-
PDF
- Split View
-
Views
-
Cite
Cite
Yongsung Kim, Chandra Kumar Krishnan, Han‐Soo Kim, Hwan Seong Cho, Ilkyu Han, Ambulation Recovery After Surgery for Metastases to the Femur, The Oncologist, Volume 25, Issue 1, January 2020, Pages e178–e185, https://doi.org/10.1634/theoncologist.2019-0107
Close - Share Icon Share
Abstract
Postoperative ambulation recovery after surgery for femur metastases has significant implications for not only the patient's quality of life but also administration of further cancer treatment. Thus, identification of preoperative predictors of ambulation recovery is necessary to set appropriate expectations and guide treatment. This study aimed to assess ambulation recovery rate and identify predictors of ambulation recovery in patients undergoing surgery for femur metastases.
A total of 244 patients who underwent surgery for femur metastases at our institution were reviewed. Patients were considered ambulatory if they were able to walk independently or walk with aids and nonambulatory if they were wheelchair bound or bedridden. The following potential clinicopathologic factors that might predict postoperative ambulation recovery were evaluated: premorbid general status, cancer burden, and local factors.
A total of 165 patients (68%) regained ambulatory status postoperatively. A multivariate analysis revealed poor Eastern Cooperative Oncology Group (ECOG) performance status (odds ratio [OR], 5.327; p < .001) and nonambulatory premorbid ambulatory status (OR, 7.459; p < .001) as independent predictors of poor ambulation recovery after surgery for femur metastases. Postoperative ambulatory status was significantly associated with postoperative survival time (p < .001).
Postoperative ambulation recovery rate in our cohort was 68%. Premorbid ambulatory status and ECOG performance status are predictors of ambulation recovery in patients undergoing surgery for femur metastases.
Implications for Practice
Postoperative ambulation recovery rate in this cohort was 68%. Premorbid ambulatory status and Eastern Cooperative Oncology Group performance status are predictors of ambulation recovery in patients undergoing surgery for femur metastases.
Introduction
The femur is one of the most common sites of metastases of the long bones [1, 2]. The hip joint and proximal femur are known to sustain more than three times a person's body weight during normal daily activities; therefore, any underlying mechanical weakness caused by metastatic disease can easily result in fracture [3]. Pathologic fractures in the femur result in loss of ambulation, and skeletal stabilization is necessary to relieve pain and regain ambulatory function [4-6].
Postoperative ambulation recovery has significant implications for not only the patient's quality of life but also for further cancer treatments such as chemotherapy and radiation therapy [7]. For example, performance status (PS) is used in the advanced cancer treatment decision making process. PS measures such as the Eastern Cooperative Oncology Group (ECOG) evaluates standing and ambulatory status in the grading scale [8-10]. Although most patients regain ambulatory function after surgery for femur metastases, some patients fail to recover ambulation. Therefore, we believe that identifying preoperative predictors of ambulation recovery would be helpful to set appropriate expectations and guide treatment in patients with femur metastases. To our knowledge, no study has investigated the predictive factors of postoperative ambulation recovery after surgery for femur metastases. With this regard, this study aimed to assess the ambulation recovery rate and identify predictors of ambulation recovery in patients undergoing surgery for femur metastases.
Material and Methods
Patients
We performed a retrospective review of 279 consecutive patients who underwent surgery for femur metastases at our institution between January 1999 and January 2016. Patients with incomplete medical records (n = 32) and uncertain survival status (n = 3) were excluded, with 244 remaining patients for the analyses. This study was approved by our institutional review board.
Ambulatory Status
Ambulatory status was classified as independent walking, assisted walking, wheelchair bound, or bedridden. [11]. Patients were considered ambulatory if they were able to walk independently or walk with aids. Patients who required a wheelchair or were bedridden were considered as wheelchair bound and bedridden, respectively. Patients were considered nonambulatory if they were wheelchair bound or bedridden. Premorbid ambulatory status was defined as the ambulatory status before the development of symptoms caused by the metastasis of femur. For the patients who underwent prophylactic skeletal stabilization of impending fracture, we assessed the ambulatory status before the patients developed functional pain. Postoperative ambulatory status was defined as the best ambulatory status after the surgery.
Predictive Factors of Ambulation Recovery
Medical records were reviewed to note the potential clinicopathologic factors that might predict postoperative ambulation recovery: (a) premorbid general status, (b) cancer burden, and (c) local factors (Table 1). For premorbid general status, age, sex, ambulatory status, and PS were analyzed. There were 99 male (41%) and 145 female (59%) patients. The median age at surgery was 59 years (range, 16–89). The mean follow‐up was 12 months (range, 1–89; Table 1). Prior to the development of morbidity caused by metastases, 77 patients (31%) were independently ambulatory, and 110 (45%) could walk with aids. Thirty‐three patients (14%) required a wheelchair, and 24 (10%) were bedridden (Table 2). To assess patients’ PS, the ECOG PS scales were used [8]. The ECOG PS scores were as follows: 0 for normal function, 1 for minimal functional impairment, 2 for impairment amounting to spend less than 50% of time in bed, 3 for impairment amounting to spend more than 50% of time in bed, and 4 for being completely bed bound. Preoperative ECOG PS was favorable (0, 1, or 2) in 167 patients (67%) and unfavorable (3 or 4) in 77 patients (31%). For cancer burden, primary cancer type, presence of visceral metastases, presence of solitary metastases, and the time from cancer diagnosis to bone metastases were evaluated. The most common primary cancer was lung cancer (n = 62), followed by hepatocellular carcinoma (50), renal cell carcinoma (33), and breast cancer (32; Table 2). For the purpose of analyses, breast cancer, multiple myeloma, prostate cancer, and thyroid cancer were grouped as cancers with favorable outcomes. The presence of visceral metastasis was confirmed by routine computed tomography of the chest, abdomen, and pelvis. Visceral metastases were present in 144 patients (59%) and solitary bone metastases in 58 patients (24%). The mean period from the diagnosis of primary cancer to the diagnosis of femur metastasis was 2.8 years (range, 0–20). The median interval from the diagnosis of femur metastasis to surgery was 1 month (range, 0–74).
