-
PDF
- Split View
-
Views
-
Cite
Cite
David Hui, Sun-Hyun Kim, Jung Hye Kwon, Kimberson Cochien Tanco, Tao Zhang, Jung Hun Kang, Wadih Rhondali, Gary Chisholm, Eduardo Bruera, Access to Palliative Care Among Patients Treated at a Comprehensive Cancer Center, The Oncologist, Volume 17, Issue 12, December 2012, Pages 1574–1580, https://doi.org/10.1634/theoncologist.2012-0192
Close - Share Icon Share
Abstract
Palliative care (PC) is a critical component of comprehensive cancer care. Previous studies on PC access have mostly examined the timing of PC referral. The proportion of patients who actually receive PC is unclear. We determined the proportion of cancer patients who received PC at our comprehensive cancer center and the predictors of PC referral.
We reviewed the charts of consecutive patients with advanced cancer from the Houston region seen at MD Anderson Cancer Center who died between September 2009 and February 2010. We compared patients who received PC services with those who did not receive PC services before death.
In total, 366 of 816 (45%) decedents had a PC consultation. The median interval between PC consultation and death was 1.4 months (interquartile range, 0.5–4.2 months) and the median number of medical team encounters before PC was 20 (interquartile range, 6–45). On multivariate analysis, older age, being married, and specific cancer types (gynecologic, lung, and head and neck) were significantly associated with a PC referral. Patients with hematologic malignancies had significantly fewer PC referrals (33%), the longest interval between an advanced cancer diagnosis and PC consultation (median, 16 months), the shortest interval between PC consultation and death (median, 0.4 months), and one of the largest numbers of medical team encounters (median, 38) before PC.
We found that a majority of cancer patients at our cancer center did not access PC before they died. PC referral occurs late in the disease process with many missed opportunities for referral.
摘要
背景. 姑息治疗(PC)是癌症综合管理的关键组成部分。先前关于PC途径的研究多侧重于分析PC转诊时机。实际接受PC的患者比例仍不清楚。本研究旨在明确在我们综合癌症中心接受PC的癌症患者比例以及PC转诊的预测因子。
方法. 我们回顾了休斯顿地区MD Anderson癌症中心死于2009年9月˜ 2010年2月的晚期癌症患者连续队列分布图表。我们在死亡之前接受过PC服务的患者与未接受过PC服务的患者之间进行了比较。
结果. 总体上,816例死亡患者中有366例(45%)曾做过PC咨询。PC咨询与死亡之间的中位间隔时间为1.4个月(四分位数间距范围,0.5 ˜4.2个月),PC之前到医生处就诊的中位次数为20次(四分位数间距范围,6˜45次)。多变量分析显示,年龄较大、已婚、特定癌症类型(妇科、肺部以及头颈部癌症)与PC转诊显著相关。血液系统肿瘤患者PC转诊比例显著降低(33%),晚期癌症确诊至PC咨询的间隔时间最长(中位值,16个月)、PC咨询与死亡之间的间隔时间最短(中位值,0.4个月)、PC之前到医生处就诊次数也属最多之列(中位值,38个月)。
结论. 本研究发现,我们分析的癌症中心癌症患者中大多数在死亡之前未获得PC途径。在疾病进展过程中PC转诊发生很晚,丧失了很多转诊时机。
Introduction
Patients with advanced cancer have many significant physical and psychological symptoms including pain, fatigue, weight loss, lack of appetite, nausea, shortness of breath, depression, anxiety, and confusion [1]. These symptoms often have a major impact on patients' quality of life [2]. Therefore, good control of these symptoms is one of the most important aspects of the care of advanced cancer patients and requires comprehensive interdisciplinary care.
Palliative care (PC) by a comprehensive interdisciplinary team has been shown to provide effective symptom management [3–5]. In fact, PC has become an important part of the continuum of care for cancer patients, and many studies have demonstrated benefits such as improvements in quality of life, various physical and psychosocial symptoms, and survival outcomes [4, 6–9].
