-
PDF
- Split View
-
Views
-
Cite
Cite
K A Turunen, A Kantele, Professor of Infectious Diseases, Revisiting travellers’ diarrhoea justifying antibiotic treatment: prospective study, Journal of Travel Medicine, Volume 28, Issue 3, April 2021, taaa237, https://doi.org/10.1093/jtm/taaa237
Close - Share Icon Share
Abstract
As antimicrobials increase the risk of acquiring multidrug-resistant (MDR) bacteria, unnecessary antibiotics should be avoided for travellers’ diarrhoea (TD). Antibiotics are recommended in TD accompanied by fever or incapacitation (TD justifying use of antibiotics, TDjuAB). Seeking tools for reducing antibiotic use, we explored factors predisposing to TDjuAB and scrutinized antibiotic treatment among those with TDjuAB [TDjuAB(+) subgroup] and those with diarrhoea not justifying antibiotics [TDjuAB(−) subgroup].
We conducted a study among 370 prospectively recruited visitors to the tropics. Stool samples and questionnaires were collected before and after travel. Enteric pathogens were analysed by qPCR for enteropathogenic (EPEC), enteroaggregative (EAEC), enterotoxigenic (ETEC), enterohaemorrhagic (EHEC) and enteroinvasive (EIEC) E. coli/Shigella, Campylobacter, Salmonella, Yersinia and Vibrio cholerae, and for ETEC’s toxins LT (heat-labile), STh (human heat-stable) and STp (porcine heat-stable). TD was defined by the WHO criteria and TDjuAB as diarrhoea accompanied by fever, and/or disrupting or preventing daily activities. Multivariable analysis was applied—separately for travel-related factors and pathogens—to identify risk factors for TDjuAB(+).
Among the 370 travellers, TD was contracted by 253 (68%), categorized as TDjuAB(+) in 93/253 (37%) and TDjuAB(−) in 160/253 (63%) of the cases. Antibiotics were used for TD by 41% in TDjuAB(+) and by 7% in the TDjuAB(−) group. Relative risk ratios (RRR)s are presented for both the TDjuAB(+) and the TDjuAB(−) groups. TDjuAB(+) was associated with long travel duration and young age. Among the 298 subjects not having taken antibiotics, increased RRRs were found e.g. for findings of Campylobacter coli/jejuni and ETEC’s STh toxin.
The first to analyse risk factors for TDjuAB, our study presents RRRs for demographic and behavioural factors and for various pathogens. Only less than half of those in the TDjuAB(+) group took antibiotics, which demonstrates that most cases meeting the current criteria recover without antimicrobial treatment.
Introduction
Visitors to emerging economies stay in conditions with weak hygiene infrastructure. Their most common health problem is travellers’ diarrhoea (TD). with attack rates varying by destination between 30 and 70%.1–3 Previously, antibiotics were the mainstay of TD treatment,4 but ever since their use was shown to predispose to acquisition of multidrug-resistant (MDR) bacteria,5–9 less liberal antibiotic practices have been recommended. With colonization rates up to 20–70%,10,11 international travellers act as transporters of MDR bacteria, contributing to the spread of antimicrobial resistance (AMR) worldwide.11,12
To prevent MDR acquisition, pre-travel advice should focus on its three leading risk factors: travel to regions with weak hygiene infrastructures,5–7,9,13–17 contracting TD5–9,13,14 and use of antimicrobials.5–9,16 Although travel destination is generally set before seeking pre-travel advice, special attention should be paid to TD prevention and avoidance of unnecessary antibiotics.
Prevention of TD appears to be an ineffective approach: there are no broadly effective vaccines against TD and, as numerous studies report, food, drink and hygiene precautions have only limited or negligible preventive effect.18–28 Indeed, for the time being, restricting antibiotic use appears to be the only realistic means available of reducing the rate of MDR bacteria acquisition. An obvious focus is TD treatment abroad, since up to 86% of all antibiotics are taken there for TD.29,30 It should be emphasized, however, that for patients with high fever or in poor clinical condition, antibiotics are often warranted.31
The current national/international guidelines recommend to consider antibiotics for healthy travellers who have TD with fever or incapacitation31 or those with TD interfering with planned activities.3,32 In a prospective study setting, seeking tools to reduce antibiotic use, we (1) evaluated RRRs of potentially predisposing travel-related factors as well as various TD pathogens with respect to the outcome as TD with symptoms justifying the use of antibiotics, TDjuAB(+), and (2) explored whether all TD cases in this group require antibiotics. We are not aware of any previous studies with this focus.
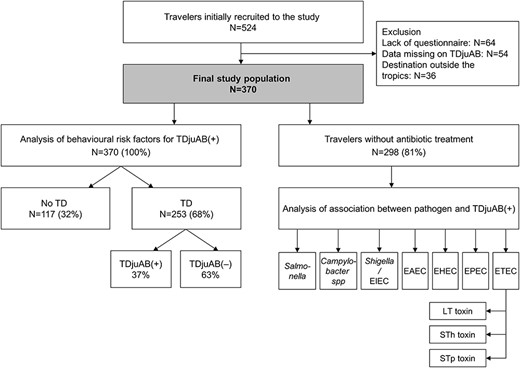
Materials and methods
Study design and subjects
Travellers were recruited prospectively at the Aava Travel Clinic, Helsinki in 2008–10 and asked to fill in pre-travel Q1 (demographics, itinerary) and post-travel Q2 questionnaires (travel-related items, symptoms, antibiotic use) and a health diary (daily TD symptoms, specifics on antibiotic use) while abroad (Figure 1), and provide pre- and post-travel stool samples; for details of the study protocol, see our previous report.6
Definitions
TD was defined by the WHO criteria as passage of three or more loose or liquid stools per day, or more frequently than is normal for the individual.33 TDjuAB was defined as diarrhoea accompanied by fever and/or diarrhoeal disease which disrupts or prevents daily activities [TDjuAB(+) subgroup]. All other types of TD were categorized into the subgroup not justifying antibiotic treatment, TDjuAB(−).
The destinations were recorded into one of six geographic regions as modified from the UN definition (see Table 1).34 Those visiting more than one region were ranked by the highest risk of TD as categorized by Steffen.35
Study among 370 prospectively recruited travellers: univariable and multivariable analyses of demographic factors correlating with the groups TDjuAB(+), TDjuAB(−) and no TD. Multivariable relative risk ratios (RRRs) are for TDjuAB(+) compared to no TD and for TDjuAB(−) compared to no TD. The data from multivariable analysis are presented in Tables 1 and 2
| . | TDjuAB(+) No. (%) . | TDjuAB(−) No. (%) . | No TDa No. (%) . | P-value . | Multivariable RRR (95% CI) for TDjuAB(+)b . | Multivariable RRR (95% CI) for TDjuAB(−)b . |
|---|---|---|---|---|---|---|
| Total, proportions of allc | 93/370 (25) | 160/370 (43) | 117/370 (32) | |||
| Genderc | 0.189 | |||||
| Male | 37 (26) | 54 (38) | 52 (36) | |||
| Female | 56 (25) | 106 (47) | 65 (29) | |||
| Age, median (IQR)cd | 31 (26–43) | 35 (27–55) | 49 (30–60) | <0.001 | 0.98 (0.96–1.00)e | 0.99 (0.98–1.01)e |
| 0–17 | 7/25 (28) | 8/25 (32) | 10/25 (40) | |||
| 18–29 | 34/97 (35) | 46/97 (47) | 17/97 (18) | |||
| 30–54 | 44/155 (28) | 66/155 (43) | 45/155 (29) | |||
| 55–64 | 4/59 (7) | 29/59 (49) | 26/59 (44) | |||
| 65– | 4/34 (12) | 11/34 (32) | 19/34 (56) | |||
| Travel duration, median (IQR)cd | 25 (15–47) | 17 (14–27) | 15 (12–19) | <0.001 | 1.03 (1.01–1.05)f | 1.01 (0.99–1.03)f |
| 4–7 days | 2/7 (29) | 2/7 (29) | 3/7 (43) | |||
| 8–29 days | 53/284 (19) | 130/284 (46) | 101/284 (36) | |||
| 30–160 days | 38/79 (48) | 28/79 (35) | 13/79 (16) | |||
| Geographic regionc | <0.001 | |||||
| South-Eastern Asia | 37/97 (38) | 36/97 (37) | 24/97 (25) | 3.7 (0.8–18.3) | 3.0 (0.8–11.6) | |
| Eastern Africa | 15/87 (17) | 48/87 (55) | 24/87 (28) | 2.0 (0.4–10.1) | 4.5 (1.2–16.8) | |
