-
PDF
- Split View
-
Views
-
Cite
Cite
Hae-Lim Lee, Robert K. Jansen, Timothy W. Chumley, Ki-Joong Kim, Gene Relocations within Chloroplast Genomes of Jasminum and Menodora (Oleaceae) Are Due to Multiple, Overlapping Inversions, Molecular Biology and Evolution, Volume 24, Issue 5, May 2007, Pages 1161–1180, https://doi.org/10.1093/molbev/msm036
Close - Share Icon Share
Abstract
The chloroplast (cp) DNA sequence of Jasminum nudiflorum (Oleaceae–Jasmineae) is completed and compared with the large single-copy region sequences from 6 related species. The cp genomes of the tribe Jasmineae (Jasminum and Menodora) show several distinctive rearrangements, including inversions, gene duplications, insertions, inverted repeat expansions, and gene and intron losses. The ycf4–psaI region in Jasminum section Primulina was relocated as a result of 2 overlapping inversions of 21,169 and 18,414 bp. The 1st, larger inversion is shared by all members of the Jasmineae indicating that it occurred in the common ancestor of the tribe. Similar rearrangements were also identified in the cp genome of Menodora. In this case, 2 fragments including ycf4 and rps4–trnS–ycf3 genes were moved by 2 additional inversions of 14 and 59 kb that are unique to Menodora. Other rearrangements in the Oleaceae are confined to certain regions of the Jasminum and Menodora cp genomes, including the presence of highly repeated sequences and duplications of coding and noncoding sequences that are inserted into clpP and between rbcL and psaI. These insertions are correlated with the loss of 2 introns in clpP and a serial loss of segments of accD. The loss of the accD gene and clpP introns in both the monocot family Poaceae and the eudicot family Oleaceae are clearly independent evolutionary events. However, their genome organization is surprisingly similar despite the distant relationship of these 2 angiosperm families.
Introduction
Chloroplast (cp) genome organization in land plants is highly conserved (Palmer 1991; Raubeson and Jansen 2005). The presence of 2 large inverted repeats (IRs), which range from 12 to 75 kb in length and separate the large and small single-copy (LSC and SSC) regions, is one of the most prominent features of these genomes (Palmer 1990, 1991). Gene order and gene/intron content are typically conserved in the majority of land plants; rearrangements have been documented in a number of groups, including Asteraceae, Campanulaceae, Fabaceae, Geraniaceae, Lobeliaceae, Onagraceae, Pinaceae, Poaceae, and Ranunculaceae (Palmer and Thompson 1981; Jansen and Palmer 1987; Palmer, Nugent, and Herbon 1987; Palmer, Osorio, et al. 1987; Hiratsuka et al. 1989; Milligan et al. 1989; Hachtel et al. 1991; Lidholm and Gustafsson 1991; Doyle et al. 1992; Knox et al. 1993; Hoot and Palmer 1994; Cosner et al. 1997, 2004; Strauss et al. 1998; Kim et al. 2005; Saski et al. 2005; Chumley et al. 2006). Several mechanisms have been implicated in generating changes in gene order and content in cp genomes, including inversion, transposition, gene duplication, gene/intron loss, insertion/deletion, and IR expansion/contraction (Palmer 1991; Raubeson and Jansen 2005). The relative frequency of each of these changes varies with transposition being the least common, and the only definitive case of cp transposition has been documented in the green alga Chlamydomonas (Fan et al. 1995), although this mechanism has been suggested in several other groups (Milligan et al. 1989; Cosner et al. 1997). Inversions are the most common mechanism for changes in gene order, and they have been fully characterized in a number of cp genomes. In several cases, dispersed repeats have been shown to promote inversions through intermolecular recombination (Ogihara et al. 1988; Palmer 1991). Several studies have demonstrated a strong correlation between the numbers and locations of repetitive sequences and the incidence of inversions (Pombert et al. 2005, 2006). The rarity of gene-order changes in cp genomes has made these events powerful phylogenetic markers in several plant groups (Jansen and Palmer 1987; Doyle et al. 1992, 1996; Raubeson and Jansen 1992).
The angiosperm family Oleaceae includes approximately 29 genera and 600 species that are widely distributed in tropical and temperate regions of the world (Heywood 1978). The tribe Jasmineae contains only 2 genera, Jasminum and Menodora (Taylor 1945; Johnson 1957; Wallander and Albert 2000). Jasminum is the largest genus of the Oleaceae with approximately 200 species (Rehder 1940; Green 2004) confined to the Old World. The genus is traditionally classified into 4 sections (Alternifolia, Unifoliolata, Pinnatifolia, and Trifoliolata) based on the leaf arrangement and the leaflet number (De Candolle 1844); however, several systematic studies have suggested that most of these sections are artificial (Rohwer 1994, 1995; Green 1997, 2001). Molecular phylogenies (Lee H-L and Kim K-J, unpublished data) support the recognition of 3 clades: an alternate-leaved group (section Alternifolia), section Primulina (Green 2001), and an opposite-leaved group that contains the majority of the species. In contrast, Menodora is a small genus with approximately 23 species (Steyermark 1932; Turner 1991) and is distributed in southwestern North America, southern South America, and southern Africa.
In this paper, we investigate the organization and evolution of cp genomes in the Oleaceae. Several unusual features of the genomes of Menodora and Jasminum are described, which provide valuable insights into cpDNA evolution. The comparisons identify multiple inversions, gene duplications, gene and intron losses, IR expansion, and a high incidence of dispersed repeats. Gene relocation caused by multiple inversions is a notable feature of the cp genomes of the Jasmineae. The phylogenetic order and timing of the major rearrangements are determined by comparative sequence analysis and phylogenetic analysis of the major lineages within the tribe.
Materials and Methods
Taxon Sampling
The taxa used in this study are listed in table 1. The cp genome of Jasminum nudiflorum was sequenced completely, whereas inversion regions only were sequenced from the following 5 species: Menodora longiflora; 1 species from each of the 3 major lineages of Jasminum (Jasminum le-ratii, Jasminum abyssinicum, Jasminum subhumile); and an outgroup species, Forsythia europaea. Seventeen species of Jasminum and 2 species of Menodora were used for comparative gene mapping. Twenty-five accessions of 24 species of Jasminum, 4 species of Menodora, and 21 species of other Oleaceae were used for the polymerase chain reaction (PCR) diagnosis of inversions and the comparative analysis of inversion endpoints. Forty-one ndhF and rbcL sequences representing all major clades of Oleaceae and outgroups were also used for estimating phylogenetic relationships and divergence times (table 1).
Taxa Used for cp DNA Sequencing, Gene Mapping, Molecular Clock Test, and PCR Diagnosis of Inversions
| Tribe Jasmineae–Specific 1st Inversionc | Section Primulina–Specific 2nd Inversionc | Menodora-Specific 3rd and 4th Inversionsc | ||||||||||||||
| Groups Based on Leaf Characters or Taxonomic Group | Scientific Name and Accession Informationa | cp Sequencingb | Gene Mapping | ndhF/rbcL Sequencesb | P1/P2 | P3/P4 | P1/P3 | P2/P4 | P5/P6 | P2/P5 | P4/P6 | P9/P10 | P7/P8 | P6/P9 | P5/P7 | P8/P10 |
| Opposite lvs. | Jasminum abyssinicum Hochst. ex DC. (Kew 335-71-03007) | DQ673258 | + | +/DQ673258 | + | + | + | + | + | |||||||
| Opposite lvs. | Jasminum angulare Vahl (Kew 367-63-36701) | + | + | + | + | + | + | |||||||||
| Opposite lvs. | Jasminum azoricum L. (Kew 000-73-12543) | + | + | + | + | + | + | |||||||||
| Opposite lvs. | Jasminum beesianum Forrest & Diels (Kew 000-69-13486) | + | + | + | + | + | + | |||||||||
| Opposite lvs. | Jasminum dallachii F. Muell. (Kew 000-73-02394) | + | + | + | + | + | + | |||||||||
| Opposite lvs. | Jasminum didymum G. Forst. (Kew 671-66-67110) | + | + | + | + | + | + | |||||||||
| Opposite lvs. | Jasminum dispermum Wall. (Kew 678-66-67818) | + | + | + | + | + | + | |||||||||
| Opposite lvs. | Jasminum floribundum R. Br. ex Fresen. (Kew 307-80-02859) | + | + | + | + | + | ||||||||||
| Opposite lvs. | Jasminum gracile Andrews (UCONN 198800144) | + | + | + | + | + | ||||||||||
| Opposite lvs. | Jasminum kedahense Ridl. (Kew 000-73-12545) | + | + | + | + | + | ||||||||||
| Opposite lvs. | Jasminum le-ratii Schltr. (Kew 083-65-08303) | DQ673257 | DQ673271/DQ673257 | + | + | + | + | + | ||||||||
| Opposite lvs. | Jasminum nervosum Lour. (Kew 220-78-06124) | + | + | + | + | + | + | |||||||||
| Opposite lvs. | Jasminum officinale L. (UCONN 198502393) | + | + | + | + | + | ||||||||||
| Opposite lvs. | Jasminum polyanthum Franch. (Edinburgh 19697043) | + | + | + | + | + | + | |||||||||
| Opposite lvs. | Jasminum pubescens (Retzius) Willd. (Fairchild PRC117) | + | + | + | + | + | ||||||||||
| Opposite lvs. | Jasminum simplicifolium G. Forst. (Kew 000-73-12904) | + | DQ673270/DQ673298 | + | + | + | + | + | ||||||||
| Opposite lvs. | Jasminum sinense Hemsl. (Kew 000-73-12548) | + | + | + | + | + | ||||||||||
| Opposite lvs. | Jasminum tortuosum Willd. (Kew 349-67-34909) | + | + | + | + | + | + | |||||||||
| Alternate lvs. | Jasminum bignoniaceum Wall. ex G. Don (Kew 000-72-12290) | + | + | + | + | + | + | |||||||||
| Alternate lvs. | Jasminum fruticans L. (Kew 448-82-04854) | + | DQ673268/DQ673297 | + | + | + | + | + | ||||||||
| Alternate lvs. | Jasminum humile L. (UCONN s.n.) | + | + | + | + | + | + | |||||||||
| Alternate lvs. | Jasminum subhumile W. W. Sm. (Kew 336-68-33602) | DQ673259 | DQ673269/DQ673259 | + | + | + | + | + | ||||||||
| Opposite lvs. -Primulina | Jasminum mesnyi Hance (Edinburgh 19697040) | + | DQ673267/DQ673296 | + | + | +* | + | + | + | + | ||||||
| Opposite lvs. -Primulina | Jasminum nudiflorum Lindl. (K.-J. Kim et al. 2004-1531, KUS) | + | + | +* | + | + | + | + | ||||||||
| Opposite lvs. -Primulina | J. nudiflorum Lindl. (UCONN 199200212) | DQ673255 | DQ673255/DQ673255 | + | + | +* | + | + | + | + | ||||||
| Menodora | Menodora longiflora Engelm. ex A. Gray (B. L. Turner 15965, TEX) | DQ673260/DQ673261 | DQ673273/DQ673260 | + | + | + | + | + | ||||||||
| Menodora | Menodora scabra Engelm. ex A. Gray (M. Neel & S. White s.n., TEX 265413) | + | + | + | + | + | + | |||||||||
| Menodora | Menodora scoparia Engelm. ex A. Gray (J. S. Miller et al. 5109, MO) | + | + | + | + | + | ||||||||||
| Menodora | Menodora spinescens A. Gray (J. D. Morefield 4399, MO) | DQ673272/DQ673299 | + | + | + | + | + | |||||||||
| Fontanesieae | Fontanesia phillyraeoides Labill. v. phillyraeoides (AA 1542-80-13) | + | DQ673263/DQ673293 | + | + | + | + | + | + | |||||||
| Forsythieae | Abeliophyllum distichum Nakai (AA 178-16-8) | + | DQ673262/DQ673292 | + | + | + | + | + | + | |||||||
| Forsythieae | Forsythia europaea Degen & Baldacci (AA 10198-1917) | DQ673256 | + | DQ673264/DQ673256 | + | + | + | + | + | + | ||||||
| Myxopyreae | Myxopyrum hainanense L. C. Chia (K.-J. Kim et al. 2000-508, KUS) | DQ673265/DQ673294 | + | + | + | + | + | + | ||||||||
