-
PDF
- Split View
-
Views
-
Cite
Cite
Sarah Barton, Eliza A. Hawkes, Andrew Wotherspoon, David Cunningham, Are We Ready To Stratify Treatment for Diffuse Large B-Cell Lymphoma Using Molecular Hallmarks?, The Oncologist, Volume 17, Issue 12, December 2012, Pages 1562–1573, https://doi.org/10.1634/theoncologist.2012-0218
Close - Share Icon Share
abstract
After completing this course, the reader will be able to:
Describe the oncogenic drivers in DLBCL, especially those that have recently been identified, and how they relate to the oncogenic DLBCL subtypes.
Describe the prognostic and potentially predictive implications of DLBCL COO subtype for chemotherapy and rituximab.
Outline the evidence for novel targeted therapies and therapeutic strategies in DLBCL, how they may be stratified by DLBCL subtype or to specific tumor molecular features, and how these strategies can be incorporated into current treatment paradigms and prospective clinical trial design.
CME This article is available for continuing medical education credit at CME.TheOncologist.com
The division of the heterogeneous entity of diffuse large B-cell lymphoma (DLBCL) into the ontogenic phenotypes of germinal center B-cell-like (GCB) and activated B-cell-like (ABC) is optimally determined by gene expression profiling (GEP), although simpler immunohistochemistry (IHC) algorithms are alternatively being used. The cell-of-origin (COO) classification assists in prognostication and may be predictive of response to therapy. Mounting data suggests that IHC methods of classifying COO may be inaccurate. GEP categorization of COO is superior in defining prognostically and biologically distinct DLBCL subtypes, but current barriers to its widescale use include inaccessibility, cost, and lack of methodological standardization and prospective validation. The poorer prognosis of ABC-DLBCL is frequently associated with constitutive activity in the NF-κB pathway and aberrations in upstream or downstream regulators of this pathway. The molecular mechanisms underlying lymphomagenesis in GCB-DLBCL are arguably less well defined, but C-REL amplification and mutations in BCL-2 and EZH2 are common. New technologies, such as next-generation sequencing, are rapidly revealing novel pathogenic genetic aberrations, and DLBCL treatment strategies are increasingly being designed focusing on distinctive pathogenic drivers within ontogenic phenotypes. This review examines emerging molecular targets and novel therapeutic agents in DLBCL, and discusses whether stratifying therapy for DLBCL using molecular features is merited by current preclinical and clinical evidence.
摘要
作为由多种成分构成的实体,弥漫性大B细胞淋巴瘤(DLBCL)可分为生发中心B细胞样(GCB)和活化B细胞样(ABC)两类个体发生表型,最佳分类方式为基因表达谱(GEP),不过更简单的免疫组化(IHC)方法也是目前的替代选择。细胞来源(COO)分类有助于判断预后,而且还可能预测治疗反应。大量数据提示,采用IHC方法对COO进行分类可能不够精确。就COO而言,GEP分类法在预后和生物学角度明确区分DLBCL亚型方面更有优势,但目前仍有很多障碍限制了其广泛应用,包括可及性差、成本高、缺乏方法论标准化以及前瞻性验证。预后较差的ABC-DLBCL常与NF-κB通路固有活性以及该通路上游或下游调节子畸变密切相关。构成GCB-DLBCL淋巴瘤发生基础的分子机制仍存在争议且尚未明确,但 BCL-2和EZH2的C-REL扩增和突变很常见。下一代测序等新的技术正在加快研究步伐以期揭示新的致病性遗传畸变,DLBCL治疗策略也在不断发展,着重于个体发生表型内独特的致病性驱动因子。本综述分析了DLBCL相关的新发现的分子靶点和新的治疗制剂,探讨了利用分子特征来对DLBCL进行治疗分层的方法能否得到当前的临床前和临床证据支持。
Introduction
There is a spectrum of malignant lymphoma composed of large B cells. The majority fall into the category of diffuse large B-cell lymphoma (DLBCL), which is the subject of this review. DLBCL comprises 30%–40% of adult lymphomas, with an incidence of around 8 cases per 100,000 [1, 2]. Although many patients with DLBCL achieve long-term remission, approximately a third of patients relapse after first-line rituximab-chemotherapy regimens, with up to 30% eventually dying of their disease [3–10]. The most established prognostic algorithm is the International Prognostic Index, which is based on clinical and biochemical parameters [11]. More recently, as with solid organ malignancies [12], there has been a shift towards incorporating tumor molecular profiling into prognostication and treatment stratification for DLBCL.
With the advent of newer technologies, the heterogeneity in clinical outcome can be increasingly attributed to DLBCL tumor biology. Pivotal studies measured gene expression using cDNA microarrays [13–15] or oligonucleotide microarrays [16, 17] to create molecular signatures characterizing individual DLBCL phenotypes. Next-generation sequencing is also revealing previously unknown pathogenic genetic alterations [18–21]. The most well-validated of the molecular phenotype classification schemas is that defining DLBCL as being representative of its cell of origin (COO) [13, 14, 18]. Using this method, approximately 50% of DLBCL are classified as germinal center B-cell (GCB) subtype, around 30% are classified as the poorer prognosis activated B-cell (ABC) subtype, and the remaining 20% of DLBCL are unclassifiable, in which case they are commonly grouped with the ABC subtype as “non-GCB” [14, 15, 22].
