-
PDF
- Split View
-
Views
-
Cite
Cite
Peter Dall, Thorsten Koch, Thomas Göhler, Johannes Selbach, Andreas Ammon, Jochen Eggert, Nidal Gazawi, Daniela Rezek, Arthur Wischnik, Carsten Hielscher, Stella Keitel, Ursula Cirrincione, Axel Hinke, Gabriele Feisel‐Schwickardi, Trastuzumab in Human Epidermal Growth Factor Receptor 2‐Positive Early Breast Cancer: Results of a Prospective, Noninterventional Study on Routine Treatment Between 2006 and 2012 in Germany, The Oncologist, Volume 22, Issue 2, February 2017, Pages 131–138, https://doi.org/10.1634/theoncologist.2016-0193
Close - Share Icon Share
Abstract
Trastuzumab is part of the standard treatment in patients with human epidermal growth factor receptor 2‐positive early breast cancer in addition to (neo)adjuvant chemotherapy. This German prospective noninterventional study, which included major patient cohorts underrepresented in the pivotal randomized studies, examined the generalizability of the results of those studies.
Between 2006 and 2012, 4,027 patients were enrolled and treated with trastuzumab; they were unselected regarding age or concomitant/sequential adjuvant chemotherapy. Long‐term outcome data were obtained in yearly intervals. All analyses were descriptive in nature.
Among 3,940 evaluable patients, 26% were elderly (older than 65 years of age). More than half of the population had pN0 tumor stage. Ninety‐four percent received chemotherapy: 78% as adjuvant treatment and 14% as neoadjuvant treatment, 2% both. Anthracyclines were administered in 87% and taxanes in 66%. Trastuzumab was stopped prematurely in 9% (because of cardiotoxicity in 3.5%). Recurrence‐free survival was 90.0% (95% confidence interval [CI], 88.9%–91.1%) and 82.8% (95% CI, 81.2%–84.4%) after 3 and 5 years, respectively. The corresponding figures for overall survival were 96.8% (95% CI, 96.1%–97.6%) and 90.0% (95% CI, 88.6%–91.4%). Pathological primary tumor size, lymph node involvement, and hormone receptor status had the greatest independent effect on recurrence risk. Cardiac function toxicity of National Cancer Institute common toxicity criteria grade ≥2 and ≥3 was observed in 2.5% and less than 1% of patients, respectively.
The maturing follow‐up data seem to confirm the beneficial results of trastuzumab treatment for early breast cancer from the randomized studies. Moreover, these findings support use of trastuzumab‐based therapy in patients groups less commonly included in the phase III trials (e.g., elderly patients and those with stage I disease).
On the basis of the results of large pivotal phase III studies, the inclusion of trastuzumab in adjuvant treatment regimens for human epidermal growth factor receptor 2‐positive breast cancer is standard of care. However, in these trials, elderly patients, those with comorbidities, and/or those with contraindications or refusal of cytotoxic chemotherapy are typically underrepresented. This study provides data on observed treatment options, outcomes, and risks in a wider, unselected patient population (including more than 1,000 patients with stage I disease), treated routinely in several institutions of varying size and location across Germany.
Introduction
More than a decade after the introduction of trastuzumab as the first monoclonal antibody in breast cancer treatment, and 10 years after its registration in the curative disease setting, this drug remains the only approved human epidermal growth factor receptor 2 (HER2)‐targeting adjuvant agent . This is predominantly based on the findings from four large randomized trials showing a robust improvement in prognosis, and even a change of the natural history [8], in patients with this high‐risk subtype of breast cancer.
However, these trial results, and thus the approval of trastuzumab (Herceptin, F. Hoffmann‐La Roche, Basel, Switzerland, http://www.roche.com), reflect the findings in a selected group of patients based on predefined inclusion criteria. This resulted in the well‐known situation of a lack of reliable data in major, and important, patient subgroups [9]. Typically, the large population of elderly patients is distinctly underrepresented in pivotal trials. Although approximately 10,000 patients were randomly assigned to these studies, only approximately 1,000 of them were age 60 years or older . Moreover, patient cohorts with small or low‐risk tumors or those with significant concomitant diseases were excluded [13].
In addition, because of individual patient requirements, the course of trastuzumab‐based treatment may often differ from the manner tested in the large trials. This may apply to the concomitant antineoplastic treatment (i.e., chemotherapy and endocrine agents), the sequence of these therapies, and their duration and dosage. During controlled trials, accompanying diagnostic procedures, such as cardiac monitoring, must be strictly followed; this may not be the case in general routine practice. Moreover, the duration of long‐term follow‐up may be limited according to protocol specifications, especially in the case of primary objectives already met at early interim analyses, as happened in the pivotal trastuzumab trials.
Postregistration observational studies are an established tool to fill these major gaps in evidence, at least to some extent [14]. Therefore, we performed a prospective, long‐term, noninterventional study to obtain data on the routine treatment of unselected patients with early stage HER2‐positive (HER2+) breast cancer in Germany, in order to examine the outcome in a wider population and in specific, hitherto insufficiently examined prognostic or treatment subgroups.
