-
PDF
- Split View
-
Views
-
Cite
Cite
Janne C. Mewes, Lotte M.G. Steuten, Maarten J. IJzerman, Wim H. van Harten, Effectiveness of Multidimensional Cancer Survivor Rehabilitation and Cost-Effectiveness of Cancer Rehabilitation in General: A Systematic Review, The Oncologist, Volume 17, Issue 12, December 2012, Pages 1581–1593, https://doi.org/10.1634/theoncologist.2012-0151
Close - Share Icon Share
Abstract
Many cancer survivors suffer from a combination of disease- and treatment-related morbidities and complaints after primary treatment. There is a growing evidence base for the effectiveness of monodimensional rehabilitation interventions; in practice, however, patients often participate in multidimensional programs. This study systematically reviews evidence regarding effectiveness of multidimensional rehabilitation programs for cancer survivors and cost-effectiveness of cancer rehabilitation in general.
The published literature was systematically reviewed. Data were extracted using standardized forms and were summarized narratively.
Sixteen effectiveness and six cost-effectiveness studies were included. Multidimensional rehabilitation programs were found to be effective, but not more effective than monodimensional interventions, and not on all outcome measures. Effect sizes for quality of life were in the range of −0.12 (95% confidence interval [CI], −0.45–0.20) to 0.98 (95% CI, 0.69–1.29). Incremental cost-effectiveness ratios ranged from −€16,976, indicating cost savings, to €11,057 per quality-adjusted life year.
The evidence for multidimensional interventions and the economic impact of rehabilitation studies is scarce and dominated by breast cancer studies. Studies published so far report statistically significant benefits for multidimensional interventions over usual care, most notably for the outcomes fatigue and physical functioning. An additional benefit of multidimensional over monodimensional rehabilitation was not found, but this was also sparsely reported on. Available economic evaluations assessed very different rehabilitation interventions. Yet, despite low comparability, all showed favorable cost-effectiveness ratios. Future studies should focus their designs on the comparative effectiveness and cost-effectiveness of multidimensional programs.
摘要
引言. 很多癌症幸存者遭受疾病相关以及治疗相关的共病困扰,经常以此为初始治疗后的主诉。越来越多的证据显示了单维度康复干预的有效性;然而,实际上患者常参与多维度管理。本研究系统性回顾了关于癌症幸存者多维度康复管理的有效性以及总体癌症康复的成本-效益。
方法. 系统回顾已发表的文献。采用标准化格式提取数据,并进行描述性总结。
结果. 共纳入16项有效性研究,6项成本-效益研究。研究表明多维度康复管理是有效的,但并未优于单维度干预,且并非所有结果测量指标都是如此。生活质量的效应量范围为-0.12[95% 可信区间(CI),-0.45 ˜ 0.20] ˜ 0.98(95% CI,0.69 ˜ 1.29)。增量成本-效益比率范围为-€16 976(表示成本节约)˜ €11 057/质量调整生命年。
结论. 康复的多维度干预与经济学影响研究非常少,且主要局限在乳腺癌领域。据迄今为止已发表的研究报告,与常规管理相比,多维度干预具有统计学意义上的显著获益,这一点尤其体现在乏力和躯体功能这两个结果上。尚未发现多维度康复比单维度康复的获益更大,但仅有极少数散在报告。现有的经济学分析评估了各种截然不同的康复干预。然而,虽然可比性较差,但均显示出良好的成本-效应比率。将来的研究设计应着重于多维度管理的有效性和成本-效益比较评估。
Introduction
Progression in screening, early detection, and effective treatment of cancer has rapidly increased the percentage of cancer survivors in developed countries. In Europe, the 5-year survival rate for all cancers has reached ≥47% for men and 56% for women, and these are expected to rise in future years [1]. In the U.S., 5-year survival rates of 68% for men and 67% for women were reported [2]. Accordingly, the demand for rehabilitation after primary cancer treatment is increasing. In The Netherlands, for example, the number of people living with or having survived cancer is estimated to be ∼692,500 (4%–5% of the population) by the year 2015 [3], a large number of whom might benefit from cancer rehabilitation.
Because of the direct and long-term effects of cancer and its treatment, most survivors do not return to their previous state of well-being [4, 5]. Cancer survivors suffer from a range of problems, varying from fatigue, reduced physical fitness, and psychological problems to symptoms related to specific cancer types, such as lymphedema or difficulties with speaking and swallowing after head and neck surgery [6]. Consequently, an important percentage of cancer survivors, ∼36% according to a recent review [7], is not able to return to work. Furthermore, cancer survivors suffer from different symptoms and complaints. Cheng et al. [8] reported an average of eight symptoms per patient. A combination of interventions, adjusted to the survivor's individual needs, is likely required to restore health-related quality of life (HRQoL). Indeed, multidimensional rehabilitation is increasingly being recommended in national and international cancer rehabilitation guidelines [9, 10].
