-
PDF
- Split View
-
Views
-
Cite
Cite
Raja Ramachandran, Vinod Kumar, Ashwani Kumar, Ashok Kumar Yadav, Ritambhra Nada, Harsha Kumar, Vivek Kumar, Manish Rathi, Harbir Singh Kohli, Krishan Lal Gupta, Vinay Sakhuja, Vivekanand Jha, PLA2R antibodies, glomerular PLA2R deposits and variations in PLA2R1 and HLA-DQA1 genes in primary membranous nephropathy in South Asians, Nephrology Dialysis Transplantation, Volume 31, Issue 9, September 2016, Pages 1486–1493, https://doi.org/10.1093/ndt/gfv399
Close - Share Icon Share
Abstract
Antibodies to M-type phospholipase A2 receptor (PLA2R) correlate with clinical activity of primary membranous nephropathy (PMN). Risk alleles in PLA2R1 and HLA-DQA1 genes are associated with PMN. Whether these alleles are associated with the development of anti-PLA2R is unknown. In this prospective study we evaluated anti-PLA2R, enhanced glomerular staining for PLA2R and variations in PLA2R1 and HLA-DQA1 genes in Indian patients with PMN and examined their association with response to treatment.
A total of 114 adult PMN patients were studied. Anti-PLA2R was estimated before treatment and after 6 and 12 months of therapy. Enhanced glomerular staining for PLA2R was assessed on fresh frozen tissue. Genotype analysis was done on recruited patients and 95 healthy controls by TaqMan assays for six single-nucleotide polymorphisms (SNPs; rs4664308, rs3749119, rs3749117, rs4664308, rs3828323 and rs2187668). Patients were followed up monthly for a period of 12 months.
Of 114 patients, 66.7% showed elevated serum anti-PLA2R by ELISA and 64.9% by indirect immunofluorescence. About 75% had enhanced glomerular staining for PLA2R. A total of 82% of patients had PLA2R-related disease. Reduction in serum anti-PLA2R titer had a significant association with remission of nephrotic syndrome (P = 0.0003) at 6 and 12 months. More than 85% of patients showing >90% reduction in the anti-PLA2R titer achieved remission of the nephrotic state, whereas of those showing <50% reduction in titers, 87.5% had persistent nephrotic state. The SNPs rs3749119, rs3749117, rs4664308 in PLA2R1 and rs2187668 in HLA-DQA1 were significantly associated with PMN. The SNP rs2187668 was associated with anti-PLA2R positivity. Patients with a high-risk genotype had higher anti-PLA2R levels.
To conclude, anti-PLA2R and enhanced glomerular PLA2R staining are found in more than two-thirds of Indian PMN cases. A reduction in the anti-PLA2R titer correlated with response to therapy.
INTRODUCTION
Membranous nephropathy is an important cause of nephrotic syndrome in adults [1]. About 30% of patients exhibit an underlying cause, and the disease is dubbed called primary in the remaining patients. The discovery of antibodies against M-type phospholipase A2 receptor (anti-PLA2R) and demonstration of their role in primary membranous nephropathy (PMN) were an important milestone in understanding this condition [2]. Reduction of anti-PLA2R with treatment, reappearance during relapse and association of variations in the PLA2R1 gene with PMN supported the experimental studies that showed a role of anti-PLA2R in the pathogenesis of PMN [3, 4]. Anti-PLA2R can be detected in serum by three methods: indirect immunofluorescence (IIF), enzyme-linked immunosorbent assay (ELISA) and western blot (WB) [2, 3]. Comparative studies have shown a good correlation between ELISA and IIF [3, 5]. Glomerular PLA2R staining is present in about three-fourths of the patients with PMN [6]. A good association between glomerular PLA2R staining and circulating anti-PLA2R has been noted [6].
A genome-wide association study (GWAS) showed an association of PMN with loci of the PLA2R1 and HLA-DQA1 genes. The odds ratio for PMN with homozygosity for risk variants at both alleles was almost 80 [4]. In a study of 1112 Chinese patients with PMN, Lv et al. [7] found an association with three single-nucleotide polymorphisms (SNPs) in PLA2R1 and one in HLA-DQA1. Coenen et al. [8] looked for rare variants in the coding sequence of the PLA2R1 gene in 95 patients with PMN and concluded that rare variants were unlikely to explain PMN.
In this study, we studied serum anti-PLA2R, examined enhanced glomerular staining for PLA2R and evaluated variations in PLA2R1 and HLA-DQA1 genes in Indian patients with PMN and the association of these parameters with disease behavior.
MATERIALS AND METHODS
Study population
This prospective study was carried out at the Postgraduate Institute of Medical Education and Research, Chandigarh, India, between July 2010 and February 2015. Patients 11–70 years of age (two <18 years) with biopsy-proven PMN with persistent nephrotic syndrome despite optimal angiotensin blockade and who received specific treatment were included. Patients with active infection, diabetes mellitus, prior treatment with any immunosuppressive therapy, secondary membranous nephropathy (MN) and those with histology showing >50% tubular atrophy and interstitial fibrosis were excluded. Age-matched voluntary kidney donors were taken as healthy controls. The study was approved by the institute's ethics committee and was conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from patients or their parents.
Treatment and monitoring
Patients were treated with either alternating monthly cycles of corticosteroid and cyclophosphamide or a combination of calcineurin inhibitors and steroids as recommended in Kidney Disease: Improving Global Outcomes guidelines [9]. All patients were followed up for at least 12 months. Blood samples for estimation of anti-PLA2R were drawn prior to initiation of treatment and at 6 and 12 months. Twenty-four-hour urine protein, serum albumin and serum creatinine were measured every month for 12 months.
Glomerular PLA2R and serum anti-PLA2R evaluation
PLA2R staining was done on fresh frozen biopsy tissue as described previously [10]. Three micrometer sections were cut and kept in phosphate buffer saline (PBS, pH 7.4) for 10 min before staining. Rabbit polyclonal anti-PLA2R antibody derived from the C-terminal domain of human PLA2R (Abcam, Cambridge, UK; ab80054) was used in 1:100 dilution. Slides were incubated for 2 h at room temperature in a humidified chamber. After primary antibody incubation, slides were washed two times with PBS (10 min each wash). This was followed by a 1-h incubation with tetramethylrhodamine isothiocyanate (TRITC) tagged secondary anti-rabbit antibody (Sigma, St Louis, MO, USA; 1:50 dilution). After a 1-h incubation, slides were washed with PBS as described above and mounted in aqueous mounting solution. Slides were viewed under a confocal microscope and examined for the presence of enhanced PLA2R staining. Twenty-three patients with other glomerular diseases (10 cases with lupus-related MN, 3 cases with hepatitis B/C-related MN, 5 cases with type 1 mesangiocapillary glomerulonephritis and 5 cases with IgA nephropathy) and 10 normal kidney tissue samples were included as controls. Staining intensity was graded semi-quantitatively on a scale of 0–3+. Cases with an intensity ≥2+ were considered positive.