This review aims to discuss established and emerging molecular hallmarks, recent developments in therapeutic strategy based on molecular phenotype, and how best to incorporate current knowledge into clinical practice in DLBCL.
Molecular Methods of Classification of DLBCL
Initial studies by the Lymphoma/Leukemia Molecular Profiling Project in the early 2000s that delineated DLBCL subtypes according to ontogenic phenotype performed gene expression profiling (GEP) on DLBCL pretreatment biopsies with the unsupervised method of hierarchical clustering (grouping according to signature similarity) to construct “lymphochip” cDNA microarrays [13, 14]. GCB-DLBCL was characterized by genes expressed in normal germinal-center B cells, whereas ABC-DLBCL was typified by genes expressed during activation of peripheral blood B cells [13]. The classification was changed to include a third unclassified group (type 3) [14] and further refined using statistical methods to resolve differences in between microarray platforms [15]. The investigators also proposed an alternative means of DLBCL classification based on both tumor microenvironment and intrinsic tumor characteristics [14, 22].
A separate research group concurrently profiled gene expression using an oligonucleotide microarray platform (Affymetrix) with a supervised clustering method to divide DLBCL into subtypes based on tumor molecular features associated with cure or refractoriness to chemotherapy [16]. Prominently overexpressed genes were NOR1, which was associated with cure, and PDE4B and PKCβ, both associated with refractory disease—the latter being a key component of the BCR and NF-κB signaling pathways [16, 23]. These authors found no difference in prognosis between COO subtypes using the classification of Alizadeh et al. [13]; however, on subsequent reanalysis of the material with refinement in COO phenotype definition, the difference in prognosis was evident (Table 1) [15]. A further study by this group used multiple unsupervised clustering methods in defining three DLBCL subtypes: OxPhos (oxidative phosphorylation), BCR/proliferation (activation of the BCR signaling cascade), and HR (presence of markers of T-cell mediated immune response) [17].
Selected studies of prognosis of diffuse large B-cell lymphoma subtype defined by either immunohistochemistry or gene expression profiling
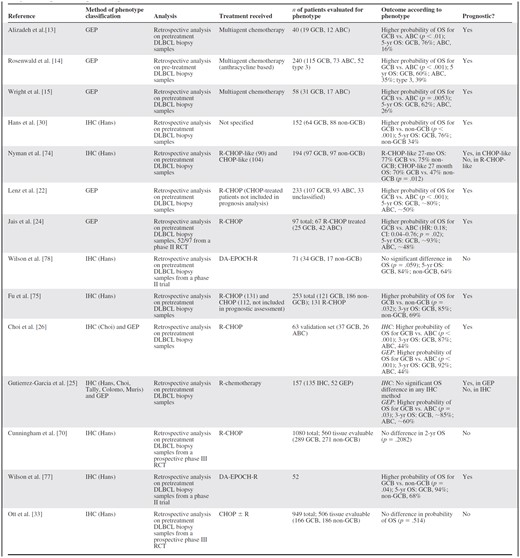
Abbreviations: ABC, activated B-cell; CHOP, rituximab, cyclophosphamide, doxorubicin, vincristine, prednisolone; CI, confidence interval; DA-EPOCH, dose-adjusted etoposide, prednisolone, vincristine, cyclophosphamide, doxorubicin; DLBCL, diffuse large B-cell lymphoma; GCB, germinal center B-cell; GEP, gene expression profiling; HR, hazard ratio; IHC, immunohistochemistry; OS, overall survival; R, rituximab; RCT, randomized controlled trial.
Selected studies of prognosis of diffuse large B-cell lymphoma subtype defined by either immunohistochemistry or gene expression profiling

Abbreviations: ABC, activated B-cell; CHOP, rituximab, cyclophosphamide, doxorubicin, vincristine, prednisolone; CI, confidence interval; DA-EPOCH, dose-adjusted etoposide, prednisolone, vincristine, cyclophosphamide, doxorubicin; DLBCL, diffuse large B-cell lymphoma; GCB, germinal center B-cell; GEP, gene expression profiling; HR, hazard ratio; IHC, immunohistochemistry; OS, overall survival; R, rituximab; RCT, randomized controlled trial.
Undoubtedly the various methods of defining DLBCL molecular phenotypes have contributed to knowledge of dysregulated pathways and potential therapeutic targets. However, lack of standardization, even within the COO phenotype definition described by the same group of authors, is an obstacle to broader clinical application. Although the GEP COO phenotype classification is the best verified as a predictor of prognosis in DLBCL [14, 22], to date independent validation has been within small retrospective analyses [24–26], with large-scale prospective clinical validation awaited.
The aforementioned studies have required fresh frozen tumor tissue samples on which to perform transcriptional profiling. This is prohibitive to wider use of GEP, as diagnostic biopsies are predominantly stored as formalin-fixed paraffin-embedded (FFPE) tissue. However, advances in technology have now permitted GEP to be successfully performed on mRNA in FFPE tissue [27], with reported high accuracy in defining COO phenotype compared to fresh frozen tissue [28, 29], although this data requires further verification.
Very recently, the exciting new technology of next-generation sequencing has enabled dramatic advances in understanding of the pathogenesis of DLBCL to be made in a short period of time through comprehensive unbiased interrogation of the DLBCL genome and transcriptome [18–21]. These methods have already revealed multiple functionally relevant mutations with a significant potential to lead to innovations in therapeutic strategy.
