-
PDF
- Split View
-
Views
-
Cite
Cite
Min‐Hee Ryu, Baek‐Yeol Ryoo, Tae Won Kim, Sung Bae Kim, Hyeong‐Seok Lim, Kyun‐Seop Bae, Sook Ryun Park, Yeong‐Woo Jo, Hyun Ju Cho, Yoon‐Koo Kang, A Phase I/IIa Study of DHP107, a Novel Oral Paclitaxel Formulation, in Patients with Advanced Solid Tumors or Gastric Cancer, The Oncologist, Volume 22, Issue 2, February 2017, Pages 129–e8, https://doi.org/10.1634/theoncologist.2016-0273
Close - Share Icon Share
Abstract
Ideally, patients should have access to an oral formulation of paclitaxel, as well as an intravenous formulation, to allow development of regimens exploring alternate schedules and to avoid reactions to Cremophor EL (BASF Corp., Ludwigshafen, Germany, https://www.basf.com).
DHP107 is a novel oral paclitaxel formulation that is a tolerable and feasible regimen for patients with gastric cancer, with data suggesting efficacy similar to that of intravenous paclitaxel.
We evaluated the maximum tolerated dose (MTD) of DHP107, a novel oral paclitaxel formulation, and the efficacy and safety of the agent in patients with advanced solid tumors.
Phase I study: cohorts of 3–6 patients with advanced solid tumors received escalating DHP107 doses. Phase IIa study: patients with measurable advanced gastric cancer received DHP107, 200 mg/m2 b.i.d., on days 1, 8, and 15 every 4 weeks. Pharmacokinetics, safety, and efficacy were analyzed.
Phase I: 17 patients received a dose‐escalating regimen of DHP107, 150–250 mg/m2 b.i.d. Dose‐limiting toxicities were neutropenia and febrile neutropenia. The MTD (recommended dose) for phase IIa was 200 mg/m2 b.i.d. Phase IIa: 11 patients with measurable advanced gastric cancer in whom first‐line therapy failed received DHP107 (MTD). Three confirmed partial responses were observed. Median progression‐free survival of gastric cancer patients (n = 16) treated at the MTD was 2.97 (95% confidence interval, 1.67–5.40) months (Fig. 1). The most frequent grade 3/4 adverse events were neutropenia (35.3%) and leukopenia (17.6%) at the MTD (phase I and IIa combined; n = 17).
DHP107 showed good antitumor efficacy and was tolerable. The MTD (200 mg/m2 b.i.d.) is recommended for use in further studies comparing DHP107 with standard intravenous paclitaxel therapy.
Discussion
DHP107, developed by Daehwa Pharmaceutical Co. Ltd., is a lipid‐based single‐agent oral paclitaxel formulation that is systemically absorbed without the need for P‐glycoprotein inhibitors or Cremophor EL (BASF Corp., Ludwigshafen, Germany, https://www.basf.com). We carried out a phase I/IIa study using a weekly regimen (days 1, 8, and 15) in which DHP107 was given b.i.d. to increase patients’ exposure to the drug to determine toxicities and the maximum tolerated dose. By using a b.i.d. regimen in this study, exposure at or above the therapeutic threshold (8.5 ng/mL) was maintained for approximately 24 hours.
In the phase I (dose‐escalation) portion, 2 of 4 patients experienced dose‐limiting toxicities (DLTs; febrile neutropenia) with DHP107, 225 mg/m2 b.i.d.; 2 of 4 patients had DLTs (neutropenia and febrile neutropenia) with DHP107, 250 mg/m2 b.i.d. No DLTs occurred among the 6 patients who received DHP107 200 mg/m2 b.i.d.; hence, this was considered the MTD. Overall, 200 mg/m2 was tolerable in the day 1, 8, and 15 schedule, with neutropenia as the main side effect; only 77% of patients had grade 1 or 2 diarrhea and 35% had grade 1 or 2 nausea. In the phase IIa study, 11 patients with measurable advanced gastric cancer were enrolled at the MTD for a total of 17 patients who received DHP107, 200 mg/m2 b.i.d., to allow preliminary evaluation of efficacy. On the basis of the optimal two‐stage design, depending on patients’ responses, we planned to enroll up to 17 gastric cancer patients in the phase IIa study. When 11 patients had been recruited, 3 showed confirmed PRs, providing an overall response rate of 27.3% (95% confidence interval [CI], 0.0%–54.9%). As a result, additional enrollment was discontinued, and the efficacy was considered adequate to support further phase III study.
The development of an oral formulation of paclitaxel is an important goal for patient convenience and lessening side effects; it could allow the development of novel regimens, for low‐dose, long exposure to paclitaxel. If oral paclitaxel is proven to deliver equally effective therapy, it could also replace intravenous paclitaxel in some regimens, thereby preventing infusion reactions due to Cremophor EL diluent. The current study indicates that DHP107 is active and safe enough for continued development.
Trial Information
- Disease
Advanced cancer/solid tumor
- Stage of disease/treatment
Metastatic/advanced
- Prior Therapy
No designated number of regimens
- Type of study
Phase I/IIa
- Primary Endpoint
Phase I: Maximum tolerated dose (MTD)
Phase IIa: Response rate (RR)
- Secondary Endpoint
Safety
- Secondary Endpoint
Efficacy
- Additional Details of Endpoints or Study Design
The aims of the current phase I/IIa study were to determine the MTD for repeated administration of DHP107 by weekly schedule in patients with metastatic solid tumors and to evaluate DHP107 efficacy in patients with advanced gastric cancer
- Investigator’s Analysis
Active and should be pursued further
Drug Information
- Generic/Working name
DHP107 (Oral paclitaxel)
- Company name
Daehwa Pharmaceutical Co. Ltd.