During previous decades, the volume of research on the rehabilitation of cancer survivors has increased faster than research on rehabilitation in general [11]. Most research conducted so far focused on physical exercise after cancer treatment, which has been shown to increase physical strength and HRQoL and to alleviate fatigue and other symptoms [12, 13]. Only a few studies suggest that cognitive and psychosocial rehabilitation interventions can reduce psychological symptoms [14–16]. Furthermore, return-to-work interventions appeared successful in helping survivors resuming work [17]. Besides alleviating particular post-treatment symptoms, rehabilitation likely mitigates cancer survivors' elevated risk for chronic diseases, such as cardiovascular disease and osteoporosis, and the risk for a second primary cancer or recurrence [6]. Performing physical activity has been shown to reduce the risk for cancer recurrence and chronic disease [18] and is suggested in several guidelines and recommendations [9, 10, 12, 19–21]. The evidence base for the long-term effects of rehabilitation is rather small because the follow-up duration is usually short [22].
Although there is a growing body of literature, to what extent the effect of multidimensional rehabilitation is greater than the effect of single interventions remains unknown, because the various interventions are mostly tested in isolation. Considering the increasing number of cancer survivors, not only the treatment effects but also the costs involved to society and health care systems become an issue, as recently underlined in leading journals [23, 24]. Data on the cost-effectiveness of rehabilitation will undoubtedly become more important to decision makers.
The first aim of this study was therefore to systematically review the research conducted on the effectiveness of multidimensional rehabilitation programs for cancer survivors. Because all rehabilitation services inevitably come at a cost, the second aim was to critically review published cost-effectiveness studies of cancer rehabilitation.
Methods
A systematic literature review was undertaken on (a) the effectiveness of multidimensional cancer survivor rehabilitation programs and (b) the cost-effectiveness of cancer rehabilitation. MEDLINE, PsycINFO, and the Cochrane Library were searched electronically using various combinations of keywords and medical subject headings (MeSH): neoplasms (MeSH), survivors, chronic cancer patients, rehabilitation, multicomponent, multidimensional, multifaceted, multitreatment, multimodal, complex, program, exercise, physical activity, physical exercise, physical therapy, return-to-work, reintegration, back to work, vocational rehabilitation, occupational rehabilitation, workplace, cognitive therapy, costs and cost analysis (MeSH), cost, and economic. Table 1 provides a detailed overview of the combinations of search terms used. Further, reference lists of included papers were hand searched.
Selection Criteria for All Studies
English language primary studies, systematic reviews, and meta-analyses were included, evaluating (a) the effectiveness of multidimensional cancer survivor rehabilitation programs and (b) the cost-effectiveness of cancer rehabilitation. Eligible study designs for primary studies were randomized controlled trials (RCTs) and quasiexperimental studies, including nonrandomized controlled studies and pretest–post-test studies. Nonsystematic reviews and qualitative and observational studies were excluded. The methodological quality of studies was assessed but did not function as a selection criterion. A rehabilitation intervention was defined as an intervention directed at enhancing the International Classification of Functioning, Disability and Health (ICF) domains in cancer survivors [25], that is, body structure and function, activity, and participation. Studies evaluating medical devices were not included. Outcome measures included clinical endpoints and intermediate endpoints that could be linked to relevant endpoints. When two articles from the same study were found, only the most recent one that included follow-up and baseline data was included.
Selection Criteria for Effectiveness Studies of Multidimensional Cancer Survivor Rehabilitation
Effectiveness studies of multidimensional rehabilitation published in January 1994 to June 2012 were considered. The participants included were adult cancer survivors with any kind of cancer. Various definitions of “cancer survivorship” exist [26]. Here, a “cancer survivor” is defined as a person diagnosed with any type of cancer who finished primary treatment either directly before the start of the study or earlier. The type of primary treatment did not function as a selection criterion. Hormone therapy could still be ongoing. The outcome of the therapy must have been positive to a degree that a survival duration ≥1 year was expected, which therefore also leads to the inclusion of chronic cancer patients. Palliative care patients, end-of-life patients, and adult survivors of pediatric cancer were excluded. Reviews, meta-analyses, and primary studies in which not all participants were cancer survivors were only considered when data were presented separately for cancer survivors.