Materials and Methods
Patient Population and Methods of Observation
The project fulfilled the criteria for a noninterventional study according to the European Community and German legislation. Therefore, it required neither an ethical committee vote nor informed consent of the patients when started in 2006 [15]. Patients with early breast cancer who received trastuzumab after its registration in Germany in 2006 were eligible for this observation study. For inclusion into the analysis, HER2 positivity had to be confirmed according to the prescribing information: as 3+ staining on immunohistochemistry (IHC) or a positive result on fluorescence in situ hybridization in case of IHC 2+ staining. There were no restrictions with respect to previous or concomitant (neo)adjuvant treatments (endocrine therapy or chemotherapy). Investigators were asked to report on a prespecified number of consecutive patients fulfilling these criteria.
Patients were treated according to routine practice of the respective institution, and findings were prospectively documented on standardized case report forms. There were no further stipulations regarding individual diagnostic and therapeutic procedures before and after patient registration. Trastuzumab was administered intravenously in all patients. The course of disease and treatment were closely monitored, either until (premature) discontinuation of trastuzumab therapy for whatever reason or for the recommended treatment period of 12 months. Major long‐term data on surviving patients were regularly retrieved thereafter via fax questionnaires. Adverse drug reactions (i.e., events with a causal relationship to trastuzumab rated as at least “possible”) were recorded according to the regulations of the German drug law. Database closure for the present analysis was October 2013, with final data on the patients’ characteristics and the treatment period available, but with long‐term follow‐up still ongoing.
Endpoint Evaluation and Statistical Analysis
The post‐therapeutic disease course, including disease recurrence, was assessed, on the basis of standard clinical procedures, at the discretion and routine of the investigators/participating institutions, without formal requirements on restaging procedures. Relapse‐free survival (RFS) and overall survival (OS) were calculated as the time between the baseline assessment before the first trastuzumab administration and the respective event. Surviving patients (without relapse, for RFS) were censored at the last valid observation point. Safety data were collected during the 12‐month period of detailed documentation, but events reported afterward were also included in the analysis.
Event‐related endpoints were analyzed by using the Kaplan‐Meier method, providing 95% confidence intervals (CIs) for event‐free proportions at specific time points. Univariable analyses comparing subgroups were performed by using the log rank test [16]; hazard ratios (HRs) with 95% CIs were derived from Cox proportional hazards models [17]. All prognostic factors with an associated p value < .1 in univariable analysis were included in a multivariable Cox proportional hazards model of RFS. By backward selection, variables with p > .05 were removed step by step so that the final model contained only covariates with a p value ≤ .05. All statistical analyses were of an exploratory nature, with p ≤ .05 considered to indicate a significant difference, with no adjustments for multiplicity applied. All reported p values are two sided. Analyses were performed using S‐PLUS (Insightful Corp., Seattle, WA, USA) and R (https://www.r-project.org)
Results
Study Population and Baseline Characteristics
Between September 2006 and July 2011, a total of 4,027 patients starting adjuvant trastuzumab treatment were prospectively identified and their data subsequently recorded in 339 clinics and practices across Germany. For the sake of homogeneity, clearly ineligible patients (26 patients with M1 disease, 64 patients subsequently assessed as HER2 negative; 3 patients meeting both of these criteria) were excluded, leaving 3,940 patients with HER2+ breast cancer for this analysis.
Patient and tumor characteristics are shown in Table 1. Of note, a high percentage of elderly patients was recruited: Twenty‐six percent were 65 years or older, and half of these were older than age 70 years. Echocardiography had been performed at baseline in 83% of patients. Quantitative data on cardiac function were reported in 64% of patients only. At least one finding rated as pathological with respect to cardiac diagnostics (echocardiography or electrocardiography) was present in 230 patients (7%).
Patient and tumor characteristics
| Characteristic . | Value . |
|---|---|
| Median age (range), yr | 56 (20–100) |
| Median body weight (range), kg | 70 (40–178) |
| ECOG performance status | |
| 0 | 2,411 (62) |
| 1 | 1,412 (36) |
| 2–4 | 70 (2) |
| Primary tumor stage | |
| pTis | 306 (8) |
| pT1 | 1,559 (40) |
| pT2 | 1,559 (40) |
| pT3 | 205 (5) |
| pT4 | 124 (3) |
| TX | 101 (3) |
| Lymph node stage | |
| pN0 | 2,065 (53) |
| pN1 | 1,056 (27) |
| pN2 | 439 (11) |
| pN3 | 272 (7) |
| NX | 94 (2) |
| Involved nodes, mean number | 2.2 |
| Grading | |
| G1 | 109 (3) |
| G2 | 1,747 (45) |
| G3 | 2,030 (52) |
| GX | 36 (1) |
| Hormone‐receptor status | |
| Estrogen receptor‐positive | 2,364 (60) |
| Progesterone receptor‐positive | 1,931 (49) |
| At least one positive | 2,490 (63) |
| IHC staining for HER2 | |
| 2+/FISH/CISH positive | 422 (11) |
| Pathological finding on echocardiography and/or ECG | 260 (7) |
| Median LVEF (range), %a | 65 (10–98) |
| Characteristic . | Value . |
|---|---|
| Median age (range), yr | 56 (20–100) |
| Median body weight (range), kg | 70 (40–178) |
| ECOG performance status | |
| 0 | 2,411 (62) |
| 1 | 1,412 (36) |
| 2–4 | 70 (2) |
| Primary tumor stage | |
| pTis | 306 (8) |
| pT1 | 1,559 (40) |
| pT2 | 1,559 (40) |
| pT3 | 205 (5) |
| pT4 | 124 (3) |
| TX | 101 (3) |
| Lymph node stage | |
| pN0 | 2,065 (53) |
| pN1 | 1,056 (27) |
| pN2 | 439 (11) |
| pN3 | 272 (7) |
| NX | 94 (2) |
| Involved nodes, mean number | 2.2 |
| Grading | |
| G1 | 109 (3) |
| G2 | 1,747 (45) |
| G3 | 2,030 (52) |
| GX | 36 (1) |
| Hormone‐receptor status | |
| Estrogen receptor‐positive | 2,364 (60) |
| Progesterone receptor‐positive | 1,931 (49) |
| At least one positive | 2,490 (63) |
| IHC staining for HER2 | |
| 2+/FISH/CISH positive | 422 (11) |
| Pathological finding on echocardiography and/or ECG | 260 (7) |
| Median LVEF (range), %a | 65 (10–98) |
Unless otherwise noted, values are the number (percentage) of patients. Total patient numbers may deviate from n=3940 in case of missing values in individual parameters.