Immunohistochemical Methods of Classification of DLBCL
Although GEP is the standard method of evaluating DLBCL COO phenotype, it is currently costly, time consuming, and inaccessible for many centers. Hence, immunohistochemistry (IHC) algorithms were developed. The Hans algorithm [30] stratifies cases as GCB or non-GCB according to protein expression of CD10, BCL-6, and MUM1. The algorithm is prognostic and correlates with GEP-defined subtype in approximately 80% of cases [30]. Subsequent IHC algorithms with minor modifications have been developed [26, 31]. The Choi algorithm incorporates FOXP1 and GCET1 [26], whereas the Tally method substitutes BCL6 for LMO2 [31].
The three algorithms were recently compared to GEP in biopsy samples from 108 patients [32], with results favoring the Hans and Choi algorithms over Tally. The positive predictive value of these IHC classification methods for identifying GEP-classified COO ranged from 0.78 to 1.0, with sensitivity of 0.58–0.83; the Tally method was the least sensitive. The Hans and Choi algorithms (but not the Tally algorithm) were significantly predictive of overall survival (OS) and progression-free survival (PFS). Although accuracy is less than that of GEP and reproducibility is variable, particularly for BCL6 staining [33], IHC phenotype classification is more readily available and cost effective; hence, it has been more widely adopted both in clinical practice and prospective trial designs.
Molecular Characteristics of ABC Cell of Origin
ABC-DLBCL is frequently associated with constitutive activation of the NF-κB pathway, resulting in enhanced cell proliferation and decreased apoptosis. NF-κB pathway signaling occurs through dimerization of NF-κB transcription factors in the cytoplasm, which migrate to activate transcription of target genes in the nucleus [34, 35]. Negative pathway regulation is controlled by IkB family members and reversed by IkB kinase (IKK) [34]. Cell-line data using RNA interference (RNAi), retroviral transduction and small molecule inhibitors as methods of repressing the NF-κB pathway show that ABC-DLBCL but not GCB-DLBCL is dependent on the NF-κB pathway for survival [34, 36–37]. Mutations affecting the NF-κB pathway are more frequent but not exclusively present in ABC-DLBCL [38].
An important source of NF-κB pathway activation in ABC-DLBCL is alteration in genes encoding components of an upstream signaling complex involving CARD11, BCL10, and MALT1 (CBM complex) [35–36, 39], which also forms part of the BCR-signaling pathway (Fig. 1). Missense mutations in CARD11 occur with higher frequency in ABC-DLBCL (10%–11%) compared to GCB-DLBCL (4%–7%) [35, 38]. CARD11 mutant but not wild-type proteins have been demonstrated to independently promote NF-κB pathway stimulation when introduced into lymphoma cell lines, indicating oncogenic functionality [35]. Furthermore, RNAi knockdown of CARD11 negatively modulates NF-κB signaling and is selectively toxic to ABC-DLBCL cells [36].
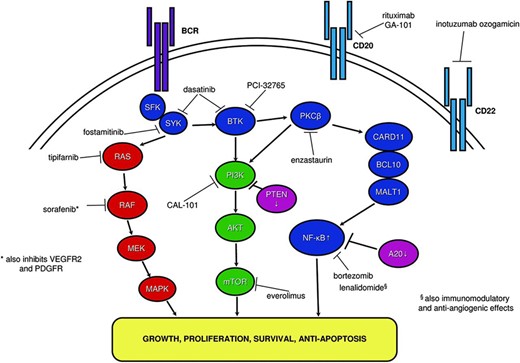
Pathogenetic signaling pathways in diffuse large B-cell lymphoma (DLBCL) and sites for exploitation with targeted therapy. Oncogenic intracellular signal transduction pathways for DLBCL including BCR signaling, RAS/RAF/MEK/MAPK, PI3K/AKT/mTOR, and NF-κB pathways are displayed. Tumor suppressors PTEN and A20 are depicted in pink, with downward arrows. Molecular targeted therapy in DLBCL and their sites of action are indicated.
Dysregulated B-cell-receptor (BCR) signaling initiated upstream of CARD11 also mediates constitutive NF-κB pathway activity and is implicated in lymphomagenesis of CARD11 wild-type (WT) ABC-DLBCL [40]. BCR-signaling is generated by ligand-induced receptor engagement and SRC family kinase phosphorylation of the CD79A and CD79B receptor subunits, leading to activation of spleen tyrosine kinase (SYK) and triggering of a downstream cascade including Bruton's tyrosine kinase (BTK) [40]. Antigenic stimulation of transient BCR-signaling is an essential physiological B-cell process [41]; however, chronic-active BCR-signaling is pathogenetic, as evidenced by the selective lethality of RNAi knockdown of key BCR pathway components in CARD11 WT ABC-DLBCL cell lines [36]. Specific mutations within the CD79B subunit that promote chronic active BCR signaling have also been demonstrated to occur in higher frequency in ABC-DLBCL than in GCB-DLBCL [40].
A20 contributes to termination of NF-κB pathway signaling and appears to have a tumour suppressor gene role [38, 42]. Inactivating mutations in A20 cause constitutive NF-κB activity through failure to abort pathway signaling; they occur in approximately 25% of ABC-DLBCL but are rare in GCB-DLBCL [38].