Multidimensional rehabilitation was defined as a rehabilitation program that consisted of two or more rehabilitation interventions directed at the ICF dimensions. Interventions typically encompassed various types of exercise (also including exercise for specific tumor type–related complaints), cognitive–behavioral therapy (CBT), psychotherapy (also including psychotherapy, counseling, and self- and symptom management), and return-to-work interventions. Therefore, a rehabilitation program that included, for example, two different kinds of exercise (e.g., walking to relieve fatigue and weight lifting to increase muscle strength) was not considered multidimensional because both interventions target the dimension “physical functioning.”
Selection Criteria for Economic Evaluations of Cancer Rehabilitation
Cost-effectiveness studies of cancer rehabilitation were those published in January 1995 to December 2011 that included adults diagnosed with any kind of cancer of any stage. In contrast to the inclusion criteria for multidimensional rehabilitation programs, cost-effectiveness studies could also include cancer patients who were still in treatment, chronic cancer patients who were not treated with curative intent, and cancer patients during or after treatment with a relatively short life expectancy. Adults experiencing late and long-term effects of pediatric cancer were excluded. Notably, economic evaluations assessing any cancer rehabilitation intervention, whether monodimensional or multidimensional, were included to obtain a sufficient evidence base for review.
Screening Process
All identified titles and abstracts were screened by two authors (J.M. and L.S.) for relevance and, if relevant, full text articles were obtained and assessed against the selection criteria (J.M. and L.S.). Disagreements were resolved by discussion or referred to a third author (W.v.H.).
Because titles and abstracts provide insufficient information to assess multidimensionality according to our definition, full text articles were obtained for all articles evaluating cancer rehabilitation effectiveness.
Data Extraction
Data were extracted using a standardized form. For effectiveness studies, study country, type of intervention, intervention design, control group, participants, methods, outcome measures, measurement instruments, retention, baseline characteristics, and results were extracted.
For economic evaluations, study country, type of intervention, design of intervention, comparator, outcome measures, measurement instruments, participants, methods, perspective, uncertainty analyses performed, effect results, cost results, economic results, results of uncertainty analyses, and results of modeling were extracted.
Data Analysis
The effect size (ES) of selected outcome measures was calculated using Cohen's d [27]. Standard deviations were calculated according to Hedges [28]. For controlled trials, the ES was expressed as the difference in the mean between the experimental and control groups at the last measurement point. For pretest–post-test studies, the ES was computed for the difference between the baseline and last measurements. In calculating 95% confidence interval (CI) for the ES, a normal distribution was assumed with standard errors calculated according to Hunter and Schmidt [29].
Methodological Quality Assessment
The methodological quality of papers was assessed using the Cochrane Collaboration's risk of bias tool [30] for multidimensional effectiveness studies and the 10-point Drummond checklist [31] for economic evaluations.
Results
In total, 4,008 citations were identified from MEDLINE, PsycINFO, and the Cochrane Library. Of these, 187 were duplicates, leading to 3,821 unique articles. By reviewing titles and abstracts, 3,607 articles were excluded; full text papers were obtained for the remaining 214 articles. Of these, 195 articles were excluded. Three articles were identified through hand searching. Common exclusion reasons were that the intervention in question was not multidimensional and that the definition of survivor was unclear. A large number of studies that possibly fulfilled the inclusion criteria were excluded because the estimated life expectancy of the patients at the end of the primary treatment was not stated. Three articles were identified through hand searching, leading to a total of 22 articles included. Sixteen articles [32–47] on the effectiveness of multidimensional cancer survivor rehabilitation were included, the data from which originated from 11 trials. The data from one of those trials were presented in two complementary articles [46, 47] and the data from another trial were used in four articles [42–45]. Six health economic evaluations [48–53] were identified. No review was identified in which the included studies fulfilled the inclusion criteria. Figure 1 depicts the study's flow chart.