| Characteristics . | (n = 244), n (%) . |
|---|---|
| Age (range), yr | 59 (16–89) |
| Sex | |
| Female | 99 (40) |
| Male | 145 (60) |
| Premorbid ambulatory status | |
| Independent ambulation | 77 (31) |
| Assisted ambulation | 110 (45) |
| Wheelchair bound | 33 (14) |
| Bedridden | 24 (10) |
| Premorbid ECOG status | |
| ECOG 0 | 21 (9) |
| ECOG 1 | 85 (34) |
| ECOG 2 | 61 (25) |
| ECOG 3 | 53 (22) |
| ECOG 4 | 24 (10) |
| Primary cancer | |
| Lung | 62 (25) |
| Liver | 50 (20) |
| Kidney | 33 (13) |
| Breast | 32 (13) |
| Stomach | 16 (7) |
| Multiple myeloma | 10 (4) |
| Colon | 10 (4) |
| Prostate | 6 (2.5) |
| Thyroid | 4 (2) |
| Lymphoma | 2 (1) |
| Gallbladder | 1 (0.5) |
| Others | 18 (8) |
| Extent of metastases | |
| Multiple | 186 (76) |
| Solitary | 58 (24) |
| Visceral metastasis | |
| Present | 144 (59) |
| Absent | 100 (41) |
| Fracture type | |
| Pathologic fracture | 130 (53) |
| Impending fracture | 114 (47) |
| Skeletal stabilization | |
| Internal fixation | 143 (58) |
| Endoprosthesis | 101 (42) |
| Contralateral metastases | |
| Present | 48 (19) |
| Absent | 196 (81) |
| Time interval to surgery | |
| >1 mo | 91 (37) |
| ≤1 mo | 153 (63) |
| Radiation therapy on surgical site | |
| Yes | 104 (42) |
| No | 140 (58) |
| Characteristics . | (n = 244), n (%) . |
|---|---|
| Age (range), yr | 59 (16–89) |
| Sex | |
| Female | 99 (40) |
| Male | 145 (60) |
| Premorbid ambulatory status | |
| Independent ambulation | 77 (31) |
| Assisted ambulation | 110 (45) |
| Wheelchair bound | 33 (14) |
| Bedridden | 24 (10) |
| Premorbid ECOG status | |
| ECOG 0 | 21 (9) |
| ECOG 1 | 85 (34) |
| ECOG 2 | 61 (25) |
| ECOG 3 | 53 (22) |
| ECOG 4 | 24 (10) |
| Primary cancer | |
| Lung | 62 (25) |
| Liver | 50 (20) |
| Kidney | 33 (13) |
| Breast | 32 (13) |
| Stomach | 16 (7) |
| Multiple myeloma | 10 (4) |
| Colon | 10 (4) |
| Prostate | 6 (2.5) |
| Thyroid | 4 (2) |
| Lymphoma | 2 (1) |
| Gallbladder | 1 (0.5) |
| Others | 18 (8) |
| Extent of metastases | |
| Multiple | 186 (76) |
| Solitary | 58 (24) |
| Visceral metastasis | |
| Present | 144 (59) |
| Absent | 100 (41) |
| Fracture type | |
| Pathologic fracture | 130 (53) |
| Impending fracture | 114 (47) |
| Skeletal stabilization | |
| Internal fixation | 143 (58) |
| Endoprosthesis | 101 (42) |
| Contralateral metastases | |
| Present | 48 (19) |
| Absent | 196 (81) |
| Time interval to surgery | |
| >1 mo | 91 (37) |
| ≤1 mo | 153 (63) |
| Radiation therapy on surgical site | |
| Yes | 104 (42) |
| No | 140 (58) |
Abbreviation: ECOG, Eastern Cooperative Oncology Group.
| Characteristics . | (n = 244), n (%) . |
|---|---|
| Age (range), yr | 59 (16–89) |
| Sex | |
| Female | 99 (40) |
| Male | 145 (60) |
| Premorbid ambulatory status | |
| Independent ambulation | 77 (31) |
| Assisted ambulation | 110 (45) |
| Wheelchair bound | 33 (14) |
| Bedridden | 24 (10) |
| Premorbid ECOG status | |
| ECOG 0 | 21 (9) |
| ECOG 1 | 85 (34) |
| ECOG 2 | 61 (25) |
| ECOG 3 | 53 (22) |
| ECOG 4 | 24 (10) |
| Primary cancer | |
| Lung | 62 (25) |
| Liver | 50 (20) |
| Kidney | 33 (13) |
| Breast | 32 (13) |
| Stomach | 16 (7) |
| Multiple myeloma | 10 (4) |
| Colon | 10 (4) |
| Prostate | 6 (2.5) |
| Thyroid | 4 (2) |
| Lymphoma | 2 (1) |
| Gallbladder | 1 (0.5) |
| Others | 18 (8) |
| Extent of metastases | |
| Multiple | 186 (76) |
| Solitary | 58 (24) |
| Visceral metastasis | |
| Present | 144 (59) |
| Absent | 100 (41) |
| Fracture type | |
| Pathologic fracture | 130 (53) |
| Impending fracture | 114 (47) |
| Skeletal stabilization | |
| Internal fixation | 143 (58) |
| Endoprosthesis | 101 (42) |
| Contralateral metastases | |
| Present | 48 (19) |
| Absent | 196 (81) |
| Time interval to surgery | |
| >1 mo | 91 (37) |
| ≤1 mo | 153 (63) |
| Radiation therapy on surgical site | |
| Yes | 104 (42) |
| No | 140 (58) |
| Characteristics . | (n = 244), n (%) . |
|---|---|
| Age (range), yr | 59 (16–89) |
| Sex | |
| Female | 99 (40) |
| Male | 145 (60) |
| Premorbid ambulatory status | |
| Independent ambulation | 77 (31) |
| Assisted ambulation | 110 (45) |
| Wheelchair bound | 33 (14) |
| Bedridden | 24 (10) |
| Premorbid ECOG status | |
| ECOG 0 | 21 (9) |
| ECOG 1 | 85 (34) |
| ECOG 2 | 61 (25) |
| ECOG 3 | 53 (22) |
| ECOG 4 | 24 (10) |
| Primary cancer | |
| Lung | 62 (25) |
| Liver | 50 (20) |
| Kidney | 33 (13) |
| Breast | 32 (13) |
| Stomach | 16 (7) |
| Multiple myeloma | 10 (4) |
| Colon | 10 (4) |
| Prostate | 6 (2.5) |
| Thyroid | 4 (2) |
| Lymphoma | 2 (1) |
| Gallbladder | 1 (0.5) |
| Others | 18 (8) |
| Extent of metastases | |
| Multiple | 186 (76) |
| Solitary | 58 (24) |
| Visceral metastasis | |
| Present | 144 (59) |
| Absent | 100 (41) |
| Fracture type | |
| Pathologic fracture | 130 (53) |
| Impending fracture | 114 (47) |
| Skeletal stabilization | |
| Internal fixation | 143 (58) |
| Endoprosthesis | 101 (42) |
| Contralateral metastases | |
| Present | 48 (19) |
| Absent | 196 (81) |
| Time interval to surgery | |
| >1 mo | 91 (37) |
| ≤1 mo | 153 (63) |
| Radiation therapy on surgical site | |
| Yes | 104 (42) |
| No | 140 (58) |
Abbreviation: ECOG, Eastern Cooperative Oncology Group.