| Western Africa, Middle Africa | 15/73 (21) | 30/73 (41) | 28/73 (38) | 2.3 (0.4–12.3) | 3.2 (0.8–12.4) | |
| Southern Asia | 18/59 (31) | 29/59 (49) | 12/59 (20) | 3.8 (0.7–20.4) | 5.5 (1.4–22.2) | |
| Latin America and the Caribbean | 5/35 (14) | 12/35 (34) | 18/35 (51) | 1.2 (0.2–7.4) | 1.9 (0.5–8.0) | |
| Southern Africa | 3/19 (16) | 5/19 (26) | 11/19 (58) | 1.0 | 1.0 |
| . | TDjuAB(+) No. (%) . | TDjuAB(−) No. (%) . | No TDa No. (%) . | P-value . | Multivariable RRR (95% CI) for TDjuAB(+)b . | Multivariable RRR (95% CI) for TDjuAB(−)b . |
|---|---|---|---|---|---|---|
| Total, proportions of allc | 93/370 (25) | 160/370 (43) | 117/370 (32) | |||
| Genderc | 0.189 | |||||
| Male | 37 (26) | 54 (38) | 52 (36) | |||
| Female | 56 (25) | 106 (47) | 65 (29) | |||
| Age, median (IQR)cd | 31 (26–43) | 35 (27–55) | 49 (30–60) | <0.001 | 0.98 (0.96–1.00)e | 0.99 (0.98–1.01)e |
| 0–17 | 7/25 (28) | 8/25 (32) | 10/25 (40) | |||
| 18–29 | 34/97 (35) | 46/97 (47) | 17/97 (18) | |||
| 30–54 | 44/155 (28) | 66/155 (43) | 45/155 (29) | |||
| 55–64 | 4/59 (7) | 29/59 (49) | 26/59 (44) | |||
| 65– | 4/34 (12) | 11/34 (32) | 19/34 (56) | |||
| Travel duration, median (IQR)cd | 25 (15–47) | 17 (14–27) | 15 (12–19) | <0.001 | 1.03 (1.01–1.05)f | 1.01 (0.99–1.03)f |
| 4–7 days | 2/7 (29) | 2/7 (29) | 3/7 (43) | |||
| 8–29 days | 53/284 (19) | 130/284 (46) | 101/284 (36) | |||
| 30–160 days | 38/79 (48) | 28/79 (35) | 13/79 (16) | |||
| Geographic regionc | <0.001 | |||||
| South-Eastern Asia | 37/97 (38) | 36/97 (37) | 24/97 (25) | 3.7 (0.8–18.3) | 3.0 (0.8–11.6) | |
| Eastern Africa | 15/87 (17) | 48/87 (55) | 24/87 (28) | 2.0 (0.4–10.1) | 4.5 (1.2–16.8) | |
| Western Africa, Middle Africa | 15/73 (21) | 30/73 (41) | 28/73 (38) | 2.3 (0.4–12.3) | 3.2 (0.8–12.4) | |
| Southern Asia | 18/59 (31) | 29/59 (49) | 12/59 (20) | 3.8 (0.7–20.4) | 5.5 (1.4–22.2) | |
| Latin America and the Caribbean | 5/35 (14) | 12/35 (34) | 18/35 (51) | 1.2 (0.2–7.4) | 1.9 (0.5–8.0) | |
| Southern Africa | 3/19 (16) | 5/19 (26) | 11/19 (58) | 1.0 | 1.0 |
Abbreviations: travellers’ diarrhoea, TD; travellers’ diarrhoea justifying use of antibiotics, TDjuAB(+); travellers’ diarrhoea not justifying antibiotic treatment, TDjuAB(−); no travellers’ diarrhoea, no TD
aTD was defined by WHO diarrhoea criteria (passage of ≥3 loose or liquid stools per day, or more frequently than normal for the individualbFactor with P-value under 0.20 in univariable analyses were chosen to the multivariable model. Multinomial logistic regression was used with multiple imputations (70 data sets). The model included gender, age (continuous), geographic region, travel duration (continuous), location, accommodation, diet, use of utensils, freshwater contact, insect stings, alcohol consumption and hypertension (for hypertension univariable P-value 0.075).
cmissing data 0; danalysed as continuous variable; eper year; fper day
Study among 370 prospectively recruited travellers: univariable and multivariable analyses of demographic factors correlating with the groups TDjuAB(+), TDjuAB(−) and no TD. Multivariable relative risk ratios (RRRs) are for TDjuAB(+) compared to no TD and for TDjuAB(−) compared to no TD. The data from multivariable analysis are presented in Tables 1 and 2
| . | TDjuAB(+) No. (%) . | TDjuAB(−) No. (%) . | No TDa No. (%) . | P-value . | Multivariable RRR (95% CI) for TDjuAB(+)b . | Multivariable RRR (95% CI) for TDjuAB(−)b . |
|---|---|---|---|---|---|---|
| Total, proportions of allc | 93/370 (25) | 160/370 (43) | 117/370 (32) | |||
| Genderc | 0.189 | |||||
| Male | 37 (26) | 54 (38) | 52 (36) | |||
| Female | 56 (25) | 106 (47) | 65 (29) | |||
| Age, median (IQR)cd | 31 (26–43) | 35 (27–55) | 49 (30–60) | <0.001 | 0.98 (0.96–1.00)e | 0.99 (0.98–1.01)e |
| 0–17 | 7/25 (28) | 8/25 (32) | 10/25 (40) | |||
| 18–29 | 34/97 (35) | 46/97 (47) | 17/97 (18) | |||
| 30–54 | 44/155 (28) | 66/155 (43) | 45/155 (29) | |||
| 55–64 | 4/59 (7) | 29/59 (49) | 26/59 (44) | |||
| 65– | 4/34 (12) | 11/34 (32) | 19/34 (56) | |||
| Travel duration, median (IQR)cd | 25 (15–47) | 17 (14–27) | 15 (12–19) | <0.001 | 1.03 (1.01–1.05)f | 1.01 (0.99–1.03)f |
| 4–7 days | 2/7 (29) | 2/7 (29) | 3/7 (43) | |||
| 8–29 days | 53/284 (19) | 130/284 (46) | 101/284 (36) | |||
| 30–160 days | 38/79 (48) | 28/79 (35) | 13/79 (16) | |||
| Geographic regionc | <0.001 | |||||
| South-Eastern Asia | 37/97 (38) | 36/97 (37) | 24/97 (25) | 3.7 (0.8–18.3) | 3.0 (0.8–11.6) | |
| Eastern Africa | 15/87 (17) | 48/87 (55) | 24/87 (28) | 2.0 (0.4–10.1) | 4.5 (1.2–16.8) | |
| Western Africa, Middle Africa | 15/73 (21) | 30/73 (41) | 28/73 (38) | 2.3 (0.4–12.3) | 3.2 (0.8–12.4) | |
| Southern Asia | 18/59 (31) | 29/59 (49) | 12/59 (20) | 3.8 (0.7–20.4) | 5.5 (1.4–22.2) | |
| Latin America and the Caribbean | 5/35 (14) | 12/35 (34) | 18/35 (51) | 1.2 (0.2–7.4) | 1.9 (0.5–8.0) | |
| Southern Africa | 3/19 (16) | 5/19 (26) | 11/19 (58) | 1.0 | 1.0 |
| . | TDjuAB(+) No. (%) . | TDjuAB(−) No. (%) . | No TDa No. (%) . | P-value . | Multivariable RRR (95% CI) for TDjuAB(+)b . | Multivariable RRR (95% CI) for TDjuAB(−)b . |
|---|---|---|---|---|---|---|
| Total, proportions of allc | 93/370 (25) | 160/370 (43) | 117/370 (32) | |||
| Genderc | 0.189 | |||||
| Male | 37 (26) | 54 (38) | 52 (36) | |||
| Female | 56 (25) | 106 (47) | 65 (29) | |||
| Age, median (IQR)cd | 31 (26–43) | 35 (27–55) | 49 (30–60) | <0.001 | 0.98 (0.96–1.00)e | 0.99 (0.98–1.01)e |
| 0–17 | 7/25 (28) | 8/25 (32) | 10/25 (40) | |||
| 18–29 | 34/97 (35) | 46/97 (47) | 17/97 (18) | |||
| 30–54 | 44/155 (28) | 66/155 (43) | 45/155 (29) | |||
| 55–64 | 4/59 (7) | 29/59 (49) | 26/59 (44) | |||
| 65– | 4/34 (12) | 11/34 (32) | 19/34 (56) | |||
| Travel duration, median (IQR)cd | 25 (15–47) | 17 (14–27) | 15 (12–19) | <0.001 | 1.03 (1.01–1.05)f | 1.01 (0.99–1.03)f |
| 4–7 days | 2/7 (29) | 2/7 (29) | 3/7 (43) | |||
| 8–29 days | 53/284 (19) | 130/284 (46) | 101/284 (36) | |||
| 30–160 days | 38/79 (48) | 28/79 (35) | 13/79 (16) | |||
| Geographic regionc | <0.001 | |||||
| South-Eastern Asia | 37/97 (38) | 36/97 (37) | 24/97 (25) | 3.7 (0.8–18.3) | 3.0 (0.8–11.6) | |
| Eastern Africa | 15/87 (17) | 48/87 (55) | 24/87 (28) | 2.0 (0.4–10.1) | 4.5 (1.2–16.8) | |
| Western Africa, Middle Africa | 15/73 (21) | 30/73 (41) | 28/73 (38) | 2.3 (0.4–12.3) | 3.2 (0.8–12.4) | |
| Southern Asia | 18/59 (31) | 29/59 (49) | 12/59 (20) | 3.8 (0.7–20.4) | 5.5 (1.4–22.2) | |
| Latin America and the Caribbean | 5/35 (14) | 12/35 (34) | 18/35 (51) | 1.2 (0.2–7.4) | 1.9 (0.5–8.0) | |
| Southern Africa | 3/19 (16) | 5/19 (26) | 11/19 (58) | 1.0 | 1.0 |
Abbreviations: travellers’ diarrhoea, TD; travellers’ diarrhoea justifying use of antibiotics, TDjuAB(+); travellers’ diarrhoea not justifying antibiotic treatment, TDjuAB(−); no travellers’ diarrhoea, no TD
aTD was defined by WHO diarrhoea criteria (passage of ≥3 loose or liquid stools per day, or more frequently than normal for the individualbFactor with P-value under 0.20 in univariable analyses were chosen to the multivariable model. Multinomial logistic regression was used with multiple imputations (70 data sets). The model included gender, age (continuous), geographic region, travel duration (continuous), location, accommodation, diet, use of utensils, freshwater contact, insect stings, alcohol consumption and hypertension (for hypertension univariable P-value 0.075).