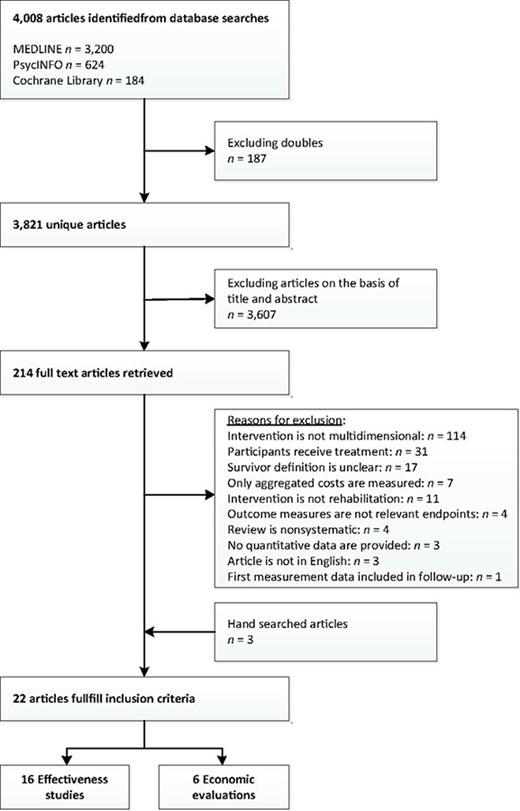
Effectiveness of Multidimensional Interventions
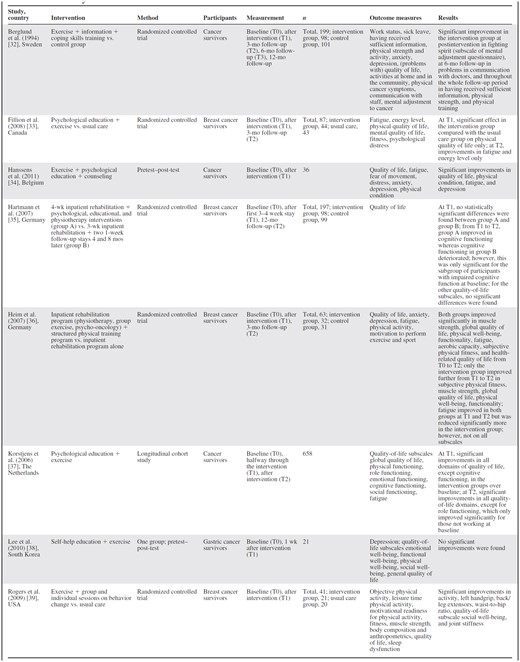
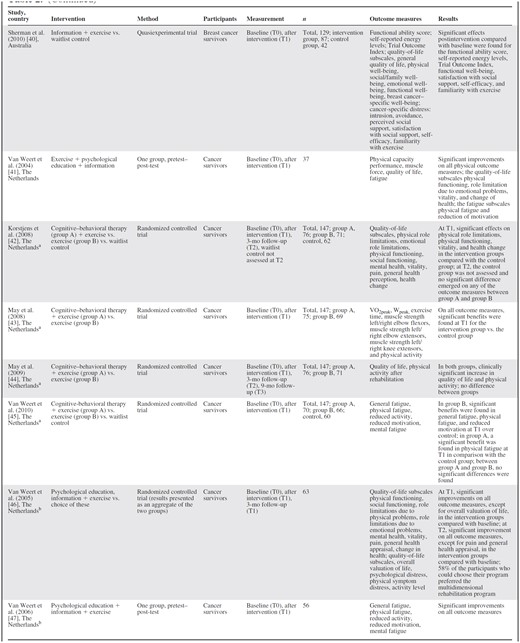
Sixteen articles met the selection criteria for multidimensional cancer rehabilitation effectiveness studies [32–47] (Table 2).

aArticles originate from the same study.
bArticles originate from the same study.

aArticles originate from the same study.
bArticles originate from the same study.
Of these, the eight Dutch articles originate from four separate trials. The evaluated interventions all consisted of exercise combined with inpatient rehabilitation programs [35, 36], CBT [39, 42–45], psychological education [33, 34, 37], psychological education and information [41, 46, 47], self-help education [38], information support [40], and information support plus CBT [32]. Interventions lasted 4–15 weeks. The designs used were RCTs [32–34, 36, 39, 41–46], pretest–post-test studies [35, 38, 47], a quasiexperiment [40], and a longitudinal study [37]. RCTs included 21–199 participants; the longitudinal study's sample size was 658. Participants were survivors of any type of cancer [32, 34, 37, 41–47], breast cancer [33, 35, 36, 39, 40], and gastric cancer [38]. Outcome measurements were performed directly at the end of the intervention [34, 37, 39–41, 43, 45, 47] or after 1 week [38], 3 months [33, 42, 46, 36], 9 months [44], or 12 months [32, 35]. Retention rates were in the range of 64%–100%.
Various outcome measures were reported (Table 2). All articles except one [38] found significantly better outcomes in the intervention group(s) for all [43, 47] or for some [32–37, 39–42, 45, 46] of the outcome measures. Articles measuring subscales of fatigue (n = 8) found statistically significant benefits, but not for all subscales [41, 46] or for all measurements [37]. For physical outcome measures, such as muscle strength, physical functioning, and energy levels, 12 of 13 articles reported significant benefits [32, 34, 37–39, 41–47]. HRQoL and emotional, cognitive, psychological, and social outcome measures, however, varied strongly among studies, although none reported significant deteriorations. Additionally, improvements observed at the end of an intervention were not always sustained on follow-up. Articles that compared monodimensional with multidimensional interventions [42–45] all originate from one trial and did not find a significant difference between the monodimensional and multidimensional intervention groups. Two articles compared a more comprehensive inpatient rehabilitation program with the standard program [35, 36]. One [35] did not find significant between-group differences and the other [36] found no significant difference at the end of the intervention but greater improvements in physical outcome measures and fatigue on follow-up. HRQoL was the only outcome measure reported in most articles (n = 13) [32–42, 44, 46]. When these could be calculated, ES values were in the range of −0.12 (95% CI, −0.45–0.20) to 0.98 (95% CI, 0.69–1.29) (Table 3). Statistically significant improvements at the last follow-up measurement were reported in three studies [36, 38, 40].
