-
PDF
- Split View
-
Views
-
Cite
Cite
Beth Sherrill, Mayur M. Amonkar, Bintu Sherif, Julie Maltzman, Lisa O'Rourke, Stephen Johnston, Quality of Life in Hormone Receptor–Positive HER-2+ Metastatic Breast Cancer Patients During Treatment with Letrozole Alone or in Combination with Lapatinib, The Oncologist, Volume 15, Issue 9, September 2010, Pages 944–953, https://doi.org/10.1634/theoncologist.2010-0012
Close - Share Icon Share
Abstract
A phase III trial compared lapatinib plus letrozole (L + Let) with letrozole plus placebo (Let) as first-line therapy for hormone receptor (HR)+ metastatic breast cancer (MBC) patients. The primary endpoint of progression-free survival (PFS) in patients whose tumors were human epidermal growth factor receptor (HER)-2+ was significantly longer for L + Let than for Let (8.2 months versus 3 months; p = .019). This analysis focuses on quality of life (QOL) in the HER-2+ population.
QOL was assessed at screening, every 12 weeks, and at withdrawal using the Functional Assessment of Cancer Therapy–Breast (FACT–B). Changes from baseline were analyzed and the proportions of patients achieving minimally important differences in QOL scores were compared. Additional exploratory analyses evaluated how QOL changes reflected tumor progression status.
Among the 1,286 patients randomized, 219 had HER-2+ tumors. Baseline QOL scores were comparable in the two arms. Mean changes in QOL scores were generally stable over time for patients who stayed on study. The average change from baseline on the FACT-B total score in both arms was positive at all scheduled visits through week 48. There was no significant difference between the two treatment arms in the percentage of QOL responders.
The addition of lapatinib to letrozole led to a significantly longer PFS interval while maintaining QOL during treatment, when compared with letrozole alone, thus confirming the clinical benefit of the combination therapy in the HR+ HER-2+ MBC patient population. This all oral regimen provides an effective option in this patient population, delaying the need for chemotherapy and its accompanying side effects.
Introduction
Breast cancer continues to remain one of the most frequently diagnosed cancers and is among the leading causes of cancer death among women across the globe. In 2009, 192,370 women in the U.S. were estimated to have received a diagnosis of breast cancer and 40,170 women were expected to die from the disease [1]. Approximately 70% of breast cancers are estrogen dependent (hormone receptor [HR] sensitive), and the majority (65%) of newly diagnosed patients are postmenopausal (≥55 years of age) with a median age at diagnosis of 60 years.
Despite recent advances in the treatment of HR+ metastatic breast cancer (MBC) patients using endocrine therapies (third-generation aromatase inhibitors, such as letrozole, anastrozole, or exemestane), resistance to these therapies limits their success. There is evidence to suggest that crosstalk between pathways involving the epidermal growth factor family of receptors—epidermal growth factor receptor (EGFR) and human epidermal growth factor receptor 2 (HER-2)—and the estrogen receptor (ER) may be a contributing factor to resistance to endocrine therapy [2–6]. This has supported a rationale for using targeted agents against EGFR pathways in combination with endocrine manipulation to overcome endocrine resistance. In a recent randomized, open-label trial for the first-line treatment of postmenopausal women with HR+ HER-2+ MBC, trastuzumab combined with anastrozole doubled the median progression-free survival (PFS) time, compared with anastrozole alone, 2.4 months versus 4.8 months [7]. This combination trastuzumab–aromatase inhibitor regimen is approved in the European Union but has not yet been approved in the U.S.