Serum anti-PLA2R was measured by IIF and ELISA using previously validated commercial kits (EUROIMMUN, Lubeck, Germany) according to the manufacturer's protocols (http://www.accessdata.fda.gov/cdrh_docs/reviews/K132379.pdf). ELISA was performed by incubating diluted (1:101) test samples in PLA2R antigen-coated ELISA plates for 30 min followed by washing and incubation with peroxidase-labeled anti-human IgG. Plates were washed and incubated for an additional 15 min with chromogen substrate and the color intensity was measured at 450 and 620 nm. Patients with antibody titer >20 RU/mL were considered positive. For IIF, diluted (1:10) samples were incubated on BIOCHIP slides for 30 min followed by washing and incubation with fluorescein-labeled anti-human globulin for 30 min. Fluorescence was read under a confocal microscope (excitation filter 450–490 nm, color separator 510 nm, blocking filter 5151 nm) (Figure 1).
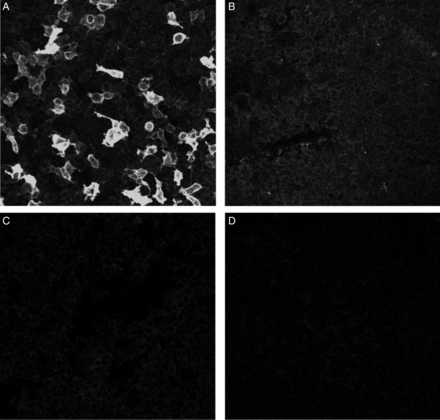
Detection of circulating anti-PLA2R using IIF. Serum of PMN patients incubated with HEK293 cells expressing (A1 and B1) and not expressing (A2 and B2) PLA2R protein incubated with sera from anti-PLA2R-positive (A1 and A2) and anti-PLA2R-negative (B1 and B2) cases (all ×20).
Genotyping
Genomic DNA was isolated from peripheral blood leukocytes (Genomic DNA isolation kit, Qiagen, Valencia, CA, USA) according to the manufacturer's instructions. Genotyping for SNPs in HLA-DQA1 (rs2187668) and PLA2R1 (rs3749119, rs3749117, rs35771982, rs3828323 and rs4664308) was performed using the ‘assay-on-demand’ allelic discrimination assay (TaqMan genotyping assay). Amplification reactions were performed on an ABI 7500 real-time PCR system (Applied Biosciences, Hamburg, Germany) according to the manufacturer's instruction.
Definitions
Nephrotic syndrome was defined as proteinuria >3.5 g/day/1.73 m2 or ≥2.0 g/day along with serum albumin <2.5 g/dL [11]. Remission was defined as complete (CR) when the urine protein excretion decreased to <500 mg/day with normal serum albumin and serum creatinine and as partial (PR) when proteinuria was 0.5–2 g/day or <50% of baseline with normal serum albumin (≥3.5 gm/dL) and serum creatinine. Subjects with anti-PLA2R (>20 RU/mL) in the serum by ELISA were defined as antibody positive. All patients with circulating anti-PLA2R and/or enhanced staining for PLA2R in the glomeruli were classified as having PLA2R-related MN.
Statistical analysis
Data are expressed as number, percentage, mean and SD or median and interquartile range, as appropriate. Student's t-test or Mann–Whitney U test was used to compare continuous date and χ2 or McNemar test was used to compare differences between proportions. Hardy–Weinberg equilibrium was tested using Michael H. Court's online calculator (http://www.tufts.edu/~mcourt01/Documents/Court%20lab%20%20HW%20calculator.xls). Haplotype analysis was done using SHEsis software (http://analysis.bio-x.cn/myAnalysis.php). Statistical analysis was performed using GraphPad Prism (version 6.0; GraphPad Software, San Diego, CA, USA). For multivariate analysis, SPSS version 16 (SPSS, Chicago, IL, USA) was used. A two-tailed P-value <0.05 was considered significant. Bonferroni correction was applied to adjust P-values for multiple comparisons. The kappa statistic was calculated to examine agreement between two antibody detection techniques.
RESULTS
Baseline characteristics
A total of 140 cases of PMN were evaluated for enrollment: 14 (10.8%) patients were excluded because they underwent spontaneous resolution of proteinuria, 3 (2.1%) because they had received prior immunosuppressive therapy and 6 (4.3%) did not consent. Thus a total of 114 patients (112 adults and 2 children) with persistent nephrotic syndrome were enrolled. The duration of nephrotic syndrome was 10.55 ± 4.37 months. A total of 106 patients received full angiotensin blockade for >6 months. Eight cases, who had illness of <6-months duration, were considered for specific therapy: six (75%) because of development of thromboembolic complications and two (25%) had severe symptomatic hypoalbuminemia nonresponsive to conservative therapy.
The baseline characteristics of the study population are described in Table 1. Enhanced glomerular PLA2R staining was seen in 86 (75.4%) patients (Figure 2). None of the samples from patients with other glomerular disease or the normal kidney tissue showed enhanced PLA2R staining. The antibody was detectable by ELISA in 76 (66.7%) patients and by IIF in 75 (64.9%). There was good agreement between the two antibody detection techniques (94.7%, 0.883). A total of 94 (82.45%) cases had PLA2R-related MN.
| Parameters . | Value . |
|---|---|
| Number of cases | 114 |
| Age (years) | 41.39 ± 13.32 (11–71) |
| Sex ratio (M:F) | 67:47 |
| Duration of illness (months) | 10.55 ± 4.37 (2–24) |
| Proteinuria (g/day) | 5.86 ± 3.14 (2–21) |
| Serum albumin (g/dL) | 2.29 ± 0.64 (0.70–4.40) |
| Serum creatinine (mg/dL) | 0.91 ± 0.27 (0.54–1.8) |
| Enhanced glomerular PLA2R staining, n (%) | 86 (75.43) |
| Anti-PLA2R positive by ELISA, n (%) | 76 (66.66) |
| Anti-PLA2R positive by IIF, n (%) | 74 (64.91) |
| Anti-PLA2R titer (RU/mL) | 321.5 ± 827.5 (1.41–313.8)* |
| Parameters . | Value . |
|---|---|
| Number of cases | 114 |
| Age (years) | 41.39 ± 13.32 (11–71) |
| Sex ratio (M:F) | 67:47 |
| Duration of illness (months) | 10.55 ± 4.37 (2–24) |
| Proteinuria (g/day) | 5.86 ± 3.14 (2–21) |
| Serum albumin (g/dL) | 2.29 ± 0.64 (0.70–4.40) |
| Serum creatinine (mg/dL) | 0.91 ± 0.27 (0.54–1.8) |
| Enhanced glomerular PLA2R staining, n (%) | 86 (75.43) |
| Anti-PLA2R positive by ELISA, n (%) | 76 (66.66) |
| Anti-PLA2R positive by IIF, n (%) | 74 (64.91) |
| Anti-PLA2R titer (RU/mL) | 321.5 ± 827.5 (1.41–313.8)* |
PLA2R, m-type phospholipase A2 receptor; ELISA, enzyme-linked immunosorbent assay; IIF, indirect immunofluorescence. Values are given as mean ± SD (IQR), unless stated otherwise. *Value in the parentheses is the interquartile range with a median of 82.43 RU/mL.