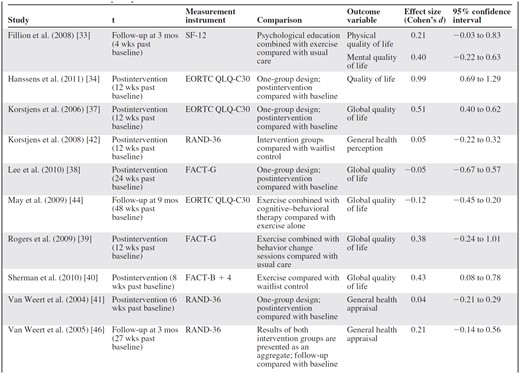
Abbreviations: EORTC QLQ, European Organization for Research and Treatment of Cancer Quality of Life Questionnaire; FACT, Functional Assessment of Cancer Therapy; SF-12, short form 12; t, measurement used for calculating effect size.

Abbreviations: EORTC QLQ, European Organization for Research and Treatment of Cancer Quality of Life Questionnaire; FACT, Functional Assessment of Cancer Therapy; SF-12, short form 12; t, measurement used for calculating effect size.
When comparing results from RCTs with those from nonrandomized studies, the findings did not differ. The only article that did not find an improvement for any of the outcome measures was a pretest–post-test study [38]. The other nonrandomized studies, in general, found improvements for a greater proportion of the reported outcome measures than did the RCTs.
The methodological assessment (Table 4) showed that the risk for bias varied widely. The categories “selective reporting,” “other source of bias,” and “allocation concealment,” when applicable, were predominantly assessed as having a low risk for bias, whereas the category “incomplete outcome data” was, in many cases, assessed as having a high risk for bias. Regarding the categories “blinding of participants and personnel” and “blinding of outcome assessment,” most articles were assessed as having an unclear risk for bias.
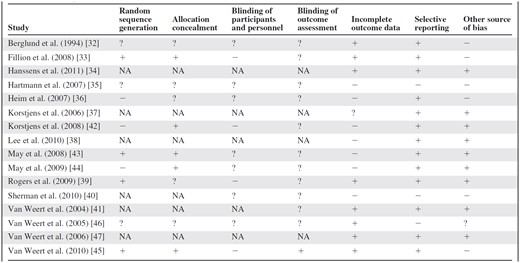
Abbreviations: −, high risk for bias; +, low risk for bias; ?, unclear risk for bias; NA, not applicable.

Abbreviations: −, high risk for bias; +, low risk for bias; ?, unclear risk for bias; NA, not applicable.
Economic Evaluations
Four articles met the inclusion criteria for economic evaluations [48–53] (Table 5).
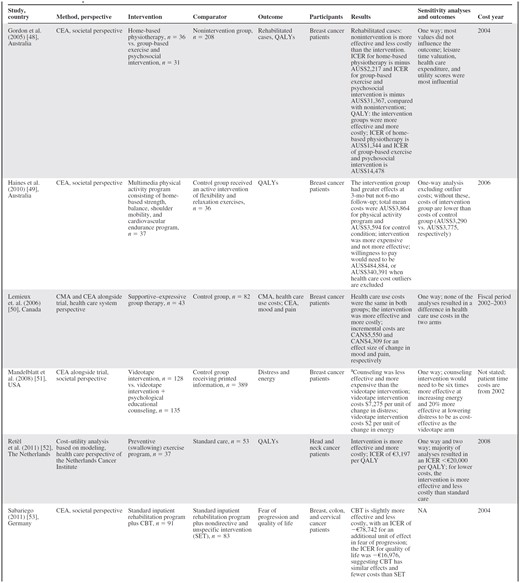
aThis evaluation used the measurement at 6 months. The original trial did not find any significant effect at the 12-month follow-up.
Abbreviations: CBT, cognitive–behavioral therapy; CEA, cost-effectiveness analysis; CMA, cost-minimization analysis; ICER, incremental cost-effectiveness ratio; QALY, quality-adjusted life year.