| Myxopyreae | Nyctanthes arbor-tristis L. (Kuriachen s.n., SPU) | DQ673266/DQ673295 | + | + | + | + | + | + | ||||||||
| Oleeae–Fraxininae | Fraxinus chinensis Roxb. (AA 8161-A) | + | DQ673275/DQ673301 | + | + | + | + | + | + | |||||||
| Oleeae–Ligustrinae | Ligustrum vulgare L. (K.-J. Kim 1997-70) | + | DQ673276/DQ673302 | + | + | + | + | + | + | |||||||
| Oleeae–Ligustrinae | Syringa vulgaris L. (Edinburgh 19644264) | + | DQ673277/DQ673303 | + | + | + | + | + | + | |||||||
| Oleeae–Oleinae | Chionanthus virginicus L. (UCONN s.n.) | + | DQ673283/DQ673309 | + | + | + | + | + | + | |||||||
| Oleeae–Oleinae | Forestiera angustifolia Torr. (B. L. Turner 755, TEX) | + | DQ673284/DQ673310 | + | + | + | + | + | + | |||||||
| Oleeae–Oleinae | Haenianthus salicifolius Griseb. (K.-J. Kim 1992-39, KUS) | DQ673282/DQ673308 | + | + | + | + | + | + | ||||||||
| Oleeae–Oleinae | Nestegis sandwicensis (A. Gray) O. Deg., I. Deg. & L. A. S. Johnson (Kew 336-68-33601) | + | DQ673279/DQ673305 | + | + | + | + | + | + | |||||||
| Oleeae–Oleinae | Noronhia emarginata Thou. (Fairchild FG-61-496) | + | DQ673281/DQ673307 | + | + | + | + | + | + | |||||||
| Oleeae–Oleinae | Notelaea ovata R. Br. (B. J. Conn. 3102, MO) | + | DQ673280/DQ673306 | + | + | + | + | + | + | |||||||
| Oleeae–Oleinae | Olea europaea L. (UCONN 198500974) | + | DQ673278/DQ673304 | + | + | + | + | + | + | |||||||
| Oleeae–Oleinae | Osmanthus americana Benth. & Hook. f. (Kew 349-67-34901) | + | DQ673285/DQ673311 | + | + | + | + | + | + | |||||||
| Oleeae–Oleinae | Phillyrea angustifolia L. (Kew 186-74-01825) | + | DQ673286/DQ673312 | + | + | + | + | + | + | |||||||
| Oleeae–Oleinae | Picconia excelsa DC. (W. R. Sykes 473/69, MO) | DQ673288/DQ673314 | + | + | + | + | + | + | ||||||||
| Oleeae–Oleinae | Priogymnanthus hasslerianus (Chodat) P. S. Green (Al. Gentry et al. 74009, MO) | + | + | + | + | + | + | |||||||||
| Oleeae–Schreberinae | Comoranthus minor H. Perrier (P. B. Phillipson et al. 3706, MO) | DQ673287/DQ673313 | + | + | + | + | + | + | ||||||||
| Oleeae–Schreberinae | Schrebera alata Welw. (Kew 000-69-18826) | + | DQ673274/DQ673300 | + | + | + | + | + | + | |||||||
| Outgroup | Carlemannia tetragona Hook. f. (K.-J. Kim et al. 2004-1433, KUS) | DQ673290/DQ673316 | + | + | + | + | + | + | ||||||||
| Outgroup | Silvianthus bracteatus Hook. f. (K.-J. Kim et al. 2004-1430, KUS) | DQ673289/DQ673315 | + | + | + | + | + | + | ||||||||
| Outgroup | Menyanthes trifoliata L. | L39388/L14006 | ||||||||||||||
| Outgroup | Aster cordifolius L. (R. K. Jansen 906, MICH) | L39449/DQ673291 | ||||||||||||||
| Outgroup | Hedera helix L. | AF130203/L01924 | ||||||||||||||
| Outgroup | Cornus florida L. | AF130220/L11215 | ||||||||||||||
| Outgroup | Nicotiana tabacum L. | N001879/N001879 | ||||||||||||||
| Outgroup | Gelsemium sempervirens Ait. | AF130170/L14397 | ||||||||||||||
| Outgroup | Clerodendrum trichotomum Thunb. ex A. Murray/Clerodendrum fragrans Willd. | AF130146/L11689 | ||||||||||||||
| Outgroup | Callicarpa dichotoma Raeusch. | L36395/L14393 | ||||||||||||||
| Outgroup | Buddleja davidii Franch. | AF130143/L14392 | ||||||||||||||
| Outgroup | Digitalis purpurea L. | AF130150/L01902 | ||||||||||||||
| Tribe Jasmineae–Specific 1st Inversionc | Section Primulina–Specific 2nd Inversionc | Menodora-Specific 3rd and 4th Inversionsc | ||||||||||||||
| Groups Based on Leaf Characters or Taxonomic Group | Scientific Name and Accession Informationa | cp Sequencingb | Gene Mapping | ndhF/rbcL Sequencesb | P1/P2 | P3/P4 | P1/P3 | P2/P4 | P5/P6 | P2/P5 | P4/P6 | P9/P10 | P7/P8 | P6/P9 | P5/P7 | P8/P10 |
| Opposite lvs. | Jasminum abyssinicum Hochst. ex DC. (Kew 335-71-03007) | DQ673258 | + | +/DQ673258 | + | + | + | + | + | |||||||
| Opposite lvs. | Jasminum angulare Vahl (Kew 367-63-36701) | + | + | + | + | + | + | |||||||||
| Opposite lvs. | Jasminum azoricum L. (Kew 000-73-12543) | + | + | + | + | + | + | |||||||||
| Opposite lvs. | Jasminum beesianum Forrest & Diels (Kew 000-69-13486) | + | + | + | + | + | + | |||||||||
| Opposite lvs. | Jasminum dallachii F. Muell. (Kew 000-73-02394) | + | + | + | + | + | + | |||||||||
| Opposite lvs. | Jasminum didymum G. Forst. (Kew 671-66-67110) | + | + | + | + | + | + | |||||||||
| Opposite lvs. | Jasminum dispermum Wall. (Kew 678-66-67818) | + | + | + | + | + | + | |||||||||
| Opposite lvs. | Jasminum floribundum R. Br. ex Fresen. (Kew 307-80-02859) | + | + | + | + | + | ||||||||||
| Opposite lvs. | Jasminum gracile Andrews (UCONN 198800144) | + | + | + | + | + | ||||||||||
| Opposite lvs. | Jasminum kedahense Ridl. (Kew 000-73-12545) | + | + | + | + | + | ||||||||||
| Opposite lvs. | Jasminum le-ratii Schltr. (Kew 083-65-08303) | DQ673257 | DQ673271/DQ673257 | + | + | + | + | + | ||||||||
| Opposite lvs. | Jasminum nervosum Lour. (Kew 220-78-06124) | + | + | + | + | + | + | |||||||||
| Opposite lvs. | Jasminum officinale L. (UCONN 198502393) | + | + | + | + | + | ||||||||||
| Opposite lvs. | Jasminum polyanthum Franch. (Edinburgh 19697043) | + | + | + | + | + | + | |||||||||
| Opposite lvs. | Jasminum pubescens (Retzius) Willd. (Fairchild PRC117) | + | + | + | + | + | ||||||||||
| Opposite lvs. | Jasminum simplicifolium G. Forst. (Kew 000-73-12904) | + | DQ673270/DQ673298 | + | + | + | + | + | ||||||||
| Opposite lvs. | Jasminum sinense Hemsl. (Kew 000-73-12548) | + | + | + | + | + | ||||||||||
| Opposite lvs. | Jasminum tortuosum Willd. (Kew 349-67-34909) | + | + | + | + | + | + | |||||||||
| Alternate lvs. | Jasminum bignoniaceum Wall. ex G. Don (Kew 000-72-12290) | + | + | + | + | + | + | |||||||||
| Alternate lvs. | Jasminum fruticans L. (Kew 448-82-04854) | + | DQ673268/DQ673297 | + | + | + | + | + | ||||||||
| Alternate lvs. | Jasminum humile L. (UCONN s.n.) | + | + | + | + | + | + | |||||||||
| Alternate lvs. | Jasminum subhumile W. W. Sm. (Kew 336-68-33602) | DQ673259 | DQ673269/DQ673259 | + | + | + | + | + | ||||||||
| Opposite lvs. -Primulina | Jasminum mesnyi Hance (Edinburgh 19697040) | + | DQ673267/DQ673296 | + | + | +* | + | + | + | + | ||||||
| Opposite lvs. -Primulina | Jasminum nudiflorum Lindl. (K.-J. Kim et al. 2004-1531, KUS) | + | + | +* | + | + | + | + | ||||||||
| Opposite lvs. -Primulina | J. nudiflorum Lindl. (UCONN 199200212) | DQ673255 | DQ673255/DQ673255 | + | + | +* | + | + | + | + | ||||||
| Menodora | Menodora longiflora Engelm. ex A. Gray (B. L. Turner 15965, TEX) | DQ673260/DQ673261 | DQ673273/DQ673260 | + | + | + | + | + | ||||||||
| Menodora | Menodora scabra Engelm. ex A. Gray (M. Neel & S. White s.n., TEX 265413) | + | + | + | + | + | + | |||||||||
| Menodora | Menodora scoparia Engelm. ex A. Gray (J. S. Miller et al. 5109, MO) | + | + | + | + | + | ||||||||||
| Menodora | Menodora spinescens A. Gray (J. D. Morefield 4399, MO) | DQ673272/DQ673299 | + | + | + | + | + | |||||||||
| Fontanesieae | Fontanesia phillyraeoides Labill. v. phillyraeoides (AA 1542-80-13) | + | DQ673263/DQ673293 | + | + | + | + | + | + | |||||||
| Forsythieae | Abeliophyllum distichum Nakai (AA 178-16-8) | + | DQ673262/DQ673292 | + | + | + | + | + | + | |||||||
| Forsythieae | Forsythia europaea Degen & Baldacci (AA 10198-1917) | DQ673256 | + | DQ673264/DQ673256 | + | + | + | + | + | + | ||||||
| Myxopyreae | Myxopyrum hainanense L. C. Chia (K.-J. Kim et al. 2000-508, KUS) | DQ673265/DQ673294 | + | + | + | + | + | + | ||||||||
| Myxopyreae | Nyctanthes arbor-tristis L. (Kuriachen s.n., SPU) | DQ673266/DQ673295 | + | + | + | + | + | + | ||||||||
| Oleeae–Fraxininae | Fraxinus chinensis Roxb. (AA 8161-A) | + | DQ673275/DQ673301 | + | + | + | + | + | + | |||||||
| Oleeae–Ligustrinae | Ligustrum vulgare L. (K.-J. Kim 1997-70) | + | DQ673276/DQ673302 | + | + | + | + | + | + | |||||||
| Oleeae–Ligustrinae | Syringa vulgaris L. (Edinburgh 19644264) | + | DQ673277/DQ673303 | + | + | + | + | + | + | |||||||
| Oleeae–Oleinae | Chionanthus virginicus L. (UCONN s.n.) | + | DQ673283/DQ673309 | + | + | + | + | + | + | |||||||
| Oleeae–Oleinae | Forestiera angustifolia Torr. (B. L. Turner 755, TEX) | + | DQ673284/DQ673310 | + | + | + | + | + | + | |||||||
| Oleeae–Oleinae | Haenianthus salicifolius Griseb. (K.-J. Kim 1992-39, KUS) | DQ673282/DQ673308 | + | + | + | + | + | + | ||||||||
| Oleeae–Oleinae | Nestegis sandwicensis (A. Gray) O. Deg., I. Deg. & L. A. S. Johnson (Kew 336-68-33601) | + | DQ673279/DQ673305 | + | + | + | + | + | + | |||||||
| Oleeae–Oleinae | Noronhia emarginata Thou. (Fairchild FG-61-496) | + | DQ673281/DQ673307 | + | + | + | + | + | + | |||||||
| Oleeae–Oleinae | Notelaea ovata R. Br. (B. J. Conn. 3102, MO) | + | DQ673280/DQ673306 | + | + | + | + | + | + | |||||||
| Oleeae–Oleinae | Olea europaea L. (UCONN 198500974) | + | DQ673278/DQ673304 | + | + | + | + | + | + | |||||||
| Oleeae–Oleinae | Osmanthus americana Benth. & Hook. f. (Kew 349-67-34901) | + | DQ673285/DQ673311 | + | + | + | + | + | + | |||||||
| Oleeae–Oleinae | Phillyrea angustifolia L. (Kew 186-74-01825) | + | DQ673286/DQ673312 | + | + | + | + | + | + | |||||||
| Oleeae–Oleinae | Picconia excelsa DC. (W. R. Sykes 473/69, MO) | DQ673288/DQ673314 | + | + | + | + | + | + | ||||||||
| Oleeae–Oleinae | Priogymnanthus hasslerianus (Chodat) P. S. Green (Al. Gentry et al. 74009, MO) | + | + | + | + | + | + | |||||||||
| Oleeae–Schreberinae | Comoranthus minor H. Perrier (P. B. Phillipson et al. 3706, MO) | DQ673287/DQ673313 | + | + | + | + | + | + | ||||||||
| Oleeae–Schreberinae | Schrebera alata Welw. (Kew 000-69-18826) | + | DQ673274/DQ673300 | + | + | + | + | + | + | |||||||
| Outgroup | Carlemannia tetragona Hook. f. (K.-J. Kim et al. 2004-1433, KUS) | DQ673290/DQ673316 | + | + | + | + | + | + | ||||||||
| Outgroup | Silvianthus bracteatus Hook. f. (K.-J. Kim et al. 2004-1430, KUS) | DQ673289/DQ673315 | + | + | + | + | + | + | ||||||||
| Outgroup | Menyanthes trifoliata L. | L39388/L14006 | ||||||||||||||
| Outgroup | Aster cordifolius L. (R. K. Jansen 906, MICH) | L39449/DQ673291 | ||||||||||||||
| Outgroup | Hedera helix L. | AF130203/L01924 | ||||||||||||||
| Outgroup | Cornus florida L. | AF130220/L11215 | ||||||||||||||
| Outgroup | Nicotiana tabacum L. | N001879/N001879 | ||||||||||||||
| Outgroup | Gelsemium sempervirens Ait. | AF130170/L14397 | ||||||||||||||
| Outgroup | Clerodendrum trichotomum Thunb. ex A. Murray/Clerodendrum fragrans Willd. | AF130146/L11689 | ||||||||||||||
| Outgroup | Callicarpa dichotoma Raeusch. | L36395/L14393 | ||||||||||||||
| Outgroup | Buddleja davidii Franch. | AF130143/L14392 | ||||||||||||||
| Outgroup | Digitalis purpurea L. | AF130150/L01902 | ||||||||||||||
Plant materials are from the living collections of Kew (Royal Botanic Garden, Kew), Edinburgh (Royal Botanic Garden, Edinburgh), Fairchild (Fairchild Tropical Botanic Garden, Miami), UCONN (Greenhouse of the University of Connecticut at Storrs), and AA (Arnold Arboretum, Boston), field collections from China, India, and United States (all voucher specimens are deposited at the indicated herbaria), and herbarium at MO (Missouri Botanic Garden) and TEX (University of Texas at Austin).