However, the appropriate timing of PC referral remains unclear. In a recent randomized controlled trial, patients with metastatic non-small cell lung cancer assigned to PC within 8 weeks of their cancer diagnosis had a better quality of life [10]. However, PC is still routinely delivered very late in the disease trajectory to patients who have advanced cancer [11]. The short amount of time for interaction with the PC team significantly limits the effectiveness of such services [6, 12, 13].
Although multiple studies have examined the timing of PC referral among patients who received PC [12, 14–16], there is a paucity of studies on the actual proportion of cancer patients who access PC and the predictors of PC referral. We only identified a few studies that explored population databases [17, 18] and surveyed families of cancer patients [19, 20], although they did not assess PC referral patterns from an institutional perspective. Our institution is a National Cancer Institute designated comprehensive cancer center and includes an active PC program consisting of three mobile teams, an acute PC unit, and a supportive care clinic. An examination of the PC referral pattern at our institution would help characterize referral patterns while controlling for an important variable, PC availability. The objective of this retrospective study is to estimate the proportion of patients seen at MD Anderson Cancer Center (MDACC) who died of cancer and had access to PC services and to identify predictors of PC referral.
Patients and Methods
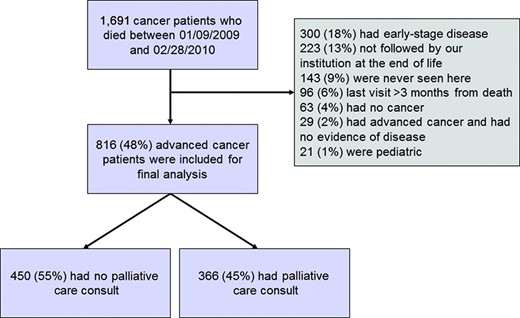
We reviewed the medical records of 1,691 consecutive MDACC patients who died as a result of advanced cancer between September 1, 2009 and February 28, 2010 and had a postal address within the seven-county Houston metropolitan area, which was defined as the central county (Harris) and the seven surrounding counties (Brazoria, Chambers, Fort Bend, Galveston, Liberty, Montgomery, and Waller). Patients aged <18 years, who did not die as a result of advanced cancer, or who had last contact with MDACC >3 months before death were excluded. The first date was chosen to ensure that all medical records would be available in the hospital's electronic medical records system. The second date was chosen to ensure that the deaths could be confirmed on the Social Security Death Index interactive search by the time we started the data collection. Geographic restriction was used to ensure that patients had the opportunity for regular follow-up at our cancer center. This retrospective study was approved by our institutional review board.
For the purpose of this study, we defined advanced cancer as locally advanced, metastatic, or recurrent disease for solid tumors and as incurable disease at presentation (e.g., myeloma, advanced stage low-grade lymphoma) or first relapse for hematologic malignancies. We also included patients who refused all curative treatments, patients who were referred to the phase I program, and patients who, according to the oncologists' notes, had an incurable (or refractory) cancer or were not eligible for potentially curative treatment (e.g., because of a low performance status or comorbidities). For patients with multiple malignancies, we used only data regarding the cancer most responsible for the patient's death.
Five PC specialists and oncologists reviewed the 1,691 medical records to identify the date of advanced cancer and the first consult date of PC services if patients had a PC consultation. Educational level was extracted manually from electronic medical records.
The information collected by informatics department staff from medical records included patient demographics (age, sex, ethnicity, religion, and marital status), date of birth, date of death, postal address and county, cancer location and type, oncology service team, date of entry into the MDACC registry, and date of cancer diagnosis. We also collected information about the number of encounters with the medical team by reviewing billing records for the intervals between the advanced cancer diagnosis and death for patients who did not receive PC services and between the advanced cancer diagnosis and first PC consultation and death for patients who received PC services.
Statistical Analysis
We summarized the baseline demographics using descriptive statistics, including medians, interquartile ranges, means, ranges, frequencies, and percentages. We compared the baseline characteristics between patients with and without PC encounters. Comparisons were made using Student's t-test for continuous variables that were normally distributed (e.g., age), the Mann-Whitney test for continuous, nonparametric variables (e.g., the interval between an advanced cancer diagnosis and death, number of clinic visits), and the χ2 test or Fisher's exact test for categorical variables (e.g., sex, race). We also compared the time interval between and the number of clinic visits before and after PC consultation using the Wilcoxon rank sum test for paired analysis.