aQuantitative data available in 2508 patients only
Abbreviations: CISH, chromogenic in situ hybridization; ECG, electrocardiography; ECOG, Eastern Cooperative Oncology Group; FISH, fluorescent in situ hybridization; HER2, human epidermal growth factor receptor 2; IHC, immunohistochemistry; LVEF, left ventricular ejection fraction.
Patient and tumor characteristics
| Characteristic . | Value . |
|---|---|
| Median age (range), yr | 56 (20–100) |
| Median body weight (range), kg | 70 (40–178) |
| ECOG performance status | |
| 0 | 2,411 (62) |
| 1 | 1,412 (36) |
| 2–4 | 70 (2) |
| Primary tumor stage | |
| pTis | 306 (8) |
| pT1 | 1,559 (40) |
| pT2 | 1,559 (40) |
| pT3 | 205 (5) |
| pT4 | 124 (3) |
| TX | 101 (3) |
| Lymph node stage | |
| pN0 | 2,065 (53) |
| pN1 | 1,056 (27) |
| pN2 | 439 (11) |
| pN3 | 272 (7) |
| NX | 94 (2) |
| Involved nodes, mean number | 2.2 |
| Grading | |
| G1 | 109 (3) |
| G2 | 1,747 (45) |
| G3 | 2,030 (52) |
| GX | 36 (1) |
| Hormone‐receptor status | |
| Estrogen receptor‐positive | 2,364 (60) |
| Progesterone receptor‐positive | 1,931 (49) |
| At least one positive | 2,490 (63) |
| IHC staining for HER2 | |
| 2+/FISH/CISH positive | 422 (11) |
| Pathological finding on echocardiography and/or ECG | 260 (7) |
| Median LVEF (range), %a | 65 (10–98) |
| Characteristic . | Value . |
|---|---|
| Median age (range), yr | 56 (20–100) |
| Median body weight (range), kg | 70 (40–178) |
| ECOG performance status | |
| 0 | 2,411 (62) |
| 1 | 1,412 (36) |
| 2–4 | 70 (2) |
| Primary tumor stage | |
| pTis | 306 (8) |
| pT1 | 1,559 (40) |
| pT2 | 1,559 (40) |
| pT3 | 205 (5) |
| pT4 | 124 (3) |
| TX | 101 (3) |
| Lymph node stage | |
| pN0 | 2,065 (53) |
| pN1 | 1,056 (27) |
| pN2 | 439 (11) |
| pN3 | 272 (7) |
| NX | 94 (2) |
| Involved nodes, mean number | 2.2 |
| Grading | |
| G1 | 109 (3) |
| G2 | 1,747 (45) |
| G3 | 2,030 (52) |
| GX | 36 (1) |
| Hormone‐receptor status | |
| Estrogen receptor‐positive | 2,364 (60) |
| Progesterone receptor‐positive | 1,931 (49) |
| At least one positive | 2,490 (63) |
| IHC staining for HER2 | |
| 2+/FISH/CISH positive | 422 (11) |
| Pathological finding on echocardiography and/or ECG | 260 (7) |
| Median LVEF (range), %a | 65 (10–98) |
Unless otherwise noted, values are the number (percentage) of patients. Total patient numbers may deviate from n=3940 in case of missing values in individual parameters.
aQuantitative data available in 2508 patients only
Abbreviations: CISH, chromogenic in situ hybridization; ECG, electrocardiography; ECOG, Eastern Cooperative Oncology Group; FISH, fluorescent in situ hybridization; HER2, human epidermal growth factor receptor 2; IHC, immunohistochemistry; LVEF, left ventricular ejection fraction.
Trastuzumab and Concomitant Antineoplastic Treatment
In agreement with the approval specification, most patients (3,703 [94%]) received trastuzumab in combination with chemotherapy, either sequentially or concomitantly. The latter was exclusively administered as adjuvant treatment in 78% of the total population, whereas neoadjuvant treatment was applied in 14%. Two percent received cytostatics both before and after surgery. A pathologically complete remission after neoadjuvant treatment had been achieved in 36%. Two hundred thirty‐two patients (6%) received trastuzumab as single‐drug adjuvant therapy or in combination with endocrine agents only.