Other pathogenic molecular characteristics that are more frequent and of selective functional significance in ABC-DLBCL, but not directly associated with the NF-κB pathway, include 19q amplification, INK4a/ARF tumour-suppressor locus deletion, trisomy 3 [43], inactivation of tumor suppressor BLIMP1 (encoded by the PRDM1 gene) [44, 45], and overexpression of PIM family serine/threonine kinases [46].
Molecular Characteristics of GCB Cell of Origin
Two early GEP-identified recurring genetic alterations exclusive to GCB-DLBCL are the t(14;18) translocation in BCL-2 and C-REL amplification, which occur with a frequency of approximately 25% and 15%, respectively [14, 43]. The t(14;18) translocation causes expression of the antiapoptotic BCL-2 protein and is a hallmark of follicular lymphoma (FL), which also arises from the germinal center—perhaps explaining why transformed DLBCL in the setting of FL is frequently of the GCB subtype. BCL2 protein expression is common in both DLBCL subtypes [47], but there are conflicting data regarding the phenotype for which it has prognostic significance. Pre-rituximab studies using GEP showed that BCL-2 expression was negatively prognostic in patients with ABC-DLBCL but not GCB-DLBCL treated with cyclophosphamide/doxorubicin/vincristine/prednisone (CHOP) [48, 49]. However, in patients treated with CHOP plus rituximab (R-CHOP), the situation appears reversed; BCL-2 expression is associated with inferior prognosis in both IHC-defined [50] and GEP-defined [49, 51] GCB-DLBCL but not in ABC (or non-GCB) DLBCL.
Variation in the mechanism causing BCL-2 expression may explain this. In a recently presented study in 483 patients treated with R-CHOP, BCL-2 protein expression correlated with BCL-2 gains in ABC-DLBCL, which was non-prognostic, but correlated instead with the t(14;18) translocation in GCB-DLBCL, which was associated with inferior prognosis [51]. However, in a smaller study of 144 patients, the t(14;18) translocation was not associated with survival outcomes in patients treated with R-CHOP for GEP-defined GCB-DLBCL, possibly due to lack of power [47]. Further prospective clinical evaluation is necessary to clarify the role of the t(14;18) translocation as a biomarker in GCB-DLBCL.
c-MYC oncogene rearrangements as detected by fluorescent in-situ hybridization (FISH) occur with a frequency of 6%–14% [52–55] in DLBCL; they appear to be more common in GCB-DLBCL using the Hans IHC classification [52–53, 55–56], although an analysis using the Choi IHC algorithm is conflicting [57]. c-MYC rearrangements cause constitutive expression and dysfunction of the transcription controller c-myc, and they have been correlated with poor prognosis in patients treated with chemotherapy both with the addition of rituximab [53–55, 58–59] and without [52, 56]. The “double-hit” gene rearrangement of both c-MYC and t(14;18) is associated with FL histologically transformed to DLBCL [60] and conveys additively inferior prognosis [54–56]. High IHC expression of both BCL-2 and MYC is also associated with a poor prognosis [61].
Exome sequencing analyses have recently revealed that mutations in genes involved in epigenetic processes are prominent lymphoma-promoting mechanisms [18–19, 21]. Inactivating mutations in key histone acetyltransferase (HAT) genes CREBBP and EP300 also occur commonly in DLBCL; they display probable haplo-insufficient tumor suppressor functionality and appear pathogenic by impeding p53 tumor suppressor activity and enhancing BCL6 oncogene activity [19]. CREBBP mutations occur more frequently in GCB-DLBCL (32%) than in ABC/nonclassified DLBCL (13%) [19]. EP300 mutations are rarer, but again occur more often in GCB-DLBCL and seldom coexist with CREBBP mutations [19].
Mutations in the mixed-lineage leukemia 2 (MLL2) gene occur in biopsy samples with frequency of 27% in GCB-DLBCL and 20% in ABC-DLBCL; the majority of these appear to be inactivating [18, 20]. MLL2 encodes a histone methyltransferase, which influences gene transcription [18]. MLL2 appears to behave as a haploinsufficient tumor suppressor gene (modified by one allelic mutation only) [18]. Mutations at codon 641 of histone methyltransferase gene EZH2 are almost exclusive to GCB-DLBCL, present in 14%–22% of cases [18, 62–63]. Other GEP-identified potential oncogenic drivers that are exclusive or almost exclusive to GCB-DLBCL are overexpression of the mir-17–92 microRNA cluster amplification in approximately 13% and PTEN deletion in 11% of cases [43].
TP53 mutations were reported to be negatively prognostic in IHC-defined GCB-DLBCL but not in non-GCB-DLBCL [64]. However, this result was not supported by a study using GEP, which failed to demonstrate a difference between subgroups [65]. TP53 mutations can provoke activation of the antiapoptotic NF-κB pathway, which may be more significant in GCB-DLBCL; in ABC-DLBCL, this pathway is already constitutively activated [64, 66].
Molecular Characteristics Shared by GCB and ABC Cell of Origin
The frequencies of other molecular alterations have either not been compared between the GCB or ABC phenotypes or are similar in both. Inactivating mutations leading to absence of expression of immune recognition molecules β2 microglobulin (β2M) and CD58 have been identified in both GCB and ABC/nonclassified DLBCL (β2M: 10%–15%; CD58: 6%–7%), indicating that cellular immune surveillance is another lymphomagenic mechanism [67].