Because HER-2 overexpression is a poor prognostic factor and HER-2+ MBC is a rapidly progressive disease, the use of anti–HER-2 therapy in combination with chemotherapy, rather than in combination with antihormonal therapy, is common in clinical practice. For patients with HER-2+ tumors, chemotherapy is unlikely to be used without an anti–HER-2 agent. Trastuzumab in combination with taxanes is the standard of care in this front-line MBC setting regardless of HR status. Although studies have indicated a longer time to tumor progression with the addition of trastuzumab to chemotherapy-based regimens in patients with HER-2+ MBC [8, 9], treating incurable patients with palliative front-line chemotherapy may be considered overly aggressive in this setting and may unnecessarily expose patients to serious toxicities associated with chemotherapy (including neutropenia, leukopenia, neuropathy, asthenia, myalgia, and arthralgia). Moreover, as a patient's disease progresses, they often experience painful and debilitating metastases to the brain, bones, and other organs, which when combined with treatment toxicities can significantly impact quality of life (QOL) [10–13]. Hence, the clinical benefit of therapy for MBC patients must be weighed against its potential negative impact on the QOL of these women.
Lapatinib, a potent, orally active, dual tyrosine kinase inhibitor of EGFR and HER-2 is currently approved for use in combination with capecitabine for the treatment of advanced or metastatic HER-2+ breast cancer in women previously treated with other anticancer drugs. A recent large, randomized, double-blind phase III trial (EGF30008) showed the combination of lapatinib with the aromatase inhibitor letrozole to delay progression significantly longer (8.2 months versus 3 months; hazard ratio, 0.71; 95% confidence interval, 0.53–0.96; p = .019) than with letrozole alone in patients with known HR+ HER-2+ MBC receiving first-line treatment [14]. This paper presents analyses evaluating QOL in patients with HR+ HER-2+ tumors receiving letrozole alone or in combination with lapatinib in clinical trial EGF30008.
Methods
Study Design and Patient Population
The EGF30008 study was a phase III, randomized, double-blinded, placebo-controlled, parallel-group, and multicenter trial. Eligible patients were postmenopausal women with histologically confirmed stage IIIB/IIIC or IV ER+ and/or progesterone receptor–positive invasive breast cancer who had not received prior therapy for advanced or metastatic disease. Details of the patient population for clinical trial EGF30008 have been previously described [14]. The protocol was approved by institutional review boards, and informed consent was obtained from all patients. Patients were randomly assigned to receive either: (a) lapatinib, 1,500 mg/day, plus letrozole, 2.5 mg/day, or (b) letrozole, 2.5 mg/day, with matching placebo. Patients were treated daily until disease progression or withdrawal from study as a result of unacceptable toxicity or other reasons. The primary objective of this clinical trial was to evaluate and compare PFS in patients with HER-2+ tumors. Secondary objectives included the overall response rate, the clinical benefit rate, the overall survival time, safety, and the PFS interval for the intent-to-treat (ITT) population. Assessing the impact of study treatments on QOL in the HER-2+ and ITT populations was one of the secondary objectives of this trial. The QOL analysis presented here uses clinical data as of the cutoff date of June 3, 2008.
QOL Instrument
QOL was assessed on day 1, every 12 weeks, and at study withdrawal using the Functional Assessment of Cancer Therapy–Breast (FACT-B) questionnaire (version 4, 1997). The FACT-B consists of the FACT–General (FACT-G) plus breast cancer subscale (BCS), which complements the general scale with items specific to QOL in breast cancer. The FACT-B is designed to measure multidimensional QOL in patients with breast cancer [15]. It is a 37-item (27 general questions and 10 breast cancer–specific questions) self-reporting instrument consisting of five dimensions/subscales: physical well-being (PWB), social/family well-being (SWB), emotional well-being (EWB), functional well-being (FWB), and the BCS. Patients assessed how true each statement had been for them in the previous 7 days on a five-point scale (0, not at all; 1, a little bit; 2, somewhat; 3, quite a bit; 4, very much). The PWB, SWB, and FWB subscales each have seven questions, whereas the EWB subscale has six questions. The BCS has 10 breast cancer–related questions and mainly asks patients to report how much they may have been bothered by various symptoms including shortness of breath, hair loss, pain, and weight change. The five subscale scores are used to derive three assessment outcomes—the FACT-B total score, FACT-G score, and trial outcome index (TOI).