The chemotherapy regimens included cyclophosphamide in 88% of patients, anthracyclines (mostly epirubicin) in 87%, taxanes (predominantly docetaxel) in 66%, antimetabolites in 54%, and platinum derivatives in 9%. Fifty‐five percent of all patients received a schedule containing both anthracyclines and taxanes. A total of 2,210 patients (56%) were treated with hormonal agents. Within this subgroup, aromatase inhibitors (53%) and tamoxifen (51%) were equally frequent, whereas gonadotropin‐releasing hormone analogs were administered in 10% only. Some sort of radiotherapy was applied in 78% of the patients.
Trastuzumab was predominantly administered after completion of chemotherapy, with concurrent administration in 16% of patients only. The overall median interval between start of chemotherapy and antibody treatment was 4.5 months (interquartile range, 2.8–6.6 months). For endocrine therapy, a median of 0 months suggests a simultaneous start with trastuzumab, but there the variability was wide and the interquartile range was −1.3 to 2.9 months. The same holds for radiotherapy, with a median of −0.1 months. A median of 18 antibody administrations was recorded in detail within the observation period, indicating the predominance of the every‐3‐weeks schedule. Trastuzumab dose was modified in only 5% of the patients, whereas dose delays were more frequent at 21%. Antibody treatment was terminated prematurely in 365 patients (9%). Forty‐six percent of these decisions were due to adverse events, followed by relapse (17%) and patient’s preference or insufficient adherence (15%).
Long‐Term Outcome
After a median follow‐up time of 39 months (interquartile range, 22–60 months), a total of 453 (11.5%) RFS events were recorded. According to the Kaplan‐Meier estimation (Fig. 1A), the 3‐year and the 5‐year RFS rates amount to 90.0% (95% CI, 88.9%–91.1%) and 82.8% (95% CI, 81.2%–84.4%), respectively. OS data are less mature, with 249 (6.3%) deaths observed, warranting further follow‐up (Fig. 1B). The OS rate was 96.8% (95% CI, 96.1%–97.6%) after 3 years and 90.0% (95% CI, 88.6%–91.4%) after 5 years.
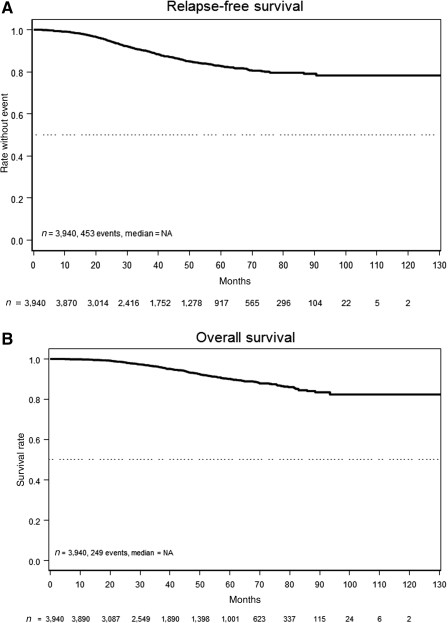
Survival rates. (A): Relapse‐free survival. (B): Overall survival.
Abbreviation: NA, not available.
Subgroups and Prognostic Analysis
The prognostic impact of major demographic and disease characteristics on RFS was assessed by univariable and multivariable analyses (Table 2). Except for body mass index, all examined parameters showed a statistically significant influence on prognosis in the expected direction. However, the overall effect of age was numerically small, and no difference was detected for the 321 youngest patients (up to 40 years of age). The most prominent impact could be shown for pT and pN tumor stage (Fig. 2A, 2B), as well as hormone receptor status (Fig. 2D). In the subgroup of 1,020 patients (26%) with a pT1N0 stage, the RFS rates after 3 and 5 years were 95.8% (95% CI, 94.3%–97.3%) and 91.1% (95% CI, 88.6%–93.7%), respectively. Among the pT1 subcategories, the corresponding RFS rates were 100% and 96% for pT1a but did not differ between pT1b and pT1c, at 95% and 90% for pT1b and 95% and 91% for pT1c. Because of the low number of patients with pT1a (n = 97), with only two events observed, the difference did not reach statistical significance (p = .24). The relapse risk grew steadily with the number of lymph nodes involved (Fig. 2C). In the multivariable regression model, only pathological primary tumor stage, lymph node involvement, and hormone receptor independently retained significance, all at p < .0001. European Cooperative Oncology Group performance status and malignancy grading had only a weak independent influence.
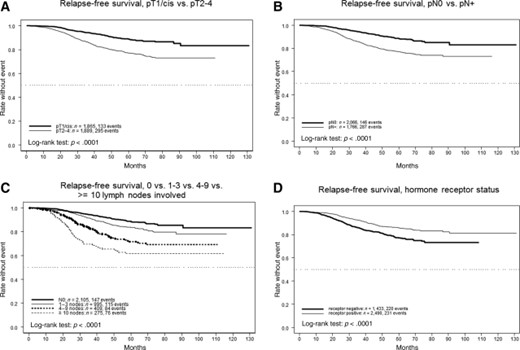
Relapse‐free survival rates per tumor stage, nodal involvement, and hormone receptor status. (A): pT1/cis versus pT2–4. (B): pN0 versus pN+. (C): 0 versus 1–3 versus 4–9 versus 10 or more lymph nodes involved. (D): Hormone receptor status.