Predictive factors of postoperative ambulation recovery after surgery for femur metastasis
| Predictive factors . | Univariate analysis . | Multivariate analysis . | ||
|---|---|---|---|---|
| Odds ratio (95% CI) . | p value . | Odds ratio (95% CI) . | p value . | |
| General status | ||||
| Age | .063 | |||
| >60 yr | Ref | |||
| ≤60 yr | 1.667 (0.97–2.86) | |||
| Sex | .043a | |||
| Female | Ref | |||
| Male | 0.555 (0.31–0.98) | |||
| Performance status | <.001a | <.001a | ||
| ECOG 3–4 | Ref | Ref | ||
| ECOG 0–2 | 14.699 (7.62–28.36) | 4.322 (1.84–10.137) | ||
| Premorbid ambulatory status | <.001a | <.001a | ||
| Nonambulatory | Ref | Ref | ||
| Ambulatory | 16.237 (8.23–32.05) | 6.427 (2.66–15.55) | ||
| Cancer burden | ||||
| Cancer type | .006a | |||
| Unfavorable | Ref | |||
| Favorable | 3.085 (1.374–6.928) | |||
| Number of metastases | .089 | |||
| Multiple | Ref | |||
| Single | 1.816 (0.91–3.61) | |||
| Visceral metastasis | .092 | |||
| Present | Ref | |||
| Absent | 1.648 (0.92–2.95) | |||
| Time from diagnosis to surgery | .18 | |||
| Delayed (>1 mo) | Ref | |||
| Immediate(≤1 mo) | 0.676 (0.38–1.19) | |||
| Local factors | ||||
| Fracture type | .055 | |||
| Complete | Ref | |||
| Impending | 1.716 (0.99–2.98) | |||
| Reconstruction type | .056a | |||
| Internal fixation | Ref | |||
| Endoprosthesis | 1.737 (0.99–3.06) | |||
| Contralateral femur metastasis | .184 | |||
| Present | Ref | |||
| Absent | 1.559 (0.81–3.0) | |||
| Pelvis metastasis | .764 | |||
| Present | Ref | |||
| Absent | 1.078 (0.63–1.87) | |||
| Predictive factors . | Univariate analysis . | Multivariate analysis . | ||
|---|---|---|---|---|
| Odds ratio (95% CI) . | p value . | Odds ratio (95% CI) . | p value . | |
| General status | ||||
| Age | .063 | |||
| >60 yr | Ref | |||
| ≤60 yr | 1.667 (0.97–2.86) | |||
| Sex | .043a | |||
| Female | Ref | |||
| Male | 0.555 (0.31–0.98) | |||
| Performance status | <.001a | <.001a | ||
| ECOG 3–4 | Ref | Ref | ||
| ECOG 0–2 | 14.699 (7.62–28.36) | 4.322 (1.84–10.137) | ||
| Premorbid ambulatory status | <.001a | <.001a | ||
| Nonambulatory | Ref | Ref | ||
| Ambulatory | 16.237 (8.23–32.05) | 6.427 (2.66–15.55) | ||
| Cancer burden | ||||
| Cancer type | .006a | |||
| Unfavorable | Ref | |||
| Favorable | 3.085 (1.374–6.928) | |||
| Number of metastases | .089 | |||
| Multiple | Ref | |||
| Single | 1.816 (0.91–3.61) | |||
| Visceral metastasis | .092 | |||
| Present | Ref | |||
| Absent | 1.648 (0.92–2.95) | |||
| Time from diagnosis to surgery | .18 | |||
| Delayed (>1 mo) | Ref | |||
| Immediate(≤1 mo) | 0.676 (0.38–1.19) | |||
| Local factors | ||||
| Fracture type | .055 | |||
| Complete | Ref | |||
| Impending | 1.716 (0.99–2.98) | |||
| Reconstruction type | .056a | |||
| Internal fixation | Ref | |||
| Endoprosthesis | 1.737 (0.99–3.06) | |||
| Contralateral femur metastasis | .184 | |||
| Present | Ref | |||
| Absent | 1.559 (0.81–3.0) | |||
| Pelvis metastasis | .764 | |||
| Present | Ref | |||
| Absent | 1.078 (0.63–1.87) | |||
aStatistically significant.
Abbreviations: CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; Ref, reference.