The FACT-B total score is calculated by summing all five unweighted subscale scores, with total scores in the range of 0–136. The FACT-G score is calculated by summing four of the five unweighted subscale scores, specifically the PWB, SWB, EWB, and FWB scores (i.e., excluding the BCS), with scores in the range of 0–108. The TOI, an efficient summary index of physical/functional outcomes, is the sum of the PWB, FWB, and BCS scores, with scores in the range of 0–84. As per FACT-B scoring guidelines, these three scores were calculated only when patients responded to at least 80% of the items that constituted the relevant score.
In a previous study, which considered both distribution- and anchor-based estimates, a minimally important difference (MID) was estimated to be 2–3 points for the BCS, 7–8 points for the FACT-B total score, and 5–6 points for the FACT-G and the TOI scores [16].
Statistical Analysis
Patients had to have completed the baseline FACT-B questionnaire and at least one follow-up questionnaire to be included in analyses. Changes from baseline in the FACT-B total score, FACT-G score, and TOI score at each scheduled visit and at withdrawal were analyzed by parametric analysis of covariance (ANCOVA) using the baseline score as a covariate. Because the duration of follow-up varied across patients, the analyses were repeated using the last observation carried forward (LOCF) approach to account for missing scores. In this approach, missing FACT-B scores from scheduled visits were imputed from the last nonmissing score at a previous visit. Results from the ITT population were considered as secondary analyses, because the HER-2+ population was identified a priori as the primary population for analysis.
A QOL responder analysis was also performed. A patient was classified as a QOL responder or nonresponder based on whether the patient achieved a MID on the relevant QOL score [16]. A MID was defined as the upper limit of the published differences for the FACT-B total, FACT-G, and TOI scores (eight points for the FACT-B total score and six points for the FACT-G and TOI scores). The best QOL response during follow-up (including scheduled and withdrawal visits) was used to determine response status. In a sensitivity analysis, an alternative definition of QOL responders, which used the lower limit of the published MIDs instead of the upper limit (seven points for the FACT-B total score and five points for the FACT-G and TOI scores), was employed.
In a post hoc exploratory analysis in the HER-2+ population, the extent to which QOL scores reflected tumor progression events was assessed by examining QOL changes at consecutive time points by progression status. At weeks 12, 24, and 36, QOL scores were pooled across treatment arms and stratified based on investigator assessment of progression prior to or up to 1 week after the scheduled assessment. For those patients who had not progressed by week 12, QOL scores were again examined at week 24, stratified by whether the patient had progressed or not in the interim. This approach was continued through week 36, after which too few patients with QOL data remained on study. The QOL score change from baseline was compared between progressors and nonprogressors using the least squared means from an ANCOVA, adjusted for baseline value. The distribution of QOL responses stratified by whether the change from baseline represented minimally important declines, increases, or stability was compared using Fisher's exact test.
Results
Patient Characteristics and QOL Completion Rates
Between December 9, 2003 and December 29, 2006, 1,286 patients in total were randomly assigned to treatment, of whom 17% (219 patients) had centrally confirmed HER-2+ tumors (n = 111 for the lapatinib plus letrozole arm, n = 108 for the letrozole plus placebo arm). Baseline patient and disease characteristics were well balanced between treatment arms for both the HER-2+ and ITT HR+ populations and have been previously reported [14].
The number of HER-2+ patients with scorable FACT-B assessments at scheduled and concluding/withdrawal visits is provided in Table 1; percentages are shown based on patients who were still on study (i.e., excluding those who had progressed, were censored for progression, or withdrew from treatment and hence were not required to complete the assessments at future visits). QOL questionnaire completion rates over the first year of follow up were 93%–100% of the available patients for both treatment arms, with the exception of the letrozole arm at week 48, which was 85%.