Multivariable regression analysis of prognostic factors for relapse‐free survival
| Prognostic factora . | Univariable HR (95% CI); p value . | Multivariable full model HR (95% CI); p value . | Multivariable reduced model HR (95% CI); p value . |
|---|---|---|---|
| Age | |||
| <65 vs. ≥65 yr | 1.23 (1.00–1.50); p = .049 | NA | NA |
| ≤40 vs. >40 yr | 1.02 (0.76–1.38); p = .88 | NA | NA |
| Per year,continuous | 1.01 (1.00–1.02); p = .036 | 1.01 (0.997–1.01); p = .24 | – |
| Primary tumor: pT1/cis vs. pT2‐4 | 2.25 (1.83–2.76); p < .0001 | 1.93 (1.55–2.40); p < .0001 | 1.92 (1.55–2.38); p < .0001 |
| Lymph nodes: pN0 vs. pN+ | 2.28 (1.86–2.78); p < .0001 | 2.11 (1.71–2.61); p < .0001 | 2.11 (1.71–2.61); p < .0001 |
| Grading: G1/2 vs. G3 | 1.40 (1.16–1.69); p = .0005 | 1.19 (0.97–1.47); p = .092 | – |
| Hormone receptor status: negative vs. positive | 0.56 (0.47–0.68); p < .0001 | 0.58 (0.47–0.71); p < .0001 | 0.55 (0.46–0.67); p < 0.0001 |
| ECOG performance status: 0 vs. 1–4 | 1.22 (1.01–1.47); p = .040 | 1.11 (0.91–1.36); p = .29 | – |
| Body mass index: <25 kg/m2 vs. 25–29 kg/m2 vs. ≥30 kg/m2 | NA; p = .24 | – | – |
| Prognostic factora . | Univariable HR (95% CI); p value . | Multivariable full model HR (95% CI); p value . | Multivariable reduced model HR (95% CI); p value . |
|---|---|---|---|
| Age | |||
| <65 vs. ≥65 yr | 1.23 (1.00–1.50); p = .049 | NA | NA |
| ≤40 vs. >40 yr | 1.02 (0.76–1.38); p = .88 | NA | NA |
| Per year,continuous | 1.01 (1.00–1.02); p = .036 | 1.01 (0.997–1.01); p = .24 | – |
| Primary tumor: pT1/cis vs. pT2‐4 | 2.25 (1.83–2.76); p < .0001 | 1.93 (1.55–2.40); p < .0001 | 1.92 (1.55–2.38); p < .0001 |
| Lymph nodes: pN0 vs. pN+ | 2.28 (1.86–2.78); p < .0001 | 2.11 (1.71–2.61); p < .0001 | 2.11 (1.71–2.61); p < .0001 |
| Grading: G1/2 vs. G3 | 1.40 (1.16–1.69); p = .0005 | 1.19 (0.97–1.47); p = .092 | – |
| Hormone receptor status: negative vs. positive | 0.56 (0.47–0.68); p < .0001 | 0.58 (0.47–0.71); p < .0001 | 0.55 (0.46–0.67); p < 0.0001 |
| ECOG performance status: 0 vs. 1–4 | 1.22 (1.01–1.47); p = .040 | 1.11 (0.91–1.36); p = .29 | – |
| Body mass index: <25 kg/m2 vs. 25–29 kg/m2 vs. ≥30 kg/m2 | NA; p = .24 | – | – |
Cells filled with a dash indicate that variable is not in model.
aFirst group mentioned is reference.
Abbreviations: CI, confidence interval; HR, hazard ratio; NA, not applicable.