Predictive factors of postoperative ambulation recovery after surgery for femur metastasis
| Predictive factors . | Univariate analysis . | Multivariate analysis . | ||
|---|---|---|---|---|
| Odds ratio (95% CI) . | p value . | Odds ratio (95% CI) . | p value . | |
| General status | ||||
| Age | .063 | |||
| >60 yr | Ref | |||
| ≤60 yr | 1.667 (0.97–2.86) | |||
| Sex | .043a | |||
| Female | Ref | |||
| Male | 0.555 (0.31–0.98) | |||
| Performance status | <.001a | <.001a | ||
| ECOG 3–4 | Ref | Ref | ||
| ECOG 0–2 | 14.699 (7.62–28.36) | 4.322 (1.84–10.137) | ||
| Premorbid ambulatory status | <.001a | <.001a | ||
| Nonambulatory | Ref | Ref | ||
| Ambulatory | 16.237 (8.23–32.05) | 6.427 (2.66–15.55) | ||
| Cancer burden | ||||
| Cancer type | .006a | |||
| Unfavorable | Ref | |||
| Favorable | 3.085 (1.374–6.928) | |||
| Number of metastases | .089 | |||
| Multiple | Ref | |||
| Single | 1.816 (0.91–3.61) | |||
| Visceral metastasis | .092 | |||
| Present | Ref | |||
| Absent | 1.648 (0.92–2.95) | |||
| Time from diagnosis to surgery | .18 | |||
| Delayed (>1 mo) | Ref | |||
| Immediate(≤1 mo) | 0.676 (0.38–1.19) | |||
| Local factors | ||||
| Fracture type | .055 | |||
| Complete | Ref | |||
| Impending | 1.716 (0.99–2.98) | |||
| Reconstruction type | .056a | |||
| Internal fixation | Ref | |||
| Endoprosthesis | 1.737 (0.99–3.06) | |||
| Contralateral femur metastasis | .184 | |||
| Present | Ref | |||
| Absent | 1.559 (0.81–3.0) | |||
| Pelvis metastasis | .764 | |||
| Present | Ref | |||
| Absent | 1.078 (0.63–1.87) | |||
| Predictive factors . | Univariate analysis . | Multivariate analysis . | ||
|---|---|---|---|---|
| Odds ratio (95% CI) . | p value . | Odds ratio (95% CI) . | p value . | |
| General status | ||||
| Age | .063 | |||
| >60 yr | Ref | |||
| ≤60 yr | 1.667 (0.97–2.86) | |||
| Sex | .043a | |||
| Female | Ref | |||
| Male | 0.555 (0.31–0.98) | |||
| Performance status | <.001a | <.001a | ||
| ECOG 3–4 | Ref | Ref | ||
| ECOG 0–2 | 14.699 (7.62–28.36) | 4.322 (1.84–10.137) | ||
| Premorbid ambulatory status | <.001a | <.001a | ||
| Nonambulatory | Ref | Ref | ||
| Ambulatory | 16.237 (8.23–32.05) | 6.427 (2.66–15.55) | ||
| Cancer burden | ||||
| Cancer type | .006a | |||
| Unfavorable | Ref | |||
| Favorable | 3.085 (1.374–6.928) | |||
| Number of metastases | .089 | |||
| Multiple | Ref | |||
| Single | 1.816 (0.91–3.61) | |||
| Visceral metastasis | .092 | |||
| Present | Ref | |||
| Absent | 1.648 (0.92–2.95) | |||
| Time from diagnosis to surgery | .18 | |||
| Delayed (>1 mo) | Ref | |||
| Immediate(≤1 mo) | 0.676 (0.38–1.19) | |||
| Local factors | ||||
| Fracture type | .055 | |||
| Complete | Ref | |||
| Impending | 1.716 (0.99–2.98) | |||
| Reconstruction type | .056a | |||
| Internal fixation | Ref | |||
| Endoprosthesis | 1.737 (0.99–3.06) | |||
| Contralateral femur metastasis | .184 | |||
| Present | Ref | |||
| Absent | 1.559 (0.81–3.0) | |||
| Pelvis metastasis | .764 | |||
| Present | Ref | |||
| Absent | 1.078 (0.63–1.87) | |||
aStatistically significant.
Abbreviations: CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; Ref, reference.
For local factors, the type of fracture, type of surgery, metastasis of contralateral femur, and administration of postoperative radiation were evaluated. A total of 130 patients (53%) had complete fracture at the time of diagnosis, and 114 (47%) underwent surgical treatment for impending fractures. Skeletal stabilizations were performed with osteosynthetic devices (n = 143, 58%) or endoprosthesis (n = 101, 42%). Osteosynthetic devices, such as plate and intramedullary nail supplemented with bone cement, were considered options of surgical treatment [12, 13]. Endoprosthesis was applied in case of severe destructive lesions with large bone defect around the proximal femur [4]. For postoperative rehabilitation, all patients were subject to inpatient rehabilitation supervised by physical therapists. Before the start of rehabilitation, patients were advised to undergo isometric muscle strengthening exercise in the bed. Rehabilitation was started after the removal of surgical drains usually within a week of surgery. Patients were allowed full weight bearing as tolerated during rehabilitation. After the achievement of assisted ambulation, most of the patients were transferred to a rehabilitation facility to continue rehabilitation. Metastatic lesion of contralateral femur was noted in 48 patients (19.7%), and 104 patients (42%) underwent radiation therapy on the operative site as adjuvant treatment.
Statistical Analysis
Frequency distributions were used to describe category‐scale measurements, and summary statistics (mean with SD or median with a 95% confidence interval [CI]) were used to describe the distributions of interval‐scale measurements. We used the chi‐square test for categorical variables. Univariate and multivariate logistic regression analyses were performed to evaluate predictive variables and related odds ratio with a 95% CI. For survival analysis, the Kaplan‐Meier survival curve with log‐rank test was used to assess the prognostic significance of factors. The Spearman correlation coefficient was used to confirm the correlation between ECOG PS and ambulatory status. Statistical significance was defined as a p value < .05. Statistical analyses were performed using SPSS software (version 18.0; SPSS Inc., Chicago, IL).
Results
Ambulation Recovery
In all, 165 patients (68%) were ambulatory postoperatively (Fig. 1). In premorbid status, 77 patients (31%) were independently ambulatory, and 110 (45%) were ambulatory with aids. Thirty‐three patients (19%) were wheelchair bound, and 24 (13%) were bedridden. Postoperatively, 86 patients (35%) were independently ambulatory, and 79 (33%) were ambulatory with aids. Forty‐seven patients (19%) were wheelchair bound, and 32 (13%) were bedridden. When premorbid status and postoperative ambulatory status were compared for each patient, 60% (n = 148) regained premorbid ambulatory status: 65% (50/77) for independent ambulators, 53% (59/110) for ambulators with aids, 69% (23/33) for wheelchair‐bound patients, and 25% (16/24) for bedridden patients. Moreover, 18% (n = 44) improved ambulatory status: 29% (32/110) for ambulators with aids, 12% (4/33) for wheelchair‐bound patients, and 33% (8/24) for bedridden patients. Deterioration of ambulatory status was noted in 52 patients (21.3%): 35% (27/77) for independent ambulators, 17% (19/110) for ambulators with aids, and 18% (6/33) for wheelchair‐bound patients. All 165 patients who gained ambulation were ambulatory by postoperative 3 months. No patient who was nonambulatory at 3 months became ambulatory afterward.