GenBank accession numbers with DQ initials are the new sequence reported from this study; other sequences are from GenBank.
Asterisk indicates nonspecific amplification. Primer sequences are P1: 5′-AAGTTTCCTCCCGTGATATGAG-3′, P2: 5′-CAAGTACGGTTCTAAGGGAAGG-3′, P3: 5′-GCGGGTCTCTTTATTAGCTCCTATTTGT-3′, P4: 5′-GGGGTGAATGGAATCCGATACATAAA-3′, P5: 5′-AATCGATCATAACCACTACCTATATTCCAG-3′, P6: 5′-CCGTAGCTAACCGAGTAGCTCTAGA-3′, P7: 5′-CTATCGCTATATTCATCGTTATGTGCTAGA-3′, P8: 5′-CATACCCAATCGAAAAAGGATGTTATCCAAAC-3′, P9: 5′-AACAGCAATCCAAGGTCGCATACCCAGACGGA-3′, and P10: 5′-CCCATTAGTAAGCCGGTTC-3′.
Taxa Used for cp DNA Sequencing, Gene Mapping, Molecular Clock Test, and PCR Diagnosis of Inversions
| Tribe Jasmineae–Specific 1st Inversionc | Section Primulina–Specific 2nd Inversionc | Menodora-Specific 3rd and 4th Inversionsc | ||||||||||||||
| Groups Based on Leaf Characters or Taxonomic Group | Scientific Name and Accession Informationa | cp Sequencingb | Gene Mapping | ndhF/rbcL Sequencesb | P1/P2 | P3/P4 | P1/P3 | P2/P4 | P5/P6 | P2/P5 | P4/P6 | P9/P10 | P7/P8 | P6/P9 | P5/P7 | P8/P10 |
| Opposite lvs. | Jasminum abyssinicum Hochst. ex DC. (Kew 335-71-03007) | DQ673258 | + | +/DQ673258 | + | + | + | + | + | |||||||
| Opposite lvs. | Jasminum angulare Vahl (Kew 367-63-36701) | + | + | + | + | + | + | |||||||||
| Opposite lvs. | Jasminum azoricum L. (Kew 000-73-12543) | + | + | + | + | + | + | |||||||||
| Opposite lvs. | Jasminum beesianum Forrest & Diels (Kew 000-69-13486) | + | + | + | + | + | + | |||||||||
| Opposite lvs. | Jasminum dallachii F. Muell. (Kew 000-73-02394) | + | + | + | + | + | + | |||||||||
| Opposite lvs. | Jasminum didymum G. Forst. (Kew 671-66-67110) | + | + | + | + | + | + | |||||||||
| Opposite lvs. | Jasminum dispermum Wall. (Kew 678-66-67818) | + | + | + | + | + | + | |||||||||
| Opposite lvs. | Jasminum floribundum R. Br. ex Fresen. (Kew 307-80-02859) | + | + | + | + | + | ||||||||||
| Opposite lvs. | Jasminum gracile Andrews (UCONN 198800144) | + | + | + | + | + | ||||||||||
| Opposite lvs. | Jasminum kedahense Ridl. (Kew 000-73-12545) | + | + | + | + | + | ||||||||||
| Opposite lvs. | Jasminum le-ratii Schltr. (Kew 083-65-08303) | DQ673257 | DQ673271/DQ673257 | + | + | + | + | + | ||||||||
| Opposite lvs. | Jasminum nervosum Lour. (Kew 220-78-06124) | + | + | + | + | + | + | |||||||||
| Opposite lvs. | Jasminum officinale L. (UCONN 198502393) | + | + | + | + | + | ||||||||||
| Opposite lvs. | Jasminum polyanthum Franch. (Edinburgh 19697043) | + | + | + | + | + | + | |||||||||
| Opposite lvs. | Jasminum pubescens (Retzius) Willd. (Fairchild PRC117) | + | + | + | + | + | ||||||||||
| Opposite lvs. | Jasminum simplicifolium G. Forst. (Kew 000-73-12904) | + | DQ673270/DQ673298 | + | + | + | + | + | ||||||||
| Opposite lvs. | Jasminum sinense Hemsl. (Kew 000-73-12548) | + | + | + | + | + | ||||||||||
| Opposite lvs. | Jasminum tortuosum Willd. (Kew 349-67-34909) | + | + | + | + | + | + | |||||||||
| Alternate lvs. | Jasminum bignoniaceum Wall. ex G. Don (Kew 000-72-12290) | + | + | + | + | + | + | |||||||||
| Alternate lvs. | Jasminum fruticans L. (Kew 448-82-04854) | + | DQ673268/DQ673297 | + | + | + | + | + | ||||||||
| Alternate lvs. | Jasminum humile L. (UCONN s.n.) | + | + | + | + | + | + | |||||||||
| Alternate lvs. | Jasminum subhumile W. W. Sm. (Kew 336-68-33602) | DQ673259 | DQ673269/DQ673259 | + | + | + | + | + | ||||||||
| Opposite lvs. -Primulina | Jasminum mesnyi Hance (Edinburgh 19697040) | + | DQ673267/DQ673296 | + | + | +* | + | + | + | + | ||||||
| Opposite lvs. -Primulina | Jasminum nudiflorum Lindl. (K.-J. Kim et al. 2004-1531, KUS) | + | + | +* | + | + | + | + | ||||||||
| Opposite lvs. -Primulina | J. nudiflorum Lindl. (UCONN 199200212) | DQ673255 | DQ673255/DQ673255 | + | + | +* | + | + | + | + | ||||||
| Menodora | Menodora longiflora Engelm. ex A. Gray (B. L. Turner 15965, TEX) | DQ673260/DQ673261 | DQ673273/DQ673260 | + | + | + | + | + | ||||||||
| Menodora | Menodora scabra Engelm. ex A. Gray (M. Neel & S. White s.n., TEX 265413) | + | + | + | + | + | + | |||||||||
| Menodora | Menodora scoparia Engelm. ex A. Gray (J. S. Miller et al. 5109, MO) | + | + | + | + | + | ||||||||||
| Menodora | Menodora spinescens A. Gray (J. D. Morefield 4399, MO) | DQ673272/DQ673299 | + | + | + | + | + | |||||||||
| Fontanesieae | Fontanesia phillyraeoides Labill. v. phillyraeoides (AA 1542-80-13) | + | DQ673263/DQ673293 | + | + | + | + | + | + | |||||||
| Forsythieae | Abeliophyllum distichum Nakai (AA 178-16-8) | + | DQ673262/DQ673292 | + | + | + | + | + | + | |||||||
| Forsythieae | Forsythia europaea Degen & Baldacci (AA 10198-1917) | DQ673256 | + | DQ673264/DQ673256 | + | + | + | + | + | + | ||||||
| Myxopyreae | Myxopyrum hainanense L. C. Chia (K.-J. Kim et al. 2000-508, KUS) | DQ673265/DQ673294 | + | + | + | + | + | + | ||||||||
| Myxopyreae | Nyctanthes arbor-tristis L. (Kuriachen s.n., SPU) | DQ673266/DQ673295 | + | + | + | + | + | + | ||||||||
| Oleeae–Fraxininae | Fraxinus chinensis Roxb. (AA 8161-A) | + | DQ673275/DQ673301 | + | + | + | + | + | + | |||||||
| Oleeae–Ligustrinae | Ligustrum vulgare L. (K.-J. Kim 1997-70) | + | DQ673276/DQ673302 | + | + | + | + | + | + | |||||||
| Oleeae–Ligustrinae | Syringa vulgaris L. (Edinburgh 19644264) | + | DQ673277/DQ673303 | + | + | + | + | + | + | |||||||
| Oleeae–Oleinae | Chionanthus virginicus L. (UCONN s.n.) | + | DQ673283/DQ673309 | + | + | + | + | + | + | |||||||
| Oleeae–Oleinae | Forestiera angustifolia Torr. (B. L. Turner 755, TEX) | + | DQ673284/DQ673310 | + | + | + | + | + | + | |||||||
| Oleeae–Oleinae | Haenianthus salicifolius Griseb. (K.-J. Kim 1992-39, KUS) | DQ673282/DQ673308 | + | + | + | + | + | + | ||||||||
| Oleeae–Oleinae | Nestegis sandwicensis (A. Gray) O. Deg., I. Deg. & L. A. S. Johnson (Kew 336-68-33601) | + | DQ673279/DQ673305 | + | + | + | + | + | + | |||||||
| Oleeae–Oleinae | Noronhia emarginata Thou. (Fairchild FG-61-496) | + | DQ673281/DQ673307 | + | + | + | + | + | + | |||||||
| Oleeae–Oleinae | Notelaea ovata R. Br. (B. J. Conn. 3102, MO) | + | DQ673280/DQ673306 | + | + | + | + | + | + | |||||||
| Oleeae–Oleinae | Olea europaea L. (UCONN 198500974) | + | DQ673278/DQ673304 | + | + | + | + | + | + | |||||||
| Oleeae–Oleinae | Osmanthus americana Benth. & Hook. f. (Kew 349-67-34901) | + | DQ673285/DQ673311 | + | + | + | + | + | + | |||||||
| Oleeae–Oleinae | Phillyrea angustifolia L. (Kew 186-74-01825) | + | DQ673286/DQ673312 | + | + | + | + | + | + | |||||||
| Oleeae–Oleinae | Picconia excelsa DC. (W. R. Sykes 473/69, MO) | DQ673288/DQ673314 | + | + | + | + | + | + | ||||||||
| Oleeae–Oleinae | Priogymnanthus hasslerianus (Chodat) P. S. Green (Al. Gentry et al. 74009, MO) | + | + | + | + | + | + | |||||||||
| Oleeae–Schreberinae | Comoranthus minor H. Perrier (P. B. Phillipson et al. 3706, MO) | DQ673287/DQ673313 | + | + | + | + | + | + | ||||||||
| Oleeae–Schreberinae | Schrebera alata Welw. (Kew 000-69-18826) | + | DQ673274/DQ673300 | + | + | + | + | + | + | |||||||
| Outgroup | Carlemannia tetragona Hook. f. (K.-J. Kim et al. 2004-1433, KUS) | DQ673290/DQ673316 | + | + | + | + | + | + | ||||||||
| Outgroup | Silvianthus bracteatus Hook. f. (K.-J. Kim et al. 2004-1430, KUS) | DQ673289/DQ673315 | + | + | + | + | + | + | ||||||||
| Outgroup | Menyanthes trifoliata L. | L39388/L14006 | ||||||||||||||
| Outgroup | Aster cordifolius L. (R. K. Jansen 906, MICH) | L39449/DQ673291 | ||||||||||||||
| Outgroup | Hedera helix L. | AF130203/L01924 | ||||||||||||||
| Outgroup | Cornus florida L. | AF130220/L11215 | ||||||||||||||
| Outgroup | Nicotiana tabacum L. | N001879/N001879 | ||||||||||||||
| Outgroup | Gelsemium sempervirens Ait. | AF130170/L14397 | ||||||||||||||
| Outgroup | Clerodendrum trichotomum Thunb. ex A. Murray/Clerodendrum fragrans Willd. | AF130146/L11689 | ||||||||||||||
| Outgroup | Callicarpa dichotoma Raeusch. | L36395/L14393 | ||||||||||||||
| Outgroup | Buddleja davidii Franch. | AF130143/L14392 | ||||||||||||||