Number of HER-2+ patients completing the Functional Assessment of Cancer Therapy–Breast questionnaire at scheduled visits
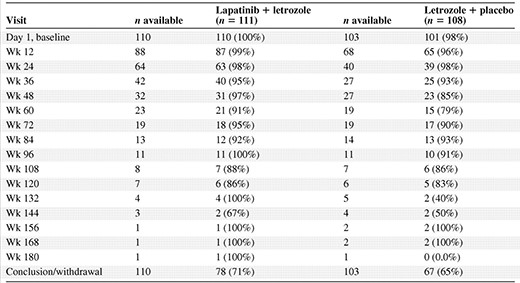
Complete was defined as completing at least one question in the quality-of-life questionnaire among those who completed a baseline questionnaire.
Percentage is of those who were scheduled to complete a questionnaire at the visit time (i.e., not progressed, censored for progression, or withdrawn from treatment).
Abbreviation: HER-2, human epidermal growth factor receptor 2.
Number of HER-2+ patients completing the Functional Assessment of Cancer Therapy–Breast questionnaire at scheduled visits

Complete was defined as completing at least one question in the quality-of-life questionnaire among those who completed a baseline questionnaire.
Percentage is of those who were scheduled to complete a questionnaire at the visit time (i.e., not progressed, censored for progression, or withdrawn from treatment).
Abbreviation: HER-2, human epidermal growth factor receptor 2.
Change in QOL from Baseline
Baseline QOL scores (FACT-B subscales and total, FACT-G, TOI) were generally comparable in the two arms (Table 2). Because QOL assessments were stopped after treatment termination or disease progression, few patients completed the questionnaire after week 48, and the results reported here are only for the visits up to week 48.
Summary of baseline FACT-B subscale, FACT-B total, FACT-G, and TOI scores by treatment arm, HER-2+ population
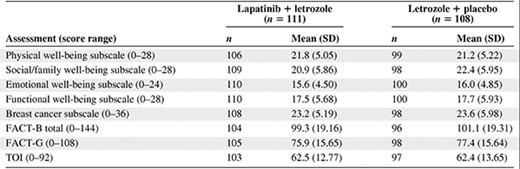
Abbreviations: FACT-B, Functional Assessment of Cancer Therapy–Breast; FACT-G, Functional Assessment of Cancer Therapy–General; HER-2, human epidermal growth factor receptor 2; SD, standard deviation; TOI, trial outcome index.
Summary of baseline FACT-B subscale, FACT-B total, FACT-G, and TOI scores by treatment arm, HER-2+ population

Abbreviations: FACT-B, Functional Assessment of Cancer Therapy–Breast; FACT-G, Functional Assessment of Cancer Therapy–General; HER-2, human epidermal growth factor receptor 2; SD, standard deviation; TOI, trial outcome index.
In general, average scores on the FACT-B, FACT-G, and TOI were unchanged during the first year of treatment (Fig. 1). In both treatment arms, small positive average changes in total FACT-B scores were observed during treatment, which did not reach MID levels until late in year 2 or 3 when few patients remained on study. As expected, average FACT-B scores at the concluding (progression or study withdrawal) visit represented minimally important decreases in QOL for both treatment arms (lapatinib plus letrozole, −8.9; letrozole plus placebo, −9.5). The same pattern was seen for both the FACT-G and TOI, with the magnitude of decline similar across treatment arms. None of the differences between groups were statistically significant. For the five dimensions/subscales, changes from baseline were small in both treatment arms. From week 12 to week 48, small differences were observed between the two arms at each follow-up, relative to baseline (Table 3). Most of the differences were not statistically significant (except for two subscales at the week 12 visit: a 1.5-point difference in the BCS in favor of the combination and a 1.5-point difference in the SWB subscale in favor of letrozole alone), and the differences did not reach MID levels.