aThis evaluation used the measurement at 6 months. The original trial did not find any significant effect at the 12-month follow-up.
Abbreviations: CBT, cognitive–behavioral therapy; CEA, cost-effectiveness analysis; CMA, cost-minimization analysis; ICER, incremental cost-effectiveness ratio; QALY, quality-adjusted life year.
All economic evaluations were published in 2005–2011. Three articles were cost-effectiveness analyses (CEA), one was a combined CEA and cost–utility analysis, one was a cost–utility analysis, and one contained both a cost-minimization analysis (CMA) and a CEA. Four articles adopted a societal perspective [48, 49, 51, 53], one used a health care system perspective [50], and one used a hospital perspective [52]. Analyses were based on multicenter RCTs [49–51, 53], a quasiexperimental design [48], and modeling [52]. Intervention patients were compared with a control group of patients who received no intervention [48], another intervention [49], or standard care [50–53]. The included patients were breast cancer patients [48–51], breast, colon, or cervical cancer patients [53], and head and neck cancer patients [52]. The effectiveness outcomes measured in the articles were, in these combinations, the number of rehabilitated cases and quality-adjusted life years (QALYs) [48], distress and energy [51], mood and pain [50], fear of regression and QALYs [53], and QALYs [49, 52]. Significant benefits over the control group were found for QALYs [48, 52, 53], energy [51], fear of regression [53], and mood and pain [50]. The costs of the interventions were in the range of €19, for a videotape intervention [41], to €793, for a group-based exercise and psychosocial intervention [48]. The incremental cost-effectiveness ratios (ICERs) when QALYs were measured and the intervention evaluated was effective were in the range of −€16,976 per QALY (which implies cost savings), for adding CBT to standard inpatient rehabilitation [53], to €11,072 per QALY, for a group-based exercise and psychosocial intervention [48]. For other outcome measures, incremental costs of −€78,742, for adding CBT to standard inpatient rehabilitation [53], to €4,098, for supportive–expressive group therapy [40], were found for one unit of difference in effect. For outcomes for which no significant effect was established, usual care and doing nothing were the most cost-effective strategies. The CMA did not find a significant difference in costs [50].
The methodological quality was moderate to good (Table 6). Positive aspects of the articles reviewed are that almost all economic evaluations gave a description of the interventions, compared relevant alternatives, and included a nonintervention or, for ethical reasons, an intervention similar to doing nothing. Five articles [48–51, 53] were CEAs alongside RCTs in the natural setting, reflecting what probably would happen in practice. A methodological limitation is that none of the economic evaluations were based on a systematic review of effectiveness data. Also, four articles assessed the cost-effectiveness of interventions that have not been shown or have only partly been shown to be effective [48–51]. Finally, two articles did not include all relevant costs and effects [50, 51] and three articles did not cover all relevant viewpoints [51–53].
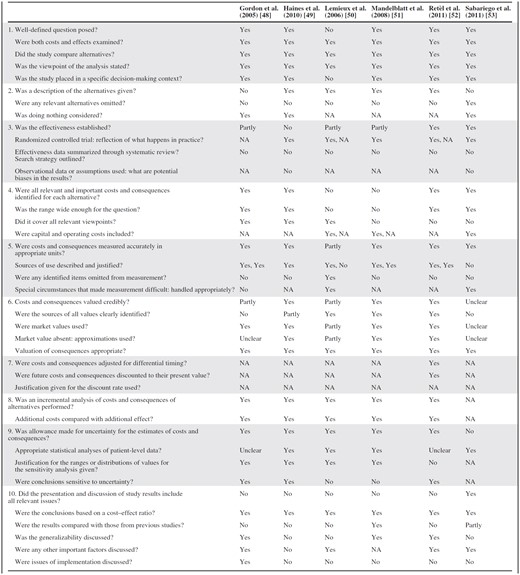
Abbreviation: NA = Not applicable.

Abbreviation: NA = Not applicable.
Discussion
Evidence on the effectiveness of multidimensional cancer survivor rehabilitation and the cost-effectiveness of cancer rehabilitation in general was systematically assessed in this review. The number of papers meeting the inclusion criteria, 16 effectiveness studies and six economic evaluations, shows that the evidence base for the effectiveness and economic impact of multidimensional cancer survivor rehabilitation and for cancer rehabilitation in general is scarce. Moreover, the majority of existing studies focused on exercise interventions plus a CBT or psychological educational intervention, whereas studies including other interventions, like return-to-work programs or patient empowerment, are lacking. A similar pattern was found for monodimensional interventions in the economic evaluations.