BCL-6 cytogenetic rearrangements are common in DLBCL [54]. There is conflicting data from studies using the Hans IHC criteria to define subtype with regard to whether these are more prevalent in non-GCB-DLBCL [68] or are similar in both subtypes [53, 54]. Uddin et al. demonstrated that PI3K pathway signaling as assessed by pAKT IHC expression occurs with a frequency of 52% in DLBCL and may represent a therapeutic target; however, the results were not analyzed according to COO subtype [69].
Therapeutic Strategy According To Molecular Profile
The enhanced delineation of tumorigenesis in DLBCL, capturing and categorizing the heterogeneous pattern of genetic alterations, has the potential to move clinicians toward stratification of treatment for DLBCL according to COO and individualized tumor signature. This knowledge has led to development of novel targeted agents, as well as to questions relating to the best use of current chemotherapy-based strategies.
Predictive and Prognostic Utility of COO for Rituximab-Treated DLBCL
Pre-rituximab analyses demonstrated superior outcomes in GEP-defined GCB-DBCL compared to ABC-DLBCL in patients receiving first-line chemotherapy [13–15]. Although a seminal study showed maintained prognostic differentiation by GEP-defined phenotype in patients treated with rituximab plus chemotherapy (R-chemotherapy) [22], retrospective analyses from large randomized controlled trials (RCTs) in R-chemotherapy-treated patients showed no significant difference in prognosis between IHC-defined ontogenic subtypes [33, 70]. This has led to the hypothesis that ABC-DLBCL may benefit more substantially from rituximab than GCB-DLBCL, and that COO may therefore have a role in prediction of benefit from rituximab [71], supported by the biological rationale of preclinical evidence of rituximab inhibiting the NF-KB pathway [72]. However, a more likely reason for the observation of lack of evidence for COO as a prognostic biomarker in rituximab-treated patients is the inferior accuracy and reproducibility of IHC-classification methods compared with GEP [73], given that studies showing lack of prognostic utility are amongst those using IHC classification methods [33, 70, 74–76].
Small retrospective studies in patients treated predominantly with R-CHOP using GEP COO classification continue to demonstrate superior prognosis for GCB compared to ABC-DLBCL in rituximab-treated patients [24, 25]. These findings require verification in prospective clinical trials designed with upfront stratification by GEP-defined ontogenic subtype. Evidence for the prognostic utility of the COO classification in clinical studies of patients with DLBCL treated with chemotherapy with or without rituximab is presented in Table 1.
GCB/ABC Subtype as a Predictive Biomarker for Chemotherapy
Evidence suggests that COO phenotype may be predictive of response to standard chemotherapy regimens. The phenotype of a subset of patients within the second-line CORAL (Collaborative Trial in Relapsed Aggressive Lymphoma) study (249 of 396 patients) [53] was retrospectively analyzed using IHC methods [30]. IHC COO classification was validated by GEP in a small sample (n = 39), with 75% concordance. Patients with Hans IHC-defined GCB-DLBCL in the R-DHAP (rituximab, dexamethasone, high-dose cytarabine, and cisplatin) arm had improved PFS compared with non-GCB (52% vs. 32%, p = .01), but no difference between subtype was seen in the R-ICE (rituximab, ifosfamide, carboplatin, and etoposide) arm (31% vs. 27%, p = .81), and OS was not significantly different between phenotype in either arm. In the small GEP-defined sample, GCB phenotype was also predictive of response to chemotherapy (3-year PFS: 100% for R-DHAP vs. 27% for R-ICE; p = .01); however, ABC phenotype was not predictive [53]. The study was not powered for comparison between phenotype. Although the authors propose that cytarabine may alter BCL-6 expression levels and facilitate p53 release as justification for the findings, they recognize that this unplanned, retrospective analysis of small subgroups can only be hypothesis generating.
Interstudy comparison has led to discussion of whether GCB-DLBCL may benefit more from nonanthracycline-based chemotherapy than from R-CHOP [22, 53], because small single-arm phase II studies suggested improved outcome for DA-EPOCH-R (dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin and rituximab) in IHC-defined GCB-DLBCL [77, 78], whereas retrospective IHC analyses of phase III clinical trials treating with CHOP with or without rituximab show no difference in outcome between GCB and non-GCB subtypes [22, 70]. Once again, the IHC classification methods used may mean that these data are unreliable and should be interpreted with caution. The results of a currently recruiting phase III RCT investigating the benefit of R-CHOP versus R-EPOCH (etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab) that incorporates GEP (ClincalTrials.gov NCT 00118209) will be interesting. Further clinical evidence from trials incorporating prospective GEP-defined COO subtype stratification is necessary to clarify the role for COO as a predictive biomarker for specific chemotherapy.