To identify independent factors associated with PC referral, all variables with a p-value < .10 on univariate analysis were included in a multivariate logistic regression model using backward selection.
The timing of PC referral was examined based on (a) the time from an advanced cancer diagnosis to PC consultation and (b) the overall survival duration from the time of PC consultation. The overall survival time was calculated from the date of PC referral to the date of death. All time-event analyses were examined using the Kaplan–Meier method and survival curves were compared using the log-rank test.
A two-sided p-value <.05 was considered to be statistically significant. The Statistical Package for the Social Sciences (IBM SPSS version 19.0, SPSS Inc., Chicago, IL) software was used for statistical analysis.
Results
Patient Characteristics and Predictors of PC Referral
Among the 1,691 patients who died in the study period, 816 (48%) met the eligibility criteria (Fig. 1). Table 1 summarizes the patient characteristics and the differences between patients with and without a PC consultation. Three hundred sixty-six (45%) patients had a PC consultation before death.
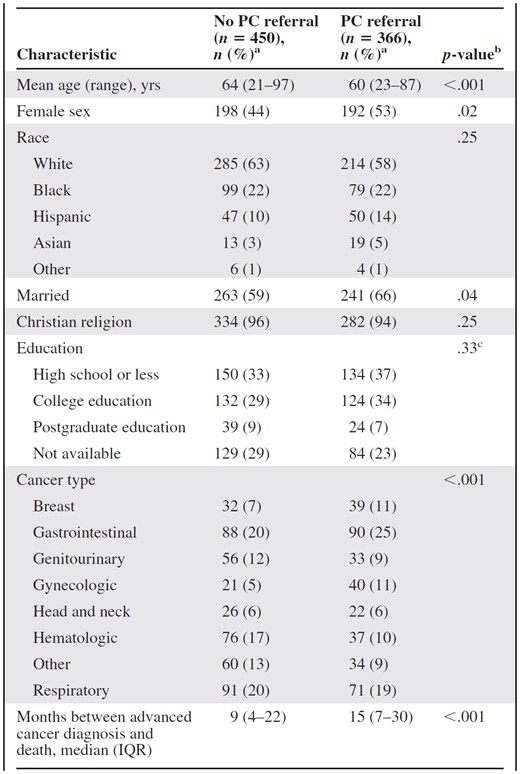
aColumn percentage unless otherwise specified.
bWe used the t-test for continuous, normally distributed variables (e.g., age), the Mann–Whitney test for continuous, nonparametric variables (e.g., duration between an advanced cancer diagnosis and death), and Pearson's χ2 test or Fisher's exact test for categorical variables (e.g., race).
cOnly patients with available data were included in the statistical analysis.
Abbreviations: IQR, interquartile range; PC, palliative care.

aColumn percentage unless otherwise specified.
bWe used the t-test for continuous, normally distributed variables (e.g., age), the Mann–Whitney test for continuous, nonparametric variables (e.g., duration between an advanced cancer diagnosis and death), and Pearson's χ2 test or Fisher's exact test for categorical variables (e.g., race).
cOnly patients with available data were included in the statistical analysis.
Abbreviations: IQR, interquartile range; PC, palliative care.
Patients who had a PC consultation were younger (mean age, 60 years vs. 64 years; p < .001), more likely to be of the female gender (53% vs. 44%; p < .02), and more likely to be married (66% vs. 59%; p < .04). There were no differences in race, education, and religion. Patients with gynecologic, breast, or gastrointestinal cancers were more likely to have PC access. In contrast, patients with hematologic or genitourinary malignancies were less likely to have had a PC consultation.