Multivariable regression analysis of prognostic factors for relapse‐free survival
| Prognostic factora . | Univariable HR (95% CI); p value . | Multivariable full model HR (95% CI); p value . | Multivariable reduced model HR (95% CI); p value . |
|---|---|---|---|
| Age | |||
| <65 vs. ≥65 yr | 1.23 (1.00–1.50); p = .049 | NA | NA |
| ≤40 vs. >40 yr | 1.02 (0.76–1.38); p = .88 | NA | NA |
| Per year,continuous | 1.01 (1.00–1.02); p = .036 | 1.01 (0.997–1.01); p = .24 | – |
| Primary tumor: pT1/cis vs. pT2‐4 | 2.25 (1.83–2.76); p < .0001 | 1.93 (1.55–2.40); p < .0001 | 1.92 (1.55–2.38); p < .0001 |
| Lymph nodes: pN0 vs. pN+ | 2.28 (1.86–2.78); p < .0001 | 2.11 (1.71–2.61); p < .0001 | 2.11 (1.71–2.61); p < .0001 |
| Grading: G1/2 vs. G3 | 1.40 (1.16–1.69); p = .0005 | 1.19 (0.97–1.47); p = .092 | – |
| Hormone receptor status: negative vs. positive | 0.56 (0.47–0.68); p < .0001 | 0.58 (0.47–0.71); p < .0001 | 0.55 (0.46–0.67); p < 0.0001 |
| ECOG performance status: 0 vs. 1–4 | 1.22 (1.01–1.47); p = .040 | 1.11 (0.91–1.36); p = .29 | – |
| Body mass index: <25 kg/m2 vs. 25–29 kg/m2 vs. ≥30 kg/m2 | NA; p = .24 | – | – |
| Prognostic factora . | Univariable HR (95% CI); p value . | Multivariable full model HR (95% CI); p value . | Multivariable reduced model HR (95% CI); p value . |
|---|---|---|---|
| Age | |||
| <65 vs. ≥65 yr | 1.23 (1.00–1.50); p = .049 | NA | NA |
| ≤40 vs. >40 yr | 1.02 (0.76–1.38); p = .88 | NA | NA |
| Per year,continuous | 1.01 (1.00–1.02); p = .036 | 1.01 (0.997–1.01); p = .24 | – |
| Primary tumor: pT1/cis vs. pT2‐4 | 2.25 (1.83–2.76); p < .0001 | 1.93 (1.55–2.40); p < .0001 | 1.92 (1.55–2.38); p < .0001 |
| Lymph nodes: pN0 vs. pN+ | 2.28 (1.86–2.78); p < .0001 | 2.11 (1.71–2.61); p < .0001 | 2.11 (1.71–2.61); p < .0001 |
| Grading: G1/2 vs. G3 | 1.40 (1.16–1.69); p = .0005 | 1.19 (0.97–1.47); p = .092 | – |
| Hormone receptor status: negative vs. positive | 0.56 (0.47–0.68); p < .0001 | 0.58 (0.47–0.71); p < .0001 | 0.55 (0.46–0.67); p < 0.0001 |
| ECOG performance status: 0 vs. 1–4 | 1.22 (1.01–1.47); p = .040 | 1.11 (0.91–1.36); p = .29 | – |
| Body mass index: <25 kg/m2 vs. 25–29 kg/m2 vs. ≥30 kg/m2 | NA; p = .24 | – | – |
Cells filled with a dash indicate that variable is not in model.
aFirst group mentioned is reference.
Abbreviations: CI, confidence interval; HR, hazard ratio; NA, not applicable.
The application of an anthracycline as part of the adjuvant chemotherapy was associated with a more favorable RFS rate of 91% after 3 years and 84% after 5 years, compared with 86% and 81% in the nonanthracycline group (HR, 0.81; 95% CI, 0.71–0.93; p = .0023). As expected because of the high degree of correlation between the two parameters, rather similar results were obtained when we assessed patients receiving chemotherapy concurrently with trastuzumab versus sequentially. The 3‐ and 5‐year RFS rates were 91% and 84% after sequential treatment compared with 86% and 79%, respectively, when the antibody treatment was started after the termination of chemotherapy (HR, 0.84; 95% CI, 0.74–0.94; p = .0032).
Cardiac Diagnostics and Safety
The HER2‐targeted antibody treatment was generally well tolerated. Adverse events causally related to trastuzumab were recorded and graded according to National Cancer Institute common toxicity criteria (CTC) version 3. The incidence of hematotoxicity categories or gastrointestinal toxicities, such as nausea, vomiting, and diarrhea, were generally very low, in the range of 1% or less, but these numbers may be less reliable because of the difficulty in differentiating from chemotherapy‐induced side effects. The criteria of serious adverse drug reactions were fulfilled for 63 event reports, recorded in 39 patients (1%).
Similar to the baseline findings, the general recommendation of performing echocardiography at least every 3 months during trastuzumab treatment was not followed in a considerable proportion of patients. When the treatment period was divided into intervals of every 3 months, the rate of patients with reported scans per period was around 60% only; the rate was 63% at the end of the adjuvant treatment. No echocardiography at all was performed in 359 patients (9%). When echocardiography and electrocardiography were considered together, pathological findings of any type and severity were reported in 8% of patients at treatment termination and in 18% at least once during the whole observation time. There was no correlation between pathologic observations (at least once or never) and the number of tests performed, with mean values of 2.8 and 2.9, respectively. Cardiac toxicity according to CTC is described in Table 3. Among 168 patients stopping trastuzumab therapy because of adverse events, cardiac side effects were most prominent (82%; 3.5% of the total population). Seventeen deaths were recorded during trastuzumab treatment: 13 of these due to tumor disease and only 1 due to a cardiac event.
Frequency of cardiac adverse reactions (highest National Institutes of Health common toxicity criteria grade per category and patient)
| Adverse reaction . | Patients with NCI CTC grade, n (%) . | ||||
|---|---|---|---|---|---|
| 1 . | 2 . | 3 . | 4 . | All grades . | |
| Heart function | 62 (1.7) | 62 (1.7) | 33 (0.9) | 2 (< 0.1) | 159 (4.4) |
| Arrhythmia | 10 (0.3) | 3 (< 0.1) | 2 (< 0.1) | 2 (< 0.1) | 17 (0.5) |
| Heart, other | 16 (0.4) | 5 (0.1) | – | – | 21 (0.6) |
| Adverse reaction . | Patients with NCI CTC grade, n (%) . | ||||
|---|---|---|---|---|---|
| 1 . | 2 . | 3 . | 4 . | All grades . | |
| Heart function | 62 (1.7) | 62 (1.7) | 33 (0.9) | 2 (< 0.1) | 159 (4.4) |
| Arrhythmia | 10 (0.3) | 3 (< 0.1) | 2 (< 0.1) | 2 (< 0.1) | 17 (0.5) |
| Heart, other | 16 (0.4) | 5 (0.1) | – | – | 21 (0.6) |
CTC, common toxicity criteria; NCI, National Cancer Institute.