| Outgroup | Digitalis purpurea L. | AF130150/L01902 | ||||||||||||||
| Tribe Jasmineae–Specific 1st Inversionc | Section Primulina–Specific 2nd Inversionc | Menodora-Specific 3rd and 4th Inversionsc | ||||||||||||||
| Groups Based on Leaf Characters or Taxonomic Group | Scientific Name and Accession Informationa | cp Sequencingb | Gene Mapping | ndhF/rbcL Sequencesb | P1/P2 | P3/P4 | P1/P3 | P2/P4 | P5/P6 | P2/P5 | P4/P6 | P9/P10 | P7/P8 | P6/P9 | P5/P7 | P8/P10 |
| Opposite lvs. | Jasminum abyssinicum Hochst. ex DC. (Kew 335-71-03007) | DQ673258 | + | +/DQ673258 | + | + | + | + | + | |||||||
| Opposite lvs. | Jasminum angulare Vahl (Kew 367-63-36701) | + | + | + | + | + | + | |||||||||
| Opposite lvs. | Jasminum azoricum L. (Kew 000-73-12543) | + | + | + | + | + | + | |||||||||
| Opposite lvs. | Jasminum beesianum Forrest & Diels (Kew 000-69-13486) | + | + | + | + | + | + | |||||||||
| Opposite lvs. | Jasminum dallachii F. Muell. (Kew 000-73-02394) | + | + | + | + | + | + | |||||||||
| Opposite lvs. | Jasminum didymum G. Forst. (Kew 671-66-67110) | + | + | + | + | + | + | |||||||||
| Opposite lvs. | Jasminum dispermum Wall. (Kew 678-66-67818) | + | + | + | + | + | + | |||||||||
| Opposite lvs. | Jasminum floribundum R. Br. ex Fresen. (Kew 307-80-02859) | + | + | + | + | + | ||||||||||
| Opposite lvs. | Jasminum gracile Andrews (UCONN 198800144) | + | + | + | + | + | ||||||||||
| Opposite lvs. | Jasminum kedahense Ridl. (Kew 000-73-12545) | + | + | + | + | + | ||||||||||
| Opposite lvs. | Jasminum le-ratii Schltr. (Kew 083-65-08303) | DQ673257 | DQ673271/DQ673257 | + | + | + | + | + | ||||||||
| Opposite lvs. | Jasminum nervosum Lour. (Kew 220-78-06124) | + | + | + | + | + | + | |||||||||
| Opposite lvs. | Jasminum officinale L. (UCONN 198502393) | + | + | + | + | + | ||||||||||
| Opposite lvs. | Jasminum polyanthum Franch. (Edinburgh 19697043) | + | + | + | + | + | + | |||||||||
| Opposite lvs. | Jasminum pubescens (Retzius) Willd. (Fairchild PRC117) | + | + | + | + | + | ||||||||||
| Opposite lvs. | Jasminum simplicifolium G. Forst. (Kew 000-73-12904) | + | DQ673270/DQ673298 | + | + | + | + | + | ||||||||
| Opposite lvs. | Jasminum sinense Hemsl. (Kew 000-73-12548) | + | + | + | + | + | ||||||||||
| Opposite lvs. | Jasminum tortuosum Willd. (Kew 349-67-34909) | + | + | + | + | + | + | |||||||||
| Alternate lvs. | Jasminum bignoniaceum Wall. ex G. Don (Kew 000-72-12290) | + | + | + | + | + | + | |||||||||
| Alternate lvs. | Jasminum fruticans L. (Kew 448-82-04854) | + | DQ673268/DQ673297 | + | + | + | + | + | ||||||||
| Alternate lvs. | Jasminum humile L. (UCONN s.n.) | + | + | + | + | + | + | |||||||||
| Alternate lvs. | Jasminum subhumile W. W. Sm. (Kew 336-68-33602) | DQ673259 | DQ673269/DQ673259 | + | + | + | + | + | ||||||||
| Opposite lvs. -Primulina | Jasminum mesnyi Hance (Edinburgh 19697040) | + | DQ673267/DQ673296 | + | + | +* | + | + | + | + | ||||||
| Opposite lvs. -Primulina | Jasminum nudiflorum Lindl. (K.-J. Kim et al. 2004-1531, KUS) | + | + | +* | + | + | + | + | ||||||||
| Opposite lvs. -Primulina | J. nudiflorum Lindl. (UCONN 199200212) | DQ673255 | DQ673255/DQ673255 | + | + | +* | + | + | + | + | ||||||
| Menodora | Menodora longiflora Engelm. ex A. Gray (B. L. Turner 15965, TEX) | DQ673260/DQ673261 | DQ673273/DQ673260 | + | + | + | + | + | ||||||||
| Menodora | Menodora scabra Engelm. ex A. Gray (M. Neel & S. White s.n., TEX 265413) | + | + | + | + | + | + | |||||||||
| Menodora | Menodora scoparia Engelm. ex A. Gray (J. S. Miller et al. 5109, MO) | + | + | + | + | + | ||||||||||
| Menodora | Menodora spinescens A. Gray (J. D. Morefield 4399, MO) | DQ673272/DQ673299 | + | + | + | + | + | |||||||||
| Fontanesieae | Fontanesia phillyraeoides Labill. v. phillyraeoides (AA 1542-80-13) | + | DQ673263/DQ673293 | + | + | + | + | + | + | |||||||
| Forsythieae | Abeliophyllum distichum Nakai (AA 178-16-8) | + | DQ673262/DQ673292 | + | + | + | + | + | + | |||||||
| Forsythieae | Forsythia europaea Degen & Baldacci (AA 10198-1917) | DQ673256 | + | DQ673264/DQ673256 | + | + | + | + | + | + | ||||||
| Myxopyreae | Myxopyrum hainanense L. C. Chia (K.-J. Kim et al. 2000-508, KUS) | DQ673265/DQ673294 | + | + | + | + | + | + | ||||||||
| Myxopyreae | Nyctanthes arbor-tristis L. (Kuriachen s.n., SPU) | DQ673266/DQ673295 | + | + | + | + | + | + | ||||||||
| Oleeae–Fraxininae | Fraxinus chinensis Roxb. (AA 8161-A) | + | DQ673275/DQ673301 | + | + | + | + | + | + | |||||||
| Oleeae–Ligustrinae | Ligustrum vulgare L. (K.-J. Kim 1997-70) | + | DQ673276/DQ673302 | + | + | + | + | + | + | |||||||
| Oleeae–Ligustrinae | Syringa vulgaris L. (Edinburgh 19644264) | + | DQ673277/DQ673303 | + | + | + | + | + | + | |||||||
| Oleeae–Oleinae | Chionanthus virginicus L. (UCONN s.n.) | + | DQ673283/DQ673309 | + | + | + | + | + | + | |||||||
| Oleeae–Oleinae | Forestiera angustifolia Torr. (B. L. Turner 755, TEX) | + | DQ673284/DQ673310 | + | + | + | + | + | + | |||||||
| Oleeae–Oleinae | Haenianthus salicifolius Griseb. (K.-J. Kim 1992-39, KUS) | DQ673282/DQ673308 | + | + | + | + | + | + | ||||||||
| Oleeae–Oleinae | Nestegis sandwicensis (A. Gray) O. Deg., I. Deg. & L. A. S. Johnson (Kew 336-68-33601) | + | DQ673279/DQ673305 | + | + | + | + | + | + | |||||||
| Oleeae–Oleinae | Noronhia emarginata Thou. (Fairchild FG-61-496) | + | DQ673281/DQ673307 | + | + | + | + | + | + | |||||||
| Oleeae–Oleinae | Notelaea ovata R. Br. (B. J. Conn. 3102, MO) | + | DQ673280/DQ673306 | + | + | + | + | + | + | |||||||
| Oleeae–Oleinae | Olea europaea L. (UCONN 198500974) | + | DQ673278/DQ673304 | + | + | + | + | + | + | |||||||
| Oleeae–Oleinae | Osmanthus americana Benth. & Hook. f. (Kew 349-67-34901) | + | DQ673285/DQ673311 | + | + | + | + | + | + | |||||||
| Oleeae–Oleinae | Phillyrea angustifolia L. (Kew 186-74-01825) | + | DQ673286/DQ673312 | + | + | + | + | + | + | |||||||
| Oleeae–Oleinae | Picconia excelsa DC. (W. R. Sykes 473/69, MO) | DQ673288/DQ673314 | + | + | + | + | + | + | ||||||||
| Oleeae–Oleinae | Priogymnanthus hasslerianus (Chodat) P. S. Green (Al. Gentry et al. 74009, MO) | + | + | + | + | + | + | |||||||||
| Oleeae–Schreberinae | Comoranthus minor H. Perrier (P. B. Phillipson et al. 3706, MO) | DQ673287/DQ673313 | + | + | + | + | + | + | ||||||||
| Oleeae–Schreberinae | Schrebera alata Welw. (Kew 000-69-18826) | + | DQ673274/DQ673300 | + | + | + | + | + | + | |||||||
| Outgroup | Carlemannia tetragona Hook. f. (K.-J. Kim et al. 2004-1433, KUS) | DQ673290/DQ673316 | + | + | + | + | + | + | ||||||||
| Outgroup | Silvianthus bracteatus Hook. f. (K.-J. Kim et al. 2004-1430, KUS) | DQ673289/DQ673315 | + | + | + | + | + | + | ||||||||
| Outgroup | Menyanthes trifoliata L. | L39388/L14006 | ||||||||||||||
| Outgroup | Aster cordifolius L. (R. K. Jansen 906, MICH) | L39449/DQ673291 | ||||||||||||||
| Outgroup | Hedera helix L. | AF130203/L01924 | ||||||||||||||
| Outgroup | Cornus florida L. | AF130220/L11215 | ||||||||||||||
| Outgroup | Nicotiana tabacum L. | N001879/N001879 | ||||||||||||||
| Outgroup | Gelsemium sempervirens Ait. | AF130170/L14397 | ||||||||||||||
| Outgroup | Clerodendrum trichotomum Thunb. ex A. Murray/Clerodendrum fragrans Willd. | AF130146/L11689 | ||||||||||||||
| Outgroup | Callicarpa dichotoma Raeusch. | L36395/L14393 | ||||||||||||||
| Outgroup | Buddleja davidii Franch. | AF130143/L14392 | ||||||||||||||
| Outgroup | Digitalis purpurea L. | AF130150/L01902 | ||||||||||||||
Plant materials are from the living collections of Kew (Royal Botanic Garden, Kew), Edinburgh (Royal Botanic Garden, Edinburgh), Fairchild (Fairchild Tropical Botanic Garden, Miami), UCONN (Greenhouse of the University of Connecticut at Storrs), and AA (Arnold Arboretum, Boston), field collections from China, India, and United States (all voucher specimens are deposited at the indicated herbaria), and herbarium at MO (Missouri Botanic Garden) and TEX (University of Texas at Austin).
GenBank accession numbers with DQ initials are the new sequence reported from this study; other sequences are from GenBank.