cmissing data 0; danalysed as continuous variable; eper year; fper day
Risk factor analyses of TDjuAB(+)
Seeking tools to avoid TDjuAB, we conducted one multivariable analysis focusing on RRRs for demographic and behavioural patterns and for various pathogen findings associated with TDjuAB(+). To allow simultaneous evaluation of the risk of contracting any TD, we also presented the respective RRRs in the TDjuAB(−) group.
As for hygienic precautions, we created a variable describing adherence to hygiene instructions and explored its association with TDjuAB(+). Compliance was evaluated by enumerating the general hygiene instructions that were followed (from among drinking bottled water, handwashing, avoiding salads, using utensils and eating cooked meat/fish). Those with unanswered questions were excluded from analyses. The rate of TDjuAB(+) was analysed between those having followed 1–3 vs 4–5 instructions.
Stool samples
DNA was isolated on arrival at the laboratory by the semi-automated protocol of easyMAG (bioMérieux, Marcy-l’Etoile, France) and stored at −80°C. The stool pathogens were examined by the previously described qPCR method.36 It covers five diarrhoeagenic Escherichia coli including enteroaggregative (EAEC), enteropathogenic (EPEC), enterotoxigenic (ETEC), enterohaemorrhagic (EHEC), enteroinvasive (EIEC) E. coli or Shigella, and Salmonella, Yersinia, Vibrio cholerae, and Campylobacter coli/jejuni. Samples with ETEC were subjected to PCR analysis of ST and LT toxins as depicted recently.37
Analyses of risk associated with various pathogens
To exclude the confounding impact of the drugs, only travellers not having used antibiotics were taken into our analyses of pathogen-associated risks by comparing the TDjuAB(+) and the TDjuAB(−) each with the no TD group. As an exception, doxycycline as antimalarial was included, since, as continuous medication, it was not expected to cause abrupt changes in the microbiota abroad.
Statistical analysis
For univariable analyses we used Pearson chi-square plus Fisher’s exact tests, and for multivariable analyses, a multinomial logistic regression model. Statistical significance was set to a P-value <0.05. Multivariable models were run for factors correlating with TD variable (TDjuAB(+)/TDjuAB(−)/no TD) with a P-value < 0.20 in univariable analysis. Multiple imputations were conducted to have 70 data sets in multivariable model of behavioural risk factors of TDjuAB(+) and TDjuAB(−), and missing values were assumed to be missing at random (MAR). Bayes’ model was used in pathogen analysis and the selection of final model was done by deviance information criterion (DIC). Two Markov chains were used and the million simulations did converge. In the final multivariable models relative risk ratios (RRR) and 95% confidence intervals plus 95% credible intervals (Bayes) were calculated. SPSS Statistics (version 25.0 64-bit, IBM Corp. in Armonk, NY) and Stata 16 (StataCorp, College Station, TX) were used in statistical analyses.
Results
Study population
As from among the 524 travellers recruited 154 were excluded, the final study population comprised 370 participants (Figure 1), all having provided post-travel stool samples (one of which was discarded for improper handling). In analyses investigating RRRs in the TDjuAB(+) and the TDjuAB(−) group with respect to each pathogen, we included findings of those 298/370 (81%) who had not used antibiotics (except doxycycline as malaria prophylaxis). For demographics, see Supplementary Table S1.
Specifics of TDjuAB(+), TDjuAB(−) and no TD groups
Of the final study population, TD was contracted by 253/370 (68%), categorized as TDjuAB(+) in 93 (37%) and TDjuAB(−) in 160 (63%) cases. In the TDjuAB(+) group, the median travel duration was 25 days and the median age 31 years, in the TDjuAB(−) group 17 days and 35 years, and among those with no TD 15 days and 49 years, respectively. There were no gender differences between the groups (Supplementary Table 1). TD was treated with antibiotics by 38/93 (41%) in the TDjuAB(+) and 11/160 (7%) in the TDjuAB(−) group (Figure 2).
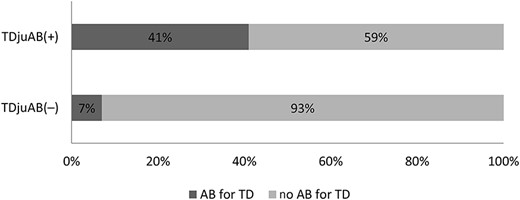
Proportions of travellers with and without antibiotic use for TD in the TDjuAB(+) and TDjuAB(−) groups
Risk factors for TDjuAB(+)
Seeking tools to avoid TDjuAB, we explored the factors associated with TDjuAB(+). To provide data on the risk of milder TD, we also show RRRs in the TDjuAB(−) group.
Univariable analysis of behavioural factors
In univariable analysis comparing the TDjuAB(+), TDjuAB(−), and no TD groups, TDjuAB(+) was associated with age, duration of travel, geographic region (Table 1), type of accommodation, freshwater contact, insect stings, use of probiotics, and healthcare visit (Table 2).