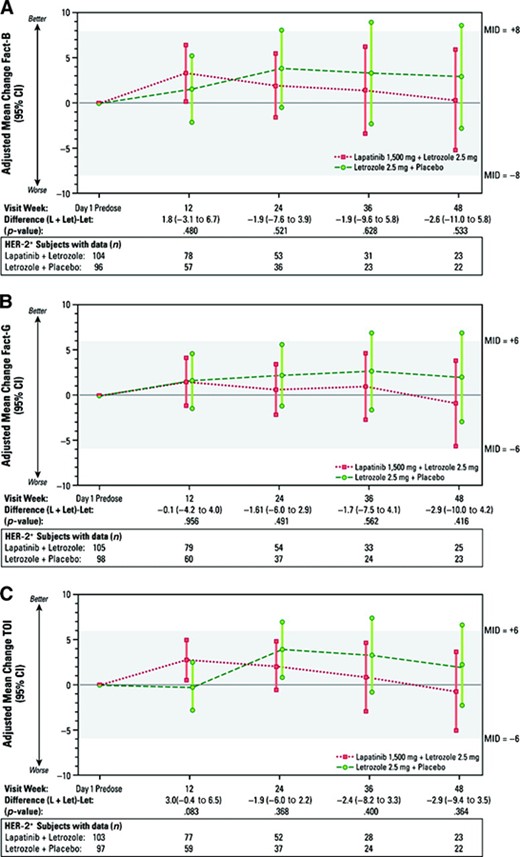
Treatment group changes from baseline for FACT-B total, FACT-G, and TOI scores, HER-2+ population. Least squares mean changes (95% CI) from analysis of covariance, adjusted for baseline score. Bars indicate ± 1.96 standard error.
Abbreviations: CI, confidence interval; FACT-B, Functional Assessment of Cancer Therapy–Breast; FACT-G, Functional Assessment of Cancer Therapy–General; HER-2, human epidermal growth factor receptor 2; L + Let, lapatinib + letrozole; Let, letrozole; MID, minimum important difference; TOI, trial outcome index.
Treatment group differences in Functional Assessment of Cancer Therapy–Breast subscales, HER-2+ population
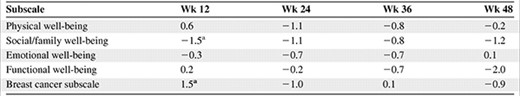
Differences were adjusted for baseline score. Positive numbers favor the lapatinib plus letrozole treatment arm.
ap < .05.
Abbreviation: HER-2, human epidermal growth factor receptor 2.
Treatment group differences in Functional Assessment of Cancer Therapy–Breast subscales, HER-2+ population

Differences were adjusted for baseline score. Positive numbers favor the lapatinib plus letrozole treatment arm.
ap < .05.
Abbreviation: HER-2, human epidermal growth factor receptor 2.
Results for the FACT-B and FACT-G using the LOCF approach on the HER-2+ population were consistent with observed data. For the TOI, a small positive increase at week 12 favored the lapatinib plus letrozole group (p = .03). Differences in declining TOI scores very late in the study also favored the combination group (.05 < p < .10 after week 144), but these differences were small and not MIDs. In the ITT population, statistically significant FACT-B, FACT-G, and TOI scores favored the letrozole plus placebo arm over the lapatinib plus letrozole arm at weeks 12, 24, and 36, in the range of −3.2 to −1.6, but none of these could be considered MIDs. As in the HER-2+ analysis, average scores in both arms were higher than baseline during the first year of treatment, except for study withdrawal assessments.
More than one third (34%–41%) of HER-2+ patients were identified as QOL responders during the treatment period (Table 4). The proportions of patients achieving MID improvements on the FACT-B, FACT-G, and TOI were equal between the two treatment groups or slightly favored the combination arm; none of the differences was statistically significant. Similar results were obtained using the lower limit of the published MIDs as a sensitivity analysis.