| Parameters . | Value . |
|---|---|
| Number of cases | 114 |
| Age (years) | 41.39 ± 13.32 (11–71) |
| Sex ratio (M:F) | 67:47 |
| Duration of illness (months) | 10.55 ± 4.37 (2–24) |
| Proteinuria (g/day) | 5.86 ± 3.14 (2–21) |
| Serum albumin (g/dL) | 2.29 ± 0.64 (0.70–4.40) |
| Serum creatinine (mg/dL) | 0.91 ± 0.27 (0.54–1.8) |
| Enhanced glomerular PLA2R staining, n (%) | 86 (75.43) |
| Anti-PLA2R positive by ELISA, n (%) | 76 (66.66) |
| Anti-PLA2R positive by IIF, n (%) | 74 (64.91) |
| Anti-PLA2R titer (RU/mL) | 321.5 ± 827.5 (1.41–313.8)* |
| Parameters . | Value . |
|---|---|
| Number of cases | 114 |
| Age (years) | 41.39 ± 13.32 (11–71) |
| Sex ratio (M:F) | 67:47 |
| Duration of illness (months) | 10.55 ± 4.37 (2–24) |
| Proteinuria (g/day) | 5.86 ± 3.14 (2–21) |
| Serum albumin (g/dL) | 2.29 ± 0.64 (0.70–4.40) |
| Serum creatinine (mg/dL) | 0.91 ± 0.27 (0.54–1.8) |
| Enhanced glomerular PLA2R staining, n (%) | 86 (75.43) |
| Anti-PLA2R positive by ELISA, n (%) | 76 (66.66) |
| Anti-PLA2R positive by IIF, n (%) | 74 (64.91) |
| Anti-PLA2R titer (RU/mL) | 321.5 ± 827.5 (1.41–313.8)* |
PLA2R, m-type phospholipase A2 receptor; ELISA, enzyme-linked immunosorbent assay; IIF, indirect immunofluorescence. Values are given as mean ± SD (IQR), unless stated otherwise. *Value in the parentheses is the interquartile range with a median of 82.43 RU/mL.
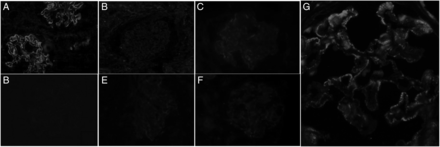
(A) Photomicrograph showing granular staining with PLA2R along glomerular capillary loops in a case of primary MN; (B) glomerular capillary loops are not stained with PLA2R in a case of MN; (C) faint staining with PLA2R in normal kidney podocytes; (D–F) lupus nephritis, IgA nephropathy and MPGN pattern, respectively, showing no glomerular capillary loops staining for PLA2R; (G) granular staining of PLA2R along capillary loops. Immunofluorescence staining, tetramithylrodamine-tagged secondary antibody, ×20 (A–F) and ×40 (G) original magnification.
A total of 68 (59.6%) patients showed enhanced staining for PLA2R in the glomeruli as well as elevated serum anti-PLA2R by ELISA (Supplementary data, Table S1). There was a strong association between the presence of glomerular PLA2R deposits and the presence of anti-PLA2R (P < 0.0001). However, there was no significant correlation between the anti-PLA2R titer and intensity of glomerular staining (r = 0.03, P = 0.76). Also, no correlation was found between the anti-PLA2R titer and proteinuria or serum albumin levels. There were no differences in the baseline parameters and outcomes of those with or without anti-PLA2R and/or enhanced staining in the glomeruli for PLA2R (data not shown).
The clinical and biochemical parameters of PLA2R-related and -unrelated PMN patients are described in Table 2. Supplementary data, Table S2 shows the clinical and biochemical parameters of anti-PLA2R-positive and -negative cases.
Comparison of clinical and serology parameters of PLA2R-related and -unrelated cases
| . | PLA2R related . | PLA2R unrelated . | P-value . |
|---|---|---|---|
| Number of cases | 94 | 20 | |
| Age (years) | 41.73 ± 12.80 (11–68) | 40 ± 15.82 (18–71) | 0.59 |
| Sex (M:F) | 56:38 | 11:09 | 0.80 |
| Time (months) | 10.52 ± 4.28 (2–24) | 10.70 ± 4.88 (4–24) | 0.86 |
| Baseline | |||
| Proteinuria (g/day) | 5.75 ± 2.90 (2–19.5) | 6.41 ± 4.12 (2.3–21) | 0.39 |
| Serum albumin (g/dL) | 2.28 ± 0.65 (0.7–4.40) | 2.35 ± 0.64 (1.50–3.60) | 0.65 |
| Serum creatinine (mg/dL) | 0.92 ± 0.27 (0.54–1.80) | 0.86 ± 0.25 (0.57–1.49) | 0.38 |
| 6 months | |||
| Proteinuria (g/day) | 1.73 ± 2.46 (0.05–19.50) | 1.12 ± 1.77 (0.10–6.00) | 0.29 |
| Serum albumin (g/dL) | 3.37 ± 0.81 (1.35–4.82) | 3.66 ± 00.81 (1.76–4.80) | 0.14 |
| Serum creatinine (mg/dL) | 0.98 ± 0.38 (0.63–4.00) | 0.90 ± 0.17 (0.60–1.20) | 0.38 |
| 12 months | |||
| Proteinuria (g/day) | 2.01 ± 2.97 (0.07–19.50) | 1.41 ± 1.75 (0.12–6.00) | 0.38 |
| Serum albumin (g/dL) | 3.59 ± 0.79 (1.35–4.82) | 3.67 ± 00.93 (1.60–5.10) | 0.69 |
| Serum creatinine (mg/dL) | 0.95 ± 0.25 (0.50–1.90) | 0.88 ± 00.15 (0.65–1.20) | 0.22 |
| Anti-PLA2R (RU/mL) | |||
| Baseline | 389.49 ± 897.49 (0.45–6528.45) | 2.01 ± 3.60 (0.45–12.94) | <0.0001a |
| 6 months | 73.85 ± 145.87b (1.01–873.36) | – | NA |
| 12 months | 79.10 ± 159.20b (0.45–873.369) | – | NA |
| Therapy, n (%) | |||
| Alkylating agent based | 55 (58.51) | 12 (60) | 1.00 |
| CNI based | 39 (41.49) | 08 (40) | |
| Remission, n (%) | 61 (64.89) | 15 (75) | 0.44 |
| . | PLA2R related . | PLA2R unrelated . | P-value . |
|---|---|---|---|
| Number of cases | 94 | 20 | |
| Age (years) | 41.73 ± 12.80 (11–68) | 40 ± 15.82 (18–71) | 0.59 |
| Sex (M:F) | 56:38 | 11:09 | 0.80 |
| Time (months) | 10.52 ± 4.28 (2–24) | 10.70 ± 4.88 (4–24) | 0.86 |
| Baseline | |||
| Proteinuria (g/day) | 5.75 ± 2.90 (2–19.5) | 6.41 ± 4.12 (2.3–21) | 0.39 |
| Serum albumin (g/dL) | 2.28 ± 0.65 (0.7–4.40) | 2.35 ± 0.64 (1.50–3.60) | 0.65 |
| Serum creatinine (mg/dL) | 0.92 ± 0.27 (0.54–1.80) | 0.86 ± 0.25 (0.57–1.49) | 0.38 |
| 6 months | |||
| Proteinuria (g/day) | 1.73 ± 2.46 (0.05–19.50) | 1.12 ± 1.77 (0.10–6.00) | 0.29 |
| Serum albumin (g/dL) | 3.37 ± 0.81 (1.35–4.82) | 3.66 ± 00.81 (1.76–4.80) | 0.14 |
| Serum creatinine (mg/dL) | 0.98 ± 0.38 (0.63–4.00) | 0.90 ± 0.17 (0.60–1.20) | 0.38 |
| 12 months | |||
| Proteinuria (g/day) | 2.01 ± 2.97 (0.07–19.50) | 1.41 ± 1.75 (0.12–6.00) | 0.38 |
| Serum albumin (g/dL) | 3.59 ± 0.79 (1.35–4.82) | 3.67 ± 00.93 (1.60–5.10) | 0.69 |
| Serum creatinine (mg/dL) | 0.95 ± 0.25 (0.50–1.90) | 0.88 ± 00.15 (0.65–1.20) | 0.22 |
| Anti-PLA2R (RU/mL) | |||
| Baseline | 389.49 ± 897.49 (0.45–6528.45) | 2.01 ± 3.60 (0.45–12.94) | <0.0001a |
| 6 months | 73.85 ± 145.87b (1.01–873.36) | – | NA |
| 12 months | 79.10 ± 159.20b (0.45–873.369) | – | NA |
| Therapy, n (%) | |||
| Alkylating agent based | 55 (58.51) | 12 (60) | 1.00 |
| CNI based | 39 (41.49) | 08 (40) | |
| Remission, n (%) | 61 (64.89) | 15 (75) | 0.44 |
aMann–Whitney test.