- Drug class
Tubulin/microtubules targeting agent
- Dose
200 mg/m2
- Route
p.o.
- Schedule of Administration
DHP107 was administered b.i.d. on days 1, 8, and 15 of a 28‐day cycle. The dose identified as the MTD was selected for the phase IIa portion of the study
Patient Characteristics (Phase I)
- Number of patients, male
10
- Number of patients, female
7
- Stage
IV
- Age
Median (range): 55 (30 – 67)
- Number of prior systemic therapies
Median (range): not collected
- Performance Status: ECOG
0 — 2
1 — 15
2 — 0
3 — 0
unknown — 0
- Cancer Types or Histologic Subtypes
Gastric 13
Colorectal 2
Parotid gland 1
Salivary gland 1
Patient Characteristics (Phase IIa)
- Number of patients, male
5
- Number of patients, female
6
- Age
52 (33 – 70)
- Performance Status: ECOG
0 — 1
1 — 10
2 — 0
3 — 0
unknown — 0
- Cancer Types or Histologic Subtypes
Gastric 11
Primary Assessment Method
- Test Arm: Total Patient Population (Phase I)
- Number of patients enrolled
17
- Number of patients evaluable for toxicity
17
- Number of patients evaluated for efficacy
17
- Test Arm: Total Patient Population (Phase IIa)
- Number of patients enrolled
11
- Number of patients evaluable for toxicity
11
- Number of patients evaluated for efficacy
11
- Response assessment CR
n = 0 (0%)
- Response assessment PR
n = 3 (27.3%)
- Response assessment SD
n = 3 (27.3%)
- Response assessment PD
n = 5 (45.5%)
- (Median) duration assessments PFS
2.97 months
| Time of Scheduled Assessment and/or Time of Event . | No. Progressed (or Deaths) . | No. Censored . | Percentage at Start of Evaluation Period . | Kaplan‐Meier % . | No. at Next Evaluation/No. at Risk . |
|---|---|---|---|---|---|
| Kaplan‐Meier Time units: months | |||||
| 0.00 | 0 | 0 | 100.00 | 100.00 | 16 |
| 0.73 | 1 | 0 | 93.75 | 94.12 | 15 |
| 0.97 | 1 | 0 | 87.50 | 88.24 | 14 |
| 1.63 | 1 | 0 | 81.25 | 82.35 | 13 |
| 1.67 | 1 | 0 | 75.00 | 76.47 | 12 |
| 1.70 | 1 | 0 | 68.75 | 70.59 | 11 |
| 1.80 | 1 | 1 | 62.50 | 64.17 | 9 |
| 2.60 | 1 | 0 | 55.56 | 57.75 | 8 |
| 2.97 | 1 | 0 | 48.61 | 51.34 | 7 |
| 3.30 | 1 | 0 | 41.67 | 44.92 | 6 |
| 4.20 | 1 | 0 | 34.72 | 38.50 | 5 |
| 5.13 | 1 | 0 | 27.78 | 32.09 | 4 |
| 5.40 | 1 | 0 | 20.83 | 25.67 | 3 |
| 5.57 | 1 | 0 | 13.89 | 19.25 | 2 |
| 8.17 | 0 | 1 | 19.25 | 19.25 | 1 |
| 13.07 | 0 | 1 | 19.25 | 19.25 | 0 |
| Time of Scheduled Assessment and/or Time of Event . | No. Progressed (or Deaths) . | No. Censored . | Percentage at Start of Evaluation Period . | Kaplan‐Meier % . | No. at Next Evaluation/No. at Risk . |
|---|---|---|---|---|---|
| Kaplan‐Meier Time units: months | |||||
| 0.00 | 0 | 0 | 100.00 | 100.00 | 16 |
| 0.73 | 1 | 0 | 93.75 | 94.12 | 15 |
| 0.97 | 1 | 0 | 87.50 | 88.24 | 14 |
| 1.63 | 1 | 0 | 81.25 | 82.35 | 13 |
| 1.67 | 1 | 0 | 75.00 | 76.47 | 12 |
| 1.70 | 1 | 0 | 68.75 | 70.59 | 11 |
| 1.80 | 1 | 1 | 62.50 | 64.17 | 9 |
| 2.60 | 1 | 0 | 55.56 | 57.75 | 8 |
| 2.97 | 1 | 0 | 48.61 | 51.34 | 7 |
| 3.30 | 1 | 0 | 41.67 | 44.92 | 6 |
| 4.20 | 1 | 0 | 34.72 | 38.50 | 5 |
| 5.13 | 1 | 0 | 27.78 | 32.09 | 4 |
| 5.40 | 1 | 0 | 20.83 | 25.67 | 3 |
| 5.57 | 1 | 0 | 13.89 | 19.25 | 2 |
| 8.17 | 0 | 1 | 19.25 | 19.25 | 1 |
| 13.07 | 0 | 1 | 19.25 | 19.25 | 0 |
Kaplan‐Meier curve shown in Figure 1.