Regarding the type of participant, five of the 16 multidimensional studies exclusively included breast cancer patients, and of the articles that aimed to include survivors of any type of cancer, the majority of participants were breast cancer survivors. This pattern applies to the economic evaluations as well, of which the majority exclusively included breast cancer patients. Therefore, generalizability of the results to other tumor groups might be limited, and when using these findings for other cancer types this should be considered carefully. In conclusion, the evidence base for cancer rehabilitation is dominated by exercise intervention studies in breast cancer survivors, whereas published evidence for other aspects of cancer rehabilitation and other tumor populations is lagging behind.
Most studies evaluating the effectiveness of multidimensional rehabilitation (i.e., programs with exercise plus another intervention) reported statistically significant [32–34, 36, 37, 39–47] and clinically relevant [44] benefits in at least one of the outcome measures reported. Although two studies found significant benefits in all outcome measures [43, 47], most studies only reported significant benefits in some outcome measures, mostly fatigue and physical outcomes [33, 37, 38, 40, 42, 44, 45]. The nonrandomized studies found significant improvements in a greater proportion of outcome measures than did RCTs, but in terms of conclusions the outcomes of randomized and nonrandomized studies were the same. When a multidimensional rehabilitation program was compared with the monodimensional interventions (i.e., exercise plus another intervention vs. exercise alone), no additional effect was reported [42–45]. Adding another exercise intervention to a rehabilitation program that was already multidimensional resulted in further benefits for physical outcome measures and quality of life [36], whereas adding longer follow-up to inpatient rehabilitation did not result in greater effectiveness [35].
Statistically significant benefits associated with multidimensional rehabilitation were reported in all the studies that employed a one-group design (pretest–post-test studies and longitudinal cohort studies), except one [38], and in all quasiexperimental and experimental studies that compared multidimensional rehabilitation with usual care without dedicated rehabilitation or with waitlist controls [32, 37, 38, 46]. The four articles, based on one trial, that compared a multidimensional rehabilitation program with a monodimensional intervention [42–45] did not observe the multidimensional intervention to be more (or less) effective than the exercise-only control for outcomes that included physical activity, muscle strength, fatigue, and HRQoL. Although not evident from the quality score, methodological aspects may have contributed to this finding, for example, the open-access character of the program and operational differences among treatment sites. Furthermore, studies might have been underpowered to evaluate secondary outcomes such as HRQoL.
The economic evaluations all assessed very different interventions. Despite the low comparability of studies, all showed acceptable cost-effectiveness ratios for interventions that produced significant health gains. The included cost–utility studies reported ICERs well below the prevailing willingness-to-pay thresholds, or even cost savings, suggesting that cancer rehabilitation is potentially a cost-effective means of allocating scarce health care resources, if effective. When interpreting the results, it should be noted that trial participants could be cancer patients during treatment, chronic cancer patients, and cancer survivors. This could influence the results considerably, because a shorter life expectancy results in a lower potential to generate QALYs, and active disease causes higher treatment costs. Also, it is increasingly argued that higher ceiling ratios should be applied for patients with a high burden of disease, although this is currently not formalized nor a prevailing practice for any of the decision-making bodies.
Our results correspond to the findings of a previous review [54] on economic evaluations of psychosocial interventions. Gordon et al. [54] also reported acceptable ICERs, although the available studies showed great heterogeneity and were of suboptimal quality. Our review included four additional studies with acceptably low ICERs, which further adds to the evidence suggesting that cancer rehabilitation can be cost-effective. However, in view of the large expected number of survivors, detailed information on cost-effectiveness from both the societal and health care perspectives is needed for making informed decisions. It might be relevant to use the experience from cardiac rehabilitation, for which multidimensional rehabilitation—though for a limited number of conditions—is recommended as standard practice and more evidence on the cost-effectiveness of multidisciplinary rehabilitation is available [55, 56]. For other diseases, such as low back pain [57], neglect [58], and traumatic brain injury [59], mixed results have been found for the greater effectiveness of multidimensional rehabilitation programs than single interventions.
Several methodological issues identified in this review give rise to recommendations for future studies. First, the underlying assumption of the need for multidimensional rehabilitation is that cancer survivors have one or more symptoms that, to be addressed appropriately, require multiple rehabilitation interventions. In addition, one would expect that the intervention or combination of interventions offered depends on the specific symptoms a patient experiences. However, in about half of the articles [32–40], it was not an explicit inclusion criterion for patients to have one or more specific symptoms. In the remaining articles [41–47], having several symptoms was specified as an inclusion criterion, but because the nature and severity of the symptoms at baseline were not described in a standardized way, it is unclear to what extent the interventions addressed the specific symptoms appropriately. Therefore, it is difficult to assess the ES that could reasonably have been expected for the chosen primary endpoints and whether or not the sample sizes were large enough to observe an incremental effect from multidimensional interventions over monodimensional interventions in a heterogeneous population.