Targeted Therapy for DLBCL According to Molecular Characteristics
NF-κB: Protease Inhibitors
NF-κB pathway targeting is logical therapeutic strategy in ABC-DLBCL because of its pivotal role in pathogenesis. In vitro research has demonstrated that inhibition of the NF-κB pathway in ABC-DLBCL cells results in cell death [34, 37]. Bortezomib is a proteasome inhibitor with multiple actions [79], including prevention of breakdown of phosphorylated IκBα, resulting in NF-κB pathway repression [34, 76, 79–80]. Bortezomib monotherapy appears to have no activity in DLBCL [76], but there is phase I/II trial evidence of efficacy in combination with chemotherapy [76, 80–81]. Bortezomib potentially acts synergistically with chemotherapy by negating the antiapoptotic, chemotherapy-resistance promoting effects of NF-κB pathway signaling.
A small nonrandomised phase II study of 27 patients with relapsed/refractory (RR)-DLBCL evaluated bortezomib plus DA-EPOCH chemotherapy in ABC-DLBCL (n = 12) versus GCB-DLBCL (n = 15). Results demonstrated greater efficacy in ABC-DLBCL (overall response rate [ORR]: 83% vs. 13%, p < .001; median OS: 10.8 vs. 3.4 months; p = .003) [76]. Phenotype was classified in approximately half of patients by GEP plus IHC and in half by IHC (Hans) alone [76].
Another nonrandomised phase I/II study of 40 patients with DLBCL treated with first-line R-CHOP plus bortezomib found no difference in outcome between GCB and non-GCB subtype using the Hans algorithm (ORR: 88%; 2-year OS: 70%). The poorer prognosis associated with non-GCB subtype and the use of IHC alone in the latter study may contribute to the discrepancy [80].
Acknowledging limitations relating to the differing phenotype classification methods and small numbers, these data have led to larger-scale research. In the U.K., the currently recruiting REMoDLB phase III trial (ClinicalTrials.gov NCT01324596) of first-line treatment of DLBCL (planned n = 940) is evaluating R-CHOP with and without bortezomib. Stratification is by COO molecular subtype incorporating an interim efficacy analysis, with a planned early stop in the GCB subgroup if bortezomib is not beneficial. A first-line U.S. phase II randomized trial is also currently recruiting, with plans to enroll 190 patients with non-GCB subtype. It will assess R-CHOP with and without bortezomib (ClinicalTrials.gov NCT00931918).
Immunomodulation: Lenalidomide
Lenalidomide, an oral immunomodulatory drug with proapoptotic and antiangiogenic properties, has demonstrated activity as monotherapy in RR-DLBCL [82, 83] and in dual combination with rituximab for elderly patients with RR-DLBCL [82]. It was safely combined with R-CHOP first-line treatment with encouraging response rates [82, 84].
Theoretically, lenalidomide may be preferentially effective in ABC-DLBCL due to preclinical evidence in cell lines demonstrating suppression of NF-κB [85, 86]. In support of this hypothesis, a retrospective clinical study of 40 patients with RR-DLBCL who were treated with lenalidomide demonstrated an improved ORR in non-GCB compared to the GCB subtype (53% vs. 9%, p = .006), using the Hans IHC classification [87].
Although these studies do not provide sufficient evidence to currently recommend stratifying treatment with lenalidomide by COO subtype, they have led to the hypothesis being tested in a randomized phase II/III trial, including patients with RR-DLBCL in third-line treatment line or who are unsuitable for transplantation. The study compares lenalidomide to the investigator's choice of cytotoxic monotherapy, with stratification for phenotype (GCB or non-GCB), again incorporating early analysis of efficacy according to subtype (ClinicalTrials.gov NCT01197560).
BCR Signaling: SYK Inhibitors
Given the evidence for the dependence of ABC-DLBCL upon BCR signaling for survival, particularly in CARD11 WT disease, targeting of the BCR-signaling pathway is a rational therapeutic strategy [35]. Experiments in cell lines and primary DLBCLs have demonstrated that sensitivity to R406, an inhibitor of SYK, is associated with BCR signaling-related factors [88].
Fostamitinib, an oral SYK inhibitor and R406 prodrug, has shown activity as monotherapy in multiply-RR DLBCL (COO subtype not reported), with an ORR of 22% in a phase II study [89]. Of 23 patients, complete remission was reported for one patient and partial remission for four patients. It is yet to be determined whether the preclinical findings will translate into greater efficacy for fostaminib in ABC-DLBCL than GCB-DLBCL in the clinical setting.
BCR Signaling: SRC and BTK Inhibitors
Bruton's tyrosine kinase (BTK) is a BCR-signaling pathway protein located upstream of CARD11, activated by SYK [40]. It is a logical target for therapeutic intervention in ABC-DLBCL given evidence that it is essential for survival in CARD11 WT cell lines [40]. Preclinical experiments revealed that a small molecule irreversible inhibitor of BTK, PCI-32765, blocks BCR signaling [90]; it is active in CARD11 WT but not in CARD11 MT ABC-DLBCL [91]. PCI-32765 has been tested in a phase I trial in RR-DLBCL, with minimal toxicity and two of seven patients showing objective responses [92]. The preliminary results of a small phase II study in RR-ABC-DLBCL reported achievement of complete response in two of eight patients treated with PCI-32765 monotherapy [93]. Other PCI-32765 phase II studies are in progress and other BTK inhibitors are in early stages of development.
Dasatinib is an inhibitor of SRC family kinases and BTK [94]. An early preclinical study found no difference in the activity of dasatinib between GCB and non-GCB cells using the Hans IHC classification [95]. However, a subsequent preclinical study using GEP demonstrated that ABC-DLBCL cells dependent on BCR signaling were selectively sensitive to dasatinib, whereas BCR-independent ABC-DLBCL and GCB-DLBCL cells were insensitive [40]. Because its targets are upstream of CARD11, dasatinib may also be more effective in CARD11 WT disease [40].
