, Eisai, Eli Lilly Oncology, F. Hoffmann-La Roche Ltd, Genentech, Inc., Ipsen, Ignyta, Innate Pharma, Loxo, Novartis, Medimmune, Merck, MSD, Pierre Fabre, Pfizer, Sanofi-Aventis, Takeda; Principal Investigator of sponsored trials (or ISR): AstraZeneca, BMS, Innate Pharma, Merck, Mirati, Pierre Fabre, F. Hoffmann-La Roche Ltd. J.E.K.: none. R.K.: Research funding: Boehringer-Ingelheim, Debiopharm, Foundation Medicine, Genentech, Grifols, Guardant, Incyte, Konica Minolta, Medimmune, Merck Serono, Omniseq, Pfizer, Sequenom, Takeda, TopAlliance and from the NCI; Consultant and/or speaker fees and/or advisory board/consultant: Actuate Therapeutics, AstraZeneca, Bicara Therapeutics, Biological Dynamics, Caris, Datar Cancer Genetics, Daiichi, EISAI, EOM Pharmaceuticals, Iylon, LabCorp, Lanuaria, Merck, NeoGenomics, Neomed, Pfizer, Precirix, Prosperdtx, Regeneron, Roche, TD2/Volastra, Turning Point Therapeutics, X-Biotech; Equity interest in CureMatch; Board member: CureMatch and CureMetrix; Co-founder: CureMatch. D.M.T.: Advisory role: F. Hoffmann-La Roche Ltd, AstraZeneca, Pfizer, Eisai, Illumina, Beigene, Elevation Oncology, RedX Pharmaceuticals, Sun Pharma, Bayer, AbbVie, George Clinical, Janssen, Merck, Kinnate, Microba, BioTessellate, Australian Unity, Foundation Medicine, Guardant, InterVenn, Amgen, Seattle Genetics, Eli Lilly; Employee of Omico; Consulting: F. Hoffmann-La Roche Ltd, AstraZeneca, Pfizer, Eisai, Illumina, BeiGene, Elevation Oncology, RedX Pharmaceuticals, Sun Pharma, Bayer, AbbVie, George Clinical, Janssen, Merck, Kinnate, Microba, BioTessellate, Australian Unity, Foundation Medicine, Guardant, InterVenn, Amgen, Seattle Genetics, Eli Lilly; Travel expenses: F. Hoffmann-La Roche Ltd; Research funding: Omico, NHMRC. R.J.: Employee of Genentech, Inc. J.N.: Employee of and holds stocks/shares in F. Hoffmann-La Roche Ltd. S.S.: Employee of and holds stocks/shares in F. Hoffmann-La Roche Ltd. C.N.: Former employee of F. Hoffmann-La Roche Ltd and current employee of GSK.
Ethics
This study used de-identified patient data from the Flatiron Health-Foundation Medicine clinico-genomic database, a US-wide longitudinal database curated through technology-enabled abstraction, and did not directly enroll patients.
Data availability
For up-to-date details on Roche’s Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see here: https://go.roche.com/data_sharing. The data that support the findings of this study were originated by and are the property of Flatiron Health, Inc. and Foundation Medicine, Inc., which has restrictions prohibiting the authors from making the data set publicly available. Requests for data sharing by license or by permission for the specific purpose of replicating results in this manuscript can be submitted to [email protected] and [email protected].
References
Author notes
Shirish M. Gadgeel and Otto Fajardo, Co-lead authors.
Christos Nikolaidis Currently an employee of GSK.