| . | TDjuAB(+) No. (%) . | TDjuAB(−) No. (%) . | No TD No. (%) . | P-value . | Multivariable RRR (95% CI) for TDjuAB(+)a . | Multivariable RRR (95% CI) for TDjuAB(−)a . |
|---|---|---|---|---|---|---|
| Locationb | 0.169 | |||||
| City | 22/102 (22) | 41/102 (40) | 39/102 (38) | |||
| Countryside/jungle | 70/254 (28) | 112/254 (44) | 72/254 (28) | |||
| Accommodationc | 0.002 | |||||
| Hotel | 25/141 (18) | 56/141 (40) | 60/141 (43) | 1.0 | 1.0 | |
| Home of a local | 16/57 (28) | 25/57 (44) | 16/57 (28) | 1.2 (0.5–3.4) | 1.1 (0.5–2.6) | |
| Guest house | 52/165 (32) | 76/165 (46) | 37/165 (22) | 1.6 (0.8–3.4) | 1.6 (0.9–2.9) | |
| Type of toiletc | 0.469 | |||||
| WC as a toilet | 75/303 (25) | 128/303 (42) | 100/303 (33) | |||
| Other type of toilet | 16/60 (27) | 29/60 (48) | 15/60 (25) | |||
| Used other than bottled waterd | 4/19 (21) | 8/19 (42) | 7/19 (37) | 0.851 | ||
| Alcohol consumptione | 0.116 | |||||
| 0–2 units per day | 68/232 (29) | 101/232 (44) | 63/232 (27) | |||
| 3– units per day | 17/85 (20) | 36/85 (42) | 32/85 (38) | |||
| Site of mealsf | 0.853 | |||||
| Restaurant >50% of meals | 79/299 (26) | 126/299 (42) | 94/299 (31) | |||
| Mostly own household | 14/61 (23) | 27/61 (44) | 20/61 (33) | |||
| Ate uncooked meat/fishd | 14/47 (30) | 14/47 (30) | 19/47 (40) | 0.127 | ||
| Did not wash hands always/ofteng | 12/49 (24) | 21/49 (43) | 16/49 (33) | 0.978 | ||
| Ate saladsh | 68/271 (25) | 122/271 (45) | 81/271 (30) | 0.567 | ||
| Dieti | 0.065 | |||||
| Omnivore | 54/232 (23) | 105/232 (45) | 73/232 (31) | 1.0 | 1.0 | |
| Vegetarian | 10/25 (40) | 12/25 (48) | 3/25 (12) | 3.2 (0.9–11.7) | 1.9 (0.5–7.0) | |
| Used milk as part of dieti | 63/252 (25) | 115/252 (46) | 74/252 (29) | 0.859 | ||
| Did not always use utensilsj | 37/107 (35) | 44/107 (41) | 26/107 (24) | 0.029 | ||
| Freshwater contactk | 40/95 (42) | 32/95 (34) | 23/95 (24) | <0.001 | ||
| Walked barefoot often/sometimesl | 74/267 (28) | 111/267 (42) | 82/267 (31) | 0.283 | ||
| Unprotected sex with localm | 3/7 (43) | 3/7 (43) | 1/7 (14) | 0.489 | ||
| Other close contact with localm | 21/67 (31) | 29/67 (43) | 17/67 (25) | 0.369 | ||
| Insect stingsn | 21/67 (31) | 29/67 (43) | 17/67 (25) | 0.024 | ||
| Behavioural advice followedo | 0.287 | |||||
| 1–3 advice | 41/138 (30) | 55/138 (40) | 42/138 (30) | |||
| 4–5 advice | 45/203 (22) | 91/203 (45) | 67/203 (33) | |||
| Probiotics/prebioticsp | 0.007 | |||||
| No | 25/138 (18) | 56/138 (41) | 57/138 (41) | |||
| Before and while travelling | 41/121 (34) | 52/121 (43) | 28/121 (23) | |||
| While abroad | 27/109 (25) | 51/109 (47) | 31/109 (28) | |||
| SBA carriedq | 18/59 (31) | 25/59 (42) | 16/59 (27) | 0.573 | ||
| Healthcare visitr | <0.001 | |||||
| No healthcare visit | 65/326 (20) | 152/326 (47) | 109/326 (33) | |||
| Healthcare visit due to TD | 21/22 (95) | 1/22 (5) | 0/22 (0) | |||
| Healthcare visit for other reason | 7/22 (32) | 7/22 (32) | 8/22 (36) | |||
| Antibiotic user | <0.001 | |||||
| No use of antibiotics | 50/298 (17) | 138/298 (46) | 110/298 (37) | |||
| Antibiotic use for diarrhoea | 38/49 (78) | 11/49 (22) | 0/49 (0) | |||
| Antibiotic use for other reason | 5/23 (22) | 11/23 (48) | 7/23 (30) |
| . | TDjuAB(+) No. (%) . | TDjuAB(−) No. (%) . | No TD No. (%) . | P-value . | Multivariable RRR (95% CI) for TDjuAB(+)a . | Multivariable RRR (95% CI) for TDjuAB(−)a . |
|---|---|---|---|---|---|---|
| Locationb | 0.169 | |||||
| City | 22/102 (22) | 41/102 (40) | 39/102 (38) | |||
| Countryside/jungle | 70/254 (28) | 112/254 (44) | 72/254 (28) | |||
| Accommodationc | 0.002 | |||||
| Hotel | 25/141 (18) | 56/141 (40) | 60/141 (43) | 1.0 | 1.0 | |
| Home of a local | 16/57 (28) | 25/57 (44) | 16/57 (28) | 1.2 (0.5–3.4) | 1.1 (0.5–2.6) | |
| Guest house | 52/165 (32) | 76/165 (46) | 37/165 (22) | 1.6 (0.8–3.4) | 1.6 (0.9–2.9) | |
| Type of toiletc | 0.469 | |||||
| WC as a toilet | 75/303 (25) | 128/303 (42) | 100/303 (33) | |||
| Other type of toilet | 16/60 (27) | 29/60 (48) | 15/60 (25) | |||
| Used other than bottled waterd | 4/19 (21) | 8/19 (42) | 7/19 (37) | 0.851 | ||
| Alcohol consumptione | 0.116 | |||||
| 0–2 units per day | 68/232 (29) | 101/232 (44) | 63/232 (27) | |||
| 3– units per day | 17/85 (20) | 36/85 (42) | 32/85 (38) | |||
| Site of mealsf | 0.853 | |||||
| Restaurant >50% of meals | 79/299 (26) | 126/299 (42) | 94/299 (31) | |||
| Mostly own household | 14/61 (23) | 27/61 (44) | 20/61 (33) | |||
| Ate uncooked meat/fishd | 14/47 (30) | 14/47 (30) | 19/47 (40) | 0.127 | ||
| Did not wash hands always/ofteng | 12/49 (24) | 21/49 (43) | 16/49 (33) | 0.978 | ||
| Ate saladsh | 68/271 (25) | 122/271 (45) | 81/271 (30) | 0.567 | ||
| Dieti | 0.065 | |||||
| Omnivore | 54/232 (23) | 105/232 (45) | 73/232 (31) | 1.0 | 1.0 | |
| Vegetarian | 10/25 (40) | 12/25 (48) | 3/25 (12) | 3.2 (0.9–11.7) | 1.9 (0.5–7.0) | |
| Used milk as part of dieti | 63/252 (25) | 115/252 (46) | 74/252 (29) | 0.859 | ||
| Did not always use utensilsj | 37/107 (35) | 44/107 (41) | 26/107 (24) | 0.029 | ||
| Freshwater contactk | 40/95 (42) | 32/95 (34) | 23/95 (24) | <0.001 | ||
| Walked barefoot often/sometimesl | 74/267 (28) | 111/267 (42) | 82/267 (31) | 0.283 | ||
| Unprotected sex with localm | 3/7 (43) | 3/7 (43) | 1/7 (14) | 0.489 | ||
| Other close contact with localm | 21/67 (31) | 29/67 (43) | 17/67 (25) | 0.369 | ||
| Insect stingsn | 21/67 (31) | 29/67 (43) | 17/67 (25) | 0.024 | ||
| Behavioural advice followedo | 0.287 | |||||
| 1–3 advice | 41/138 (30) | 55/138 (40) | 42/138 (30) | |||
| 4–5 advice | 45/203 (22) | 91/203 (45) | 67/203 (33) | |||
| Probiotics/prebioticsp | 0.007 | |||||
| No | 25/138 (18) | 56/138 (41) | 57/138 (41) | |||
| Before and while travelling | 41/121 (34) | 52/121 (43) | 28/121 (23) | |||
| While abroad | 27/109 (25) | 51/109 (47) | 31/109 (28) | |||
| SBA carriedq | 18/59 (31) | 25/59 (42) | 16/59 (27) | 0.573 | ||
| Healthcare visitr | <0.001 | |||||
| No healthcare visit | 65/326 (20) | 152/326 (47) | 109/326 (33) | |||
| Healthcare visit due to TD | 21/22 (95) | 1/22 (5) | 0/22 (0) | |||
| Healthcare visit for other reason | 7/22 (32) | 7/22 (32) | 8/22 (36) | |||
| Antibiotic user | <0.001 | |||||
| No use of antibiotics | 50/298 (17) | 138/298 (46) | 110/298 (37) | |||
| Antibiotic use for diarrhoea | 38/49 (78) | 11/49 (22) | 0/49 (0) | |||
| Antibiotic use for other reason | 5/23 (22) | 11/23 (48) | 7/23 (30) |
Abbreviations: SBA, stand-by antibiotics
aFactor with P-value < 0.20 in univariable analyses were chosen to the multivariable model. Multinomial logistic regression was used with multiple imputations (70 data sets). The model included gender, age (continuous), geographic region, travel duration (continuous), location, accommodation, diet, use of utensils, freshwater contact, insect stings, alcohol consumption and hypertension (for hypertension univariable P-value 0.075).