Adverse Events
| All Cycles Grade . | |||||||
|---|---|---|---|---|---|---|---|
| Name . | *NC/NA . | 1 . | 2 . | 3 . | 4 . | 5 . | All Grades . |
| Neutrophil count decreased | 12% | 0% | 35% | 29% | 24% | 0% | 88% |
| White blood cell decreased | 29% | 0% | 47% | 18% | 6% | 0% | 71% |
| Anemia | 94% | 0% | 0% | 6% | 0% | 0% | 6% |
| Febrile neutropenia | 94% | 0% | 0% | 0% | 6% | 0% | 6% |
| Abdominal pain | 41% | 53% | 6% | 0% | 0% | 0% | 59% |
| Alopecia | 6% | 65% | 29% | 0% | 0% | 0% | 94% |
| Anorexia | 53% | 41% | 6% | 0% | 0% | 0% | 47% |
| Diarrhea | 23% | 59% | 18% | 0% | 0% | 0% | 77% |
| Dyspepsia | 70% | 12% | 18% | 0% | 0% | 0% | 30% |
| Fatigue | 70% | 24% | 6% | 0% | 0% | 0% | 30% |
| Fever | 70% | 24% | 6% | 0% | 0% | 0% | 30% |
| Flu‐like symptoms | 88% | 6% | 6% | 0% | 0% | 0% | 12% |
| Myalgia | 53% | 41% | 6% | 0% | 0% | 0% | 47% |
| Nausea | 65% | 29% | 6% | 0% | 0% | 0% | 35% |
| Peripheral sensory neuropathy | 82% | 12% | 6% | 0% | 0% | 0% | 18% |
| Pruritus | 82% | 18% | 0% | 0% | 0% | 0% | 18% |
| Mucositis oral | 82% | 6% | 0% | 12% | 0% | 0% | 18% |
| Vomiting | 59% | 41% | 0% | 0% | 0% | 0% | 41% |
| All Cycles Grade . | |||||||
|---|---|---|---|---|---|---|---|
| Name . | *NC/NA . | 1 . | 2 . | 3 . | 4 . | 5 . | All Grades . |
| Neutrophil count decreased | 12% | 0% | 35% | 29% | 24% | 0% | 88% |
| White blood cell decreased | 29% | 0% | 47% | 18% | 6% | 0% | 71% |
| Anemia | 94% | 0% | 0% | 6% | 0% | 0% | 6% |
| Febrile neutropenia | 94% | 0% | 0% | 0% | 6% | 0% | 6% |
| Abdominal pain | 41% | 53% | 6% | 0% | 0% | 0% | 59% |
| Alopecia | 6% | 65% | 29% | 0% | 0% | 0% | 94% |
| Anorexia | 53% | 41% | 6% | 0% | 0% | 0% | 47% |
| Diarrhea | 23% | 59% | 18% | 0% | 0% | 0% | 77% |
| Dyspepsia | 70% | 12% | 18% | 0% | 0% | 0% | 30% |
| Fatigue | 70% | 24% | 6% | 0% | 0% | 0% | 30% |
| Fever | 70% | 24% | 6% | 0% | 0% | 0% | 30% |
| Flu‐like symptoms | 88% | 6% | 6% | 0% | 0% | 0% | 12% |
| Myalgia | 53% | 41% | 6% | 0% | 0% | 0% | 47% |
| Nausea | 65% | 29% | 6% | 0% | 0% | 0% | 35% |
| Peripheral sensory neuropathy | 82% | 12% | 6% | 0% | 0% | 0% | 18% |
| Pruritus | 82% | 18% | 0% | 0% | 0% | 0% | 18% |
| Mucositis oral | 82% | 6% | 0% | 12% | 0% | 0% | 18% |
| Vomiting | 59% | 41% | 0% | 0% | 0% | 0% | 41% |
* No change from baseline/no adverse event
Hematologic adverse events occurring in >5% of patients in all cycles and nonhematologic adverse events occurring in >10% of patients in all cycles at the 200 mg/m2 dose level (n = 17).
Dose‐Limiting Toxicities
| Dose Level . | Dose of Drug: DHP107 . | Number Enrolled . | Number Evaluable for Toxicity . | Number with a Dose‐Limiting Toxicity . | Dose‐Limiting Toxicity Information . |
|---|---|---|---|---|---|
| 1 | 150 mg/m2 | 3 | 3 | 0 | |
| 2 | 200 mg/m2 | 3 | 3 | 0 | |
| 3 | 250 mg/m2 | 4 | 4 | 2 | Grade 4 neutropenia over 5 days, Grade 3 febrile neutropenia |
| 3A | 225 mg/m2 | 4 | 4 | 2 | Grade 3 febrile neutropenia |
| 2 | 200 mg/m2 | 3 | 3 | 0 |
| Dose Level . | Dose of Drug: DHP107 . | Number Enrolled . | Number Evaluable for Toxicity . | Number with a Dose‐Limiting Toxicity . | Dose‐Limiting Toxicity Information . |
|---|---|---|---|---|---|
| 1 | 150 mg/m2 | 3 | 3 | 0 | |
| 2 | 200 mg/m2 | 3 | 3 | 0 | |
| 3 | 250 mg/m2 | 4 | 4 | 2 | Grade 4 neutropenia over 5 days, Grade 3 febrile neutropenia |
| 3A | 225 mg/m2 | 4 | 4 | 2 | Grade 3 febrile neutropenia |
| 2 | 200 mg/m2 | 3 | 3 | 0 |
Assessment, Analysis, and Discussion
Paclitaxel has proven efficacy in treating a variety of cancers and is widely used to treat ovarian, gastric, breast, and non‐small cell lung cancers . Because paclitaxel has poor solubility in water, pharmaceutical agents, such as Cremophor EL (BASF Corp., Ludwigshafen, Germany), are used as a vehicle to aid intravenous administration . However, Cremophor EL can have biological implications, including hypersensitivity reactions [9]. Furthermore, it alters the pharmacokinetics of paclitaxel, causing it to have a nonlinear profile .