Asterisk indicates nonspecific amplification. Primer sequences are P1: 5′-AAGTTTCCTCCCGTGATATGAG-3′, P2: 5′-CAAGTACGGTTCTAAGGGAAGG-3′, P3: 5′-GCGGGTCTCTTTATTAGCTCCTATTTGT-3′, P4: 5′-GGGGTGAATGGAATCCGATACATAAA-3′, P5: 5′-AATCGATCATAACCACTACCTATATTCCAG-3′, P6: 5′-CCGTAGCTAACCGAGTAGCTCTAGA-3′, P7: 5′-CTATCGCTATATTCATCGTTATGTGCTAGA-3′, P8: 5′-CATACCCAATCGAAAAAGGATGTTATCCAAAC-3′, P9: 5′-AACAGCAATCCAAGGTCGCATACCCAGACGGA-3′, and P10: 5′-CCCATTAGTAAGCCGGTTC-3′.
DNA Extraction, Enzyme Digestion, Filter Hybridization, and Restriction Site Mapping
Total DNA was isolated from the fresh leaves using the hexadecyltrimethylammonium bromide method (Doyle JJ and Doyle JA 1987), and DNA was further purified by ultracentrifugation in cesium chloride/ethidium bromide gradients. Purified total DNA was digested with 22 restriction endonucleases (AvaI, AvaII, AseI, BamHI, BanI, BanII, BclI, BglII, BstNI, BstXI, ClaI, DraI, EcoRI, Eco0109I, EcoRV, HaeII, HincII, HindIII, NciI, NsiI, XbaI, and XmnI), and the resulting fragments were separated on 1–1.6% agarose gels depending on the number of expected restriction fragments. Bidirectional transfer of the DNA from agarose gels to Zetabind nylon filters (AMF Cuno, Meriden, CT), radioactive labeling of 43 cloned tobacco cpDNA probes (Olmstead and Palmer 1992), filter hybridization, and autoradiography were performed as described in Palmer (1986). In addition, DNA from Olea europaea, Menodora scabra, Jasminum humile, and J. nudiflorum were digested with the same 22 enzymes, transferred to nylon membranes, and hybridized to 106 small cpDNA probes from tobacco (Palmer et al. 1994) to construct detailed gene and restriction site maps.
Genome Sequencing and Annotation
For the complete sequencing of the J. nudiflorum cp genome, cpDNA was isolated and purified following the methods in Palmer (1986). The cpDNA was digested with a combination of 3 restriction enzymes (BamHI, SacI, and ClaI), and the resulting fragments were cloned into a pBluescript II vector. Inserted cpDNA fragments were shotgun sequenced using the BigDye3.0 terminal cycle sequencing kit (Applied Biosystems, Foster City, CA) and an ABI 3700 sequencer. Sequences were assembled using Sequencher (version 4.1; Gene Codes Corporation, Ann Arbor, MI). In order to fill gap regions, primers were designed using the complete cp sequences of Nicotiana and Panax (Shinozaki et al. 1986; Kim and Lee 2004).
A series of PCR products spanning the entire region containing the inversions were generated for J. le-ratii, J. abyssinicum, J. subhumile, M. longiflora, and F. europaea. The PCR cycle was as follows: 94 °C for 3 min, then 30 cycles of 94 °C for 30 s, 50–68 °C for 1 min, and 68–72 °C for 1–8 min (depending upon the size of the target region). PCR-amplified DNA was then purified using the QIAquick PCR purification kit (Qiagen, Hilden, Germany). Amplified cpDNA fragments were sequenced directly.
Gene annotations and comparative sequence analyses were performed using the current versions of Blast (Altschul et al. 1990), ORF finder programs from National Center for Biotechnology Information, and DOGMA (Wyman et al. 2004). The locations and secondary structures of tRNA genes were determined using tRNAscan-SE version 1.21 (Lowe and Eddy 1997), DOGMA (Wyman et al. 2004), and MFOLD version 3.0 (Zuker 2003). Repeated sequences were identified using REPuter (Kurtz et al. 2001).
PCR Diagnosis of Inversions and Determination of Inversion Endpoints
Ten PCR primers (table 1) were designed based on the sequence comparisons among 3 cp genome sequences of Jasminum (this study), Nicotiana (Shinozaki et al. 1986), and Panax (Kim and Lee 2004). Different combinations of these primers were used to test for the presence or absence of certain rearrangements. Positive PCR amplifications were sequenced and aligned to determine inversion endpoints. The standard PCR-amplification reactions were 30 cycles of 1 min denaturation at 94 °C, 1 min annealing at 50–68 °C, and 2–8 min extension at 68–72 °C.
Phylogenetic Analyses and Estimation of Divergence Times of Inversions
DNA sequence data of ndhF and rbcL for 41 taxa and the complete inversion regions (31–35 kb) of 6 taxa were used for phylogenetic analysis. Forty-one ndhF and rbcL sequences representing all major clades of Oleaceae and outgroups were generated by methods described elsewhere (Olmstead et al. 1992; Kim and Jansen 1995). Sequence alignment, base substitution analysis, and phylogenetic analyses were performed using ClustalX (Higgins et al. 1996), MEGA2 (Kumar et al. 2001), PAUP 4.0 (Swofford 2002), and MacClade Version 4.06 (Maddison WP and Maddison DR 2003). The model of evolution was selected using the hierarchical likelihood ratio test as implemented in Modeltest 3.06 (Posada and Crandall 1998).
Divergence times were estimated using the combined ndhF and rbcL data. A likelihood ratio test (Felsenstein 1981) was performed on the maximum likelihood (ML) tree by comparing the scores of the ML tree with and without a molecular clock. This test rejected a molecular clock hypothesis; therefore, the penalized likelihood (PL) method (Sanderson 2002) in the program r8s version 1.60 (Sanderson 2003) was used. The size of the roughness penalty was specified by a smoothing parameter obtained from a cross-validation procedure. After cross-validation, the smoothing parameter was set to an absolute value of 1.0. The ML tree was converted to an ultrametric tree in PL using the truncated network (TN) algorithm, and in the process, zero length branches were collapsed. Divergence times were calibrated using Cornaceae and Oleaceae fossils (Kavadas 1956; Muller 1981; Takahashi et al. 2002).
Results
The cp Genome Organization of J. nudiflorum
The cp genome of J. nudiflorum (GenBank accession number NC_008407) is 165,121-bp long and is divided into 4 regions by a pair of IRs (IRa and IRb) of 29,486 bp each. The LSC and SSC regions are 92,877 and 13,272 bp, respectively. The locations of the 133 genes in the J. nudiflorum cp genome are presented in figure 1. Nineteen genes (7 tRNAs, 4 rRNAs, and 8 protein-coding genes) are duplicated in the IR. In addition, a duplicate copy of trnG-GCC is located in the middle of the LSC region. Eleven protein-coding genes and a tRNA gene are located in the SSC region, whereas 61 protein-coding genes and 23 tRNA genes are located in the LSC region. Nine protein-coding genes and 6 tRNA genes each contain a single intron, and 2 protein-coding genes (rps12 and ycf3) have 2 introns (supplementary material SM 1, Supplementary Material online).
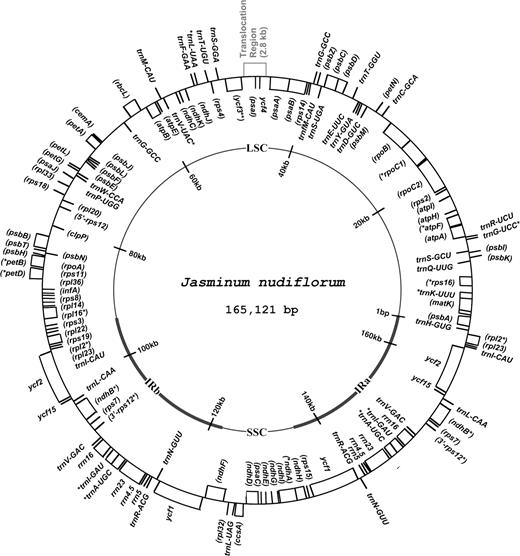
Gene map of the Jasminum nudiflorum cp genome based on the complete sequence. A pair of thick lines on the inside circle represents the IRs (IRa and IRb: 29,486 bp each), which separate the LSC region (92,877 bp) from the SSC region (13,272 bp). Genes drawn inside the circle are transcribed clockwise, whereas those outside are transcribed counterclockwise. Asterisks indicate intron-containing genes. Protein-coding gene names are in parentheses. The relocated region is located in the middle of the LSC region.
The J. nudiflorum cp genome consists of 57% coding sequence (50% protein and 7% RNA) and 43% noncoding sequences, including introns (12%) and intergenic spacers (31%). The overall A-T content of the J. nudiflorum cp genome is 62%. The A-T content in the noncoding region (65%) is higher than in the coding region (60%). The A-T content of the IR is 58%, whereas the A-T content in the LSC and SSC regions is 64% and 67%, respectively. The lower A-T content of the IR reflects the low A-T content in the 4 rRNA (45%) genes in this region.
The gene and intron content and gene order of J. nudiflorum differ from Nicotiana tabacum in several ways. For example, the cp genome of J. nudiflorum is missing most of accD and the 2 introns of clpP. Additionally, duplicate copies of trnG-GCC and ycf1 are present. The extra copy of ycf1 is due to the expansion of the IR at the IRb–SSC boundary, accounting for much of the increase in size of the J. nudiflorum cp genome in comparison with tobacco. In contrast, rps19 is located on the LSC region because of the contraction of IR. One notable rearrangement of the J. nudiflorum cp genome is the movement of a 2.8-kb region containing ycf4 and psaI to the middle of the LSC region (fig. 1).
Comparative Gene Mapping of Oleaceae cp Genomes
The cp genomes were mapped for 32 representative species of all major genera of Oleaceae and 12 closely related outgroup species (table 1). The gene maps (not shown) indicate that all genera of the Oleaceae except Jasminum and Menodora have an identical gene order to N. tabacum. We sequenced the rearranged portions of the LSC region of the cp genomes for the representative species of Jasminum, Menodora, and the closely related genus Forsythia to better characterize the rearrangements in members of the Jasmineae.
Identification of Inversions among Members of the Oleaceae
Complete sequences for the region between psaA and psbB in the LSC region were generated from F. europaea, J. subhumile, J. le-ratii, and J. abyssinicum (fig. 2) and are approximately 32-kb long for each species. For M. longiflora, 2 additional regions of the cp genome extending from psbA to psaA (15,387 bp) and from trnK to rpl20 (18,522 bp) were sequenced because mapping data (not shown) suggested the presence of inversions in these regions (fig. 3).
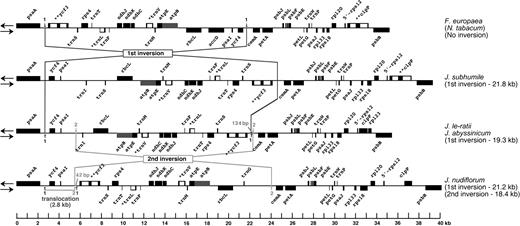
Comparative gene maps showing the 1st and 2nd inversions in the Jasminum cp genome compared with those of the Nicotiana and Forsythia genomes.
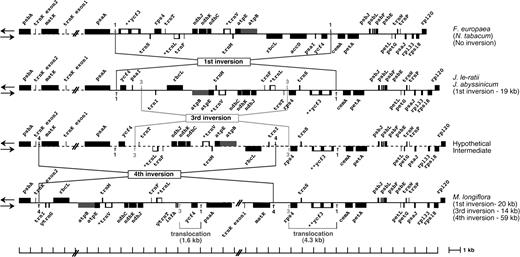
Comparative gene maps showing the 1st, 3rd, and 4th inversions in the Menodora cp genome compared with those of the Nicotiana, Forsythia, and Jasminum genomes.
The sequenced region of the outgroup F. europaea (tribe Forsythieae) is 31,495-bp long and includes 25 protein-coding and 8 tRNA genes (fig. 2). The gene content and order are identical to the Nicotiana cp genome. The sequence of J. subhumile (section Alternifolia) between psbA and psbB is 35,515-bp long, including an inversion of 21.8 kb not present in Nicotiana or Forsythia. Relative to the genomes of Nicotiana and Forsythia, one endpoint of the inversion is located between psaA and ycf3, and the other endpoint is located between ycf4 and cemA. In addition, the J. subhumile cp genome lacks more than 50% of accD but includes trnI-CAU and trnS-GGA in this region.
Sequences between psaA and psbB genes of J. le-ratii and J. abyssinicum (both members of the opposite-leaved group of Jasminum) are 31,507- and 31,044-bp long, respectively. These species have a similar gene order to J. subhumile, including one inversion of 19.3 kb possessing the same endpoints. Gene and intron content of this group are slightly different from F. europaea; they both lack most of accD and 2 introns of clpP but include trnI-CAU downstream from rbcL and rpl23 downstream from clpP.