On multivariate logistic regression analysis, age (p < .001), marital status (p = .005), and cancer type (p < .001) were found to be significantly associated with PC encounters (Table 2). Specifically, patients with gynecologic cancers (odds ratio [OR], 2.2; 95% confidence interval [CI], 1.2–4.1) had greater PC access and those with hematologic malignancies (OR, 0.6; 95% CI, 0.3–0.9) were less likely to have access to PC than lung cancer patients (Table 2).
Multivariate logistic regression analysis for predictors associated with palliative care referral (n = 816)
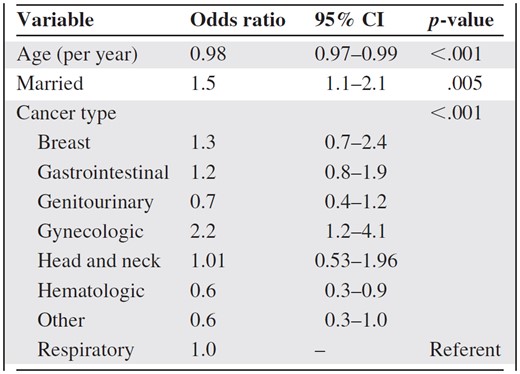
Variables included were age, sex, marital status, and cancer type.
Abbreviation: CI, confidence interval.
Multivariate logistic regression analysis for predictors associated with palliative care referral (n = 816)

Variables included were age, sex, marital status, and cancer type.
Abbreviation: CI, confidence interval.
Figure 2 shows the percentage of PC access according to tumor type. Only 37 patients (33%) with hematologic malignancies had PC encounters.
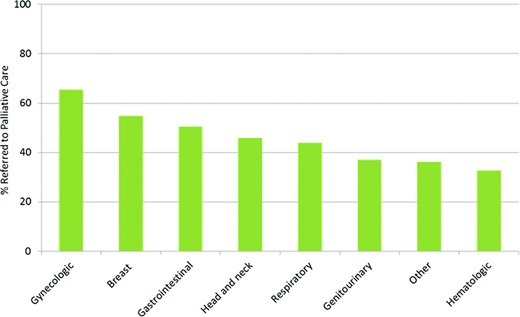
Percentage of patients with access to palliative care according to tumor type. We found significant differences in palliative care access by tumor type (p < .001).
Time Intervals and Number of Medical Team Encounters
Table 3 illustrates the significant differences in the intervals between advanced cancer and death (p < .001), advanced cancer and PC consultation (p < .001), and PC consultation and death (p < .001) and in the number of encounters with the medical team (p < .001) among patients with various types of cancer. The interval between an advanced cancer diagnosis and death and the interval between an advanced cancer diagnosis and PC consultation were longest for hematologic patients (median, 13 months and 16 months, respectively), and the interval between PC consultation and death was shortest for these patients (median, 0.4 months). The interval between an advanced cancer diagnosis and death and the interval between an advanced cancer diagnosis and PC consultation were shortest for patients with lung malignancies (median, 7 months and 5 months, respectively), and the interval between PC consultation and death was longest for patients with lung (median, 2 months), head and neck (median, 2 months), and gynecologic (median, 92 days) cancers. Patients with breast and hematologic cancers had the largest number of medical encounters (median, 41 and 38, respectively) before PC and respiratory cancer patients had the fewest (median, 11).
Time intervals and encounters according to tumor type among patients who had a PC referral (n = 366)
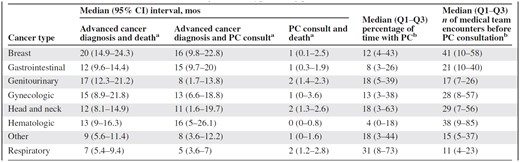
ap < .001 by the log-rank test.
bp < .001 by the Kruskal-Wallis test.
Abbreviations: CI, confidence interval; PC, palliative care; Q1–Q3, interquartile range.
Time intervals and encounters according to tumor type among patients who had a PC referral (n = 366)

ap < .001 by the log-rank test.
bp < .001 by the Kruskal-Wallis test.
Abbreviations: CI, confidence interval; PC, palliative care; Q1–Q3, interquartile range.