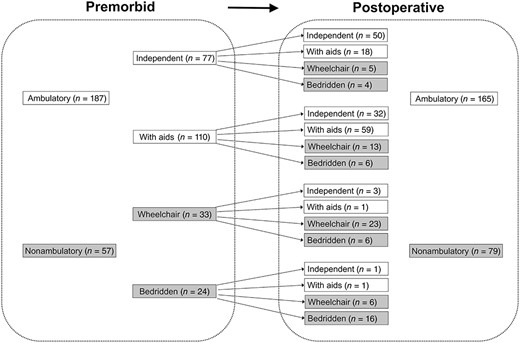
Change in ambulatory status after surgery for femur metastases.
Predictive Factors of Ambulation Recovery
Premorbid ambulatory status and ECOG PS were significant and independent predictive factors of postoperative restoration of ambulatory status. In premorbid general status, female sex (odds ratio [OR], 1.818; p = .043), favorable premorbid PS (OR, 14.699; p < .001), and premorbid ambulatory status (OR, 16.237; p < .001) were found to be predictive of ambulation recovery. In cancer burden, favorable cancer type (OR, 3.085; p = .006) was a predictive factor that was not statistically significant in the multivariate analysis. In local factors, even though it was not statistically significant, impending fracture showed a favorable tendency for ambulation recovery (OR, 1.716, p = .055). In contrast, immediate operation after diagnosis of the fracture was adversely favorable for recovery without statistical significance. We performed a multivariate logistic regression analysis with the four above‐mentioned candidates to elaborate the predictive factors. Finally, premorbid ambulatory status (OR, 7.459; p < .001) and favorable ECOG PS (OR, 5.327; p < .001) were identified as independent predictive factors of ambulation recovery (Table 4).
Association of Ambulation Recovery and Survival
At the last follow‐up, 181 patients died of the disease, and 63 survived. The median and mean survival times of expired patients were 7.0 months (95% CI, 4.0–8.0) and 15 months (range, 1–88), respectively. The mean follow‐up period of patients who survived at the last follow‐up was 33 months (range, 1–111). The survival rate was 41% after 1 year and 28% after 2 years postoperatively. Postoperative ambulatory status was significantly associated with the postoperative survival time (p < .001; Fig. 2; Table 3). The median survival time of patients who regained postoperative ambulatory function was 14.0 months (95% CI, 10.4–17.6), and that of patients without ambulatory function was 3.0 months (95% CI, 2.0–4.0). In the multivariate analyses of factors associated with survival, ambulation recovery was an independent prognostic factor for survival after surgical treatment of femur metastasis (OR, 0.477; p < .001) along with premorbid PS (p = .003), favorable cancer type (p < .001), solitary bone metastasis (p < .001), impending fracture (p = .01), and reconstruction with endoprosthesis (p = .047; Table 4).
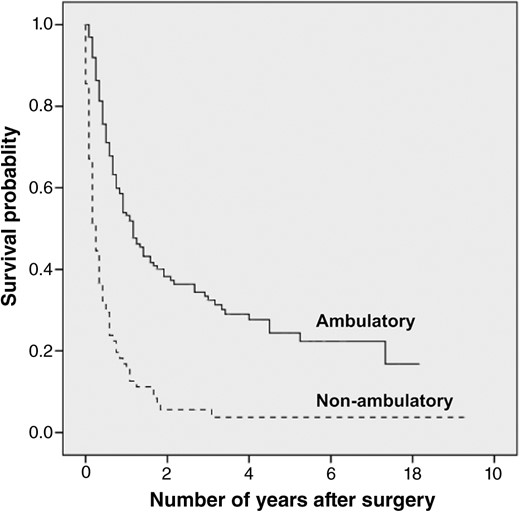
Comparison of survival between the ambulatory group and nonambulatory group. On Kaplan‐Meier analysis, the ambulatory group showed significantly better postoperative survival than the nonambulatory group (p < .001).
| Factors . | 6 mo (%) . | 1 y (%) . | 2 y (%) . | 3 y (%) . | p value . |
|---|---|---|---|---|---|
| General status | |||||
| Age | .243 | ||||
| >60 yr | 61 | 46 | 30 | 27 | |
| ≤60 yr | 35 | 35 | 25 | 22 | |
| Sex | .126 | ||||
| Male | 31 | 31 | 25 | 23 | |
| Female | 96 | 91 | 81 | 72 | |
| Premorbid performance status | <.001a | ||||
| Unfavorable (ECOG 3–4) | 32 | 18 | 6 | 6 | |
| Favorable (ECOG 0–2) | 70 | 52 | 37 | 32 | |
| Premorbid ambulatory status | <.001a | ||||
| Nonambulatory | 34 | 20 | 9 | 9 | |
| Ambulatory | 73 | 50 | 35 | 30 | |
| Cancer burden | |||||
| Primary cancer | <.001a | ||||
| Unfavorable | 50 | 32 | 20 | 17 | |
| Favorable | 84 | 75 | 55 | 50 | |
| Number of metastases | .008a | ||||
| Multiple | 54 | 37 | 25 | 20 | |
| Single | 71 | 60 | 37 | 37 | |
| Visceral metastasis | .106 | ||||
| Present | 57 | 38 | 25 | 18 | |
| Absent | 68 | 46 | 31 | 30 | |
| Local factors | |||||
| Fracture type | .003a | ||||
| Complete | 50 | 33 | 22 | 19 | |
| Impending | 66 | 50 | 34 | 30 | |
| Reconstruction type | .014a | ||||
| Internal fixation | 52 | 36 | 23 | 19 | |
| Endoprosthesis | 66 | 48 | 35 | 30 | |
| Contralateral femur metastasis | .366 | ||||
| Present | 58 | 41 | 17 | 17 | |
| Absent | 63 | 41 | 30 | 25 | |
| Pelvis metastasis | .827 | ||||
| Present | 58 | 39 | 25 | 22 | |
| Absent | 62 | 42 | 29 | 25 | |
| Time from diagnosis to surgery | .244 | ||||
| Delayed (>1 mo) | 58 | 37 | 23 | 19 | |
| Immediate (≤1 mo) | 62 | 45 | 30 | 26 | |
| Ambulation recovery | <.001a | ||||
| Failed | 32 | 15 | 6 | 6 | |
| Recovered | 71 | 53 | 38 | 32 | |
| Factors . | 6 mo (%) . | 1 y (%) . | 2 y (%) . | 3 y (%) . | p value . |
|---|---|---|---|---|---|
| General status | |||||
| Age | .243 | ||||
| >60 yr | 61 | 46 | 30 | 27 | |
| ≤60 yr | 35 | 35 | 25 | 22 | |
| Sex | .126 | ||||
| Male | 31 | 31 | 25 | 23 | |
| Female | 96 | 91 | 81 | 72 | |
| Premorbid performance status | <.001a | ||||
