-
PDF
- Split View
-
Views
-
Cite
Cite
Howard A. Burris, Jeffrey R. Infante, Roxanne C. Jewell, David R. Spigel, F. Anthony Greco, Dana S. Thompson, Suzanne F. Jones, A Phase I Study of Weekly Topotecan in Combination with Pemetrexed in Patients with Advanced Malignancies, The Oncologist, Volume 15, Issue 9, September 2010, Pages 954–960, https://doi.org/10.1634/theoncologist.2010-0006
Close - Share Icon Share
Abstract
This phase I study evaluated the safety, tolerability, preliminary antitumor activity, and pharmacokinetic interaction of weekly topotecan (days 1 and 8) in combination with pemetrexed (day 1 only) in patients with advanced solid tumors.
Patients received topotecan (3.0–4.0 mg/m2 i.v. days 1 and 8) and pemetrexed (375–500 mg/m2 i.v. day 1) over 21-day cycles. Patients were accrued across five different dose levels and were observed for safety, tolerability, and preliminary activity.
Twenty-six patients received 120 cycles of pemetrexed and topotecan, including five patients who received 8, 8, 10, 12, and 17 cycles without dose reductions, confirming a lack of cumulative myelosuppression. Four patients received topotecan (4.0 mg/m2 i.v.) and pemetrexed (500 mg/m2 i.v.), but experienced two dose-limiting toxicities (febrile neutropenia, grade 4 thrombocytopenia). As a result, the topotecan (3.5 mg/m2 i.v.) and pemetrexed (500 mg/m2 i.v.) group was expanded to 12 patients. The only grade 3 or 4 nonhematologic toxicity was one episode of grade 3 fatigue; no grade 3 or 4 nausea/vomiting/diarrhea, mucositis, or rash was reported. One non-small cell lung cancer (NSCLC) patient (12 months) and one soft tissue sarcoma patient (6 months) achieved a partial response.
Weekly topotecan plus every-3-week pemetrexed was well tolerated and active. Full doses of topotecan plus pemetrexed caused brief reversible myelosuppression with minimal dose delays/reductions; no grade 3 or 4 nausea/vomiting/diarrhea, mucositis, or rash was reported. All six NSCLC patients at the recommended phase II dose had at least stable disease as a best response, including one partial response lasting 12 months. There was no evidence of an effect of pemetrexed on topotecan pharmacokinetics. Collectively, these data suggest that further phase II exploration of weekly topotecan plus every-3-week pemetrexed for advanced malignancies is indicated.
Introduction
Topotecan (Hycamtin®; GlaxoSmithKline, Philadelphia) is a topoisomerase I inhibitor that has been demonstrated to have a broad spectrum of antitumor activity in a variety of tumor types, including ovarian cancer, endometrial cancer, cervical cancer, non-small cell lung cancer (NSCLC), and small cell lung cancer, as well as in brain metastases from a variety of tumor types. The i.v. formulation of the drug is currently approved by the U.S. Food and Drug Administration (FDA) for the treatment of patients with ovarian or small cell lung cancer. The approved dosing schedule is 1.5 mg/m2 administered as a 30-minute infusion daily for five consecutive days, with treatment cycles repeated every 3 weeks. Alternative dosing schedules, such as 3-day dosing regimens, continuous infusion, and weekly administration schedules, have also been evaluated in the clinical trial setting. An oral formulation of topotecan was also approved by the FDA in October of 2007 for the treatment of relapsed small cell lung cancer. Myelosuppression is the most common toxicity of topotecan, particularly when the drug is administered on consecutive days. Nonhematologic toxicities are generally mild to moderate in severity and consist primarily of nausea, vomiting, and diarrhea.
Based on the activity of topotecan in patients with small cell lung cancer, the drug has also been investigated for the treatment of NSCLC. Several trials have been conducted with single-agent i.v. or oral topotecan for the first- or second-line treatment of NSCLC. Those trials produced a response rate of 0%–15% with median survival times of 26–41 weeks [1–7].