Table 4 shows the time intervals and number of medical appointments in relation to PC consultation. PC referral remained limited to the last 1.4 months of life for a majority of patients, and the average patient had 20 medical team encounters prior to being referred. After PC consultation, patients only had a median of one clinic visit, suggesting limited time for intervention. Patients without PC also had a median of 20 (interquartile range, 7–38) visits between their advanced cancer diagnosis and death, representing the number of missed opportunities for making a PC referral.
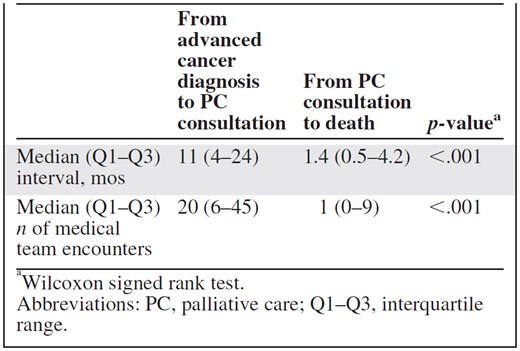
aWilcoxon signed rank test.
Abbreviations: PC, palliative care; Q1–Q3, interquartile range.

aWilcoxon signed rank test.
Abbreviations: PC, palliative care; Q1–Q3, interquartile range.
Discussion
In this retrospective study, we found that the proportion of patients referred for PC is small (45%), particularly among hematologic patients (33%). We also found that younger patients and married people were more likely to have access to PC. Among patients seen in PC, the referral occurred very late in the disease trajectory.
This study examines multiple indices of PC access, including the proportion of patients who received PC and the timing of PC access, while controlling for another important factor, PC program availability. It further introduces a new index—the number of appointments with oncology services prior to PC referral—which represents the number of missed opportunities for PC involvement. This was particularly high among hematologic patients (Table 3). This is not surprising, because of the delayed referral and the fact that patients with hematologic malignancies generally require more hospital visits to address their supportive care needs (e.g., transfusions).
In a previous study, we examined predictors of PC referral and identified that having a hematologic malignancy was associated with lower PC access; however, that study only included hospitalized patients who died at our cancer center, a highly select patient population [21]. In the current study, we examined the predictors of PC referral among all decedents who were followed by our institution at the end of life and identified several other predictive factors for PC referral, including younger age and marital status. Younger age is an important predictor for PC referral, which may be because illness is physically and emotionally more traumatic and requires greater support among those who are younger. Older patients might experience less pain and less severe symptoms [1]. There is evidence that younger patients have higher symptom expression and reporting than elderly cancer patients [22, 23]. Caregivers may also perceive greater health care needs among younger patients [24]. Further studies are needed to examine the unique needs of young cancer patients.
We found that patients who had a PC referral were more often married than were patients without a PC referral, which has also been reported in other studies [17, 20, 25, 26]. The reason for this observation is unknown, but it may be possible that a spouse acts as an advocate, lending a second voice to issues of symptom management at the end of life.
We found that the vast majority of patients diagnosed with a hematologic malignancy do not access PC, and that even if they have a PC consultation it happens a median of 0.4 months before death. Our results are consistent with other studies demonstrating that these patients are more likely to receive aggressive therapy at the end of life [14], to die in an intensive care unit [5], and to have late PC referral [21]. Our finding raises important concerns regarding the quality of end-of-life care for patients with hematologic malignancies. In contrast, we found that gynecologic cancer patients had higher rates of access to PC (66%) and were generally referred earlier in the disease trajectory (median interval from PC consultation to death was 2 months). This may be related to the high symptom burden among patients with gynecologic malignancies, the relative lack of systemic therapy options for patients with resistant gynecologic cancers, and perhaps most importantly, a culture of PC referral among gynecologic oncologists. Further research is needed to determine why PC referral differs among various oncology subspecialties.