Frequency of cardiac adverse reactions (highest National Institutes of Health common toxicity criteria grade per category and patient)
| Adverse reaction . | Patients with NCI CTC grade, n (%) . | ||||
|---|---|---|---|---|---|
| 1 . | 2 . | 3 . | 4 . | All grades . | |
| Heart function | 62 (1.7) | 62 (1.7) | 33 (0.9) | 2 (< 0.1) | 159 (4.4) |
| Arrhythmia | 10 (0.3) | 3 (< 0.1) | 2 (< 0.1) | 2 (< 0.1) | 17 (0.5) |
| Heart, other | 16 (0.4) | 5 (0.1) | – | – | 21 (0.6) |
| Adverse reaction . | Patients with NCI CTC grade, n (%) . | ||||
|---|---|---|---|---|---|
| 1 . | 2 . | 3 . | 4 . | All grades . | |
| Heart function | 62 (1.7) | 62 (1.7) | 33 (0.9) | 2 (< 0.1) | 159 (4.4) |
| Arrhythmia | 10 (0.3) | 3 (< 0.1) | 2 (< 0.1) | 2 (< 0.1) | 17 (0.5) |
| Heart, other | 16 (0.4) | 5 (0.1) | – | – | 21 (0.6) |
CTC, common toxicity criteria; NCI, National Cancer Institute.
Discussion
The results of our observation study, conducted in a large, representative number of clinics and practices across Germany, reflect routine trastuzumab treatment and its outcome in almost 4,000 patients with HER2+ early breast cancer during a 5‐year recruitment period between 2006 and 2011. The cohort comprises only patients who actually received trastuzumab. However, epidemiological data from Germany suggest that most HER2+ patients received the trastuzumab treatment according to guidelines early after the time point of approval for treatment of early breast cancer [18]. Comparison of the patient characteristics to those in the large pivotal trials reveal the expected differences: Most important, there is a major shift in patients’ age. In the published randomized studies, the overall rate of patients older than 60 years of age was only approximately 10% . Even in the European Herceptin Adjuvant (HERA) study, with comparatively unrestricted inclusion criteria, the proportion was only 16% [4], contrasting with 39% in our population. Consequently, subgroup analyses performed in HERA focused on very young patients rather than on the elderly [19]. The specific results in our large subgroup of elderly patients (≥65 years) have been reported in detail elsewhere [20].
Disease‐related characteristics also differ. Whereas in HERA and in Breast Cancer International Research Group (BCIRG)‐006 [6], the nodal status was negative in less than one third of patients, this subgroup makes up the majority in our cohort (53%). On the other hand, the proportion of pT1 primaries was rather similar. In the randomized trials, tumors tended to be more aggressive, indicated by poor differentiation in 60% versus 52% and hormone receptor negativity in 47% versus 37% in HERA and our study, respectively. The same dissimilarities can also be found for the U.S. trials, with the major exception that pN0 cases were very rare (6%) because of entry criteria restrictions [5].
This confirms the necessity of large postregistration studies on the widespread use and daily practice of drug treatment. It is unlikely that the groups underrepresented in the pivotal trials will ever be examined in randomized experiments , and thus, data from this area of subjective judgment‐based medicine should be collected and analyzed. Hitherto, few studies have assessed the routine implementation of adjuvant trastuzumab (e.g., small series from the Netherlands and the U.K. suggesting similar extended use ). To our knowledge, our cohort is by far the largest in this setting.
In most patients, trastuzumab was given sequentially after a conventional chemotherapy with anthracyclines, often combined with a taxane. Platinum combinations were used in less than 10% of patients, but with growing frequency over the years, especially in elderly patients [20]. In contrast to the registered label, 6% received trastuzumab without previous or concomitant chemotherapy, a patient group nearly absent in all other series. A detailed analysis of these 232 patients will be provided separately.
Our data suggest a somewhat suboptimal intensity of cardiac diagnostics in routine practice, well in agreement with a recent U.S. registry study in older patients detecting adequate monitoring in only 36% [25]. Nevertheless, trastuzumab‐based adjuvant treatment proved to be a safe and highly feasible treatment also in this setting. In only 3.5% of patients was trastuzumab stopped prematurely because of cardiac events. This is much less than 13.8%, as reported in the U.K. registry. However, the latter rate decreased markedly to 5.2% in the last year of observation (2008) [24]. In the Dutch study on the 2005–2007 period, the rate was also high at 15.6%. The respective proportion in HERA was 5.1%, similar to our findings, as was the overall cardiac function toxicity rate of 3.6% . Within the limited period of intensive observation, we cannot support the findings of much higher rates detected in Medicare data [27].