Although many studies are available that report the prevalence of the symptoms from which cancer survivors suffer, a more detailed description of the prevalence of combinations of symptoms is lacking [6]. Because trial participants might suffer from a great variety of symptoms, patient samples may actually be more heterogeneous than the predominantly clinical baseline measures show. In future studies on both multidimensional and monodimensional interventions, the match between the nature and severity of specific patient symptoms and the interventions offered should be described more explicitly.
Another potential methodological flaw in the current evidence base is the length of patient follow-up. In most articles, the follow-up duration was very short and the measurement was performed directly at the end of the intervention [34, 37–41, 43, 45, 47]. Therefore, some effects that may typically occur over the longer run (e.g., changes in emotional distress, depression) may not have been observed within these short follow-up times and could interfere, even if not chosen as the primary endpoint.
Moreover, the incorporated studies included cancer survivors with an estimated life expectancy ≥1 year. This can lead to the inclusion of chronic cancer patients with a life expectancy of 1–2 years or more and also cured cancer survivors in whom the disease will not return. Depending on life expectancy (and potentially other factors), these patients and survivors may have very different rehabilitation needs. However, from the included studies, it is not possible to distinguish firmly to which group the results apply—cancer survivors with a very good life expectancy outlook or chronic cancer patients. Future studies should provide more detailed descriptions of the treatment outcomes and the estimated life expectancy of the participants.
The quality assessment showed that future trials could be better by using blinding in outcome assessments when possible. Regarding trial design, future studies should compare the multidimensional intervention with the single components it consists of and with usual care to detect any additional effect of multidimensional rehabilitation. An example of this design is a trial by Duijts et al. [60], in which CBT and relaxation combined with exercise was compared with CBT and relaxation, exercise, and a waitlist control for relieving symptoms of treatment-induced menopause. In addition, pragmatic trials, which are more suitable for providing information for clinical and policy decision making [61], could form a valuable addition to the evidence derived from RCTs. Research should also involve cancer types other than breast cancer, and if all types of cancer are to be included in studies, greater effort should be made to obtain samples representative of the various subgroups and tumor types.
Regarding economic evaluations, it is recommended to use QALYs for enabling comparability among various rehabilitation programs and with other health services. Furthermore, the scarcity of economic evaluations should lead researchers to routinely include cost data in their studies.
Conclusion
Cancer rehabilitation guidelines are increasingly being implemented in many western countries and the evidence base is growing rapidly. Evidence for multidimensional interventions and the economic impact of rehabilitation interventions is still scarce, but studies published so far report statistically significant benefits for multidimensional interventions over usual care, particularly for fatigue and physical outcome measures. No additional benefit of multidimensional rehabilitation over monodimensional intervention was found, but this was also sparsely reported on. These findings should be interpreted with caution because the existing literature base is dominated by exercise intervention studies in breast cancer survivors. Published evidence for combinations with other interventions in cancer rehabilitation, like return-to-work programs, and evidence for other cancer populations are lagging behind. The available economic evaluations assessed very different rehabilitation interventions. Yet, despite their low comparability, all showed acceptable cost-effectiveness ratios or cost savings for those interventions that produced significant health gains. Recommendations for future studies include better targeting of all available interventions to patients' needs, longer trial follow-up, and the use of QALYs and cost data in all studies.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Acknowledgments
This study is part of the A-CaRe Program (http://www.a-care.org). The authors acknowledge the A-CaRe2Move Research Group. This study is supported by Alpe d'HuZes, a foundation that is part of the Dutch Cancer Society (KWF Kankerbestrijding).
Author Contributions
Conception/Design: Janne C. Mewes, Lotte M.G. Steuten, Wim H. van Harten
Collection and/or assembly of data: Janne C. Mewes, Lotte M.G. Steuten
Data analysis and interpretation: Janne C. Mewes, Lotte M.G. Steuten, Maarten J. IJzerman, Wim H. van Harten
Manuscript writing: Janne C. Mewes, Lotte M.G. Steuten, Maarten J. IJzerman, Wim H. van Harten
Final approval of manuscript: Janne C. Mewes, Lotte M.G. Steuten, Maarten J. IJzerman, Wim H. van Harten
References
Author notes
Disclosures: Lotte M.G. Steuten: PANAXEA b.v.: Biomedical product innovation (C/A); Maarten J. IJzerman: PANAXEA b.v.: Biomedical product innovation (C/A); Wim H. van Harten: Netherlands Cancer Institute (E). The other authors indicated no financial relationships.