PKCβ Inhibition
Enzastaurin is an oral inhibitor of protein kinase Cβ (PKCβ), which has the knock-on effects of PI3K/AKT and mTOR pathway inhibition [96]. PKCβ indirectly stimulates IKK activity, promoting constitutive NF-κB signaling [97], so notionally it may be more effective in ABC-DLBCL. Enzastaurin shows activity in preclinical studies [98, 99], and 4E-BP1 function has been identified as a potential biomarker [96].
A phase II study reported activity of enzastaurin monotherapy in RR-DLBCL (COO subtype unreported), with minimal toxicity and 4 of 55 patients achieving durable remissions [100]. A phase III study assessing the benefit of enzastaurin in maintenance of remission after first-line R-CHOP in DLBCL has completed accrual and the results are awaited (ClinicalTrials.gov NCT00332202).
Epigenetic Strategies
The histone deacetylase inhibitor (HDACI) vorinostat has limited single-agent activity in DLBCL [101]. However, the combination of HDACI pabinostat and hypomethylating agent decitabine is synergistic in DLBCL in preclinical studies [102]. GEP analysis has revealed genes with altered methylation as a result of the combination drug therapy, such as VHL methylation, a known poor prognostic factor in DLBCL, which may be worthy of evaluation in clinical studies [102, 103].
Angiogenesis Inhibition
Continuing on from the recognition that the DLBCL stromal-2 phenotype has an abundance of vessels and angiogenic factors [22], VEGF-targeting with bevacizumab has been tested in DLBCL, although without evidence of benefit. A phase II trial in first-line treatment of DLBCL with bevacizumab plus R-CHOP was negative. Additionally, 30 of the 64 patients treated had serious adverse events, including increased gastrointestinal perforation and cardiac dysfunction [104]. A phase III trial of R-CHOP with and without bevacizumab was stopped early due to an unfavorable risk-benefit assessment (ClinicalTrials.gov NCT00486759).
PI3K/mTOR Inhibition
The PI3K/AKT/mTOR pathway is a key physiological and oncogenic lymphocyte intracellular signaling pathway [105]. In DLBCL, dysregulated signaling through phosphatidylinositol-3-kinase (PI3K) may be propagated by BCR signaling, stimuli from the tumor microenvironment, or PTEN deletion [105, 106]. CAL-101 is a selective small-molecule inhibitor of the hematopoietic cell-specific p110δ isoform of PI3K and is active in DLBCL cell lines [106]. CAL 101 has promising clinical activity in non-Hodgkin lymphoma and chronic lymphocytic leukemia [80, 107], but clinical activity in DLBCL is yet to be demonstrated [108].
Based on evidence of efficacy of mTOR inhibition in other types of lymphoma, oral everolimus, a selective mTORC1 inhibitor, has been tested in small nonrandomized phase I/II clinical trials for RR-DLBCL, with ORR of 30%–31% [109, 110]. There is phase II evidence for the activity of temsirolimus, another mTORC1 inhibitor, in RR-DLBCL, with ORR of 28% (n = 32) [111]. Dual-targeting strategies may have the potential to improve on single-agent efficacy by overcoming resistance mechanisms, such as escape signaling via mTORC2 [109, 112].
Ras/Raf/MEK/ERK Pathway Inhibition
Oral tipifarnib is a farnesyltransferase inhibitor that has multiple effects. A central mechanism of action is inhibition of the Ras signaling pathway, leading to upregulation of the proapoptotic BH-3 protein BIM [113, 114]. A phase II trial including 37 patients with RR-DLBCL treated with tipifarnib reported ORR of 17% [113]. Upregulation of BIM in association with low BCL-2 expression (BCL-2 inhibits BIM) was predictive for response [113].
Other Potential Targeted Therapies in Early Development
Many other potential therapies remain under early evaluation, including JAK1/JAK2 inhibitors [115], MDM2 inhibitor nutlin-3a [116], AKT inhibitor MK-2206 [103], a small-molecule MEK inhibitor [117], CHK1/2 inhibitor PF-0477736 [118], AAK inhibitor MLN8237 [119], anti-CD 22 immunoconjugate inotuzumab ozogamicin [120], CD19 targeting [121, 122], and type II anti-CD20 monoclonal antibody GA-101 [123]. A selection of key emerging targeted therapies are summarized in Table 2.
Selected clinical studies of targeted therapy in patients with diffuse large B-cell lymphoma subtype
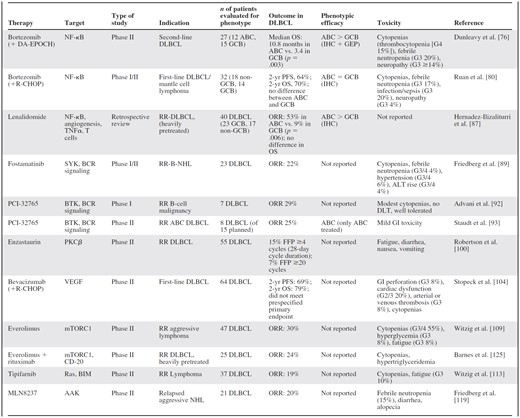
Abbreviations: ABC, activated B-cell; ALT, alanine aminotransferase; CHOP, rituximab, cyclophosphamide, doxorubicin, vincristine, prednisolone; DA-EPOCH, dose-adjusted etoposide, prednisolone, vincristine, cyclophosphamide, doxorubicin; DLBCL, diffuse large B-cell lymphoma; DLT, dose-limiting toxicity; G, grade; GCB, germinal center B-cell; GEP, gene expression profiling; GI, gastrointestinal; IHC, immunohistochemistry; NHL, non-Hodgkin lymphoma; ORR, overall response rate; OS, overall survival; PFS, progression-free survival; R, rituximab; RCT, randomized controlled trial; RR, relapsed/refractory.