bIncluded only 76 cases positive for anti-PLA2R at baseline. Values given as mean ± SD (IQR) unless stated otherwise. NA, not applicable.
Comparison of clinical and serology parameters of PLA2R-related and -unrelated cases
| . | PLA2R related . | PLA2R unrelated . | P-value . |
|---|---|---|---|
| Number of cases | 94 | 20 | |
| Age (years) | 41.73 ± 12.80 (11–68) | 40 ± 15.82 (18–71) | 0.59 |
| Sex (M:F) | 56:38 | 11:09 | 0.80 |
| Time (months) | 10.52 ± 4.28 (2–24) | 10.70 ± 4.88 (4–24) | 0.86 |
| Baseline | |||
| Proteinuria (g/day) | 5.75 ± 2.90 (2–19.5) | 6.41 ± 4.12 (2.3–21) | 0.39 |
| Serum albumin (g/dL) | 2.28 ± 0.65 (0.7–4.40) | 2.35 ± 0.64 (1.50–3.60) | 0.65 |
| Serum creatinine (mg/dL) | 0.92 ± 0.27 (0.54–1.80) | 0.86 ± 0.25 (0.57–1.49) | 0.38 |
| 6 months | |||
| Proteinuria (g/day) | 1.73 ± 2.46 (0.05–19.50) | 1.12 ± 1.77 (0.10–6.00) | 0.29 |
| Serum albumin (g/dL) | 3.37 ± 0.81 (1.35–4.82) | 3.66 ± 00.81 (1.76–4.80) | 0.14 |
| Serum creatinine (mg/dL) | 0.98 ± 0.38 (0.63–4.00) | 0.90 ± 0.17 (0.60–1.20) | 0.38 |
| 12 months | |||
| Proteinuria (g/day) | 2.01 ± 2.97 (0.07–19.50) | 1.41 ± 1.75 (0.12–6.00) | 0.38 |
| Serum albumin (g/dL) | 3.59 ± 0.79 (1.35–4.82) | 3.67 ± 00.93 (1.60–5.10) | 0.69 |
| Serum creatinine (mg/dL) | 0.95 ± 0.25 (0.50–1.90) | 0.88 ± 00.15 (0.65–1.20) | 0.22 |
| Anti-PLA2R (RU/mL) | |||
| Baseline | 389.49 ± 897.49 (0.45–6528.45) | 2.01 ± 3.60 (0.45–12.94) | <0.0001a |
| 6 months | 73.85 ± 145.87b (1.01–873.36) | – | NA |
| 12 months | 79.10 ± 159.20b (0.45–873.369) | – | NA |
| Therapy, n (%) | |||
| Alkylating agent based | 55 (58.51) | 12 (60) | 1.00 |
| CNI based | 39 (41.49) | 08 (40) | |
| Remission, n (%) | 61 (64.89) | 15 (75) | 0.44 |
| . | PLA2R related . | PLA2R unrelated . | P-value . |
|---|---|---|---|
| Number of cases | 94 | 20 | |
| Age (years) | 41.73 ± 12.80 (11–68) | 40 ± 15.82 (18–71) | 0.59 |
| Sex (M:F) | 56:38 | 11:09 | 0.80 |
| Time (months) | 10.52 ± 4.28 (2–24) | 10.70 ± 4.88 (4–24) | 0.86 |
| Baseline | |||
| Proteinuria (g/day) | 5.75 ± 2.90 (2–19.5) | 6.41 ± 4.12 (2.3–21) | 0.39 |
| Serum albumin (g/dL) | 2.28 ± 0.65 (0.7–4.40) | 2.35 ± 0.64 (1.50–3.60) | 0.65 |
| Serum creatinine (mg/dL) | 0.92 ± 0.27 (0.54–1.80) | 0.86 ± 0.25 (0.57–1.49) | 0.38 |
| 6 months | |||
| Proteinuria (g/day) | 1.73 ± 2.46 (0.05–19.50) | 1.12 ± 1.77 (0.10–6.00) | 0.29 |
| Serum albumin (g/dL) | 3.37 ± 0.81 (1.35–4.82) | 3.66 ± 00.81 (1.76–4.80) | 0.14 |
| Serum creatinine (mg/dL) | 0.98 ± 0.38 (0.63–4.00) | 0.90 ± 0.17 (0.60–1.20) | 0.38 |
| 12 months | |||
| Proteinuria (g/day) | 2.01 ± 2.97 (0.07–19.50) | 1.41 ± 1.75 (0.12–6.00) | 0.38 |
| Serum albumin (g/dL) | 3.59 ± 0.79 (1.35–4.82) | 3.67 ± 00.93 (1.60–5.10) | 0.69 |
| Serum creatinine (mg/dL) | 0.95 ± 0.25 (0.50–1.90) | 0.88 ± 00.15 (0.65–1.20) | 0.22 |
| Anti-PLA2R (RU/mL) | |||
| Baseline | 389.49 ± 897.49 (0.45–6528.45) | 2.01 ± 3.60 (0.45–12.94) | <0.0001a |
| 6 months | 73.85 ± 145.87b (1.01–873.36) | – | NA |
| 12 months | 79.10 ± 159.20b (0.45–873.369) | – | NA |
| Therapy, n (%) | |||
| Alkylating agent based | 55 (58.51) | 12 (60) | 1.00 |
| CNI based | 39 (41.49) | 08 (40) | |
| Remission, n (%) | 61 (64.89) | 15 (75) | 0.44 |
aMann–Whitney test.
bIncluded only 76 cases positive for anti-PLA2R at baseline. Values given as mean ± SD (IQR) unless stated otherwise. NA, not applicable.