In an effort to enhance antitumor activity, topotecan has also been combined with various other chemotherapeutic agents, including cisplatin, carboplatin, vinorelbine, gemcitabine, etoposide, paclitaxel, and docetaxel, in the first- and second-line treatment of NSCLC patients [8–18]. The combination regimens had superior antitumor activity, when compared with studies using single-agent topotecan.
Alternative topotecan dosing schedules have been explored in an attempt to ameliorate treatment-related myelosuppression, increase antitumor activity, enhance drug incorporation into combination regimens, and improve patient convenience. Myelotoxicity is no longer dose limiting with the weekly administration schedule, and with the exception of fatigue, the dosing regimen is very well tolerated. Two small phase I/II trials have been conducted with weekly topotecan combination regimens in NSCLC patients. The first trial was a dose escalation study of weekly topotecan, cisplatin, and gemcitabine (days 1, 8, and 15 every 28 days) in the first-line treatment of patients with inoperable NSCLC [12]. The triple drug regimen was extremely well tolerated, with minimal myelosuppression, and resulted in a response rate of 38% with a median survival duration of 38 weeks and a 1-year survival rate of 33%. The second trial was a phase I/II study of weekly topotecan and gemcitabine (days 1, 8, and 15 every 28 days) in patients with advanced NSCLC [19]. Again, the regimen was extremely well tolerated, with a 21% response rate and 22-week median survival time. These preliminary studies suggest that weekly topotecan administration does ameliorate treatment-related myelosuppression, allowing the drug to be easily incorporated into combination regimens. Patient convenience and quality of life should also be improved with the weekly administration schedule. However, further studies evaluating the efficacy of weekly topotecan administration in a variety of tumor types are certainly warranted.
Pemetrexed (Alimta®; Eli Lilly and Company, Indianapolis) is a multitargeted antifolate antineoplastic agent that has been demonstrated to have antitumor activity in a variety of tumor types, including nonsquamous NSCLC, mesothelioma, breast cancer, colorectal cancer, bladder cancer, cervical cancer, gastric cancer, and pancreatic cancer. The drug is currently approved by the FDA for the treatment of patients with malignant pleural mesothelioma or locally advanced or metastatic NSCLC. The approved dosing regimen is 500 mg/m2 administered i.v. over 10 minutes on day 1 of a 21-day cycle. Premedication with corticosteroids and vitamin supplementation are required to minimize the skin, mucosal, and bone marrow toxicities related to pemetrexed administration. The most common toxicities reported with pemetrexed therapy include myelosuppression, stomatitis, and rash, all of which are ameliorated with the administration of appropriate premedications.
Pemetrexed has been demonstrated to have significant antitumor activity as a single agent in the first- or second-line treatment of NSCLC patients. In four trials using single-agent pemetrexed in NSCLC patients, response rates of 9%–21% and median survival durations of 4–9.2 months were reported [20–23]. Pemetrexed has also been combined with other chemotherapy agents, including cisplatin, carboplatin, oxaliplatin, gemcitabine, and vinorelbine, for the treatment of advanced NSCLC. These chemotherapy doublets have produced response rates and median survival times that are very similar to those seen with the platinum- and nonplatinum-based doublet regimens used for the treatment of NSCLC.
Prior to this trial, no data existed regarding the safety and tolerability of a combination regimen using weekly topotecan in combination with pemetrexed. Although both drugs are associated with myelosuppression, it was hoped that use of the weekly topotecan dosing schedule would allow the drugs to be easily combined. This phase I trial evaluated the safety, tolerability, preliminary antitumor activity, and pharmacokinetic interaction of weekly topotecan (days 1 and 8) in combination with pemetrexed (day 1 only) in patients with advanced solid tumors.