bMissing data 14; cmissing data 7; dmissing data 3; emissing data 53; fmissing data 10; gmissing data 8; hmissing data 18; imissing data 113; jmissing data 15; kmissing data 126; lmissing data 6; mmissing data 19; nmissing data 16; omissing data 29; pmissing data 2; qmissing data 69; rmissing data 0
| . | TDjuAB(+) No. (%) . | TDjuAB(−) No. (%) . | No TD No. (%) . | P-value . | Multivariable RRR (95% CI) for TDjuAB(+)a . | Multivariable RRR (95% CI) for TDjuAB(−)a . |
|---|---|---|---|---|---|---|
| Locationb | 0.169 | |||||
| City | 22/102 (22) | 41/102 (40) | 39/102 (38) | |||
| Countryside/jungle | 70/254 (28) | 112/254 (44) | 72/254 (28) | |||
| Accommodationc | 0.002 | |||||
| Hotel | 25/141 (18) | 56/141 (40) | 60/141 (43) | 1.0 | 1.0 | |
| Home of a local | 16/57 (28) | 25/57 (44) | 16/57 (28) | 1.2 (0.5–3.4) | 1.1 (0.5–2.6) | |
| Guest house | 52/165 (32) | 76/165 (46) | 37/165 (22) | 1.6 (0.8–3.4) | 1.6 (0.9–2.9) | |
| Type of toiletc | 0.469 | |||||
| WC as a toilet | 75/303 (25) | 128/303 (42) | 100/303 (33) | |||
| Other type of toilet | 16/60 (27) | 29/60 (48) | 15/60 (25) | |||
| Used other than bottled waterd | 4/19 (21) | 8/19 (42) | 7/19 (37) | 0.851 | ||
| Alcohol consumptione | 0.116 | |||||
| 0–2 units per day | 68/232 (29) | 101/232 (44) | 63/232 (27) | |||
| 3– units per day | 17/85 (20) | 36/85 (42) | 32/85 (38) | |||
| Site of mealsf | 0.853 | |||||
| Restaurant >50% of meals | 79/299 (26) | 126/299 (42) | 94/299 (31) | |||
| Mostly own household | 14/61 (23) | 27/61 (44) | 20/61 (33) | |||
| Ate uncooked meat/fishd | 14/47 (30) | 14/47 (30) | 19/47 (40) | 0.127 | ||
| Did not wash hands always/ofteng | 12/49 (24) | 21/49 (43) | 16/49 (33) | 0.978 | ||
| Ate saladsh | 68/271 (25) | 122/271 (45) | 81/271 (30) | 0.567 | ||
| Dieti | 0.065 | |||||
| Omnivore | 54/232 (23) | 105/232 (45) | 73/232 (31) | 1.0 | 1.0 | |
| Vegetarian | 10/25 (40) | 12/25 (48) | 3/25 (12) | 3.2 (0.9–11.7) | 1.9 (0.5–7.0) | |
| Used milk as part of dieti | 63/252 (25) | 115/252 (46) | 74/252 (29) | 0.859 | ||
| Did not always use utensilsj | 37/107 (35) | 44/107 (41) | 26/107 (24) | 0.029 | ||
| Freshwater contactk | 40/95 (42) | 32/95 (34) | 23/95 (24) | <0.001 | ||
| Walked barefoot often/sometimesl | 74/267 (28) | 111/267 (42) | 82/267 (31) | 0.283 | ||
| Unprotected sex with localm | 3/7 (43) | 3/7 (43) | 1/7 (14) | 0.489 | ||
| Other close contact with localm | 21/67 (31) | 29/67 (43) | 17/67 (25) | 0.369 | ||
| Insect stingsn | 21/67 (31) | 29/67 (43) | 17/67 (25) | 0.024 | ||
| Behavioural advice followedo | 0.287 | |||||
| 1–3 advice | 41/138 (30) | 55/138 (40) | 42/138 (30) | |||
| 4–5 advice | 45/203 (22) | 91/203 (45) | 67/203 (33) | |||
| Probiotics/prebioticsp | 0.007 | |||||
| No | 25/138 (18) | 56/138 (41) | 57/138 (41) | |||
| Before and while travelling | 41/121 (34) | 52/121 (43) | 28/121 (23) | |||
| While abroad | 27/109 (25) | 51/109 (47) | 31/109 (28) | |||
| SBA carriedq | 18/59 (31) | 25/59 (42) | 16/59 (27) | 0.573 | ||
| Healthcare visitr | <0.001 | |||||
| No healthcare visit | 65/326 (20) | 152/326 (47) | 109/326 (33) | |||
| Healthcare visit due to TD | 21/22 (95) | 1/22 (5) | 0/22 (0) | |||
| Healthcare visit for other reason | 7/22 (32) | 7/22 (32) | 8/22 (36) | |||
| Antibiotic user | <0.001 | |||||
| No use of antibiotics | 50/298 (17) | 138/298 (46) | 110/298 (37) | |||
| Antibiotic use for diarrhoea | 38/49 (78) | 11/49 (22) | 0/49 (0) | |||
| Antibiotic use for other reason | 5/23 (22) | 11/23 (48) | 7/23 (30) |
| . | TDjuAB(+) No. (%) . | TDjuAB(−) No. (%) . | No TD No. (%) . | P-value . | Multivariable RRR (95% CI) for TDjuAB(+)a . | Multivariable RRR (95% CI) for TDjuAB(−)a . |
|---|---|---|---|---|---|---|
| Locationb | 0.169 | |||||
| City | 22/102 (22) | 41/102 (40) | 39/102 (38) | |||
| Countryside/jungle | 70/254 (28) | 112/254 (44) | 72/254 (28) | |||
| Accommodationc | 0.002 | |||||
| Hotel | 25/141 (18) | 56/141 (40) | 60/141 (43) | 1.0 | 1.0 | |
| Home of a local | 16/57 (28) | 25/57 (44) | 16/57 (28) | 1.2 (0.5–3.4) | 1.1 (0.5–2.6) | |
| Guest house | 52/165 (32) | 76/165 (46) | 37/165 (22) | 1.6 (0.8–3.4) | 1.6 (0.9–2.9) | |
| Type of toiletc | 0.469 | |||||
| WC as a toilet | 75/303 (25) | 128/303 (42) | 100/303 (33) | |||
| Other type of toilet | 16/60 (27) | 29/60 (48) | 15/60 (25) | |||
| Used other than bottled waterd | 4/19 (21) | 8/19 (42) | 7/19 (37) | 0.851 | ||
| Alcohol consumptione | 0.116 | |||||
| 0–2 units per day | 68/232 (29) | 101/232 (44) | 63/232 (27) | |||
| 3– units per day | 17/85 (20) | 36/85 (42) | 32/85 (38) | |||
| Site of mealsf | 0.853 | |||||
| Restaurant >50% of meals | 79/299 (26) | 126/299 (42) | 94/299 (31) | |||
| Mostly own household | 14/61 (23) | 27/61 (44) | 20/61 (33) | |||
| Ate uncooked meat/fishd | 14/47 (30) | 14/47 (30) | 19/47 (40) | 0.127 | ||
| Did not wash hands always/ofteng | 12/49 (24) | 21/49 (43) | 16/49 (33) | 0.978 | ||
| Ate saladsh | 68/271 (25) | 122/271 (45) | 81/271 (30) | 0.567 | ||
| Dieti | 0.065 | |||||
| Omnivore | 54/232 (23) | 105/232 (45) | 73/232 (31) | 1.0 | 1.0 | |
| Vegetarian | 10/25 (40) | 12/25 (48) | 3/25 (12) | 3.2 (0.9–11.7) | 1.9 (0.5–7.0) | |
| Used milk as part of dieti | 63/252 (25) | 115/252 (46) | 74/252 (29) | 0.859 | ||
| Did not always use utensilsj | 37/107 (35) | 44/107 (41) | 26/107 (24) | 0.029 | ||
| Freshwater contactk | 40/95 (42) | 32/95 (34) | 23/95 (24) | <0.001 | ||
| Walked barefoot often/sometimesl | 74/267 (28) | 111/267 (42) | 82/267 (31) | 0.283 | ||
| Unprotected sex with localm | 3/7 (43) | 3/7 (43) | 1/7 (14) | 0.489 | ||
| Other close contact with localm | 21/67 (31) | 29/67 (43) | 17/67 (25) | 0.369 | ||
| Insect stingsn | 21/67 (31) | 29/67 (43) | 17/67 (25) | 0.024 | ||
| Behavioural advice followedo | 0.287 | |||||
| 1–3 advice | 41/138 (30) | 55/138 (40) | 42/138 (30) | |||
| 4–5 advice | 45/203 (22) | 91/203 (45) | 67/203 (33) | |||
| Probiotics/prebioticsp | 0.007 | |||||
| No | 25/138 (18) | 56/138 (41) | 57/138 (41) | |||
| Before and while travelling | 41/121 (34) | 52/121 (43) | 28/121 (23) | |||
| While abroad | 27/109 (25) | 51/109 (47) | 31/109 (28) | |||
| SBA carriedq | 18/59 (31) | 25/59 (42) | 16/59 (27) | 0.573 | ||
| Healthcare visitr | <0.001 | |||||
| No healthcare visit | 65/326 (20) | 152/326 (47) | 109/326 (33) | |||
| Healthcare visit due to TD | 21/22 (95) | 1/22 (5) | 0/22 (0) | |||
| Healthcare visit for other reason | 7/22 (32) | 7/22 (32) | 8/22 (36) | |||
| Antibiotic user | <0.001 | |||||
| No use of antibiotics | 50/298 (17) | 138/298 (46) | 110/298 (37) | |||
| Antibiotic use for diarrhoea | 38/49 (78) | 11/49 (22) | 0/49 (0) | |||
| Antibiotic use for other reason | 5/23 (22) | 11/23 (48) | 7/23 (30) |
Abbreviations: SBA, stand-by antibiotics
aFactor with P-value < 0.20 in univariable analyses were chosen to the multivariable model. Multinomial logistic regression was used with multiple imputations (70 data sets). The model included gender, age (continuous), geographic region, travel duration (continuous), location, accommodation, diet, use of utensils, freshwater contact, insect stings, alcohol consumption and hypertension (for hypertension univariable P-value 0.075).