Sixty-seven (58.8%) patients were treated with alkylating agent–based therapy and 47 (41.2%) received CNI-based therapy. There was no difference in the cumulative dose of individual drugs between the four groups (enhanced glomerular staining for PLA2R positive and serum anti-PLA2R positive, enhanced glomerular staining for PLA2R positive and serum anti-PLA2R negative, enhanced glomerular staining for PLA2R negative and serum anti-PLA2R positive and enhanced glomerular staining for PLA2R negative and serum anti-PLA2R negative; data not shown). A total of 76 (66.7%) cases exhibited therapeutic response, with 49 (43%) in complete and 27 (23.7%) in partial remission at 12 months. The time to remission after starting therapy was 1–15 months. Thirty-eight out of 68 (55.8%) cases with both enhanced glomerular staining and serum positivity, 7 of 8 (87.5%) with serum antibodies but no enhanced glomerular staining, 16 of 18 (88.8%) with enhanced glomerular staining and no antibodies and 15 of 20 (75%) without enhanced staining or the presence of serum antibodies achieved remission.
Relationship between anti-PLA2R positivity and treatment response
Anti-PLA2R-positive cases showed a parallel reduction in antibody titer and proteinuria (Figure 3). Patients with anti-PLA2R at baseline were more likely to be treatment resistant than antibody-negative cases (P = 0.02). Among patients with >90% reduction in their anti-PLA2R titer at 12 months from baseline, 85.4% achieved remission of the nephrotic state, whereas of those showing <50% reduction in titers, 87.5% had persistent nephrotic state. In patients with a baseline titer >300 RU/mL, 55.2% had resistant disease, and in those with a baseline titer <100 RU/mL, 68.2% achieved remission. On univariate analysis, urine protein (6 months r = 0.43, P < 0.0001 and 12 months r = 0.52, P < 0.0001, respectively) and serum albumin at 6 and 12 months (6 months r = 0.37, P = 0.001 and 12 months r = 0.58, P < 0.0001, respectively) correlated with the anti-PLA2R titer at 6 and 12 months. On multivariate analysis, the anti-PLA2R at 12 months correlated with remission of the nephrotic state (β = 0.326, P = 0.004).
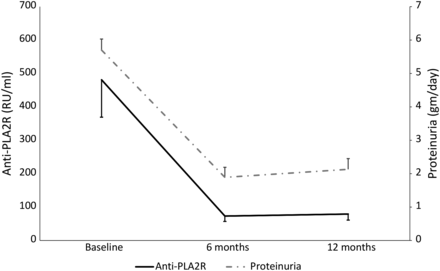
Graphical representation of anti-PLA2R and proteinuria at baseline, 6 months and 12 months.
The clinical and biochemical parameters of therapy-responsive and -resistant cases are shown in Supplementary data, Table S3. The antibody levels declined below the threshold in 46 (60.5%) patients at both 6 and 12 months. No difference was noted in the clinical behavior with respect to the two treatment regimens and their association with anti-PLA2R positivity.
Comparison of glomerular PLA2R-positive and -negative PMN
Comparison of the clinical and biochemical parameters showed no differences between the enhanced glomerular PLA2R-positive and -negative patients, except for a greater proportion of enhanced glomerular PLA2R-positive patients showing circulating anti-PLA2R (P < 0.0001). Similarly, no difference was noted when patients were grouped into four categories according to serum and enhanced glomerular PLA2R staining (data not shown).
Anti-PLA2R level and outcome
Antibody-positive cases were divided into tertiles based on anti-PLA2R titers. There were no differences in the baseline clinical parameters between the three groups. However, patients in the highest tertile had a trend toward a greater number of therapy-resistant cases compared with the other two groups (P = 0.06). Significantly, a greater proportion of cases in the first two tertiles had CR compared with patients in the third tertile (P = 0.04) (Supplementary data, Table S4).
Association of polymorphisms with PMN
The allele frequency and genotype distribution of studied SNPs in PMN patients and controls are depicted in Table 3 and Supplementary data, Tables S5 and S6. The genotype distributions of the studied SNPs were in Hardy–Weinberg equilibrium. Variations at three SNPs in PLA2R1 (rs3749119, rs35771982 and rs4664308) and one in HLA-DQA1 (rs2187668) were associated with PMN. The presence of the AA or AG genotype at rs2187668 conferred an increased risk of developing PMN compared with those with GG [OR 5.36 (CI 2.83–9.82), P < 0.0001]. At the rs35771982 locus, the GG genotype was encountered more frequently among subjects with PMN compared with those with CC or CG [OR 3.17 (CI 1.75–5.75), P < 0.0001]. Similarly, the presence of AA at rs4664308 was associated with PMN compared with AG or GG [OR 3.10 (CI 1.69–5.67), P = 0.0003]. Haplotype analysis revealed a strong association of the CACGCA haplotype at rs3749119, rs2187668, rs3749117, rs35771982, rs3828323 and rs4664308 with PMN [OR 64.48 (95% CI 7.32–567.30), P < 0.0001] (Supplementary data, Table S7). A combination of AA genotypes at both HLA-DQA1 (rs2187668) and PLA2R1 (rs4664308) conferred a 58.33-fold (95% CI 7.3–567.30) increased risk (Table 4). When only PLA2R-related cases were compared with healthy subjects, greater differences in the distribution of PLA2R1 rs3749119, rs35771982, rs3828323 and rs4664308 and HLA-DQA1 rs2187668 SNPs became evident (Table 4). However, there were no significant variations at any of the six loci between PLA2R-related and -unrelated cases (Supplementary data, Table S8), likely due to the small sample size in the PLA2R-unrelated group.