Patients and Methods
Patient Selection
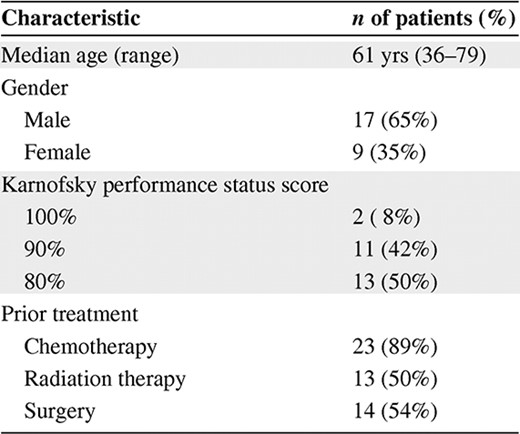
Patients aged ≥18 years with histologically proven advanced solid tumors and an Eastern Cooperative Oncology Group performance status score of 0 or 1 were enrolled. Patients must have received no more than three previous chemotherapy regimens in the metastatic setting and must have recovered from the toxicities of previous regimens prior to enrollment. Patients must not have previously received topotecan or pemetrexed. Patients must have completed prior chemotherapy regimens >3 weeks or prior investigational therapy >4 weeks before the first day of treatment. Patients must have completed previous radiotherapy at least 4 weeks prior to study entry and recovered from any toxicity associated with the radiotherapy. The following baseline laboratory values were required: absolute neutrophil count (ANC) >1,500/μl, hemoglobin >9.0 g/dl, platelet count >100,000/μl, serum bilirubin <1.5× the upper limit of normal (ULN), serum glutamic oraloacetic transaminase and serum glutamic pyruvic transaminase <3× ULN (5× ULN if liver disease was documented), serum creatinine ≤1.5 mg/dl, and estimated creatinine clearance ≥45 ml/minute. Patients with any of the following were excluded from the trial: clinically significant third space fluid (effusion or ascites), inability to interrupt aspirin or nonsteroidal anti-inflammatory drugs around pemetrexed dosing, inability to take steroid premedications or vitamin supplementation required for pemetrexed, active brain metastases, and prior surgery within 3 weeks of treatment. Female patients who were pregnant or lactating were ineligible for the study and male and female patients of child-bearing potential not practicing adequate contraception were excluded. The study was approved by the local institutional review board and written informed consent was obtained from all patients prior to enrollment.
Treatment Plan
Table 1 describes the dose levels that were explored. Because of the similarity in dose levels, patients were accrued to dose levels 2 and 3 simultaneously. Three patients were accrued at each individual dose level and all three patients were observed for an entire treatment cycle before subsequent dose escalation could proceed. Dose level −1 was only used for purposes of dose reductions in the event that dose level 1 was not tolerated. If dose-limiting toxicities were observed in one of three patients, the dose level was expanded to six patients. If dose-limiting toxicities (DLTs) were reported in < two of six patients, then dose escalation was continued. If more than or equal to two of three or more than or equal to two of six patients experienced a DLT, the next lower dose was considered the maximum-tolerated dose. No intrapatient dose escalations were permitted. In total, 12 patients were treated at the maximum-tolerated dose to further assess the toxicity of the dosing regimen and its suitability for use in subsequent trials.
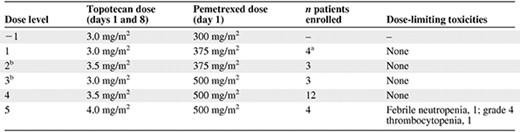
aOne patient was unevaluable for dose-limiting toxicity assessment because of underlying illness.
bPatients were enrolled simultaneously on dose levels 2 and 3.

aOne patient was unevaluable for dose-limiting toxicity assessment because of underlying illness.
bPatients were enrolled simultaneously on dose levels 2 and 3.