Treatment group comparison of quality of life responders during study, HER-2+ population
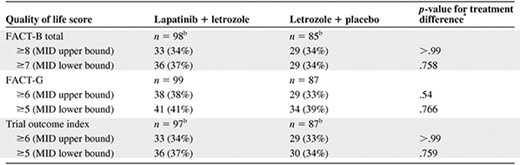
ap-values are from Fisher's exact test.
bn is number of subjects with baseline score and at least one postbaseline score.
Abbreviations: FACT-B, Functional Assessment of Cancer Therapy–Breast; FACT-G, Functional Assessment of Cancer Therapy–General; HER-2+, human epidermal growth factor receptor 2; MID = minimum important difference.
Treatment group comparison of quality of life responders during study, HER-2+ population

ap-values are from Fisher's exact test.
bn is number of subjects with baseline score and at least one postbaseline score.
Abbreviations: FACT-B, Functional Assessment of Cancer Therapy–Breast; FACT-G, Functional Assessment of Cancer Therapy–General; HER-2+, human epidermal growth factor receptor 2; MID = minimum important difference.
Relationship Between QOL and Tumor Response
Between randomization and the week 12 visit (including up to 1 week beyond the scheduled assessment), 72 HER-2+ patients experienced disease progression events (including one death) and 144 patients had tumors that did not meet progression criteria. Of these 144, 10 were censored, 37 experienced disease progression (including one death) by the week 24 visit, and 97 still did not have disease progression at week 24. By the time of the week 36 visit, another seven patients were censored, 21 had tumors with disease progression (including one death), and 69 remained progression free.
For patients who stayed on treatment and did not progress over a 36-week period, changes from baseline in FACT-B total scores remained stable and within MID levels (represented by the highlighted area in Figure 2). Average QOL declines reached clinically and statistically significant levels for patients whose tumors progressed after week 12, but not for patients whose tumors progressed earlier. Differences in average changes from baseline in FACT-B total scores were statistically and clinically significant between patients who experienced progression events and those who did not at week 24 (p < .0001) and week 36 (p = 0.04) but not at week 12 (p = .16).
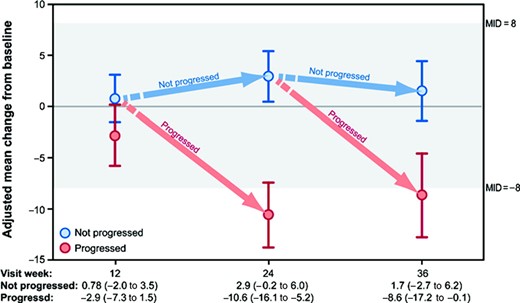
Adjusted mean change from baseline for FACT-B total scores, by progression status, HER-2+ population. Least squares mean changes (95% confidence interval) from analysis of covariance, adjusted for baseline score.
Abbreviations: FACT-B, Functional Assessment of Cancer Therapy–Breast; HER-2, human epidermal growth factor receptor 2; MID, minimum important difference.
At week 12, the distribution of patients by FACT-B response level was similar between patients whose disease progressed and those whose disease did not progress (p = .69) (Fig. 3). For patients with disease progression between week 12 and week 24, 65% had FACT-B declines more than seven points from baseline, versus 18% of patients without disease progression (p < .0001). Results were similar at week 36, but not statistically significant (p = .15). The pattern of QOL changes and distribution of MID response levels was consistent for the TOI and BCS scores (data not shown).
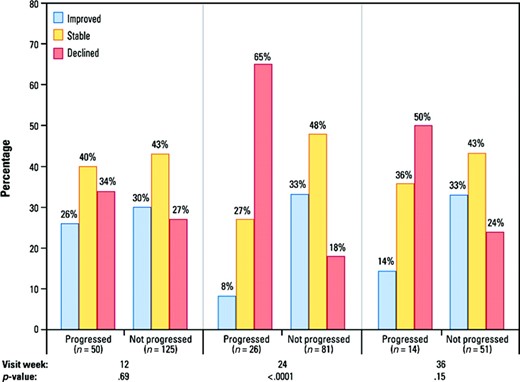
Distribution of QOL response based on minimum important difference FACT-B change from baseline, by progression status, HER-2+ population. Declined represents decrease from baseline of ≥7 points; improved is ≥7-point increase from baseline. p-values are from Fisher's exact test using patients with baseline and postbaseline QOL scores.