The region between psaA and psbB in J. nudiflorum (section Primulina) is 36,404-bp long, and the gene order is similar to Forsythia and Nicotiana, except for the relocation of ycf4 and psaI. The 2.8-kb region that includes ycf4 and psaI is almost 18 kb upstream from its position in Forsythia and Nicotiana (fig. 1). One endpoint of this region is located between psaA and ycf4, and the other is between psaI and ycf3. Comparison of Jasminum, Forsythia, and Nicotiana cp genomes reveals that movement of this 2.8-kb region in J. nudiflorum is caused by 2 unequal, sequential inversions of 21 and 18 kb. The 21-kb inversion in J. nudiflorum is also present in other Jasminum species. One endpoint of this inversion is located between psaA and ycf3, and the other endpoint is between ycf4 and cemA. In addition, we hypothesize a 2nd 18-kb inversion in J. nudiflorum. One endpoint of the 2nd inversion is between ycf3 and cemA and is only 134 bp from the endpoint of the 21-kb inversion. The other endpoint of the 18-kb inversion is 2.8 kb from the 21-kb inversion endpoint. Therefore, the 2 inversions represent independent evolutionary events, even though 1 endpoint of each inversion is found in nearly the identical location. The incorporation of 134 bp of sequence outside of the 1st inversion endpoint at one end and the inclusion of 42 bp of this sequence at the other endpoint during the 2nd inversion clearly support the evolutionary ordering of these 2 inversions. The distribution pattern of these remnant sequences indicates that the 18-kb inversion occurred after the 21-kb inversion.
The gene order in the Menodora cp genome is distinct from Jasminum species as well as other genera of Oleaceae. Three inversions can explain the differences in the cp genome organization of Menodora (fig. 3). The 1st is 20 kb and is shared with all sampled species of Jasminum. Therefore, this represents a shared derived character among members of the tribe Jasmineae. The 2 endpoints of this inversion are identical to the 1st inversion (21 kb) in Jasminum (fig. 2). Two additional inversions (3rd and 4th inversions in fig. 3) are unique to Menodora. The 3rd inversion is approximately 14 kb, and the 2 endpoints are located between psaI and trnI-CAU and between trnT-UGU and rps4, and this inversion is nested within the 1st inversion (fig. 3, the trnI is truncated). The 4th inversion is the largest one at 59 kb. Because one endpoint of the 4th inversion is located within trnK-UUU, the 2 exons of trnK-UUU are located almost 59 kb apart. The remaining endpoint is between trnI-CAU and rps4 (fig. 3). The 3rd inversion occurred prior to the 4th inversion.
Distribution of Inversions in Oleaceae
We designed 10 primers (P1–P10) to amplify the inversion endpoint regions (table 1 and fig. 4), and different combinations of these primers were used in PCR reactions to determine the phylogenetic distribution of the 4 Oleaceae inversions among 52 species (table 1).
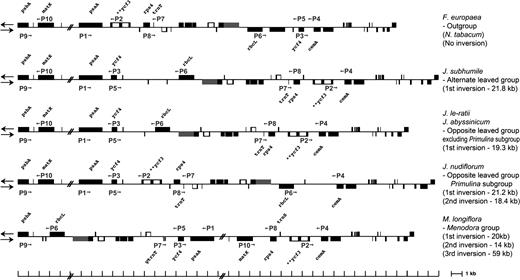
Location of 10 diagnostic PCR primers for identification of 4 inversion endpoints. Sequences for the 10 primers (P1–P10) are given in table 1.
A positive PCR amplification would be expected from the primer combinations of P1/P3 and P2/P4 for species with the 1st 21-kb inversion. In contrast, for the species without the 1st inversion, a positive PCR reaction would result from the primer combinations of P1/P2 and P3/P4. For the species with both the 1st and the 2nd inversions, the primer combinations of P2/P5 and P4/P6 would produce positive PCR reactions, whereas the primer combination of P4/P6 would produce a positive PCR reaction in the species without both inversions. For the 3rd and 4th inversions, a series of positive amplifications would be expected from the primer combinations of P6/P9, P5/P7, P1/P3, P8/P10, and P2/P4.
Representative positive and negative PCR results for 9 species are shown in figure 5. Lanes 1–4 represent species with only the 1st inversion (all members of Jasminum except section Primulina), lanes 5–6 represent species with the 1st and the 2nd inversions (section Primulina), lanes 7–8 are species with the 1st, 3rd, and 4th inversions (Menodora), and lane 9 represents the species without any inversions (Forsythia).
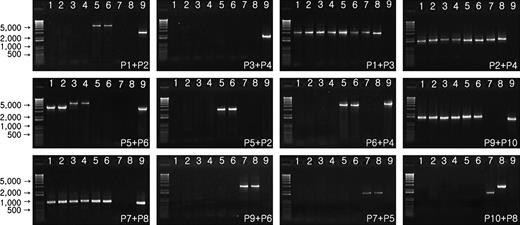
Selected examples of positive/negative PCR amplifications for the presence/absence of inversions using various combinations of primers. Nine representative taxa are shown: left most lane—100 bp marker ladder; 1, Jasminum le-ratii; 2, Jasminum abyssinicum; 3, Jasminum subhumile; 4, Jasminum bignoniaceum; 5, Jasminum nudiflorum; 6, Jasminum mesnyi; 7, Menodora scabra; 8, Menodara longiflora; and 9, Forsythia europaea.
The PCR diagnosis (table 1 and fig. 5) indicates that the 1st inversion is shared by all sampled members of the tribe Jasmineae, whereas all other tribes of the Oleaceae lack this inversion. Our findings also indicate that the 2nd inversion is restricted to the section Primulina of Jasminum and that the 3rd and 4th inversions are unique to Menodora.
All PCR results agreed with our expectations with one exception (fig. 5); an unexpected amplification was observed for the primer combination of P2/P4 in J. nudiflorum. Based on the complete cp sequence, the primer combination of P2/P4 should not generate a PCR product; however, an amplicon was produced from total DNA. This region is between ycf3 and cemA, and we sequenced the fragment and compared it with sequences of this region from other species. The PCR product contains many base substitutions and insertions/deletions, suggesting that the fragments may not be cpDNA. The amplicon apparently came from the primer-specific sites (see table 1), but from cpDNA that underwent intracellular gene transfer prior to the 2nd inversion and is no longer resident in the cp genome. Filter hybridizations of J. nudiflorum in this region also showed weak hybridizing fragments (data not shown). These results suggest that the unexpected fragment of J. nudiflorum might be due to the transfer of this region to the nucleus or mitochondrion. Transfers of cp genes to the mitochondrion and nucleus are well documented in several lineages (Blanchard and Schmidt 1995; Martin et al. 1998; Cummings et al. 2003; Matsuo et al. 2005).
Identification of Inversion Endpoints
To identify the precise location of endpoints of the 4 inversions (figs. 2 and 3) in Jasminum and Menodora, amplified fragments of each primer pair were sequenced and aligned from representative species (table 1 and figs. 5 and 6). The sequences between primer pairs P1/P2 and P3/P4 for F. europaea, primer pairs P1/P3 and P2/P4 for J. le-ratii, J. abyssinicum, J. subhumile, M. longiflora, and primer pairs P1/P3, P2/P5, and P4/P6 for J. nudiflorum were aligned (fig. 6) for the 1st and 2nd inversion endpoints. Endpoints of the 1st inversion were easily identified from the aligned sequences because of the high level of sequence conservation across species. Distinctive, short, reverse complementary sequences are associated with both inversion endpoints. The sequence AAAAGAAA overlaps on the 2 different orientations of alignments, although the P2/P5 fragment of J. nudiflorum and the P2/P4 fragment of M. longiflora appear to have lost this overlapping sequence by deletion. This overlapping sequence corresponds to base positions 580–587 upstream of psaA in the J. nudiflorum cp genome. Endpoints for the 2nd inversion in J. nudiflorum were also revealed by comparison of P4/P6 and P2/P5 fragments (fig. 6). The 2nd inversion is also associated with distinctive, short duplicated sequences of AAARGAAY. These sequence elements are duplicated at the endpoints and correspond to base positions 1,425–1,431 upstream of psaI and 444–450 upstream of cemA in the J. nudiflorum genome. Three bases are deleted on one endpoint. This sequence shows high identity to the 1st inversion endpoint (AAAAGAAA). One endpoint of the 2nd inversion is located 42 bp upstream of the 1st inversion endpoint (figs. 2 and 6).

Sequence alignments showing 1 of 4 inversion endpoints (shaded area). Eight of 52 sequences are shown.
The endpoints of the 3rd inversion in Menodora were identified by comparative alignment of sequences between P7/P8 of J. nudiflorum and P5/P7 or P8/P10 of M. longiflora. The endpoints of the 4th inversion in Menodora were identified by comparative sequence alignments between P9/P10 of J. nudiflorum and P6/P9 or P8/P10 of M. longiflora (figs. 3 and 4). One endpoint of the 3rd inversion is located 530 bp downstream of trnT-UGU, and the other endpoint is located 144 bp upstream of rps4 (arrow 3 in fig. 3; alignment not shown). The 3rd inversion is nested within the 1st inversion. Relocations of ycf4 and the rps4–trnS-GGA–ycf3 regions between endpoints 1 and 3 were caused by the 1st and 3rd inversions, and this region was further modified by the 4th inversion. The distance between endpoint 3 and ycf4 is 779 bp, and the distance between endpoints 3 and 4 is 1,343 bp. The 1,343 bp consist of 403 bp of noncoding sequence and a 939-bp repeat. This repeat is essentially the same as the 779 bp of sequence except for a 160-bp insertion in the middle of the 779-bp fragment. One endpoint of the 4th inversion (4 in fig. 3) is located 266 bp (±73 bp) upstream of trnK-UUU exon 2, and the other endpoint is located 135 bp (±73 bp) downstream of matK (fig. 3). Size variation between P10 and P8 in Menodora species (lanes 7 and 8 of fig. 6) is due to an 852-bp insertion that is associated with the direct repeats of GARGAAGAAA at the endpoint of the 4th inversion.
Phylogenetic Distribution and Timing of Inversions
In order to determine the phylogenetic distribution and times of origin of the 4 inversions, we reconstructed a phylogeny of the Oleaceae using sequences of rbcL and ndhF. Thirty species representing most of the genera in the family were sequenced, and 10 outgroup sequences were downloaded from GenBank (table 1). The gene sequences were aligned, and phylogenetic trees were reconstructed from the combined gene sequences using ML methods with the transversion (TVM) + gamma distribution (G) model.
Among the outgroups, 2 genera of Carlemanniaceae (Carlemannia and Silvianthus) are sister to Oleaceae (fig. 7). The Oleaceae is monophyletic with the 3 tribes Myxopyreae, Fontanesieae, and Forsythieae forming the earliest diverging lineages. The tribe Jasmineae forms a strongly supported monophyletic group. Within tribe Jasmineae, 3 lineages are evident, and they correspond to 3 well-recognized taxonomic groups: the alternate-leaved Jasminum (section Alternifolia), the opposite-leaved Jasminum, and Menodora. Within the opposite-leaved Jasminum, 2 subgroups are present, one of which corresponds to section Primulina (J. nudiflorum and Jasminum mesnyi) and the other including Jasminum simplicifolium and J. le-ratii.
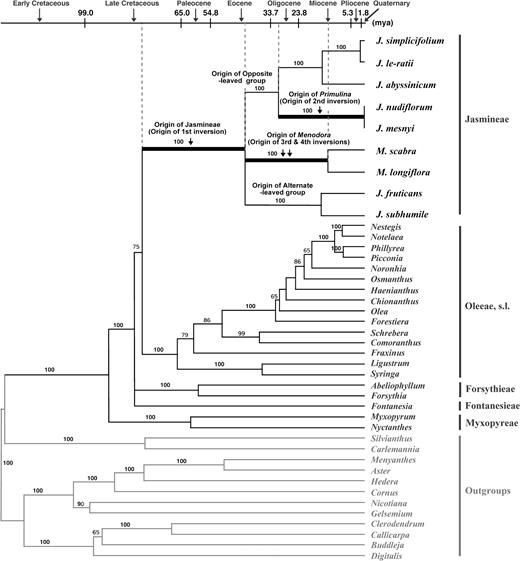
Phylogenetic tree of the Oleaceae and related families with ages estimated according to the PL method. Four inversion events are mapped on the tree, and the times of inversions are estimated. Numbers above nodes are bootstrap values.
All sampled members of the Jasmineae share the 1st 21-kb inversion. The 2 species of section Primulina have a 2nd inversion that results in the relocation of the 2.8-kb ycf4–psaI region. All sampled species of Menodora share the 3rd and the 4th inversions. The phylogenetic tree based on the gene sequence data clearly suggests that the 1st inversion originated prior to the other 3 inversions. The data also suggest that the 2nd inversion was independent of the 3rd and 4th inversions.