Comparison of PLA2R1 and HLA-DQA1 polymorphism in patients with PLA2R-related PMN (n = 94) and healthy controls (n = 95)
| Gene variation . | Genotype . | Controls . | PMN . | P-value . | Allele . | Controls . | PMN . | OR (95% CI) . | P-value . |
|---|---|---|---|---|---|---|---|---|---|
| HLADQ1 rs2187668 | AA | 00 (00) | 16 (17) | <0.0001a | A | 23 (12.11) | 79 (42.02) | 5.26 (3.11–8.88) | <0.0001 |
| AG | 23 (24.2) | 47 (50) | G | 167 (87.89) | 109 (57.98) | ||||
| GG | 72 (75.8) | 31 (33) | |||||||
| PLA2R | |||||||||
| rs3749119 | CC | 55 (57.9) | 80 (85.2) | 9.4 × 10−5b | C | 131 (68.95) | 166 (88.3) | 2.86 (1.25–6.52) | <0.0001 |
| CT | 21 (22.1) | 06 (6.4) | T | 59 (31.05) | 22 (11.7) | ||||
| TT | 19 (20) | 8 (8.4) | |||||||
| rs3749117 | CC | 14 (14.7) | 11 (11.7) | 0.11c | T | 124 (65.26) | 115 (61.17) | 0.84 (0.55–1.27) | 0.40 |
| CT | 38 (40) | 51 (54.3) | C | 66 (34.74) | 73 (38.83) | ||||
| TT | 43 (45.3) | 32 (34) | |||||||
| rs35771982 | CC | 05 (5.3) | 02 (2.1) | 9.4 × 10−5d | G | 139 (73.16) | 166 (88.3) | 2.77 (1.6–4.7) | <0.0002 |
| CG | 41 (43.2) | 18 (19.2) | C | 51 (26.84) | 22 (11.7) | ||||
| GG | 49 (51.6) | 74 (78.7) | |||||||
| rs3828323 | CC | 59 (62.1) | 75 (79.8) | 0.007e | C | 147 (77.37) | 166 (88.3) | 2.20 (1.26–3.86) | 0.005 |
| CT | 29 (30.5) | 16 (17) | T | 43 (22.63) | 22 (11.7) | ||||
| TT | 07 (7.4) | 03 (3.2) | |||||||
| rs4664308 | AA | 52 (54.7) | 75 (79.8) | 2.4 × 10−4f | A | 143 (75.26) | 168 (89.34) | 2.76 (1.56–4.87) | 0.0003 |
| AG | 39 (41.1) | 18 (19.2) | G | 47 (24.74) | 20 (10.64) | ||||
| GG | 04 (04.2) | 01 (1) | |||||||
| Gene variation . | Genotype . | Controls . | PMN . | P-value . | Allele . | Controls . | PMN . | OR (95% CI) . | P-value . |
|---|---|---|---|---|---|---|---|---|---|
| HLADQ1 rs2187668 | AA | 00 (00) | 16 (17) | <0.0001a | A | 23 (12.11) | 79 (42.02) | 5.26 (3.11–8.88) | <0.0001 |
| AG | 23 (24.2) | 47 (50) | G | 167 (87.89) | 109 (57.98) | ||||
| GG | 72 (75.8) | 31 (33) | |||||||
| PLA2R | |||||||||
| rs3749119 | CC | 55 (57.9) | 80 (85.2) | 9.4 × 10−5b | C | 131 (68.95) | 166 (88.3) | 2.86 (1.25–6.52) | <0.0001 |
| CT | 21 (22.1) | 06 (6.4) | T | 59 (31.05) | 22 (11.7) | ||||
| TT | 19 (20) | 8 (8.4) | |||||||
| rs3749117 | CC | 14 (14.7) | 11 (11.7) | 0.11c | T | 124 (65.26) | 115 (61.17) | 0.84 (0.55–1.27) | 0.40 |
| CT | 38 (40) | 51 (54.3) | C | 66 (34.74) | 73 (38.83) | ||||
| TT | 43 (45.3) | 32 (34) | |||||||
| rs35771982 | CC | 05 (5.3) | 02 (2.1) | 9.4 × 10−5d | G | 139 (73.16) | 166 (88.3) | 2.77 (1.6–4.7) | <0.0002 |
| CG | 41 (43.2) | 18 (19.2) | C | 51 (26.84) | 22 (11.7) | ||||
| GG | 49 (51.6) | 74 (78.7) | |||||||
| rs3828323 | CC | 59 (62.1) | 75 (79.8) | 0.007e | C | 147 (77.37) | 166 (88.3) | 2.20 (1.26–3.86) | 0.005 |
| CT | 29 (30.5) | 16 (17) | T | 43 (22.63) | 22 (11.7) | ||||
| TT | 07 (7.4) | 03 (3.2) | |||||||
| rs4664308 | AA | 52 (54.7) | 75 (79.8) | 2.4 × 10−4f | A | 143 (75.26) | 168 (89.34) | 2.76 (1.56–4.87) | 0.0003 |
| AG | 39 (41.1) | 18 (19.2) | G | 47 (24.74) | 20 (10.64) | ||||
| GG | 04 (04.2) | 01 (1) | |||||||
PMN, primary membranous nephropathy, PLA2R, m-type phospholipase A2 receptor.
aAA + AG versus GG.
bCC versus CT + TT.
cCC + CT versus TT.
dCC + CG versus GG.
eCC versus CT + TT.
fAA versus AG + GG.
Comparison of PLA2R1 and HLA-DQA1 polymorphism in patients with PLA2R-related PMN (n = 94) and healthy controls (n = 95)
| Gene variation . | Genotype . | Controls . | PMN . | P-value . | Allele . | Controls . | PMN . | OR (95% CI) . | P-value . |
|---|---|---|---|---|---|---|---|---|---|
| HLADQ1 rs2187668 | AA | 00 (00) | 16 (17) | <0.0001a | A | 23 (12.11) | 79 (42.02) | 5.26 (3.11–8.88) | <0.0001 |
| AG | 23 (24.2) | 47 (50) | G | 167 (87.89) | 109 (57.98) | ||||
| GG | 72 (75.8) | 31 (33) | |||||||
| PLA2R | |||||||||
| rs3749119 | CC | 55 (57.9) | 80 (85.2) | 9.4 × 10−5b | C | 131 (68.95) | 166 (88.3) | 2.86 (1.25–6.52) | <0.0001 |
| CT | 21 (22.1) | 06 (6.4) | T | 59 (31.05) | 22 (11.7) | ||||
| TT | 19 (20) | 8 (8.4) | |||||||
| rs3749117 | CC | 14 (14.7) | 11 (11.7) | 0.11c | T | 124 (65.26) | 115 (61.17) | 0.84 (0.55–1.27) | 0.40 |
| CT | 38 (40) | 51 (54.3) | C | 66 (34.74) | 73 (38.83) | ||||
| TT | 43 (45.3) | 32 (34) | |||||||
| rs35771982 | CC | 05 (5.3) | 02 (2.1) | 9.4 × 10−5d | G | 139 (73.16) | 166 (88.3) | 2.77 (1.6–4.7) | <0.0002 |
| CG | 41 (43.2) | 18 (19.2) | C | 51 (26.84) | 22 (11.7) | ||||
| GG | 49 (51.6) | 74 (78.7) | |||||||
| rs3828323 | CC | 59 (62.1) | 75 (79.8) | 0.007e | C | 147 (77.37) | 166 (88.3) | 2.20 (1.26–3.86) | 0.005 |
| CT | 29 (30.5) | 16 (17) | T | 43 (22.63) | 22 (11.7) | ||||
| TT | 07 (7.4) | 03 (3.2) | |||||||
| rs4664308 | AA | 52 (54.7) | 75 (79.8) | 2.4 × 10−4f | A | 143 (75.26) | 168 (89.34) | 2.76 (1.56–4.87) | 0.0003 |
| AG | 39 (41.1) | 18 (19.2) | G | 47 (24.74) | 20 (10.64) | ||||
| GG | 04 (04.2) | 01 (1) | |||||||
| Gene variation . | Genotype . | Controls . | PMN . | P-value . | Allele . | Controls . | PMN . | OR (95% CI) . | P-value . |
|---|---|---|---|---|---|---|---|---|---|