bMissing data 14; cmissing data 7; dmissing data 3; emissing data 53; fmissing data 10; gmissing data 8; hmissing data 18; imissing data 113; jmissing data 15; kmissing data 126; lmissing data 6; mmissing data 19; nmissing data 16; omissing data 29; pmissing data 2; qmissing data 69; rmissing data 0
Over half (60%) of all participants complied with four to five hygiene instructions; hardly any differences were seen between the three subgroups: 52% in the group TDjuAB(+), 62% in TDjuAB(−), and 61% in no TD (P = 0.287; Table 2).
Multivariable analysis of demographics and behavioural factors
For multivariable analysis we selected factors that showed in univariable analysis a P-value < 0.2 for correlation with TDjuAB variable (Tables 1 and 2). Comparisons were made between the groups TDjuAB(+) vs no TD and TDjuAB(−) vs no TD. As factors with increased risk in the TDjuAB(+) group, we identified longer travel duration (RRR/day 1.028; 95% 1.009–1.048) and younger age (RRR/year 0.979; 95% 0.961–0.998). Vegetarian diet may also be linked with TDjuAB(+) (RRR 3.2; 95% 0.85–12) as well as Southern Asia (RRR 3.8; 95% 0.71–20) and South-eastern Asia as destination (RRR 3.7; 95% 0.75–18; compared to Southern Africa). Increased RRRs were also found in the TDjuAB(−) group as shown in Tables 1 and 2.
Stool bacteria associated with TDjuAB(+)
Among the travellers who had not taken antibiotics, 50/298 (17%) belonged to the subgroup TDjuAB(+), 138/298 (46%) to TDjuAB(−) and 110/298 (37%) to no TD. The stool findings were compared between non-antibiotic users of TDjuAB(+) vs no TD and TDjuAB(−) vs no TD groups.
In univariable analyses an increased RRR was found in the TDjuAB(+) group for Campylobacter coli/jejuni (8/25; 32%; P = 0.048) and ETEC’s toxin STh (STh; 6/11; 55%; P = 0.004) (Table 3). All travellers with STh ETEC and all except one with Campylobacter coli/jejuni had TD. Likewise, all travellers with Shigella/EIEC had TD, yet their number was low and statistical significance was not reached.
Univariable and multivariable model on pathogen findings correlating with TDjuAB(+), TDjuAB(−), and no TD among 298 travellers without antimicrobial treatment (doxycycline excluded)
| . | TDjuAB(+) No. (%) . | TDjuAB(−) No. (%) . | No TD No. (%) . | P-value . | Multivariable RRR (95% CI) for TDjuAB(+)a . | Multivariable RRR (95% CI) for TDjuAB(−)b . |
|---|---|---|---|---|---|---|
| Total, proportions of all | 50/298 (17) | 138/298 (46) | 110/298 (37) | |||
| Salmonellac | 1/6 (17) | 2/6 (33) | 3/6 (50) | 0.861 | ||
| Campylobacterc | 8/25(32) | 16/25(64) | 1/25 (4) | 0.001 | 85 (3.2–460) | 62 (2.8–340) |
| Shigella/EIECc | 2/4 (50) | 2/4 (50) | 0/4 (0) | 0.126 | ||
| EAECc | 25/136 (18) | 74/136 (54) | 37/136 (27) | 0.007 | 2.3 (1.03–4.6) | 2.4 (1.3–4.0) |
| EHECc | 7/25 (28) | 11/25 (44) | 7/25 (28) | 0.270 | ||
| EPECc | 22/148 (15) | 71/148 (48) | 55/148 (37) | 0.656 | ||
| ETECc | 13/68 (19) | 43/68 (63) | 12/68 (18) | 0.001 | ||
| LTd | 10/52 (19) | 32/52 (62) | 10/52 (19) | 0.013 | 1.7 (0.49–4.5) | 2.5 (1.04–5.5) |
| SThd | 6/11 (55) | 5/11 (45) | 0/11 (0) | 0.002 | 15 000 (12–120 000) | 2500 (2.5–18 000) |
| STpd | 4/19 (21) | 12/19 (63) | 3/19 (16) | 0.144 | ||
| Any pathogenc | 41/233 (18) | 117/233 (50) | 75/233 (32) | 0.008 | ||
| Multiple pathogensc | 26/130 (20) | 71/130 (55) | 33/130 (30) | 0.002 |
| . | TDjuAB(+) No. (%) . | TDjuAB(−) No. (%) . | No TD No. (%) . | P-value . | Multivariable RRR (95% CI) for TDjuAB(+)a . | Multivariable RRR (95% CI) for TDjuAB(−)b . |
|---|---|---|---|---|---|---|
| Total, proportions of all | 50/298 (17) | 138/298 (46) | 110/298 (37) | |||
| Salmonellac | 1/6 (17) | 2/6 (33) | 3/6 (50) | 0.861 | ||
| Campylobacterc | 8/25(32) | 16/25(64) | 1/25 (4) | 0.001 | 85 (3.2–460) | 62 (2.8–340) |
| Shigella/EIECc | 2/4 (50) | 2/4 (50) | 0/4 (0) | 0.126 | ||
| EAECc | 25/136 (18) | 74/136 (54) | 37/136 (27) | 0.007 | 2.3 (1.03–4.6) | 2.4 (1.3–4.0) |
| EHECc | 7/25 (28) | 11/25 (44) | 7/25 (28) | 0.270 | ||
| EPECc | 22/148 (15) | 71/148 (48) | 55/148 (37) | 0.656 | ||
| ETECc | 13/68 (19) | 43/68 (63) | 12/68 (18) | 0.001 | ||
| LTd | 10/52 (19) | 32/52 (62) | 10/52 (19) | 0.013 | 1.7 (0.49–4.5) | 2.5 (1.04–5.5) |
| SThd | 6/11 (55) | 5/11 (45) | 0/11 (0) | 0.002 | 15 000 (12–120 000) | 2500 (2.5–18 000) |
| STpd | 4/19 (21) | 12/19 (63) | 3/19 (16) | 0.144 | ||
| Any pathogenc | 41/233 (18) | 117/233 (50) | 75/233 (32) | 0.008 | ||
| Multiple pathogensc | 26/130 (20) | 71/130 (55) | 33/130 (30) | 0.002 |
aReference category no TD. Variables with P < 0.20 were chosen to the multivariable model: Campylobacter coli/jejuni, Shigella/EIEC, EAEC, LT, STh, STp, gender (priori), age (continuous, standard deviation, priori) and travel duration (continuous, standard deviation, priori). Result for age RRR 0.67 (95% CI 0.44–0.96) and for travel duration RRR 1.9 (95% CI 1.05–3.2).