At present, we observed an RFS rate of 90.0% and an OS rate of 96.8% after 3 years. This compares favorably to 81% and 92% in the HERA study, suggesting that the trastuzumab treatment benefit shown in controlled trials is retained in the general setting. The Dutch and the U.K. registries correspond to our findings, with 3‐year RFS rates of 89% and 95% and OS rates of 90% and 98.5%, respectively.
With a high level of significance, our univariable and multivariable analyses confirm the validity of conventional prognostic parameters (i.e., T and N stage and hormone receptor status) in the HER2+ subpopulation treated with adjuvant trastuzumab. This is in line with subgroup analyses from the HERA study. The latter showed a 3‐year RFS of 91% in node‐negative patients [28], quite similar to 94% in our respective subgroup.
There is much debate on the necessity of trastuzumab‐based chemotherapy in T1N0 disease . A recent subgroup meta‐analysis of the pivotal trastuzumab trials showed a relevant benefit in the total group of pT1 tumor patients (all nodal groups), suggesting a constant relative effect across all extents of tumor [31]. Although data on the natural course of small HER2+ tumors are sparse, our favorable results of 95.8% of the more than 1,000 stage I patients remaining free of disease after 3 years confirm the recommendations drawn from smaller prospective and retrospective series of patients treated with the chemotherapy‐antibody combination .
Because of the nature of our noninterventional observation study, it has relevant limitations. These include the lack of any control group and the lack of information on any patients who had HER2+ tumors but were not selected for trastuzumab treatment. In particular, the exclusion of patients stopping initial adjuvant chemotherapy due to an unfavorable course may have led to a slight positive bias.
Conclusion
Several further HER2‐targeting agents proved effective in advanced disease and are now under development in early breast cancer ; pertuzumab is already approved for the neoadjuvant setting, in combination with trastuzumab and chemotherapy. However, for the time being, trastuzumab remains the only generally used and approved HER2‐directed agent after primary surgery. Our data show its favorable efficacy and feasibility in the everyday treatment of localized disease and, thus, confirm the current worldwide recommendations for trastuzumab as the standard of care for a wide population of patients in this setting.
Acknowledgments
This study was sponsored and supported by Roche Pharma AG, Grenzach‐Wyhlen, Germany. Interim results were presented at the 47th Annual Meeting of the American Society of Clinical Oncology, 2011, and at the 33rd, 34th, 35th, and 36th San Antonio Breast Cancer Symposia (2010–2013).
Author Contributions
Conception/Design: Peter Dall, Stella Keitel, Axel Hinke
Provision of study material or patients: Peter Dall, Thorsten Koch, Johannes Selbach, Andreas Ammon, Jochen Eggert, Nidal Gazawi, Daniela Rezek, Arthur Wischnik, Carsten Hielscher, Gabriele Feisel‐Schwickardi
Collection and/or assembly of data: Peter Dall, Thorsten Koch, Johannes Selbach, Andreas Ammon, Jochen Eggert, Nidal Gazawi, Daniela Rezek, Arthur Wischnik, Carsten Hielscher, Ursula Cirrincione, Gabriele Feisel‐Schwickardi
Data analysis and interpretation: Peter Dall, Stella Keitel, Ursula Cirrincione, Axel Hinke
Manuscript writing: Peter Dall, Thorsten Koch, Johannes Selbach, Andreas Ammon, Jochen Eggert, Nidal Gazawi, Daniela Rezek, Arthur Wischnik, Carsten Hielscher, Stella Keitel, Ursula Cirrincione, Axel Hinke, Gabriele Feisel‐Schwickardi
Final approval of manuscript: Peter Dall, Thorsten Koch, Johannes Selbach, Andreas Ammon, Jochen Eggert, Nidal Gazawi, Daniela Rezek, Arthur Wischnik, Carsten Hielscher, Stella Keitel, Ursula Cirrincione, Axel Hinke, Gabriele Feisel‐Schwickardi
Disclosures
Peter Dall: Roche Pharma AG, Novartis, Astra Zeneca (H); Carsten Hielscher: Roche, Celgene, Oncovis (H); Stella Keitel: Roche Pharma AG (E); Axel Hinke: Roche Pharma AG (H). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
For Further Reading: Shanly C. Seferina, Dorien J.A. Lobbezoo, Maaike de Boer et al. Real‐Life Use and Effectiveness of Adjuvant Trastuzumab in Early Breast Cancer Patients: A Study of the Southeast Netherlands Breast Cancer Consortium. The Oncologist 2015; 20:856‐863; first published on June 22, 2015.
Implications for Practice: When new drugs are introduced, knowledge about the correct prescription and impact on health care resources is much appreciated. In the first years after the introduction of trastuzumab in The Netherlands, its use was in accordance with the eligibility criteria of the randomized trials. However, currently almost all patients with HER2‐positive disease will be offered trastuzumab. This study shows that real‐life studies can provide insight into smaller specific patient groups that might benefit from trastuzumab. This may quicken the expansion of indications for drug use, as real life tends to become less strict with growing expertise. Therefore, real‐life data are of additional value for informative decision‐making, as they provide insight on the outcome of patients considered ineligible for treatment in initial clinical trials.
References
Author notes
Disclosures of potential conflicts of interest may be found at the end of this article.