Although PC has made major progress over the past decade and gained increasing recognition as an essential part of comprehensive cancer care [27, 28], our results show that there are still many missed opportunities for early and integrated PC [29]. Delayed referral for PC has a negative impact not only on the management of various physical and psychosocial symptoms but also on the timing of hospice referral, which is one of the key quality of care indicators. The median length of stay in hospice was 20 days in 2010, with 34% of patients referred to hospice in the last week of life [30]. We recently reported that the term “supportive care” caused less distress than “palliative care” among oncologists and midlevel providers and that a name change to supportive care was associated with a higher number of and earlier referrals [31, 32]. Further research is needed to identify other potential barriers for PC referral and to overcome these barriers through targeted education and the establishment of clinical pathways.
Limitations of the present study include its retrospective design and the study setting. Given that we needed to examine PC access throughout the patient's entire lifespan, a retrospective case–control design is the only feasible methodology. It is not clear if these findings from our tertiary care cancer center are generalizable to other populations such as community-based practices and other cancer centers. We recently conducted a survey of PC services at cancer centers in the U.S., and found late referrals to be a common issue [11]. Second, we used all patients who died as a denominator for PC access, which was also the assumption used in other studies [18, 19]. Some investigators may argue that not every patient may need PC before they die; however, given that a substantial proportion of patients with advanced cancer experiences physical and psychological distress at the end of life [33, 34], we believe that examining PC access as a percentage of the total number of deaths is an appropriate indicator. A recent study also suggests significant clinical benefit when patients with advanced lung cancer were referred universally and early in the disease trajectory [10, 35]. Third, some patients may have been referred for PC by the oncology team but declined the PC consultation. Thus, the absence of a PC encounter does not always translate to a lack of PC referral. Finally, we did not include some important clinical outcomes, such as symptom management and quality of life. Unfortunately, these important outcomes are not routinely collected in the majority of cancer centers. Nevertheless, multiple studies have demonstrated the effectiveness of PC in improving symptoms and quality of life and reducing the cost of care [36, 37].
A recent provisional clinical opinion published by the American Society of Clinical Oncology supports routine PC referral for patients with incurable cancer [35]. However, earlier and more frequent PC access is limited by the fact that a vast majority of cancer centers in the U.S. do not have adequate staffing and resources to enable a dramatic increase in patient referral [11]. Until PC programs are widely available, it would be helpful to develop clinical care pathways to address the generally low and unpredictable rates of PC referral. Under this system, cancer patients with various sentinel events, such as a poor performance status, severe symptom distress on screening, and brain metastases, would routinely be referred for PC [38]. Oncologists should also be educated and encouraged to make PC referrals, ideally by their “palliphilic” colleagues [29]. More research is needed to determine which patients are most likely to benefit from specialist PC services.
Conclusion
We found that the majority of cancer patients at our comprehensive cancer center did not receive PC before they died despite the presence of an active interdisciplinary PC team. We also identified hematologic malignancies, older age, and an unmarried status as significant barriers to PC referral. Further research and education is needed to overcome these challenges and to ensure that cancer patients have access to high-quality comprehensive cancer care.
Acknowledgments
The authors take full responsibility for the content of the paper but thank Ms. Virginia M. Mohlere, M.S., M.L.I.S., a scientific editor of the Department of Scientific Publications at the MD Anderson Cancer Center, for copyediting of the manuscript.
David Hui and Sun-Hyun Kim contributed equally.
Author Contributions
Conception/Design: David Hui, Kermerson Tanco, Sun-Hyun Kim, Wadih Rhondali, Jung Hun Kang, Gary Chisholm, Tao Zhang, Jung Hye Kwon, Eduardo Bruera
Collection and/or assembly of data: David Hui, Kermerson Tanco, Sun-Hyun Kim, Wadih Rhondali, Jung Hun Kang, Tao Zhang, Jung Hye Kwon
Data analysis and interpretation: David Hui, Gary Chisholm, Eduardo Bruera
Manuscript writing: David Hui, Sun-Hyun Kim, Eduardo Bruera
Final approval of manuscript: David Hui, Kermerson Tanco, Sun-Hyun Kim, Wadih Rhondali, Jung Hun Kang, Gary Chisholm, Tao Zhang, Jung Hye Kwon, Eduardo Bruera
References
Author notes
Disclosures: The authors indicated no financial relationships.