bReference category no TD. Variables with P < 0.20 were chosen to the multivariable model: Campylobacter coli/jejuni, Shigella/EIEC, EAEC, LT, STh, STp, gender (priori), age (continuous, standard deviation, priori) and travel duration (continuous, standard deviation, priori). Result for age RRR 0.73 (95% CI 0.55–0.96) and for travel duration RRR 1.4 (95% CI 0.86–2.4)
cmissing data 1; dmissing data 4
Univariable and multivariable model on pathogen findings correlating with TDjuAB(+), TDjuAB(−), and no TD among 298 travellers without antimicrobial treatment (doxycycline excluded)
| . | TDjuAB(+) No. (%) . | TDjuAB(−) No. (%) . | No TD No. (%) . | P-value . | Multivariable RRR (95% CI) for TDjuAB(+)a . | Multivariable RRR (95% CI) for TDjuAB(−)b . |
|---|---|---|---|---|---|---|
| Total, proportions of all | 50/298 (17) | 138/298 (46) | 110/298 (37) | |||
| Salmonellac | 1/6 (17) | 2/6 (33) | 3/6 (50) | 0.861 | ||
| Campylobacterc | 8/25(32) | 16/25(64) | 1/25 (4) | 0.001 | 85 (3.2–460) | 62 (2.8–340) |
| Shigella/EIECc | 2/4 (50) | 2/4 (50) | 0/4 (0) | 0.126 | ||
| EAECc | 25/136 (18) | 74/136 (54) | 37/136 (27) | 0.007 | 2.3 (1.03–4.6) | 2.4 (1.3–4.0) |
| EHECc | 7/25 (28) | 11/25 (44) | 7/25 (28) | 0.270 | ||
| EPECc | 22/148 (15) | 71/148 (48) | 55/148 (37) | 0.656 | ||
| ETECc | 13/68 (19) | 43/68 (63) | 12/68 (18) | 0.001 | ||
| LTd | 10/52 (19) | 32/52 (62) | 10/52 (19) | 0.013 | 1.7 (0.49–4.5) | 2.5 (1.04–5.5) |
| SThd | 6/11 (55) | 5/11 (45) | 0/11 (0) | 0.002 | 15 000 (12–120 000) | 2500 (2.5–18 000) |
| STpd | 4/19 (21) | 12/19 (63) | 3/19 (16) | 0.144 | ||
| Any pathogenc | 41/233 (18) | 117/233 (50) | 75/233 (32) | 0.008 | ||
| Multiple pathogensc | 26/130 (20) | 71/130 (55) | 33/130 (30) | 0.002 |
| . | TDjuAB(+) No. (%) . | TDjuAB(−) No. (%) . | No TD No. (%) . | P-value . | Multivariable RRR (95% CI) for TDjuAB(+)a . | Multivariable RRR (95% CI) for TDjuAB(−)b . |
|---|---|---|---|---|---|---|
| Total, proportions of all | 50/298 (17) | 138/298 (46) | 110/298 (37) | |||
| Salmonellac | 1/6 (17) | 2/6 (33) | 3/6 (50) | 0.861 | ||
| Campylobacterc | 8/25(32) | 16/25(64) | 1/25 (4) | 0.001 | 85 (3.2–460) | 62 (2.8–340) |
| Shigella/EIECc | 2/4 (50) | 2/4 (50) | 0/4 (0) | 0.126 | ||
| EAECc | 25/136 (18) | 74/136 (54) | 37/136 (27) | 0.007 | 2.3 (1.03–4.6) | 2.4 (1.3–4.0) |
| EHECc | 7/25 (28) | 11/25 (44) | 7/25 (28) | 0.270 | ||
| EPECc | 22/148 (15) | 71/148 (48) | 55/148 (37) | 0.656 | ||
| ETECc | 13/68 (19) | 43/68 (63) | 12/68 (18) | 0.001 | ||
| LTd | 10/52 (19) | 32/52 (62) | 10/52 (19) | 0.013 | 1.7 (0.49–4.5) | 2.5 (1.04–5.5) |
| SThd | 6/11 (55) | 5/11 (45) | 0/11 (0) | 0.002 | 15 000 (12–120 000) | 2500 (2.5–18 000) |
| STpd | 4/19 (21) | 12/19 (63) | 3/19 (16) | 0.144 | ||
| Any pathogenc | 41/233 (18) | 117/233 (50) | 75/233 (32) | 0.008 | ||
| Multiple pathogensc | 26/130 (20) | 71/130 (55) | 33/130 (30) | 0.002 |
aReference category no TD. Variables with P < 0.20 were chosen to the multivariable model: Campylobacter coli/jejuni, Shigella/EIEC, EAEC, LT, STh, STp, gender (priori), age (continuous, standard deviation, priori) and travel duration (continuous, standard deviation, priori). Result for age RRR 0.67 (95% CI 0.44–0.96) and for travel duration RRR 1.9 (95% CI 1.05–3.2).
bReference category no TD. Variables with P < 0.20 were chosen to the multivariable model: Campylobacter coli/jejuni, Shigella/EIEC, EAEC, LT, STh, STp, gender (priori), age (continuous, standard deviation, priori) and travel duration (continuous, standard deviation, priori). Result for age RRR 0.73 (95% CI 0.55–0.96) and for travel duration RRR 1.4 (95% CI 0.86–2.4)
cmissing data 1; dmissing data 4
In multivariable analysis, STh ETEC (RRR 15000, 95% CI 12–120 000), Campylobacter coli/jejuni (RRR 85, 95% CI 3.2–460), and EAEC (RRR 2.3, 95% CI 1.02–4.6) were associated with TDjuAB(+) (Table 3). They were also associated with TDjuAB(−), the RRRs provided in Table 3. It is noteworthy that the RRR for EAEC was very similar in the TDjuAB(+) and the TDjuAB(−) groups, whereas for the first two pathogens, there appeared to be a difference. Priors for the parameters gender, age and travel duration were non-informative. Behavioural factors were added to the model, yet they were conditional to the pathogens and no longer associated with the outcome, thus the direct effect of behavioural factors did not predict the severity of TD, whereas pathogens did.
Discussion
As antibiotic use is a major factor predisposing travellers to acquisition of MDR bacteria,5–9,16 we sought means to decrease the consumption. While abroad, antibiotics are mainly used for TD.29,30 Therefore, so as to provide tools to reduce antibiotic use for TD, we (i) conducted a multivariable analysis evaluating the RRRs of potential factors predisposing to TDjuAB, and in addition, (ii), to see if antibiotics are always needed in TDjuAB, we explored whether part of travellers with TDjuAB actually could manage without antibiotics. Not only did we evaluate the RRRs for various factors potentially contributing to TDjuAB, but we also observed that antibiotics were not always needed for TDjuAB.
Definitions and recommendations for antibiotic use
The recommendations concerning indications for antibiotic use vary somewhat. As an example from North America, the recommendation of the US Center for Disease control and Prevention (CDC) advise that ‘Antibiotics should be used to treat severe travelers’ diarrhea’. Severe TD is defined as ‘diarrhea that is incapacitating or completely prevents planned activities; all dysentery ––.3 In Europe, the National Health Service (NHS) Scotland recommends antibiotics to be considered only for severe diarrhoea, ‘depending on the cause’. Severe diarrhoea is defined as a disease with > 6 diarrhoea stools/24 h which causes incapacitation, or there is blood/mucus in the stools, or marked vomiting, fever, and/or stomach ache.31 An expert panel supported by the International Society of Travel Medicine (ISTM) states that antibiotics can be considered for moderate TD and they should be used for severe TD, which is defined as in CDC’s recommendation.32 TD justifying use of antibiotics as used in the present study—denoting incapacitating diarrhoeal disease and/or TD accompanied with fever—was included both in the NHS listing when to consider antibiotic treatment and the CDC and ISTM recommendation for when antibiotics should be used for TD. The present study yielded findings described below which favour the former recommendation.
Rate of TDjuAB(+) and antibiotic use
The TDjuAB(+) rate (37%) among our travellers with diarrhoea accords with earlier studies. Among 784 US travellers, Hill reports of 35% of those with TD having to alter their travel plans.29 Similarly, Soonawala et al. found that 33% of Dutch travellers with TD had to change their programme or were confined to accommodation.38 Thus, these three studies suggest that if all those with disabling disease would resort to antibiotics, every third traveller with TD would use antibiotics, which would imply for 2019—before Covid-19 outbreak—300 million travellers with TD (600 million visitors to emerging economies39 with 50% TD frequency) around 100 million antibiotic courses.