For the purposes of this study, acute DLT was defined as any of the following: (a) grade 4 neutropenia lasting ≥7 days or febrile neutropenia, (b) grade 4 thrombocytopenia or requirement for a platelet transfusion, (c) any grade 3 or 4 treatment-related nonhematologic toxicity considered clinically significant by the investigator (warranting a dose reduction or discontinuation of therapy), (4) grade 3 or 4 nausea, vomiting, or diarrhea that occurred despite the use of maximal supportive therapy, (e) inability to administer all doses in a treatment cycle at the full dose (100%) or inability to start cycle 2 of treatment as scheduled because of treatment-related toxicity. The National Cancer Institute Common Toxicity Criteria, version 3.0, was used to grade treatment-related toxicities.
Drug Dosing and Administration
Treatment cycles were repeated every 21 days. Topotecan and pemetrexed were administered on day 1. On days when both drugs were administered, the pemetrexed infusion was administered first followed immediately by the administration of topotecan. The topotecan dose was repeated on day 8 of the treatment cycle if the patient's ANC was ≥500/μl, platelet count was ≥50,000/μl, and there were no treatment-related nonhematologic toxicities of grade >2 present. If these criteria were not met, the day 8 dose of topotecan was omitted for that treatment cycle and was not made up at a later time.
All topotecan and pemetrexed doses were based on body surface area calculated at the beginning of each treatment cycle. Actual weight was used to calculate body surface area for all patients, including obese patients. An investigational supply of topotecan was provided by GlaxoSmithKline. The drug was diluted in 100 ml 0.9% normal saline or 5% dextrose and infused over 30 minutes. An investigational supply of pemetrexed was provided by Lilly. The drug was diluted in 100 ml 0.9% normal saline and was infused over 10 minutes. Antiemetics were administered at the discretion of the treating physician. All patients were instructed to take a low-dose oral folic acid preparation or multivitamin with folic acid (350–1,000 μg) on a daily basis. At least five daily doses of folic acid were taken during the 7-day period preceding the first dose of pemetrexed and dosing was continued throughout therapy. Patients also received an i.m. injection of vitamin B12 (1,000 μg) during the week preceding the first dose of pemetrexed, with subsequent doses repeated approximately every 9 weeks (three cycles). For pemetrexed rash prophylaxis, patients also received dexamethasone (4 mg orally twice daily) the day before, the day of, and the day after pemetrexed administration.
Dose Modification/Reduction Guidelines
Patients were required to meet all the following criteria in order to begin a new cycle of treatment: (a) platelet count ≥100,000/μl, (b) ANC ≥1,500/μl, and (c) resolution of clinically significant nonhematologic toxicities to grade ≤2. If a patient did not meet these criteria at the end of a treatment cycle, the next cycle of treatment was delayed until all criteria were met. Patients requiring a delay in dosing of >2 weeks were removed from study because of poor tolerability.
Any patient experiencing a treatment-related DLT as described above had subsequent doses of topotecan and/or pemetrexed reduced by one dose level. If the treatment-related toxicity could be specifically attributable to only one drug, then the treating physician could reduce the dose of the attributable drug and the dose of the other drug could remain the same. Any required dose reductions were maintained throughout all subsequent cycles. The potential dose levels for topotecan were 3.0 mg/m2, 3.5 mg/m2, and 4.0 mg/m2 and they were 300 mg/m2, 375 mg/m2, and 500 mg/m2 for pemetrexed.
Disease Assessment
Disease assessments were performed within 4 weeks of the first day of treatment. Repeat assessments were performed after the completion of every two cycles of treatment (approximately every 6 weeks). Response was evaluated according to the Response Evaluation Criteria in Solid Tumors.
Pharmacokinetics
To evaluate the effect of pemetrexed administration on the pharmacokinetics of topotecan, blood samples were collected predose and at 0.25, 0.5 (end of topotecan infusion), 0.75, 1, 1.5, 2.5, 4.5, 6.5, 8.5, 12, and 24 hours from the start of the 30-minute topotecan infusion on day 1 (topotecan and pemetrexed) and day 8 (topotecan alone) of cycle 1. Total topotecan concentrations were quantified by a validated high-performance liquid chromatography/mass spectrometry/mass spectrometry method after protein precipitation using acetonitrile (GlaxoSmithKline, data on file).