| Unfavorable (ECOG 3–4) | 32 | 18 | 6 | 6 | |
| Favorable (ECOG 0–2) | 70 | 52 | 37 | 32 | |
| Premorbid ambulatory status | <.001a | ||||
| Nonambulatory | 34 | 20 | 9 | 9 | |
| Ambulatory | 73 | 50 | 35 | 30 | |
| Cancer burden | |||||
| Primary cancer | <.001a | ||||
| Unfavorable | 50 | 32 | 20 | 17 | |
| Favorable | 84 | 75 | 55 | 50 | |
| Number of metastases | .008a | ||||
| Multiple | 54 | 37 | 25 | 20 | |
| Single | 71 | 60 | 37 | 37 | |
| Visceral metastasis | .106 | ||||
| Present | 57 | 38 | 25 | 18 | |
| Absent | 68 | 46 | 31 | 30 | |
| Local factors | |||||
| Fracture type | .003a | ||||
| Complete | 50 | 33 | 22 | 19 | |
| Impending | 66 | 50 | 34 | 30 | |
| Reconstruction type | .014a | ||||
| Internal fixation | 52 | 36 | 23 | 19 | |
| Endoprosthesis | 66 | 48 | 35 | 30 | |
| Contralateral femur metastasis | .366 | ||||
| Present | 58 | 41 | 17 | 17 | |
| Absent | 63 | 41 | 30 | 25 | |
| Pelvis metastasis | .827 | ||||
| Present | 58 | 39 | 25 | 22 | |
| Absent | 62 | 42 | 29 | 25 | |
| Time from diagnosis to surgery | .244 | ||||
| Delayed (>1 mo) | 58 | 37 | 23 | 19 | |
| Immediate (≤1 mo) | 62 | 45 | 30 | 26 | |
| Ambulation recovery | <.001a | ||||
| Failed | 32 | 15 | 6 | 6 | |
| Recovered | 71 | 53 | 38 | 32 | |
Data are expressed as percentage unless otherwise specified.
aStatistically significant.
Abbreviation: ECOG, Eastern Cooperative Oncology Group.
| Factors . | 6 mo (%) . | 1 y (%) . | 2 y (%) . | 3 y (%) . | p value . |
|---|---|---|---|---|---|
| General status | |||||
| Age | .243 | ||||
| >60 yr | 61 | 46 | 30 | 27 | |
| ≤60 yr | 35 | 35 | 25 | 22 | |
| Sex | .126 | ||||
| Male | 31 | 31 | 25 | 23 | |
| Female | 96 | 91 | 81 | 72 | |
| Premorbid performance status | <.001a | ||||
| Unfavorable (ECOG 3–4) | 32 | 18 | 6 | 6 | |
| Favorable (ECOG 0–2) | 70 | 52 | 37 | 32 | |
| Premorbid ambulatory status | <.001a | ||||
| Nonambulatory | 34 | 20 | 9 | 9 | |
| Ambulatory | 73 | 50 | 35 | 30 | |
| Cancer burden | |||||
| Primary cancer | <.001a | ||||
| Unfavorable | 50 | 32 | 20 | 17 | |
| Favorable | 84 | 75 | 55 | 50 | |
| Number of metastases | .008a | ||||
| Multiple | 54 | 37 | 25 | 20 | |
| Single | 71 | 60 | 37 | 37 | |
| Visceral metastasis | .106 | ||||
| Present | 57 | 38 | 25 | 18 | |
| Absent | 68 | 46 | 31 | 30 | |
| Local factors | |||||
| Fracture type | .003a | ||||
| Complete | 50 | 33 | 22 | 19 | |
| Impending | 66 | 50 | 34 | 30 | |
| Reconstruction type | .014a | ||||
| Internal fixation | 52 | 36 | 23 | 19 | |
| Endoprosthesis | 66 | 48 | 35 | 30 | |
| Contralateral femur metastasis | .366 | ||||
| Present | 58 | 41 | 17 | 17 | |
| Absent | 63 | 41 | 30 | 25 | |
| Pelvis metastasis | .827 | ||||
| Present | 58 | 39 | 25 | 22 | |
| Absent | 62 | 42 | 29 | 25 | |
| Time from diagnosis to surgery | .244 | ||||
| Delayed (>1 mo) | 58 | 37 | 23 | 19 | |
| Immediate (≤1 mo) | 62 | 45 | 30 | 26 | |
| Ambulation recovery | <.001a | ||||
| Failed | 32 | 15 | 6 | 6 | |
| Recovered | 71 | 53 | 38 | 32 | |
| Factors . | 6 mo (%) . | 1 y (%) . | 2 y (%) . | 3 y (%) . | p value . |
|---|---|---|---|---|---|
| General status | |||||
| Age | .243 | ||||
| >60 yr | 61 | 46 | 30 | 27 | |
| ≤60 yr | 35 | 35 | 25 | 22 | |
| Sex | .126 | ||||
| Male | 31 | 31 | 25 | 23 | |
| Female | 96 | 91 | 81 | 72 | |
| Premorbid performance status | <.001a | ||||
| Unfavorable (ECOG 3–4) | 32 | 18 | 6 | 6 | |
| Favorable (ECOG 0–2) | 70 | 52 | 37 | 32 | |
| Premorbid ambulatory status | <.001a | ||||
| Nonambulatory | 34 | 20 | 9 | 9 | |
| Ambulatory | 73 | 50 | 35 | 30 | |
| Cancer burden | |||||
| Primary cancer | <.001a | ||||
| Unfavorable | 50 | 32 | 20 | 17 | |
| Favorable | 84 | 75 | 55 | 50 | |
| Number of metastases | .008a | ||||
| Multiple | 54 | 37 | 25 | 20 | |
| Single | 71 | 60 | 37 | 37 | |
| Visceral metastasis | .106 | ||||
| Present | 57 | 38 | 25 | 18 | |
| Absent | 68 | 46 | 31 | 30 | |
| Local factors | |||||
| Fracture type | .003a | ||||
| Complete | 50 | 33 | 22 | 19 | |
| Impending | 66 | 50 | 34 | 30 | |
| Reconstruction type | .014a | ||||
| Internal fixation | 52 | 36 | 23 | 19 | |
| Endoprosthesis | 66 | 48 | 35 | 30 | |
| Contralateral femur metastasis | .366 | ||||
| Present | 58 | 41 | 17 | 17 | |
| Absent | 63 | 41 | 30 | 25 | |
| Pelvis metastasis | .827 | ||||
| Present | 58 | 39 | 25 | 22 | |
| Absent | 62 | 42 | 29 | 25 | |
| Time from diagnosis to surgery | .244 | ||||
| Delayed (>1 mo) | 58 | 37 | 23 | 19 | |
| Immediate (≤1 mo) | 62 | 45 | 30 | 26 | |
| Ambulation recovery | <.001a | ||||
| Failed | 32 | 15 | 6 | 6 | |
| Recovered | 71 | 53 | 38 | 32 | |
Data are expressed as percentage unless otherwise specified.