A number of attempts have been made to reformulate paclitaxel to make it a more convenient and safer medication. Oral administration of paclitaxel is problematic because of low bioavailability related to P‐glycoprotein (P‐gp) and other membrane proteins in the gastrointestinal mucosa, which inhibit absorption. Moreover, cytochrome P450 isoenzymes in gastrointestinal tract and liver rapidly metabolize the drug . Development of an oral formulation has focused on improving the solubility and oral bioavailability of paclitaxel. To increase systemic exposure of oral paclitaxel, it has been coadministered with an orally applicable P‐gp blocker, such as cyclosporine A . However, the oral formulation of a cytotoxic agent combined with a P‐gp blocker has disadvantages because of potential interactions with concomitant medications, including substrates for P‐gp and/or with cytochrome P450 3A [14].
DHP107, developed by Daehwa Pharmaceutical Co. Ltd., is a lipid‐based single‐agent oral paclitaxel formulation that is systemically absorbed without the need for P‐gp inhibitors or Cremophor EL [15]. An animal study of DHP107 showed it has a similar antitumor effect compared with intravenous paclitaxel in human gastric cancer xenografts [16]. A previous phase I study in patients with advanced solid tumors refractory to all standard treatments showed no dose‐limiting toxicities (DLTs) with a single dose of DHP107 ranging from 60 to 600 mg/m2. DHP107 pharmacokinetics did not increase proportionally, and pharmacokinetic profiles, including area under the plasma concentration–time curve (AUC) and maximum plasma concentration (Cmax), plateaued at doses above 250 mg/m2 [17].
Intravenous paclitaxel has been one of the most commonly used salvage chemotherapies in gastric cancer. Although there has been no phase III comparative study of weekly paclitaxel versus every‐3‐weeks paclitaxel in gastric cancer, a phase II study of weekly paclitaxel showed antitumor effects similar to historical data for a every‐3‐weeks regimen as salvage chemotherapy [18]. With frequent use of weekly intravenous paclitaxel in gastric cancer and with the lower AUC and Cmax of a single dose of DHP107 compared with every‐3‐weeks intravenous paclitaxel [17], a weekly schedule of DHP107 was adopted in the current study.
The aims of the current phase I/IIa study were to determine the maximum tolerated dose (MTD) for repeated administration of DHP107 by weekly schedule in patients with metastatic solid tumors and to evaluate DHP107 efficacy in patients with advanced gastric cancer. The results of this study will guide phase III studies to compare the safety and efficacy of DHP107 versus intravenous paclitaxel.
Therefore, we carried out this phase I/IIa study using a weekly regimen (days 1, 8, and 15) in which DHP107 was given as a divided dose on the treatment day to increase patients’ exposure to the drug to allow determination of DLTs and the MTD.
In the phase I (dose‐escalation) portion of our study, 2 of 4 patients experienced DLTs (febrile neutropenia) with DHP107, 225 mg/m2 b.i.d., and 2 of 4 patients had DLTs (neutropenia and febrile neutropenia) with DHP107, 250 mg/m2 b.i.d. No DLTs occurred among the 6 patients who received DHP107, 200 mg/m2 b.i.d.; hence, this was determined as the MTD. After enrollment of additional patients, a total of 17 patients received DHP107, 200 mg/m2 b.i.d. At this dose level, 1 patient experienced febrile neutropenia and only 3 experienced grade 3/4 neutropenia in whole cycles. As a result, the dose was deemed tolerable. The most frequent non‐hematologic toxicities at the MTD were alopecia, diarrhea, and anorexia, which were generally of mild severity (grade 1/2). In the previously reported phase I study, 4 of 21 patients (19.0%) receiving a single administration of DHP107 at doses above 300 mg/m2 experienced grade 3 diarrhea; the grade of diarrhea seemed to increase with dose [17]. It is likely that the lipid‐based formulation of DHP107 leads to the increased incidence of diarrhea.
The pharmacokinetic parameters of DHP107, such as Cmax and AUCinf, were not linear in the dose range of 150–250 mg/m2 b.i.d. However, when the values of AUCinf in the phase IIa study were standardized with administered dose and compared with the data from the previous phase I study [17], there was no significant difference between dose‐normalized AUCinf values (Student’s t test, p = 0.954). Mean Tmax was 2.7 hours; hence, the first dose will not interfere with the pharmacokinetics—in particular Cmax—of a second dose given after a 10‐hour interval. These pharmacokinetic characteristics are thought to be related to the specific absorption mechanism of DHP107 based on the lipid drug‐delivery system. The muco‐adhesiveness of the formulation in the gastrointestinal tract—especially in the stomach and upper intestine—inhibits absorption of the second dose of paclitaxel [20]. However, by using a b.i.d. regimen in this study, exposure at or above the therapeutic threshold (8.5 ng/mL) was maintained for approximately 24 hours [20]. In addition, no patients in the current study experienced severe diarrhea (grade 3/4) with DHP107, 200 mg/m2, a dose which is far below the dose that induced severe diarrhea in the previous phase I study. Therefore, this divided regimen of DHP107 is recommended in terms of efficacy and safety.
Gastrectomy is widely used as a standard therapy for patients with gastric cancer, and many patients with advanced gastric cancer will have undergone partial or total gastrectomy. The pharmacokinetic parameters of DHP107 were compared between gastrectomy and nongastrectomy patients with gastric cancer who received the 200 mg/m2 b.i.d. dose. Tmax values in the gastrectomy group were significantly lower (i.e., the drug was absorbed more rapidly) than in the nongastrectomy group; however, AUCinf and Cmax showed no significant difference by gastrectomy status. Therefore, the bioavailability of DHP107 is considered not to be affected by gastrectomy.