Topotecan pharmacokinetic parameter values were determined using standard noncompartmental methods with WinNonlin Professional, version 5.2 (Pharsight, Mountain View, CA). For the determination of the area under the concentration–Time curve (AUC), the linear-up/log-down trapezoidal rule was used. Pharmacokinetic parameters included AUC0-∞, maximum concentration (Cmax), maximum time (tmax), clearance (CL), volume of distribution at steady state (Vss), and half-life (t1/2). The same topotecan dose in mg and in mg/m2 was given to each patient on the two pharmacokinetic sampling days. Because different topotecan doses were given to different patients, AUC0-∞ and Cmax values were normalized by dose in mg prior to further analysis.
The geometric mean and coefficient of variation were calculated for all pharmacokinetic parameter values except tmax, for which the median and range were determined. All other pharmacokinetic parameter values were natural log (ln) transformed prior to statistical analysis. The ln-transformed values were tested using a paired t-test. tmax values were tested without transformation using a Wilcoxon signed rank test.
Results
A total of 26 patients with advanced solid tumors received the combination of pemetrexed and topotecan at all five dose levels outlined in Table 1. The demographics of the patient population are outlined in Table 2. These patients were treated with 120 cycles of therapy, including five patients who received 8, 8, 10, 12, and 17 cycles without dose reductions, confirming a lack of cumulative myelosuppression. Four patients were dosed at level 5—topotecan, 4.0 mg/m2, and pemetrexed, 500 mg/m2—but experienced two DLTs, one each of febrile neutropenia and grade 4 thrombocytopenia. As a result, dose level 4 (topotecan, 3.5 mg/m2 on days 1 and 8, plus pemetrexed, 500 mg/m2 on day 1 every 21 days) was expanded to a total of 12 patients. Interestingly, only one episode of grade 3 or 4 nonhematologic toxicity, a case of grade 3 fatigue, was observed; no grade 3 or 4 diarrhea, mucositis, nausea/vomiting, or rash were reported. The grade 3 or 4 hematologic toxicities are outlined in Table 3.

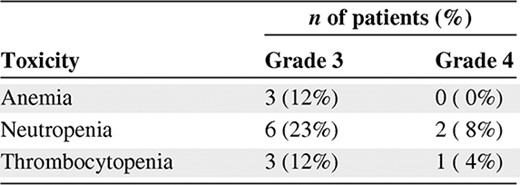

Antitumor Activity
Objective antitumor activity was demonstrated in one patient with NSCLC (12 months) and one patient with soft tissue sarcoma (6 months) who each achieved a partial response. The NSCLC responder (previously treated with paclitaxel and carboplatin) had a near complete remission, including a positron emission tomography scan with no evidence of hypermetabolic activity, and stopped treatment for what appeared to be maximum benefit. He subsequently relapsed <3 months after halting therapy and resumed additional chemotherapy. Eleven patients (42%) experienced stable disease as their best response, nine patients (35%) had progressive disease at the first disease assessment, and four patients (15%) were unevaluable for response.
Pharmacokinetics
Figure 1 shows concentration–time curves for total topotecan after i.v. administration with pemetrexed (day 1) and after i.v. administration alone (day 8) in a patient who received topotecan at 3.5 mg/m2, and Table 4 provides the summary pharmacokinetic parameter values for all patients. There were no statistically significant differences between total topotecan pharmacokinetic parameters with and without concomitant pemetrexed administration.