Our data clearly show that antibiotics are not necessary in all cases of disabling diarrhoea: only 41% used the drugs for TDjuAB. In other words, most of our participants with disabling TD managed without antibiotics. Our results appear to accord with those of Lalani et al., who report among 123 travellers with moderate/severe TD an antibiotic consumption rate of 41%.40 However, their definition of moderate/severe TD covers acute watery diarrhoea with decreased ability/complete inability to participate in daily activities, whereas in our study the term TDjuAB only refers to severe TD. Therefore, the results of these two studies cannot be directly compared. Even considerably lower antibiotic use rates for severe TD (10%) are reported by Belderok et al. who define severe TD differently (diarrhoea with blood and/or mucus),41 the findings thus not quite comparable with ours. However, as all these data show antibiotics not to be needed in all cases of severe/incapacitating disease, the guidelines stating that antibiotics can be considered31 appear more reasonable than those recommending they should be used.32 It appears justified that the guidelines be revised accordingly, i.e. to heed this data calling for stricter, more prudent use of antibiotics.
In addition to data demonstrating that severe TD can often be managed without antibiotics, more precise recommendations are also grounded by studies questioning their actual benefits in TD.42 Genton et al., by extrapolating the Cochrane data on antibiotic treatment of TD43 into general traveller population, conclude that three of six travellers with TD recover without antibiotics within 72 h, whereas if all six were treated, four will be cured, but at the cost of an adverse event to one traveller.42 When antibiotics are taken, the median post-treatment diarrhoea is 0.7–1.5 days shorter, and the number of unformed stools per 24 h marginally lower (0–24 h − 1.6, 25–48 h − 2.1, and 49–72 h − 1.4), yet with higher incidence (OR 2.4) of adverse effects.43 They conclude that recommendations ‘rely on low-quality evidence and are based on expert opinions or – even worse – on commercial interest’.42
We only found one prospective investigation, a military cohort study, looking at antibiotic treatment of moderate/severe TD.40 It reports no difference between the antibiotic treatment and non-treatment groups regarding time to last unformed stool (TLUS) (P = 0.97), clinical cure at 24 h (P = 0.92), or clinical cure at 48 h (P = 0.70). Rather, nausea was experienced by 30% of travellers with moderate/severe TD using antibiotics plus loperamide, compared to 0% of those only taking loperamide (P = 0.005); for vomiting, the respective rates were 14% and 0% (P = 0.15). The poor efficacy of antibiotics in severe TD may at least partly be related to viral aetiology often linked to an exceptionally vigorous clinical picture.44–47 Clinical decision-making frequently has to rely on clinical picture—in lack of aetiological data, antimicrobials are warranted in the most severe cases.
It is not rare that travel medicine practitioners prescribe stand-by antimicrobials (SBA) as a precaution for (severe) TD. Unfortunately, however, prescription of SBA appears to lead to overuse of antibiotics: we recently reported that those carrying SBA resorted to them even in cases of mild/moderate diarrhoea, whereas those not carrying SBA mainly took antibiotics for severe disease.48 As a recent development, the rate of SBA prescriptions has decreased in the USA over the past decade.49
Behavioural risk factors for TDjuAB(+)
To get an overall picture of factors associated with any type of TD, the RRRs of both the TDjuAB(+) and the TDjuAB(−) group should be considered. However, as the present study is primarily concerned with need for antibiotics as presumed in the guidelines, the following discussion only looks at TDjuAB(+). For those interested in TDjuAB(−), the Tables provide the respective figures.
Multivariable analysis showed TDjuAB(+) to be associated with long travel duration and younger age. Vegetarian diet and Southern Asia plus South-eastern Asia as destinations also appeared as risk factors, but did not reach statistical significance. Although we found no previous studies exploring the risk factors for TDjuAB(+), longer duration of travel and younger age have been shown in several investigations to be risk factors for TD in general.23,29,50–54 The association between TDjuAB(+) and Southern Asia plus South-Eastern Asia may be linked to the high TD risk in these destinations.29,38,54 Although our results suggest vegetarian diet to be a risk factor of TDjuAB(+) (RRR 3.2; 95% CI 0.9–11.7; P = 0.085), we found no data in the literature to support this. Such a finding might, however, be explained by the relatively large proportion of uncooked food in the vegetarian diet.
We found no strong correlation between behavioural factors and TDjuAB(+). According with this, numerous studies searching for tools to prevent TD have failed to prove the benefits of hygienic and food/drink precautions.18–28 Mattila et al. found no significant association between dietary errors and TD.21 In the investigation by Cavalcanti et al. no single food or beverage item could be linked to increased risk of diarrhoea.23 Hillel et al., report among long-term travellers that adherence to WHO’s precautions (avoiding unsafe food and drinks) did not correlate with occurrence of diarrhoea.25 Likewise, in the study by Dia et al. food hygiene did not prove efficient in preventing diarrhoea.27 Interestingly, the oral cholera vaccine Dukoral® has in some countries as indication prevention of both cholera and TD: oral B subunit—whole-cell cholera vaccine induces a cross-reactive immune response to LT toxin of ETEC and has shown some efficacy against ETEC TD in a field trial in Morocco.55 Furthermore, investigation into the composition of microbiota has been suggested to provide tools for treatment and prevention of TD.56
Association between TDjuAB(+) and various microbes
A possible association between the nine individual stool pathogens or the three different ETEC (by toxin type) and TDjuAB(+) was of special interest. Campylobacter coli/jejuni, STh ETEC, and EAEC were found to be associated with TDjuAB(+). However, the RRRs for EAEC were similar in the TDjuAB(+) and the TDjuAB(−) group, whereas for Campylobacter and STh ETEC, the RRRs appeared higher in the TDjuAB(+) group. The link between STh ETEC and TDjuAB(+) proved particularly strong: all of those with STh had TD; TDjuAB(+) 55%, TDjuAB(−) 45%.
Our data accord with previous literature. Sanders et al. show Campylobacter coli/jejuni to be associated with severe TD and decreased functional ability among military personnel.57 Likewise, in Mattila’s investigation among tourists to Morocco, those with diarrhoea caused by Campylobacter spp had the most severe disease.58 In two paediatric studies STh ETEC proved more pathogenic than STp ETEC59,60 and in one STh was the most common toxin among hospitalized children with diarrhoea.61 Liu et al. investigating moderate to severe diarrhoea among African and Asian children under 5 years found Shigella spp, rotavirus, adenovirus 40/41, ST-ETEC, Cryptosporidium spp, and Campylobacter spp to be the six major attributable pathogens.62
We have earlier reported no association between pathogens and severity of TD among travellers with symptoms ongoing at return.63 Differing definitions may account for differing results: instead of incapacitation used in the current study, the earlier study categorized TD by number of stools.63 If judged by travellers’ antibiotic use for TD, the experience of severity appears to be connected with incapacitation rather than number of stools.48
Limitations
Some limitations of the study deserve to be discussed. First, some subgroup analyses were not powered enough to yield reliable statistics (e.g. the low number of Shigella/EIEC cases). Second, the same samples may have contained other pathogen findings influencing the interpretation of association between pathogen findings and TDjuAB(+). Indeed, analyses of bacterial pathogens often showed multiple findings, whereas parasites and viruses were not covered.
Conclusion
About one third of the travellers with diarrhoea had TD justifying antibiotic treatment. Although the factors associated with TDjuAB(+) (e.g. Campylobacter coli/jejuni and STh ETEC as pathogens plus longer journey duration and younger age as predisposing factors) may not offer any remarkable novel tools for pre-travel counselling, the result that over half of those with TDjuAB(+) managed without antibiotics bears considerable weight. In addition to indicating that the current categorization of TDjuAB is invalid, these data show that treatment guidelines should be revised and a stricter definition of cases justifying antibiotic treatment should be introduced.
Abbreviations
- TD
travellers’ diarrhoea
- TDjuAB
TD justifying use of antibiotics
- ETEC
enterotoxigenic Escherichia coli
- LT
heat-labile toxin
- ST
heat-stable toxin
- STh
human heat-stable toxin
- STp
porcine heat-stable toxin
- SBA
stand-by antimicrobials
- AMR
antimicrobial resistance
- MDR
multidrug-resistant bacteria
- md
missing data
Authors’ contributions
Study concept and design was performed by AK; acquisition of data was done by KT and AK; analysis of data was performed by KT; KT and AK had drafted the first version of manuscript; statistical analysis was performed by KT; final approval of version published was done by KT and AK.
Funding
This work was supported by a Finnish government subsidy for health science research (grant numbers: TYH 2012141, TYH 2013218 and TYH 2014216), the SSAC Foundation (grant number SLS-504141), and University-Funded Doctoral Candidate Positions in the Doctoral Programmes of the Doctoral School in Health Sciences, University of Helsinki.
Conflict of interest
AK has received investigator-initiated grants from Valneva and Pfizer, neither of which is relevant to the current manuscript. KT declares no conflicts of interest.