The efficacy results suggested that DHP107 is comparable to intravenous paclitaxel as a second‐line treatment in patients with advanced gastric cancer. Generally, the objective of a phase IIa study is to establish whether an intervention has sufficient efficacy against the disease to ensure further research [21]. Although the population of this study was small, based on an optimal two‐stage design, this regimen showed encouraging efficacy in poor‐prognosis patients. The overall response rate was 27.3% in the 11 patients with measurable disease; median progression‐free survival (PFS) was 2.97 months in the 16 patients with gastric cancer who received DHP107, 200 mg/m2 b.i.d. These results are in line with previous studies of intravenous weekly paclitaxel, in which response rates of 16%–24% and median PFS of 2.1–2.6 months were reported .
Paclitaxel is a cell cycle‐specific agent. Accordingly, it is expected that paclitaxel is more effective with increasing exposure time than with increasing maximum concentration. Indeed, cell line experiments demonstrated that paclitaxel is more effective with increasing exposure time . In future studies, it is anticipated that oral administration of paclitaxel may make the development of a continuous low dose regimen possible. Such regimen would allow plasma concentration to be maintained above the therapeutic threshold over extended period without the need for a break during the therapy . Positive results have also been reported for low‐dose continuous chemotherapy regimens in patients with recurrent ovarian cancer and advanced cancers of various tumor types . It is hoped that oral DHP107 will allow the development of a low‐dose continuous schedule that will reduce toxicity and increase antitumor effects.
In conclusion, DHP107 is a novel oral paclitaxel formulation that is mixed with edible oils without the need for absorption enhancers, such as P‐gp inhibitors. DHP107 is a potent and convenient chemotherapeutic agent for patients. In this study, DHP107 was a tolerable and feasible regimen for patients with gastric cancer, with efficacy that would seem to be similar to that of intravenous paclitaxel. On the basis of the results from this study, a phase III trial to assess the efficacy and safety of DHP107 compared with intravenous paclitaxel was conducted in patients with previously treated advanced gastric cancers (clinicaltrials.gov NCT01839773).
Acknowledgment
This research was supported by a grant from Daehwa Pharmaceutical Co. Ltd., and Gangwon Institute for Regional Program Evaluation by Korean government. Medical editing support was provided by Lee Miller from Miller Medical Communications Ltd. Funding for medical editing work was provided by Daehwa Pharmaceutical Co. Ltd.
Disclosures
Tae Won Kim: Merck Serono, Bayer, Roche (RF), Amgen, Eli Lilly (H); Yeong-Woo Jo: Daehwa Pharmaceutical Co. Ltd. (E); Hyun Ju Cho: Daehwa Pharmaceutical Co. Ltd. (E); Yoon-Koo Kang: Novartis (RF), Novartis, Bayer, Lilly, Sanofi, Taiho, Pfizer (H). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Figures and Tables
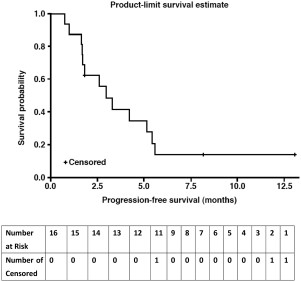
Kaplan‐Meier curve for progression‐free survival in patients with gastric cancer in the efficacy‐evaluable population (n = 16). These were patients with gastric cancer.
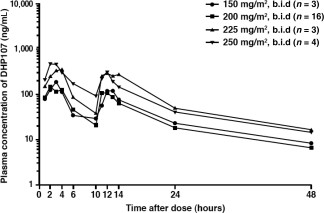
Plasma concentration of paclitaxel after oral administration of DHP107.
Unless otherwise noted, values are number (percentage) of patients.
In total, 24 patients had gastric cancer (phase I, n = 13; phase IIa, n = 11), which was classified by disease location and extent.
Abbreviation: ECOG, Eastern Cooperative Oncology Group.
Unless otherwise noted, values are number (percentage) of patients.
In total, 24 patients had gastric cancer (phase I, n = 13; phase IIa, n = 11), which was classified by disease location and extent.
Abbreviation: ECOG, Eastern Cooperative Oncology Group.
Dose‐limiting toxicity.
Dose‐limiting toxicity.
Antitumor efficacy in patients with measurable lesions in the phase IIa study (n = 11)
| Outcome . | Patients, n (%) . | 95% CI . |
|---|---|---|
| Overall response rate | 3 (27.3) | 0.0–54.9 |
| Complete response | 0 (0) | 0.0–0.0 |
| Partial response | 3 (27.3) | 0.0–54.9 |
| Stable disease | 3 (27.3) | 0.0–54.9 |
| Progressive disease | 5 (45.5) | 14.6–76.3 |
| Outcome . | Patients, n (%) . | 95% CI . |
|---|---|---|
| Overall response rate | 3 (27.3) | 0.0–54.9 |
| Complete response | 0 (0) | 0.0–0.0 |
| Partial response | 3 (27.3) | 0.0–54.9 |
| Stable disease | 3 (27.3) | 0.0–54.9 |
| Progressive disease | 5 (45.5) | 14.6–76.3 |
Abbreviation: CI, confidence interval.