Selected clinical studies of targeted therapy in patients with diffuse large B-cell lymphoma subtype

Abbreviations: ABC, activated B-cell; ALT, alanine aminotransferase; CHOP, rituximab, cyclophosphamide, doxorubicin, vincristine, prednisolone; DA-EPOCH, dose-adjusted etoposide, prednisolone, vincristine, cyclophosphamide, doxorubicin; DLBCL, diffuse large B-cell lymphoma; DLT, dose-limiting toxicity; G, grade; GCB, germinal center B-cell; GEP, gene expression profiling; GI, gastrointestinal; IHC, immunohistochemistry; NHL, non-Hodgkin lymphoma; ORR, overall response rate; OS, overall survival; PFS, progression-free survival; R, rituximab; RCT, randomized controlled trial; RR, relapsed/refractory.
Dual-Targeting Strategies
There is no single driving mutation in DLBCL; hence, a multitargeted strategy is required. Several combinations of targeted agents are being evaluated prospectively in early-phase trials due to promising synergistic activity, such as sorafenib (an inhibitor of Raf/VEGF) plus everolimus (ClinicalTrials.gov NCT00474929) [124], everolimus plus rituximab [125], and pabinostat plus everolimus [126]. Other combinations with preclinical evidence of synergy include NVP-BEZ235 (a dual PI3K/mTOR inhibitor) plus enzastaurin [127], lenalidomide and the BTK inhibitor ibrutinib [86], and the BCL-2 inhibitor obatoclax plus proteosome inhibitor carfilzomib [128].
Future Directions
It is important to recognize that the majority of these novel drugs are currently in early stages of development. Many of the targets at which these therapies are directed have been developed based on preclinical science in cell lines rather than in primary tumor specimens, making it difficult to be certain that the dyregulated pathways identified represent authentic oncogenic driving mechanisms in DLBCL. At the time of writing, the reported prospective clinical studies have contained small nonrandomized populations, and clearly further verification of their efficacy is required.
To truly personalize medicine for DLBCL, not only does efficacy of these novel agents need validation, but their role needs to be established within the current treatment paradigm. Adaptive trial designs with enriched patient populations and markers of early response, marrying translational and clinical knowledge, must remain a key focus for future research. Preplanned subgroup analyses and stratification should be based on not only clinical characteristics but also on tumor biology.
Current treatment paradigms do not distinguish between COO phenotype, and this can be readily justified. Several large analyses have shown no difference in prognosis between phenotypes using IHC classification methods, indicating a lack of precision and reliability that dissuades against routine clinical use. GEP has superior accuracy in classifying DLBCL into COO subtypes with different biology and prognosis. It holds promise for future clinical application once issues of availability, cost, preference for analyses to be performed on fresh frozen tissue, lack of standardization in COO phenotype classification methods, and lack of prospective randomized clinical evidence validating its use can be overcome.
Conclusion
Biologically distinct ontogenic phenotypes with distinct pathogenic driving mechanisms are now well recognized in DLBCL, offering potential opportunities to individualize therapy based on molecular characteristics. Although GEP is more accurate than IHC in determining COO, it is associated with issues of accessibility, methodology, and lack of robust evidence. Therefore, a change in clinical practice with stratification of treatment according to molecular hallmarks is not presently vindicated. However, given the prognostic and therapeutic implications, prudence is suggested with respect to recognizing GEP-determined COO and key molecular targets within all future therapeutic trial designs.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Acknowledgments
This work was supported by National Health Service funding to the National Institute for Health Research Biomedical Research Centre.
Author Contributions
Conception and design: Sarah Barton, Eliza A. Hawkes, David Cunningham
Collection and/or assembly of data: Sarah Barton, Eliza A. Hawkes, Andrew Wotherspoon
Data analysis and interpretation: Sarah Barton, Eliza A. Hawkes, Andrew Wotherspoon, David Cunningham
Manuscript writing: Sarah Barton, Eliza A. Hawkes, Andrew Wotherspoon
Final approval of manuscript: Sarah Barton, Eliza A. Hawkes, David Cunningham
References
Author notes
Disclosures: David Cunningham: Roche, Amgen (C/A); Roche, Merck, Amgen (RF). The other authors indicated no financial relationships.
Section Editors: George P. Canellos: Celgene Business Advisory Board (C/A)
Reviewer “A”: None
Reviewer “B”: Gilead Sciences, Allos Therapeutics, Algeta, Millennium, Genentech, Chesapeake Biotech (C/A); Roche (H)
Reviewer “C”: NIH-RO1 grant (RF)