| HLADQ1 rs2187668 | AA | 00 (00) | 16 (17) | <0.0001a | A | 23 (12.11) | 79 (42.02) | 5.26 (3.11–8.88) | <0.0001 |
| AG | 23 (24.2) | 47 (50) | G | 167 (87.89) | 109 (57.98) | ||||
| GG | 72 (75.8) | 31 (33) | |||||||
| PLA2R | |||||||||
| rs3749119 | CC | 55 (57.9) | 80 (85.2) | 9.4 × 10−5b | C | 131 (68.95) | 166 (88.3) | 2.86 (1.25–6.52) | <0.0001 |
| CT | 21 (22.1) | 06 (6.4) | T | 59 (31.05) | 22 (11.7) | ||||
| TT | 19 (20) | 8 (8.4) | |||||||
| rs3749117 | CC | 14 (14.7) | 11 (11.7) | 0.11c | T | 124 (65.26) | 115 (61.17) | 0.84 (0.55–1.27) | 0.40 |
| CT | 38 (40) | 51 (54.3) | C | 66 (34.74) | 73 (38.83) | ||||
| TT | 43 (45.3) | 32 (34) | |||||||
| rs35771982 | CC | 05 (5.3) | 02 (2.1) | 9.4 × 10−5d | G | 139 (73.16) | 166 (88.3) | 2.77 (1.6–4.7) | <0.0002 |
| CG | 41 (43.2) | 18 (19.2) | C | 51 (26.84) | 22 (11.7) | ||||
| GG | 49 (51.6) | 74 (78.7) | |||||||
| rs3828323 | CC | 59 (62.1) | 75 (79.8) | 0.007e | C | 147 (77.37) | 166 (88.3) | 2.20 (1.26–3.86) | 0.005 |
| CT | 29 (30.5) | 16 (17) | T | 43 (22.63) | 22 (11.7) | ||||
| TT | 07 (7.4) | 03 (3.2) | |||||||
| rs4664308 | AA | 52 (54.7) | 75 (79.8) | 2.4 × 10−4f | A | 143 (75.26) | 168 (89.34) | 2.76 (1.56–4.87) | 0.0003 |
| AG | 39 (41.1) | 18 (19.2) | G | 47 (24.74) | 20 (10.64) | ||||
| GG | 04 (04.2) | 01 (1) | |||||||
PMN, primary membranous nephropathy, PLA2R, m-type phospholipase A2 receptor.
aAA + AG versus GG.
bCC versus CT + TT.
cCC + CT versus TT.
dCC + CG versus GG.
eCC versus CT + TT.
fAA versus AG + GG.
Analysis of gene–gene interaction: odds ratios for PMN, according to SNP and genotype combinations
| HLA-DQA1 (rs2187668) . | PLA2R1 (rs4664308) . | ||
|---|---|---|---|
| GG . | GA . | AA . | |
| GG | 01/03 | 09/28 0.96 (0.08–10.47) | 32/41 2.34 (0.23–23.60) |
| GA | 01/01 3.0 (0.08–107.5) | 07/11 1.90 (0.16–22) | 46/11 12.55 (1.18–132.50) |
| AA | 00/00 00 | 06/00 30.33 (0.95–960.5) | 12/00 58.33 (1.92–1772) |
| HLA-DQA1 (rs2187668) . | PLA2R1 (rs4664308) . | ||
|---|---|---|---|
| GG . | GA . | AA . | |
| GG | 01/03 | 09/28 0.96 (0.08–10.47) | 32/41 2.34 (0.23–23.60) |
| GA | 01/01 3.0 (0.08–107.5) | 07/11 1.90 (0.16–22) | 46/11 12.55 (1.18–132.50) |
| AA | 00/00 00 | 06/00 30.33 (0.95–960.5) | 12/00 58.33 (1.92–1772) |
Cases with low-risk allele (GG at rs2187668 with GG at rs4664308) were taken as a reference.
PMN, primary membranous nephropathy; SNP, single-nucleotide polymorphism.
Analysis of gene–gene interaction: odds ratios for PMN, according to SNP and genotype combinations
| HLA-DQA1 (rs2187668) . | PLA2R1 (rs4664308) . | ||
|---|---|---|---|
| GG . | GA . | AA . | |
| GG | 01/03 | 09/28 0.96 (0.08–10.47) | 32/41 2.34 (0.23–23.60) |
| GA | 01/01 3.0 (0.08–107.5) | 07/11 1.90 (0.16–22) | 46/11 12.55 (1.18–132.50) |
| AA | 00/00 00 | 06/00 30.33 (0.95–960.5) | 12/00 58.33 (1.92–1772) |
| HLA-DQA1 (rs2187668) . | PLA2R1 (rs4664308) . | ||
|---|---|---|---|
| GG . | GA . | AA . | |
| GG | 01/03 | 09/28 0.96 (0.08–10.47) | 32/41 2.34 (0.23–23.60) |
| GA | 01/01 3.0 (0.08–107.5) | 07/11 1.90 (0.16–22) | 46/11 12.55 (1.18–132.50) |
| AA | 00/00 00 | 06/00 30.33 (0.95–960.5) | 12/00 58.33 (1.92–1772) |
Cases with low-risk allele (GG at rs2187668 with GG at rs4664308) were taken as a reference.
PMN, primary membranous nephropathy; SNP, single-nucleotide polymorphism.
The presence of an A allele at HLA-DQA1 rs2187668 was associated with anti-PLA2R positivity (P = 0.0006). Variation at all the SNPs in PLA2R1 was associated with anti-PLA2R positivity, but the difference was not statistically significant after Bonferroni correction (P > 0.008).
DISCUSSION
This is the first study to evaluate the prevalence of circulating anti-PLA2R, enhanced glomerular PLA2R staining and variations in PLA2R1 and HLA-DQA1 genes in South Asian subjects with PMN. We found elevated anti-PLA2R in more than two-thirds and enhanced glomerular PLA2R staining in ∼75% patients. Follow-up anti-PLA2R titers were associated with clinical outcome. These findings are consistent with previous reports from other parts of the world [12]. Anti-PLA2R has been reported in 52–74% of Caucasian and Chinese patients with PMN using WB, IIF or ELISA [5, 7, 12–14]. As noted by others, we also observed a good correlation between the two anti-PLA2R detection techniques—ELISA and IIF [3, 5].
Similar to the findings of Debiec et al. [12] and Svobodova et al. [15], we found a significant association between glomerular PLA2R staining and circulating anti-PLA2R. We did not note any quantitative association between enhanced glomerular PLA2R staining and serum anti-PLA2R. This on–off phenomenon was described previously [6]. The presence of circulating anti-PLA2R without detectable glomerular PLA2R may be explained by poorly accessible antigen epitopes in the glomeruli at the time of staining, while glomerular staining without circulating antibodies may be due to the fact that antibodies are detectable only after the buffering capacity of the kidney is saturated, which was described by Van De Logt et al. [16] as the ‘kidney as a sink’ hypothesis.