Antitumor efficacy in patients with measurable lesions in the phase IIa study (n = 11)
| Outcome . | Patients, n (%) . | 95% CI . |
|---|---|---|
| Overall response rate | 3 (27.3) | 0.0–54.9 |
| Complete response | 0 (0) | 0.0–0.0 |
| Partial response | 3 (27.3) | 0.0–54.9 |
| Stable disease | 3 (27.3) | 0.0–54.9 |
| Progressive disease | 5 (45.5) | 14.6–76.3 |
| Outcome . | Patients, n (%) . | 95% CI . |
|---|---|---|
| Overall response rate | 3 (27.3) | 0.0–54.9 |
| Complete response | 0 (0) | 0.0–0.0 |
| Partial response | 3 (27.3) | 0.0–54.9 |
| Stable disease | 3 (27.3) | 0.0–54.9 |
| Progressive disease | 5 (45.5) | 14.6–76.3 |
Abbreviation: CI, confidence interval.
| Adverse event . | Grade 1 . | Grade 2 . | Grade 3 . | Grade 4 . |
|---|---|---|---|---|
| Hematologic toxicity | ||||
| Anemia | 0 (0.0) | 0 (0) | 1 (5.9) | 0 (0) |
| Febrile neutropenia | 0 (0.0) | 0 (0) | 0 (0) | 1 (5.9) |
| Leukopenia | 0 (0.0) | 8 (47.1) | 3 (17.6) | 1 (5.9) |
| Neutropenia | 0 (0.0) | 6 (35.3) | 5 (29.4) | 4 (23.5) |
| Nonhematologic toxicity | ||||
| Abdominal distension | 0 (0.0) | 1 (5.9) | 0 (0.0) | 0 (0.0) |
| Abdominal pain | 5 (29.4) | 1 (5.9) | 0 (0.0) | 0 (0.0) |
| Abdominal pain, upper | 4 (23.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Alopecia | 11 (64.7) | 5 (29.4) | 0 (0.0) | 0 (0.0) |
| Anorexia | 7 (41.2) | 1 (5.9) | 0 (0.0) | 0 (0.0) |
| Burn | 0 (0.0) | 1 (5.9) | 0 (0.0) | 0 (0.0) |
| Chill | 1 (5.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Cough | 1 (5.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Diarrhea | 10 (58.8) | 3 (17.6) | 0 (0.0) | 0 (0.0) |
| Dyspepsia | 2 (11.8) | 3 (17.6) | 0 (0.0) | 0 (0.0) |
| Edema limbs | 1 (5.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Fatigue | 4 (23.5) | 1 (5.9) | 0 (0.0) | 0 (0.0) |
| Fever | 4 (23.5) | 1 (5.9) | 0 (0.0) | 0 (0.0) |
| Flu‐like symptoms | 1 (5.9) | 1 (5.9) | 0 (0.0) | 0 (0.0) |
| Hypertension | 0 (0.0) | 1 (5.9) | 0 (0.0) | 0 (0.0) |
| Hyponatremia | 0 (0.0) | 0 (0.0) | 1 (5.9) | 0 (0.0) |
| Insomnia | 0 (0.0) | 1 (5.9) | 0 (0.0) | 0 (0.0) |
| Myalgia | 7 (41.2) | 1 (5.9) | 0 (0.0) | 0 (0.0) |
| Nausea | 5 (29.4) | 1 (5.9) | 0 (0.0) | 0 (0.0) |
| Neurodermatitis | 0 (0.0) | 1 (5.9) | 0 (0.0) | 0 (0.0) |
| Obstruction gastric | 0 (0.0) | 0 (0.0) | 1 (5.9) | 0 (0.0) |
| Peripheral sensory neuropathy | 2 (11.8) | 1 (5.9) | 0 (0.0) | 0 (0.0) |
| Pruritus | 3 (17.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Stomatitis | 1 (5.9) | 0 (0.0) | 2 (11.8) | 0 (0.0) |
| Vomiting | 7 (41.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Adverse event . | Grade 1 . | Grade 2 . | Grade 3 . | Grade 4 . |
|---|---|---|---|---|
| Hematologic toxicity | ||||
| Anemia | 0 (0.0) | 0 (0) | 1 (5.9) | 0 (0) |
| Febrile neutropenia | 0 (0.0) | 0 (0) | 0 (0) | 1 (5.9) |
| Leukopenia | 0 (0.0) | 8 (47.1) | 3 (17.6) | 1 (5.9) |
| Neutropenia | 0 (0.0) | 6 (35.3) | 5 (29.4) | 4 (23.5) |
| Nonhematologic toxicity | ||||
| Abdominal distension | 0 (0.0) | 1 (5.9) | 0 (0.0) | 0 (0.0) |
| Abdominal pain | 5 (29.4) | 1 (5.9) | 0 (0.0) | 0 (0.0) |
| Abdominal pain, upper | 4 (23.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Alopecia | 11 (64.7) | 5 (29.4) | 0 (0.0) | 0 (0.0) |
| Anorexia | 7 (41.2) | 1 (5.9) | 0 (0.0) | 0 (0.0) |
| Burn | 0 (0.0) | 1 (5.9) | 0 (0.0) | 0 (0.0) |
| Chill | 1 (5.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Cough | 1 (5.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Diarrhea | 10 (58.8) | 3 (17.6) | 0 (0.0) | 0 (0.0) |
| Dyspepsia | 2 (11.8) | 3 (17.6) | 0 (0.0) | 0 (0.0) |
| Edema limbs | 1 (5.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Fatigue | 4 (23.5) | 1 (5.9) | 0 (0.0) | 0 (0.0) |
| Fever | 4 (23.5) | 1 (5.9) | 0 (0.0) | 0 (0.0) |
| Flu‐like symptoms | 1 (5.9) | 1 (5.9) | 0 (0.0) | 0 (0.0) |
| Hypertension | 0 (0.0) | 1 (5.9) | 0 (0.0) | 0 (0.0) |
| Hyponatremia | 0 (0.0) | 0 (0.0) | 1 (5.9) | 0 (0.0) |
| Insomnia | 0 (0.0) | 1 (5.9) | 0 (0.0) | 0 (0.0) |
| Myalgia | 7 (41.2) | 1 (5.9) | 0 (0.0) | 0 (0.0) |
| Nausea | 5 (29.4) | 1 (5.9) | 0 (0.0) | 0 (0.0) |
| Neurodermatitis | 0 (0.0) | 1 (5.9) | 0 (0.0) | 0 (0.0) |
| Obstruction gastric | 0 (0.0) | 0 (0.0) | 1 (5.9) | 0 (0.0) |
| Peripheral sensory neuropathy | 2 (11.8) | 1 (5.9) | 0 (0.0) | 0 (0.0) |
| Pruritus | 3 (17.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Stomatitis | 1 (5.9) | 0 (0.0) | 2 (11.8) | 0 (0.0) |
| Vomiting | 7 (41.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
Values are expressed as number (percentage) of patients.