aStatistically significant.
Abbreviation: ECOG, Eastern Cooperative Oncology Group.
Multivariate analysis for factors associated with survival after surgery for femur metastasis
| Factors . | Odds ratio (95% CI) . | p value . |
|---|---|---|
| Cancer type | <.001a | |
| Favorable | .416 (0.27–0.63) | |
| Unfavorable | Ref | |
| Metastasis | .001a | |
| Single | .527 (0.36–0.76) | |
| Multiple | Ref | |
| Fracture type | .01a | |
| Impending | .670 (0.49–0.90) | |
| Complete | Ref | |
| Type of surgery | .047a | |
| Endoprosthesis | .731 (0.53–0.99) | |
| Internal fixation | Ref | |
| Ambulation recovery | <.001a | |
| Recovered | .477 (0.32–0.69) | |
| Failed | Ref | |
| Premorbid ECOG | .003a | |
| Favorable | .572 (0.39–0.82) | |
| Unfavorable | Ref |
| Factors . | Odds ratio (95% CI) . | p value . |
|---|---|---|
| Cancer type | <.001a | |
| Favorable | .416 (0.27–0.63) | |
| Unfavorable | Ref | |
| Metastasis | .001a | |
| Single | .527 (0.36–0.76) | |
| Multiple | Ref | |
| Fracture type | .01a | |
| Impending | .670 (0.49–0.90) | |
| Complete | Ref | |
| Type of surgery | .047a | |
| Endoprosthesis | .731 (0.53–0.99) | |
| Internal fixation | Ref | |
| Ambulation recovery | <.001a | |
| Recovered | .477 (0.32–0.69) | |
| Failed | Ref | |
| Premorbid ECOG | .003a | |
| Favorable | .572 (0.39–0.82) | |
| Unfavorable | Ref |
aStatistically significant.
Abbreviations: CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; Ref, reference.
Multivariate analysis for factors associated with survival after surgery for femur metastasis
| Factors . | Odds ratio (95% CI) . | p value . |
|---|---|---|
| Cancer type | <.001a | |
| Favorable | .416 (0.27–0.63) | |
| Unfavorable | Ref | |
| Metastasis | .001a | |
| Single | .527 (0.36–0.76) | |
| Multiple | Ref | |
| Fracture type | .01a | |
| Impending | .670 (0.49–0.90) | |
| Complete | Ref | |
| Type of surgery | .047a | |
| Endoprosthesis | .731 (0.53–0.99) | |
| Internal fixation | Ref | |
| Ambulation recovery | <.001a | |
| Recovered | .477 (0.32–0.69) | |
| Failed | Ref | |
| Premorbid ECOG | .003a | |
| Favorable | .572 (0.39–0.82) | |
| Unfavorable | Ref |
| Factors . | Odds ratio (95% CI) . | p value . |
|---|---|---|
| Cancer type | <.001a | |
| Favorable | .416 (0.27–0.63) | |
| Unfavorable | Ref | |
| Metastasis | .001a | |
| Single | .527 (0.36–0.76) | |
| Multiple | Ref | |
| Fracture type | .01a | |
| Impending | .670 (0.49–0.90) | |
| Complete | Ref | |
| Type of surgery | .047a | |
| Endoprosthesis | .731 (0.53–0.99) | |
| Internal fixation | Ref | |
| Ambulation recovery | <.001a | |
| Recovered | .477 (0.32–0.69) | |
| Failed | Ref | |
| Premorbid ECOG | .003a | |
| Favorable | .572 (0.39–0.82) | |
| Unfavorable | Ref |
aStatistically significant.
Abbreviations: CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; Ref, reference.
The Influence of Ambulation Recovery on Further Treatment
Patients who recovered ambulation were more likely to undergo chemotherapy postoperatively than patients who failed to recover ambulation without statistical significance (79 of 165 patients [47.9%] vs. 29 of 79 patients [36.7%] respectively, p = .066). In the group that regained ambulatory function, 93 of 167 patients (47.9%, p = .066) underwent adjuvant chemotherapy after the surgical intervention. Of 93 patients with ambulation recovery, 54 (58%) resumed their previous chemotherapy or changed the regimen, and 38 (41%) began their first cycle of chemotherapy after surgical treatment. Seventy‐four patients (44%) did not undergo postoperative chemotherapy, 34 of whom (46%) (n = 34) did not have chemotherapy before the fracture. Ten (29%) of these patients had hepatocellular carcinoma and were under local treatment such as transarterial chemoembolization. Four patients (12%) presented with thigh pain as their first symptoms and were diagnosed with lung cancer after evaluation. The remaining patients had been off therapy after treatment for primary cancer and had been diagnosed with femur metastasis as their first distant metastasis. In contrast, in the group that failed to regain ambulatory function, 29 of 79 patients (36.7% p = .066) were treated with adjuvant chemotherapy. Of the 29 patients, 21 (72%) were able to resume their previous chemotherapy, and only 8 began their first chemotherapy. In this group, only two patients completed their cycle of chemotherapy, and the rest of them failed to finish the cycle.
Discussion
This study retrospectively reviewed predictors of ambulation recovery in patients who had surgical treatment for pathologic or impending fracture of femur metastases under the premise that postoperative restoration of ambulatory function is a fundamental and important factor for further management and, eventually, survival. By means of a multivariate analysis, premorbid ambulation status and ECOG PS were found to be independent predictive factors of ambulation recovery. Postoperatively, patients who regained ambulatory function showed longer survival than patients who could not.