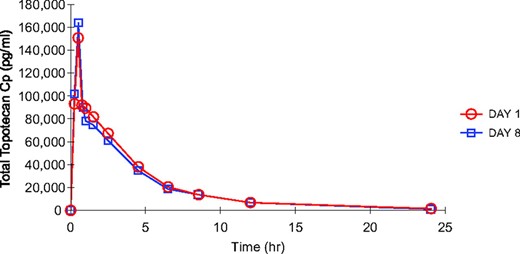
Total topotecan concentration–time curves for a patient who received topotecan at 3.5 mg/m2 (day 1, topotecan plus pemetrexed; day 8, topotecan alone).
Abbreviation: Cp, plasma concentration.
Geometric mean (percent coefficient of variation) total topotecan pharmacokinetic parameter values
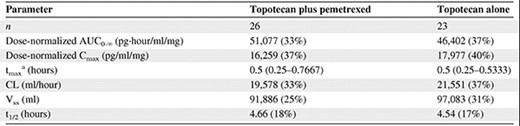
There were no statistically significant differences.
aMedian (minimum–maximum).
Abbreviations: AUC, area under the concentration–time curve; CL, clearance; Cmax, maximum concentration; t1/2, half-life; tmax, maximum time; Vss, volume of distribution at steady state.
Geometric mean (percent coefficient of variation) total topotecan pharmacokinetic parameter values

There were no statistically significant differences.
aMedian (minimum–maximum).
Abbreviations: AUC, area under the concentration–time curve; CL, clearance; Cmax, maximum concentration; t1/2, half-life; tmax, maximum time; Vss, volume of distribution at steady state.
Conclusion
The combination of weekly topotecan plus every-3-week pemetrexed was reasonably well tolerated and demonstrated evidence of antitumor activity. Full doses of pemetrexed—500 mg/m2 i.v. every 21 days—and reasonably high doses of weekly topotecan—3.5 mg/m2 i.v. on days 1 and 8 every 21 days—resulted in brief reversible myelosuppression with minimal dose delays and reductions. No incidents of grade 3 or 4 nonhematologic toxicities of mucositis, diarrhea, or nausea/vomiting were observed. All six NSCLC patients at the recommended phase II dose had at least stable disease as a best response, including one partial response lasting 12 months. There was no evidence of an effect of pemetrexed on topotecan pharmacokinetics. A formal phase II study of pemetrexed and weekly topotecan, combining two independent cytotoxic mechanisms of action, is recommended in patients with NSCLC with minimal prior therapy to better define antitumor activity.
Acknowledgment
Supported in part by grants from GlaxoSmithKline and Eli Lilly and Company.
Author Contributions
Conception/Design: Suzanne F. Jones, Roxanne C. Jewell, David R. Spigel, Howard A. Burris III
Provision of study material or patients: Jeffrey R. Infante, David R. Spigel, F. Anthony Greco, Dana S. Thompson, Howard A. Burris III
Collection and/or assembly of data: Suzanne F. Jones, Roxanne C. Jewell, David R. Spigel, Howard A. Burris III
Data analysis and interpretation: Suzanne F. Jones, Roxanne C. Jewell, David R. Spigel, Howard A. Burris III
Manuscript writing: Suzanne F. Jones, Roxanne C. Jewell, David R. Spigel, Howard A. Burris III
Final approval of manuscript: Suzanne F. Jones, Jeffrey R. Infante, David R. Spigel, F. Anthony Greco, Dana S. Thompson, Howard A. Burris III
References
Author notes
Disclosures: Howard A. Burris: None; Jeffrey R. Infante: Consultant/advisory role: Mek Development; None; Roxanne C. Jewell: Employment/leadership position: GlaxoSmithKline; Ownership interest: GlaxoSmithKline; David R. Spigel: None; F. Anthony Greco: None; Dana S. Thompson: None; Suzanne F. Jones: None.
The combination of weekly topotecan plus pemetrexed in patients with advanced malignancies is investigational.
The content of this article has been reviewed by independent peer reviewers to ensure that it is balanced, objective, and free from commercial bias. No financial relationships relevant to the content of this article have been disclosed by the independent peer reviewers.