| Adverse event . | Grade 1 . | Grade 2 . | Grade 3 . | Grade 4 . |
|---|---|---|---|---|
| Hematologic toxicity | ||||
| Anemia | 0 (0.0) | 0 (0) | 1 (5.9) | 0 (0) |
| Febrile neutropenia | 0 (0.0) | 0 (0) | 0 (0) | 1 (5.9) |
| Leukopenia | 0 (0.0) | 8 (47.1) | 3 (17.6) | 1 (5.9) |
| Neutropenia | 0 (0.0) | 6 (35.3) | 5 (29.4) | 4 (23.5) |
| Nonhematologic toxicity | ||||
| Abdominal distension | 0 (0.0) | 1 (5.9) | 0 (0.0) | 0 (0.0) |
| Abdominal pain | 5 (29.4) | 1 (5.9) | 0 (0.0) | 0 (0.0) |
| Abdominal pain, upper | 4 (23.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Alopecia | 11 (64.7) | 5 (29.4) | 0 (0.0) | 0 (0.0) |
| Anorexia | 7 (41.2) | 1 (5.9) | 0 (0.0) | 0 (0.0) |
| Burn | 0 (0.0) | 1 (5.9) | 0 (0.0) | 0 (0.0) |
| Chill | 1 (5.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Cough | 1 (5.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Diarrhea | 10 (58.8) | 3 (17.6) | 0 (0.0) | 0 (0.0) |
| Dyspepsia | 2 (11.8) | 3 (17.6) | 0 (0.0) | 0 (0.0) |
| Edema limbs | 1 (5.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Fatigue | 4 (23.5) | 1 (5.9) | 0 (0.0) | 0 (0.0) |
| Fever | 4 (23.5) | 1 (5.9) | 0 (0.0) | 0 (0.0) |
| Flu‐like symptoms | 1 (5.9) | 1 (5.9) | 0 (0.0) | 0 (0.0) |
| Hypertension | 0 (0.0) | 1 (5.9) | 0 (0.0) | 0 (0.0) |
| Hyponatremia | 0 (0.0) | 0 (0.0) | 1 (5.9) | 0 (0.0) |
| Insomnia | 0 (0.0) | 1 (5.9) | 0 (0.0) | 0 (0.0) |
| Myalgia | 7 (41.2) | 1 (5.9) | 0 (0.0) | 0 (0.0) |
| Nausea | 5 (29.4) | 1 (5.9) | 0 (0.0) | 0 (0.0) |
| Neurodermatitis | 0 (0.0) | 1 (5.9) | 0 (0.0) | 0 (0.0) |
| Obstruction gastric | 0 (0.0) | 0 (0.0) | 1 (5.9) | 0 (0.0) |
| Peripheral sensory neuropathy | 2 (11.8) | 1 (5.9) | 0 (0.0) | 0 (0.0) |
| Pruritus | 3 (17.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Stomatitis | 1 (5.9) | 0 (0.0) | 2 (11.8) | 0 (0.0) |
| Vomiting | 7 (41.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Adverse event . | Grade 1 . | Grade 2 . | Grade 3 . | Grade 4 . |
|---|---|---|---|---|
| Hematologic toxicity | ||||
| Anemia | 0 (0.0) | 0 (0) | 1 (5.9) | 0 (0) |
| Febrile neutropenia | 0 (0.0) | 0 (0) | 0 (0) | 1 (5.9) |
| Leukopenia | 0 (0.0) | 8 (47.1) | 3 (17.6) | 1 (5.9) |
| Neutropenia | 0 (0.0) | 6 (35.3) | 5 (29.4) | 4 (23.5) |
| Nonhematologic toxicity | ||||
| Abdominal distension | 0 (0.0) | 1 (5.9) | 0 (0.0) | 0 (0.0) |
| Abdominal pain | 5 (29.4) | 1 (5.9) | 0 (0.0) | 0 (0.0) |
| Abdominal pain, upper | 4 (23.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Alopecia | 11 (64.7) | 5 (29.4) | 0 (0.0) | 0 (0.0) |
| Anorexia | 7 (41.2) | 1 (5.9) | 0 (0.0) | 0 (0.0) |
| Burn | 0 (0.0) | 1 (5.9) | 0 (0.0) | 0 (0.0) |
| Chill | 1 (5.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Cough | 1 (5.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Diarrhea | 10 (58.8) | 3 (17.6) | 0 (0.0) | 0 (0.0) |
| Dyspepsia | 2 (11.8) | 3 (17.6) | 0 (0.0) | 0 (0.0) |
| Edema limbs | 1 (5.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Fatigue | 4 (23.5) | 1 (5.9) | 0 (0.0) | 0 (0.0) |
| Fever | 4 (23.5) | 1 (5.9) | 0 (0.0) | 0 (0.0) |
| Flu‐like symptoms | 1 (5.9) | 1 (5.9) | 0 (0.0) | 0 (0.0) |
| Hypertension | 0 (0.0) | 1 (5.9) | 0 (0.0) | 0 (0.0) |
| Hyponatremia | 0 (0.0) | 0 (0.0) | 1 (5.9) | 0 (0.0) |
| Insomnia | 0 (0.0) | 1 (5.9) | 0 (0.0) | 0 (0.0) |
| Myalgia | 7 (41.2) | 1 (5.9) | 0 (0.0) | 0 (0.0) |
| Nausea | 5 (29.4) | 1 (5.9) | 0 (0.0) | 0 (0.0) |
| Neurodermatitis | 0 (0.0) | 1 (5.9) | 0 (0.0) | 0 (0.0) |
| Obstruction gastric | 0 (0.0) | 0 (0.0) | 1 (5.9) | 0 (0.0) |
| Peripheral sensory neuropathy | 2 (11.8) | 1 (5.9) | 0 (0.0) | 0 (0.0) |
| Pruritus | 3 (17.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Stomatitis | 1 (5.9) | 0 (0.0) | 2 (11.8) | 0 (0.0) |
| Vomiting | 7 (41.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
Values are expressed as number (percentage) of patients.