Abbreviations: FACT-B, Functional Assessment of Cancer Therapy–Breast; HER-2, human epidermal growth factor receptor 2; QOL, quality of life.
Discussion
In addition to clinical benefits such as delayed tumor progression, higher tumor response rates, and longer survival, another goal of treating women with MBC is to provide palliation and maintain or even improve their QOL. QOL was measured in this study because it was important to investigate whether additive therapy (lapatinib plus letrozole), compared with letrozole alone, caused any detrimental effects on QOL in this patient population with metastatic disease. Results suggest that the addition of lapatinib to a currently accepted regimen for HER-2+ MBC patients did not deteriorate patient QOL.
Analyses of QOL scores and changes from baseline in the HER-2+ and ITT populations in the EGF30008 trial showed that mean changes in subscale and total QOL scores were generally stable over time in both treatment groups for patients who stayed on treatment. The fact that no study difference in QOL was observed between treatment groups was an expected finding. Current oncology study designs make it difficult to evaluate the full magnitude of the effect on QOL over time, because assessments are typically stopped after withdrawal of the randomized treatment, which usually coincides with progression. Although the current study had high response rates on QOL assessments, limited data were available postprogression because QOL assessments were not required during that time, as per the study protocol. As a consequence, few patients, particularly in the placebo arm, had QOL data available over the entire follow-up. Hence, any postprogression QOL benefit resulting from delaying progression with lapatinib and letrozole treatment could not be evaluated. Thus, the QOL analysis that was conducted based on available data was conservative and possibly biased against lapatinib, because postprogression patients were missing from the placebo arm at a higher rate. Even if data were available postprogression, it is likely that these data would have been confounded by subsequent treatments that the patients received.
In another study of women with HER-2+ MBC, who had been previously treated and hence had higher disease severity, the addition of lapatinib to capecitabine was shown to significantly delay disease progression without evidence of a deleterious effect on patients' QOL [17]. When toxicities were accounted for, lapatinib combined with capecitabine provided more quality-adjusted survival than capecitabine monotherapy [18]. Although the mechanism for this effect is likely a result of the better tumor status of patients in the combination arm, the opportunity to delay or avoid the toxic effects of chemotherapy is desirable.
Results shown here demonstrate clinically meaningful declines in QOL scores associated with tumor progression in patients with HR+ HER-2+ MBC. These hypothesis-generating analyses suggest that these declines may take more than a few months to evolve. The exploratory analyses show that patients who remain on treatment and experience delayed progression have stable QOL and that a higher percentage will show meaningful improvements in QOL during the study period than patients whose disease progresses. These findings are not particularly surprising, but the QOL benefits of delayed progression have not been previously demonstrated so clearly. Based on these findings, we anticipate that the QOL decline associated with progression is delayed when disease progression can be delayed for several months, as seen with combination lapatinib and letrozole treatment. If future study designs continue QOL assessments after disease progression, it would be possible to more fully evaluate the true value of treatments that delay or interrupt the disease process. A full year of assessments would directly demonstrate the degree to which delayed progression translates into a QOL benefit. Not only could we determine the timing of the QOL changes for the patient, but we could also begin to answer questions about whether lingering effects of treatment accrue after treatment end. For example, does delay of tumor progression equate to a slower decline for the patient? Analysis approaches such as time to QOL decline would be more meaningful because the QOL measure would not have to be censored precisely at progression. In the meantime, we rely on QOL assessments during the treatment phase and at study withdrawal to infer that patient QOL is served by slowing tumor growth.