Sequence data for ndhF and rbcL were used to estimate the time of origin of the 4 inversions using a PL analysis. These estimates indicate that the Jasmineae originated in the upper Cretaceous (78.3 MYA) and that the extant species of Jasmineae diverged from a common ancestral species in the middle Eocene (42.1 MYA). The 1st cpDNA inversion occurred during this same time period (fig. 7). The 2nd inversion of the Primulina subgroup cp genome originated more recently, sometime between the Oligocene (30.3 MYA) and Quaternary (0 MYA). The 3rd and 4th inversions in Menodora occurred sometime between the middle Eocene (42.1 MYA) and middle Miocene (12.6 MYA).
In order to more accurately estimate the order and timing of the 4 inversions, we performed phylogenetic analyses of the tribe Jasmineae using cpDNA sequences between psaA and 5′-rps12, which includes the inversion regions for J. nudiflorum, J. le-ratii, J. abyssinicum, J. subhumile, M. longiflora, and F. europaea. The 6 aligned sequences were 34,607-bp long, and phylogenetic analyses using ML and general time reversible (GTR) + invariable site proportion (I) + G resulted in the tree in figure 8a. We also performed phylogenetic analyses of the accD gene region after excluding unalignable noncoding regions and all small gaps from the data matrix; the resulting matrix included 23,217 aligned base pairs, and the resulting ML tree using GTR + I + G is shown in figure 8b.
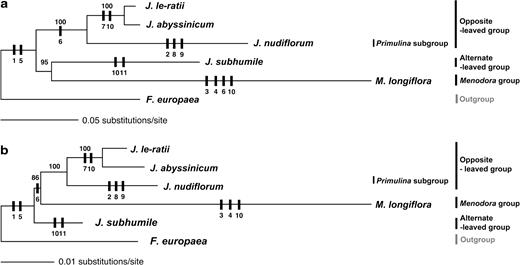
Two different phylogenetic trees illustrating the evolution of various cp genome rearrangements. Trees were generated from the aligned sequences of 31-kb regions of cpDNA using ML methods. Numbers above nodes are bootstrap values. (a) Tree was generated by ML methods with GTR + I + G model from 34,607 bp of aligned sequences between the psaA and 5′-rps12 genes. (b) Tree was generated by ML methods with GTR + I + G model from 23,217 bp excluding accD gene regions, unalignable noncoding regions, and all small gaps. Genome rearrangement characters are the following: 1, inversion one; 2, inversion two; 3, inversion three; 4, inversion four; 5, accD loss; 6, intron losses in clpP; 7, rpl23 duplication and relocation; 8: trnT duplication and relocation; 9, trnG duplication; 10, trnI duplication; and 11, trnS duplication.
The topologies of the 2 trees (figs. 8a and b) differ in the position of Menodora. Menodora longifolia is sister to an alternate-leaved J. subhumile in tree A, whereas in tree B, M. longiflora is sister to the clade comprising the other 3 Jasminum species. The ML values (lnL) of tree A and tree B with gaps are −89806.04 and −89810.90, respectively. The lnL of the 2 trees without gaps are −48182.76 and −48177.34, respectively. The P values for the Kishino–Hasegawa test on tree A and tree B are 1.000 and 0.139, respectively. The P values for the Kishino–Hasegawa test on trees A and B without gaps are 0.320 and 1.000, respectively. Thus, the Kishino–Hasegawa test indicates that the 2 trees are not significantly different.
The phylogenetic analyses confirm our hypotheses regarding the evolutionary order of the inversion events. The 1st inversion arose prior to the other 3 inversions (character 1 in fig. 8). The 2nd inversion (character 2 in fig. 8) is independent of the 3rd and 4th inversions (characters 3 and 4 in fig. 8). The 3rd and the 4th inversions are restricted to the Menodora.
Other Rearrangements in Jasmineae cp Genomes
Duplicated Insertions of 3 tRNA Genes
Two copies of trnG-GCC are located in the LSC region of J. nudiflorum. The copy near psbZ (coordinate 39,000 in fig. 1) is at the same location in tobacco, and the copy downstream of rbcL (coordinate 63,000 in fig. 1) is the duplicate. The 2 copies have 100% sequence identity but are in reverse orientations. The noncoding sequences 42 bp upstream and 54 bp downstream of the gene are also identical in both locations, suggesting that trnG-GCC and the surrounding noncoding sequence are duplicated and inserted into a new location.
Similar duplicated insertions of tRNA genes are found in other lineages of Jasminum and Menodora. The trnI-CAU, which is usually located in the IR, also occurs downstream of rbcL in all species of Menodora and Jasminum except section Primulina. In addition, a duplicate copy of trnS-GCU, which is normally located near psbI (near coordinate 10,000; fig. 1), also occurs downstream of rbcL in the alternate-leaved species of Jasminum (represented by J. subhumile in fig. 2). All 3 duplicated insertions are located downstream of rbcL and are correlated with the reduction or loss of accD, even though the 3 tRNA genes were originally in several different regions of the cp genome.
Duplicated Insertion of rpl23
An extra copy of rpl23 is inserted downstream of clpP in the LSC region of J. le-ratii and J. abyssinicum. This gene is located between trnI-CAU and rpl2 in the IR region in tobacco. Copies of these genes in the LSC and IR regions show minor differences in length and sequence identity (supplementary material SM 2, Supplementary Material online). The IR copy is 282-bp long, and a 3-bp deletion as well as 4 base substitutions are present in the LSC region of J. le-ratii. In addition, 79 and 15 bp of noncoding sequence located upstream and downstream of both copies of rpl23, respectively, are duplicated. Different species of Jasminum exhibit variation between the 2 copies of rpl23. For example, the LSC region of J. abyssinicum terminates at position 228 because of a 2-bp deletion, causing a frameshift mutation. The duplicated segments of rpl23 and associated noncoding regions have identical sequences in all surveyed species, suggesting that this is a shared derived character of the opposite-leaved species of Jasminum.
Duplication of ycf1 by Expansion of the IR
Detailed comparisons of the IR–SSC boundaries of Nicotiana, Panax, Arabidopsis, and J. nudiflorum (fig. 9) indicate that ycf1 is completely incorporated into the IR region in J. nudiflorum. This expansion makes the IR region of J. nudiflorum approximately 4 kb longer than the other species compared, and the SSC region of Jasminum is consequently shorter than these other genomes.
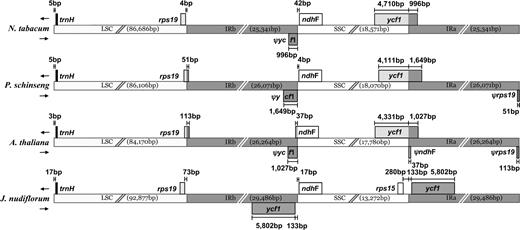
Comparison of boundaries of the SSC, LSC, and IR regions among 4 cp genomes. Length variation of ycf1 pseudogenes (5′ portions) occurs at the IRb–SSC boundary. The IR region extends into the ycf1 gene resulting in 2 complete copies in Jasminum nudiflorum.
Small Inversion of trnT-UGU
The trnT-UGU is normally located on the opposite strand and upstream of trnL-UAA. However, in J. nudiflorum, it is located on the same strand as trnL-UAA (fig. 1, near coordinate 55,000). This small inversion is only present in J. nudiflorum and J. mesnyi. The change in strand of trnT-UGU is due to a small 219-bp inversion. In addition to the trnT-UGU gene, the inversion includes 24 bp upstream and 122 bp downstream of the gene sequence. Short IR sequences are conserved (GAA-TTCCTAT and ATAGGAACTTC) except one base indel at both ends of the inversion.
Interruption of trnK-UUU by Inversion
The trnK-UUU is unusual because it contains matK within its intron in most cp genomes. Yet, the cp genomes of Menodora have an interrupted trnK-UUU gene due to an inversion endpoint within the intron between matK and the 3′ exon of trnK-UUU (fig. 3, inversion 4). As a result, the 5′ exon is located upstream of matK, whereas the 3′ exon is located upstream of the psaA gene. The 2 exons of the trnK-UUU gene are separated by 59 kb because of this large inversion (fig. 3).
Serial Loss of accD
All sampled species of Jasminum and Menodora are missing some or all of accD. The serial loss of this gene is restricted to the members of the tribe Jasmineae, thus this character supports the monophyly of the tribe Jasmineae (fig. 8, character 5). The accD is usually located between rbcL and psaI in cp genomes of land plants. Therefore, we sequenced the region from rbcL to psaI for 5 Jasmineae species that have lost portions of accD gene and compared these sequences with 6 other species in which complete accD is present. The length of this region in species with accD is conserved, ranging from 2,835 to 3,068 bp in 6 phylogenetically diverse species (Nicotiana, Arabidopsis, Panax, Spinacia, Atropa, and Forsythia). In contrast, the size of the region that corresponds to the rbcL–psaI intergenic spacer region among 5 Jasmineae species ranges from 2,922 to 5,271 bp. Furthermore, average sequence divergence for this region among Jasmineae species is 0.4428, which is far higher than the average of 0.2407 seen among the 6 diverse outgroups.
Distinctive sequence elements are present in the region that corresponds to rbcL and psaI of the Jasminum species (fig. 10). Nearly half of the 3′ end of accD (947–1,449 bp) remains in the alternate-leaved clade of Jasminum (represented by J. subhumile). In addition, a small fragment from the middle of accD (822–907 bp) remains in all members of Jasminum (fig. 10). However, the middle section of the accD sequence is completely absent in Menodora. The data suggest that the loss of accD occurred sequentially with the 5′ portion first, the 3′ portion next, and finally the middle portion of the gene. Complete or partial gene fragments from other parts of cp genomes are inserted into this region (fig. 10). These fragments include an atpE gene fragment (172–290 bp) in all species; a complete copy of trnI-CAU in all Jasminum except the Primulina subgroup (supplementary material SM 3, Supplementary Material online); complete trnS-GCU, partial trnP-UGG, and partial 5′-rps12 for the alternate-leaved species of Jasminum; and complete trnG-GCC for all species of Jasminum except the alternate-leaved Jasminum. The trnI-CAU and trnG-GCC genes are also inserted into this region in Menodora. In addition, a portion of the rpl23 gene is found in this region in Menodora and the Primulina subgroup of Jasminum. A massive duplication of a 12-bp repeated sequence in the middle of the noncoding region between rbcL and psaI occurs in J. nudiflorum (fig. 10). The consensus 12-bp sequence of MNNMAYYDGNNV is repeated 100 times in the region where accD has been reduced (supplementary material SM 4, Supplementary Material online). The insertion of these repeats probably originated a long time ago because the individual repeating units are very divergent. Another highly repetitive element occurs downstream of clpP in J. nudiflorum. In this case, there are 56 copies of a 27-bp repeat (YWTTTTYTTTWGDRAHHRWYAYTTHHT), and this insertion coincides with the loss of 2 introns in clpP (supplementary material SM 5, Supplementary Material online). These repeats probably originated more recently because there are no base substitutions among the individual copies of the repeat elements.
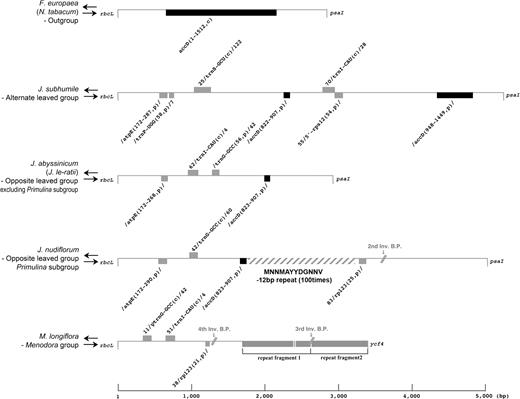
Detailed gene map between rbcL and psaI for 5 representative species of Jasmineae. Numbers before and after the gene name indicate the coordinate of the gene in reference to Nicotiana tabacum. C, completed gene; p, partial gene.
Intron Losses in clpP
Two introns in clpP are missing in the cp genome of J. nudiflorum. Filter hybridization and sequencing results suggest that the losses of 2 introns in clpP are confined to opposite-leaved Jasminum, with both introns being well conserved in alternate-leaved Jasminum (fig. 8, character 6). The clpP introns are also absent in Menodora cpDNA. Two independent intron losses are hypothesized in figure 8A, whereas single intron loss is supported in figure 8B. In addition to the clpP intron loss, further rearrangements are located downstream of clpP. The gene rpl23 typically found in the IR in other angiosperm cp genomes has been duplicated, and one copy occurs downstream of clpP in Menodora and the opposite-leaved species of Jasminum (figs. 2 and 3; supplementary material SM. 2, Supplementary Material online). In J. nudiflorum, the above-mentioned 27-bp sequence is repeated 56 times in this region (supplementary material SM 5, Supplementary Material online).