| DHP107 dose (mg/m2 b.i.d.) . | Patients (n) . | AUCinf (ng·h/mL) . | Cmax, 0–10h (ng/mL) . | CL/F (L/h/m2) . | Vz/F (L/m2) . |
|---|---|---|---|---|---|
| 150 | 3 | 2,064.11 (541.85) | 200.33 (52.52) | 152.61 (42.01) | 2,778.37 (1,290.61) |
| 200 | 17 | 2,180.44 (1,708.05) | 213.42 (108.49) | 265.20 (135.40) | 5,112.16 (2,975.55) |
| 225 | 3 | 5,088.09 (4,546.75) | 381.87 (300.32) | 136.32 (80.39) | 2,318.43 (1,660.95) |
| 250 | 4 | 4,830.53 (1,853.75) | 574.97 (468.68) | 114.69 (40.59) | 2,249.34 (1,826.00) |
| DHP107 dose (mg/m2 b.i.d.) . | Patients (n) . | AUCinf (ng·h/mL) . | Cmax, 0–10h (ng/mL) . | CL/F (L/h/m2) . | Vz/F (L/m2) . |
|---|---|---|---|---|---|
| 150 | 3 | 2,064.11 (541.85) | 200.33 (52.52) | 152.61 (42.01) | 2,778.37 (1,290.61) |
| 200 | 17 | 2,180.44 (1,708.05) | 213.42 (108.49) | 265.20 (135.40) | 5,112.16 (2,975.55) |
| 225 | 3 | 5,088.09 (4,546.75) | 381.87 (300.32) | 136.32 (80.39) | 2,318.43 (1,660.95) |
| 250 | 4 | 4,830.53 (1,853.75) | 574.97 (468.68) | 114.69 (40.59) | 2,249.34 (1,826.00) |
Unless otherwise noted, data are mean (standard deviation).
Abbreviations: AUC, area under the plasma concentration‐time curve; CL/F, apparent total clearance of the drug from plasma after oral administration; Cmax, maximum plasma concentration; Vz/F, apparent volume of distribution during terminal phase after non‐intravenous administration.
| DHP107 dose (mg/m2 b.i.d.) . | Patients (n) . | AUCinf (ng·h/mL) . | Cmax, 0–10h (ng/mL) . | CL/F (L/h/m2) . | Vz/F (L/m2) . |
|---|---|---|---|---|---|
| 150 | 3 | 2,064.11 (541.85) | 200.33 (52.52) | 152.61 (42.01) | 2,778.37 (1,290.61) |
| 200 | 17 | 2,180.44 (1,708.05) | 213.42 (108.49) | 265.20 (135.40) | 5,112.16 (2,975.55) |
| 225 | 3 | 5,088.09 (4,546.75) | 381.87 (300.32) | 136.32 (80.39) | 2,318.43 (1,660.95) |
| 250 | 4 | 4,830.53 (1,853.75) | 574.97 (468.68) | 114.69 (40.59) | 2,249.34 (1,826.00) |
| DHP107 dose (mg/m2 b.i.d.) . | Patients (n) . | AUCinf (ng·h/mL) . | Cmax, 0–10h (ng/mL) . | CL/F (L/h/m2) . | Vz/F (L/m2) . |
|---|---|---|---|---|---|
| 150 | 3 | 2,064.11 (541.85) | 200.33 (52.52) | 152.61 (42.01) | 2,778.37 (1,290.61) |
| 200 | 17 | 2,180.44 (1,708.05) | 213.42 (108.49) | 265.20 (135.40) | 5,112.16 (2,975.55) |
| 225 | 3 | 5,088.09 (4,546.75) | 381.87 (300.32) | 136.32 (80.39) | 2,318.43 (1,660.95) |
| 250 | 4 | 4,830.53 (1,853.75) | 574.97 (468.68) | 114.69 (40.59) | 2,249.34 (1,826.00) |
Unless otherwise noted, data are mean (standard deviation).
Abbreviations: AUC, area under the plasma concentration‐time curve; CL/F, apparent total clearance of the drug from plasma after oral administration; Cmax, maximum plasma concentration; Vz/F, apparent volume of distribution during terminal phase after non‐intravenous administration.
ClinicalTrials.gov Identifier: NCT02890511
Sponsor: Daehwa Pharmaceutical Co. Ltd.
Principal Investigator: Yoon-Koo Kang
IRB Approved: Yes