We did not find any difference in the baseline clinical parameters of patients with or without demonstrable circulating anti-PLA2R or enhanced glomerular PLA2R staining. However, anti-PLA2R-positive patients had a lower remission rate at 12 months compared with antibody-negative cases. Patients with a higher antibody titer had poor remission rates. Ruggenenti et al. [17] did not find any difference in the outcome between antibody-positive and -negative cases. In another study, Bech et al. [18] also failed to find a relation between the presence or level of antibody and response to treatment. In part, the difference could be explained by the different definitions of remission and anti-PLA2R cutoffs in different studies. With respect to the relationship between the antibody titer and likelihood of achieving remission, the results of the present study are similar to the results by Ruggenenti et al. [17], who also found that patients with antibody levels in the higher tertile had a lower probability of remission compared with the first two tertiles. In terms of association between the glomerular staining and clinical parameters, our results are similar to those reported by Svobodova et al. [15], who did not find any difference in clinical parameters between glomerular PLA2R-positive or -negative cases [15].
There was a significant reduction in the anti-PLA2R titer with treatment at 6 and 12 months, which paralleled the decline in proteinuria and improvement in serum albumin. Hoxha et al. [3] serially evaluated anti-PLA2R titers in 133 cases of anti-PLA2R-positive PMN over 24 months. Patients with good clinical response had lower baseline anti-PLA2R titers. They also noted a weak, but significant, correlation between baseline proteinuria values and anti-PLA2R titers. We did not find such a correlation and suspect that the presence of hypoalbuminemia in the majority of patients could have resulted in decreased urine protein excretion, leading to confounding [11, 19]. About 64% of our patients had severe hypoalbuminemia (<2.5 g/dL) at the time of starting therapy. The result of the present study is similar to the study by Ruggenenti et al. [17], which enrolled PMN patients of European ancestry and found no association of baseline proteinuria with antibody titer [17]. Consistent with previous reports, we found a consistent decline in serum anti-PLA2R in patients who responded to treatment. Unfortunately, we are unable to comment on the predictive ability of a change in antibody titer on disease remission since the antibody levels were not measured at earlier time points. Tentative support, however, can be suggested, as patients with complete remission had lower anti-PLA2R antibody titers at 6 months compared with those with partial remission. Similar observations were made by Hoxha et al. [3]. Patients with >80% reduction in the anti-PLA2R titer by 6 months of therapy had a very high probability of remission and patients with a <50% reduction in their titers were more likely to have resistant disease. Similar results regarding titer reduction before proteinuria have been demonstrated previously [3, 17]. A reduction in anti-PLA2R titer at 12 months was the only predictive parameter in multivariate analysis for remission among all the baseline parameters, which included urine protein, serum albumin and anti-PLA2R titer at baseline, 6 and 12 months. Similar observations were also made by Hoxha et al. [3].
This study also defines the nature of risk alleles in the HLA-DQA1 and PLA2R1 genes in South Asian subjects with PMN. In a GWAS, Stanescu et al. [4] reported the association between rs2187668 on HLA-DQA1 and rs4664308 on PLA2R1 with PMN in the European Caucasian population. The risk association was stronger for HLA-DQA1 compared with the PLA2R1 gene. Lv et al. [7] investigated the association of PMN with variations at three loci each in the HLA-DQA1 and PLA2R1 genes in a Chinese population. The strongest associations of PMN were with SNPs in the PLA2R1 gene [rs3749117: OR 2.32 (95% CI 2–2.69) and rs4664308: OR 2.35 (95% CI 2.02–2.73)]. In the present study, the strongest association was with variation at rs2187668 on the HLA-DQA1 gene.
We found that a combination of variations in HLA-DQA1 (rs2187668) and PLA2R1 (rs4664308) was overrepresented in PMN patients. The association was stronger only in PLA2R-related cases. Previous studies have shown evidence of gene–gene interaction between HLA-DQA1 (rs2187668) and PLA2R1 (rs4664308) as determinants of PMN risk [4, 7]. Homozygosity for the rare variants at both loci conferred a 78.5-fold (range 34.55–178.17) higher risk of developing PMN in the Caucasian population [4]. In contrast, the risk was higher in the Chinese PMN patients bearing AA at the rs4664308 locus in HLA-DQA1 and GA at the rs2187668 locus in PLA2R1 [OR 12.33 (95% CI 1.38–110.04)] [7]. The results of the present study demonstrate that the genetic susceptibility for PMN in South Asians is closer to that of Caucasians than Chinese [4].
Lv et al. [7] reported an increase in the frequency of anti-PLA2R positivity in patients with high-risk genotypes, which included variations in both PLA2R1 and HLA-DQA1. Our results extend these findings by documenting genetic variation in anti-PLA2R-positive PMN cases. In the present study, we found an association of HLA-DQA1 (rs2187668) with anti-PLA2R positivity. Variations at PLA2R1 loci were not significantly associated with antibody positivity. The difference in the present observation with that of the study on Chinese PMN [7] cases can be partly explained by racial differences, small sample size, probable gene–gene interaction between PLA2R1 and HLA-DQA1, the effect of an as yet unknown modifier genes or differential environmental influences in the causation of PMN.
This study confirms three facts: (i) the strong association of anti-PLA2R and enhanced glomerular PLA2R with PMN, (ii) the excellent association between anti-PLA2R levels with clinical response and (iii) the association of genetic variants in HLA-DQA1 and PLA2R1 with PMN in South Asian patients. Our study provides further support for the diagnostic utility of anti-PLA2R for PMN and suggests its importance in assessing treatment response. At present, all patients receive similar immunosuppressive therapy for a fixed period. It is possible that in the future, individualization may be possible by stopping therapy early in patients who show an immunological response. On the other hand, an alternative therapy might be considered earlier in those who do not show a significant reduction in antibody titers.
Our study has several limitations. We studied only those patients who needed therapy, thus we are unable to comment on the relationship of anti-PLA2R titer and spontaneous remission. Also, the duration of follow-up was limited and the sample size for the genetic analysis was small. The absence of more frequent antibody testing precluded greater elucidation of the relationship between immunological and clinical remission. Treatment was as per the current clinical practice guidelines and not targeted to anti-PLA2R. Comparison between subgroups (enhanced glomerular staining for PLA2R positive and serum anti-PLA2R positive, enhanced glomerular staining for PLA2R positive and serum anti-PLA2R negative, enhanced glomerular staining for PLA2R negative and serum anti-PLA2R positive and enhanced glomerular staining for PLA2R negative and serum anti-PLA2R negative) is limited by the small subject numbers, making it difficult to draw definitive conclusions on differences in clinical behavior of patients with various combinations. Finally, we tested only reported candidate SNPs in HLA-DQA1 and PLA2R1.
To conclude, we show that ∼82% of Indian patients with PMN have PLA2R-related disease. Baseline serum anti-PLA2R levels are not useful for predicting disease behavior. A decrease with treatment, however, is associated with remission. Variation in HLA-DQA1 (rs2187668) is independently associated with risk for PMN, and a combination of this variation along with PLA2R1 (rs4664308, rs3749119 and rs35771982) confers a high risk of development of PMN.
FUNDING
This study was supported by the ISN-LaRenon research grant of the Indian Society of Nephrology.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
Author notes
These authors contributed equally to this work.





Comments