Discussion
The cp genomes of the Oleaceae tribe Jasmineae exhibit a number of unusual rearrangements; together these changes make the Jasminum and Menodora cp genomes unique among land plants. The most notable changes concern the relocation of genes due to multiple, overlapping inversions, and the separation of the 2 exons of trnK-UUU by 59 kb. Below, we discuss several unusual features of Oleaceae cp genomes and compare them with other rearranged land plant cp genomes.
Gene Relocations Caused by Multiple Inversions
The cp genomes of the tribe Jasmineae have experienced multiple movements of gene segments. In Jasminum section Primulina (J. mesnyi and J. nudiflorum), a 2.8-kb region containing ycf4, psaI, and associated noncoding sequences has been moved 18 kb by 2 consecutive inversions of 21 and 18 kb. The phylogenetic distribution of these 2 inversions (fig. 8, characters 1 and 2) suggests that the 21-kb inversion originated prior to the 18-kb inversion (figs. 2, 3, and 7). Divergence time estimates also indicate that the 1st inversion originated between the upper Cretaceous (78.3 MYA) and the middle Eocene (42.1 MYA), whereas the 2nd inversion originated between the lower Oligocene (30.3 MYA) and Quaternary (0 MYA). In Menodora, 2 regions, rps4–trnS-GGA–ycf3 and ycf4, were relocated by 3 different inversions (fig. 3). The 1st inversion is shared among all members of Jasmineae, and the other 2 (inversions 3 and 4 in fig. 3) are unique to Menodora. The 3rd inversion likely occurred prior to the 4th inversion even though this inversion is not present in any species examined.
Relocations of genes have been documented in other angiosperm lineages, and 2 different mechanisms have been proposed to cause these events. In legumes (Palmer et al. 1988; Perry et al. 2002), successive expansion and contraction of the IR have resulted in the relocation of a block of 6 ribosomal protein genes (rps19–rps8) from one end of the LSC region to the other end. Gene movements in Pelargonium can be attributed to both IR expansion and contraction and multiple inversions (Chumley et al. 2006). In grasses (Quigley and Weil 1985; Hiratsuka et al. 1989; Doyle et al. 1992), 3 inversions have occurred, which have caused the relocation of several genes, including trnG-GCC through psbD.
Short dispersed repeats have been suggested as one of the major factors promoting inversions (Palmer 1991; Knox et al. 1993). Such repeats are common in cp genomes that are highly rearranged, including algae, grasses, conifers, geranium, subclover, and Campanulaceae (Bowman and Dyer 1986; Palmer, Nugent, and Herbon 1987; Bowman et al. 1988; Milligan et al. 1989; Lidholm and Gustafsson 1991; Doyle et al. 1992; Knox et al. 1993; Cosner et al. 1997; Chumley et al. 2006). Inversions have been shown to occur through intramolecular recombination between repeat elements (Palmer, Nugent, and Herbon 1987; Ogihara et al. 1988; Achaz et al. 2003; Rocha 2003; Pombert et al. 2006). Both endpoints of the 1st inversion in Jasminum and Menodora are terminated by the short repeats of AAAAGAAA (fig. 6). The 2nd inversion is also associated with the similar short repeated sequences of AAARGAAY in J. nudiflorum, and 100 copies of MNNMAYYDGNNV sequence elements occur near one endpoint of this inversion (supplementary material SM 4, Supplementary Material online). The endpoints of the 1st inversion in Menodora are also associated with short repeats of AAAAGAAA. In contrast to the short repeats at the endpoints of the 1st and 2nd inversions, the ends of the 3rd and the 4th inversions have much longer repeat elements of 779 and 939 bp (fig. 10). These long duplicated sequences likely facilitated the 3rd and 4th inversions by intramolecular recombination in Menodora.
Several studies have also suggested that tRNAs play a role in promoting gene-order changes by nonhomologous recombination (Hiratsuka et al. 1989; Knox et al. 1993; Turmel et al. 2002). Duplicated copies of 2 tRNAs, trnG-GCC and trnT-UGU, are located near the endpoints of inversions 3 and 4 in Menodora (fig. 3). Thus, the widespread occurrence of repeats and tRNAs near the inversion endpoints both in Menodora and Jasminum cp genomes is coincident with a series of inversions caused by intramolecular recombination.
Gene and Intron Loss
Gene and intron losses have been documented in a number of land plant cp genomes (Downie and Palmer 1992; Raubeson and Jansen 2005). Within the Jasmineae, accD has a series of losses in the species of Jasminum and Menodora examined, supporting the possible phylogenetic relationship within the tribe (figs. 7, 8, and 10). This gene has been lost completely in 4 other lineages of angiosperms, including the grasses (Hiratsuka et al. 1989; Maier et al. 1995; Katayama and Ogihara 1996), Campanulaceae (Cosner et al. 1997), Geraniaceae (Palmer, Nugent, and Herbon 1987; Chumley et al. 2006), and Lobeliaceae (Knox and Palmer 1999). The loss of accD in Campanulaceae and Lobeliaceae is a shared derived feature of these families and supports their close phylogenetic relationship (Knox and Palmer 1999). It is notable that the loss of accD is associated with hotspots of rearrangements in each of the families in which it has been lost. In the grasses, Menodora, and the Primulina subgroup of Jasminum in the region where accD normally resides, a pseudogene of rpl23 is inserted into the region between rbcL and psaI (fig. 10). The mechanism for insertion of different sequence elements in this region is uncertain. The accD gene loss may either accelerate gene relocations by unknown mechanisms, or various movements of genes may induce the sequential loss of accD.
Most cp genomes of land plants include 18 intron-containing genes, 15 of which have 2 exons and 3 (ycf3, rps12, and clpP) with 3 exons. A number of independent losses of introns have been detected in various genes, including rpoC1 (Downie et al. 1996, 1998; Hansen et al. 2006), rpl16 (Downie and Palmer 1992), clpP (Maier et al. 1995; Stefanovic and Olmstead 2005), rpl2 (Downie et al. 1991; Doyle et al. 1995; Stefanovic and Olmstead 2005), rps12 (Hoot and Palmer 1994; Freyer et al. 1995), trnK (Wolfe et al. 1992; Turmel et al. 2005), and ycf3 (Stefanovic and Olmstead 2005). In the Oleaceae, the loss of the 2 introns in clpP is confined to opposite-leaved Jasminum and Menodora (figs. 3 and 8).
Gene Duplications
Most gene duplications in cp genomes are caused by the expansion of the IR (Goulding et al. 1996). These expansions tend to be relatively small and result in the duplication of only single genes or parts of genes, which often produce pseudogenes at the IR–SC boundaries (Kim and Lee 2004). In a number of cases, the IR has greatly expanded, including a 12-kb expansion in Nicotiana acuminata (Solanaceae; Goulding et al. 1996), 11.5 kb in Mahonia (Berberidaceae; Kim and Jansen 1994), 11 kb in Lobelia thuliana (Lobeliaceae; Knox and Palmer 1999), and a remarkable 50 kb in Pelargonium (Geraniaceae; Palmer, Nugent, and Herbon 1987; Chumley et al. 2006). In J. nudiflorum, expansion of the IR has caused a duplication of the normally single-copy gene ycf1 (fig. 9). The duplication ycf1 of Jasminum is analogous to the rps15 duplication in the rye cp genome (Prombona and Subramanian 1989).
Duplications of cp genes that do not involve the IR are uncommon, and only a few have been documented in cp genomes of Arabidopsis (Brassicaceae; Koch et al. 2005), Pelargonium (Geraniaceae; Chumley et al. 2006), Pinus (Pinaceae; Wakasugi et al. 1994), and Trachelium (Campanulaceae; Cosner et al. 1997). These duplications have involved mostly tRNAs and some protein-coding genes. The only duplication of a protein-coding gene in the Oleaceae involves rpl23, which has resulted in a 2nd copy of this gene in the LSC region of the opposite-leaved species of Jasminum. This gene is normally located between trnI-CAU and rpl2 (supplementary material SM 2, Supplementary Material online) in the IR region (supplementary material SM 3, Supplementary Material online). A very similar duplication of rp123 has been documented in the Poaceae, and in both the Oleaceae and Poaceae, the duplicated copy has been inserted into the intergenic region between rbcL and psaI where accD normally resides. Most cp gene duplications outside of the IR involve tRNAs, and this is certainly the case in the Oleaceae. Several tRNA duplications have occurred, including trnG-GCC in the LSC region of J. nudiflorum (fig. 1), trnI-CAU in the LSC species of Menodora and Jasminum except section Primulina, and trnS-GCU in the LSC region of alternate-leaved species of Jasminum (represented by J. subhumile) (fig. 10). In the Oleaceae, most of these tRNA duplications are associated with other rearrangements in the cp genomes. The mechanism for tRNA duplications is unknown, but they could be caused by duplicative transpositions.
Interruption of trnK-UUU
The most unusual rearrangement in the Oleaceae involves the separation of the 2 exons of trnK-UUU by 59 kb. The cp genomes of Menodora have an interrupted trnK-UUU gene because of an inversion breakpoint within the intron between the matK gene and the trnK-UUU 3′ exon (fig. 3). The only other gene with exons that are separated by such a large distance is rps12, which has 3 exons. The 5′ exon for this gene is located in the LSC region within an operon that includes 2 other genes, clpP and rpl20, and the two 3′ exons are in the IR, approximately 30 kb from the 5′ exon. In several cp genomes, transsplicing has been demonstrated for rps12 to produce a transcript (Fukuzawa et al. 1986; Torazawa et al. 1986; Giese et al. 1987). Separation of the 2 trnK-UUU exons suggests 2 possible alternatives for the functionality of this gene in M. longiflora. Either the gene must be transspliced so that it can function as it does in other cp genomes or the gene is nonfunctional. We have no experimental evidence demonstrating transsplicing of trnK-UUU in Menodora. However, this gene is absent in 2 cp genomes, the fern Adiantum (Wolf et al. 2003) and the holoparasite Epifagus (Wolfe et al. 1992). In both of these genomes, matK, which is encoded in the trnK-UUU intron and is involved in intron splicing of trnK-UUU, is present and functional (Hauser et al. 2006). The situation in Adiantum is very similar to Menodora because a large inversion in the cp genome has one endpoint in the trnK-UUU intron, but in this case the 2 exons were not found (Wolf et al. 2003). In Epifagus, Wolfe et al. (1992) suggested that matK may have an alternative function, which most likely involves splicing of other group II introns (Hauser et al. 2006). Experimental studies of both trnK-UUU and matK are needed to investigate these 2 alternatives in Menodora.
In summary, cpDNA rearrangements in Jasminum and Menodora are confined to several regions of the genome. Gene duplications, insertions of highly repetitive DNA, and gene and intron losses are concentrated between the rbcL and psaI genes and near clpP. These rearrangements also correlate with 4 large inversions in the LSC regions. Overall, the combination of these molecular evolutionary events makes the Jasmineae genomes distinct from all previously examined land plant cp genomes. Duplicated insertions of coding or noncoding sequences into sites where genes or introns have been lost may compensate for the organizational instability caused by these rearrangements. Alternatively, insertions of duplicated elements may be caused by gene or intron losses. Although the genome organization of grasses and the Jasmineae is very similar, losses of major portions of accD and the clpP introns represent parallel evolutionary events. This is also the case for the occurrence of a large inversion that splits the trnK-UUU intron in Menodora and the fern Adiantum.
We thank several botanical gardens for providing plant material, including Kew Gardens, University of Connecticut, Fairchild Tropical Garden, The Royal Botanic Garden, Edinburgh, Arnold Arboretum, and Sisanbana Tropical Garden. All DNA materials were obtained from the plant DNA Bank in Korea. We also thank Mary Guisinger-Bellan, Katie Hansen, and 2 anonymous reviewers for comments on an earlier version of the manuscript. This research was supported by a grant (C00449) from Korea Research Foundation to K.J.K. and a National Science Foundation grant (DEB-0120709) to R.K.J.
Funding to pay the Open Access publication charges for this article was provided by the BK21 School of Life Sciences and Biotechnology Initiative, Korea University, Seoul, Korea.
References
Author notes
Charles Delwiche, Associate Editor