Premorbid ambulatory status was strongly correlated with postoperative ambulatory status. Of premorbid ambulatory patients, 85% (159 of 187) remained ambulatory postoperatively, whereas only 10% (6 of 57) of premorbid nonambulatory patients became ambulatory postoperatively (p < .001; Fig. 1). Of 77 patients who were independently ambulatory in premorbid status, 68 (88%) were ambulatory postoperatively. In contrast, of 24 patients who were premorbid bedridden, only 1 (4%) was ambulatory postoperatively. Evaluation of premorbid ambulation status is a convenient and effective way of predicting postoperative ambulation recovery.
ECOG PS is a comprehensive and efficient modality for assessing the PS of patients. Nathan et al. suggested that ECOG PS is an independently significant predictor of survival after a prospective review of 191 patients who underwent surgery for metastatic bone disease [14, 15]. It reflects patients’ functional level including ability to care for themselves, daily activities, and physical ability. Among them, ambulation occupies an important part of this evaluation form, and it is natural to expect that a patient with good ECOG score is expected to have a high level of ambulatory function. From our study, this patient would have higher chance of restoration to premorbid ambulation level after surgical treatment. Lower premorbid ECOG status was also prognostic of survival after surgical treatment in a multivariate analysis (p = .003). Therefore, favorable ECOG status is a useful predictive factor of ambulatory recovery and a prognostic factor of postoperative survival. Of note, the recovery rate of good ECOG (ECOG >2) was 69% (169/244), which was similar to the recovery rate of ambulation (68%; supplemental online Table 1).
From our results, postoperative ambulation status, as a part of PS, contributes to decision making of further treatment. The surgical goal of bone metastases is durable skeletal stabilization, which reduces pain and enables immediate weight bearing, and achieving bone union at the fracture site, osteosynthesis, is not an ultimate goal. The surgeon should also consider surgical options that could minimize the impact on future treatments, such as chemotherapy and radiation therapy postoperatively [2, 3]. Forsberg et al. recently confirmed that their Bayesian model‐based survival estimation system, which could assess postoperative 1‐month and 6‐month survival, is valid for clinical use [16].
Regarding the effect of prophylactic fixation for impending fracture, even though it was not statistically significant, we could find a tendency that impending fracture group had a better outcome with ambulation recovery than pathologic fracture group (p = .055). Many studies suggested the advantage of prophylactic fixation of impending fracture in perspective of orthopedic surgeon [16-19]. Mirels suggested a scoring system with criteria such as lesion site, characteristic, and size and degree of pain. When the lesion has a score of more than 8, the metastatic lesion requires prophylactic internal fixation because of a higher risk of pathologic fracture [18]. In Arvinius et al. 's retrospective study with 65 femoral metastasis cases, preventive fixation with intramedullary nailing showed both a higher survival and ambulation rate at 6 months postoperatively [20]. Moreover, we found that patients with impending fracture had better survival compared with patients with complete pathologic fracture (Table 4). Therefore, early detection and surgery of femoral impending fractures seems important for ambulation recovery and subsequent survival in patients with femur metastases.
There was no statistical correlation between time to surgical intervention and postoperative ambulation recovery (p = .18). We also could not find statistical difference between time to surgical intervention and survival outcome (p = .244). The heterogeneity of primary cancers with different prognoses can be a possible explanation for this result. In patients with unfavorable primary cancer, 64.1% (n = 123/192) underwent surgery within a month after diagnosis of metastases, whereas 57.6% (n = 30/52) of patients with favorable primary cancers had surgery within a month of detection (p = .399). In addition, when we compared premorbid nonambulatory patients with unfavorable cancer and patients with favorable cancer, they were 31.3% (n = 60/192) and 17.3% (n = 9/52), respectively (p = .048). Therefore, it is reasonable to explain that patients with unfavorable cancer types tend to have more aggressive progression of systemic disease, which results in higher risk of loss of function. Because these patients are usually under thorough surveillance, they tend to be diagnosed earlier than patients with unfavorable cancer types and have a higher chance to immediately receive intervention, which would not necessarily promise successful ambulation recovery.
This study has several limitations. First, it was a retrospective study involving a review of medical records. Evaluation of ambulatory status was based on description of medical records, and there were excluded cases owing to lack of definite premorbid ambulatory status. Second, we defined postoperative ambulatory status as optimal status achieved postoperatively regardless of the period. We did not consider time as a criterion of postoperative ambulatory status because of the insufficient evaluation form and system of ambulation status. Therefore, we used a linear regression method to evaluate predictive factors of ambulation recovery and attempted to overcome the inability to use the Cox proportional hazard method. Third, this study includes patients treated in a relatively long period, during which the treatment and prognoses of bone metastases could have changed. For treatment, the proportion of endoprosthetic reconstruction has increased over osteosynthetic reconstruction. When we compared the three periods from 1999 to 2016 in a 5‐year interval, the proportion of endoprosthesis increased from 23.4% in 2000–2005, to 43.8% in 2006–2010, and eventually to 47.4% in 2011–2016, respectively. As for the prognoses, there was a significant increase in postoperative survival (p = .015). Finally, the measure we used to assess the patient's ambulatory status may not reflect the ambulatory function of a patient in its entirety. The use of a validated and functional measure, such as 6‐minute walk test, would have provided a better reference [21].
Conclusion
Postoperative ambulation recovery rate in our cohort was 68%. Premorbid ambulatory status and ECOG PS are predictors of ambulation recovery in patients undergoing surgery for femur metastases.
Author Contributions
Conception/design: Yongsung Kim, Ilkyu Han
Provision of study material or patients: Yongsung Kim, Chandra Kumar Krishnan, Ilkyu Han
Collection and/or assembly of data: Yongsung Kim, Chandra Kumar Krishnan, Hwan Seong Cho, Ilkyu Han
Data analysis and interpretation: Yongsung Kim, Han‐Soo Kim, Ilkyu Han
Manuscript writing: Yongsung Kim
Final approval of manuscript: Yongsung Kim, Chandra Kumar Krishnan, Han‐Soo Kim, Hwan Seong Cho, Ilkyu Han
Disclosures
The authors indicated no financial relationships.
References
Author notes
Disclosures of potential conflicts of interest may be found at the end of this article.