Our current understanding of the most aggressive form of MBC suggests that combination treatment provides positive clinical results and interrupts signaling pathways that could have a multiplicative effect on tumor growth. Because MBC is not curable, maintenance of QOL is an important goal. Treatment with an anti-HER-2 agent in combination with endocrine therapy may help support this goal while reserving chemotherapy for when the disease stops responding to endocrine modulation. Furthermore, the combination of antiendocrine and anti–HER-2 therapies may delay tumor endocrine resistance via cointerruption of the ER and EGFR networks [19]. The National Comprehensive Cancer Network guidelines support the use of endocrine therapy and only recommend chemotherapy with trastuzumab for patients with visceral crisis. Thus, effective treatment of patients with HR+ HER-2+ tumors relies on concurrent inhibition of the ER and HER-2 networks.
In summary, the addition of lapatinib to letrozole led to a significantly longer PFS interval than with antiestrogen therapy (letrozole) alone, whereas the average QOL was unchanged for patients who remained on study, thus confirming the clinical benefit of the combination therapy in the HR+ HER-2+ MBC patient population. This combination provides an effective option in this patient population by maintaining QOL during treatment and delaying the need for chemotherapy and its accompanying side effects. As an all oral regimen, it also has the potential to offer benefits to patients in terms of convenience and preference, at a stage in their disease in which their time and energy is likely to be limited.
Acknowledgments
We thank the patients who participated in the study and their families; the medical, nursing, and research staff at the study centers; the independent data and safety monitoring committee; and the monitors, clinical operations staff, data managers, statisticians, and programmers at GlaxoSmithKline. Preliminary results of analyses were presented as posters at conferences [20–22]. The EGF30008 study and the QOL analyses reported in this paper were funded by GlaxoSmithKline. The study is registered in ClinicalTrials.gov (NCT00073528).
Author Contributions
Conception/Design: Beth Sherrill, Mayur M. Amonkar
Financial support: Mayur M. Amonkar
Provision of study material or patients: Mayur M. Amonkar, Julie Maltzman, Lisa O'Rourke, Stephen Johnston
Collection and/or assembly of data: Mayur M. Amonkar, Julie Maltzman, Lisa O'Rourke
Data analysis and interpretation: Beth Sherrill, Bintu Sherif
Manuscript writing: Beth Sherrill, Mayur M. Amonkar, Bintu Sherif
Final approval of manuscript: Beth Sherrill, Mayur M. Amonkar, Julie Maltzman, Lisa O'Rourke, Stephen Johnston
The authors take full responsibility for the content of the paper but thank Adele Monroe (medical editor) and Candy Webster (graphic artist) at RTI Health Solutions for their editorial and production assistance. Ms. Monroe provided copyediting (e.g., grammatical and punctuation assistance) and editorial assistance (e.g., preparing references, labeling tables). Ms. Webster is a graphic artist who converted SAS-generated figures into JPG files (i.e. production assistance, assembling tables, graphs, figures).
References
Author notes
Disclosures: Beth Sherrill: Research funding/contracted research: GlaxoSmithKline; Mayur M. Amonkar: Employment/leadership position: GlaxoSmithKline; Ownership interest: GlaxoSmithKline; Bintu Sherif: None; Lisa O'Rourke: Employment/leadership position: GlaxoSmithKline; Ownership interest: GlaxoSmithKline; Julie Maltzman: Employment/leadership position: GlaxoSmithKline; Ownership interest: GlaxoSmithKline; Stephen Johnston: Research funding/contracted research: GlaxoSmithKline.
The article discusses results from the clinical trial EGF30008 that studied the combination of letrozole plus lapatinib in metastatic breast cancer treatment.
The content of this article has been reviewed by independent peer reviewers to ensure that it is balanced, objective, and free from commercial bias. No financial relationships relevant to the content of this article have been disclosed by the independent peer reviewers.



