-
PDF
- Split View
-
Views
-
Cite
Cite
Neil Dufton, Robert Hannon, Vincenzo Brancaleone, Jesmond Dalli, Hetal B Patel, Mohini Gray, Fulvio D'Acquisto, Julia C Buckingham, Mauro Perretti, Roderick J Flower, Corrections: Anti-Inflammatory Role of the Murine Formyl-Peptide Receptor 2: Ligand-Specific Effects on Leukocyte Responses and Experimental Inflammation, The Journal of Immunology, Volume 186, Issue 4, February 2011, Pages 2684–2685, https://doi.org/10.4049/jimmunol.1090139
Close - Share Icon Share
Dufton, N., R. Hannon, V. Brancaleone, J. Dalli, H. B. Patel, M. Gray, F. D'Acquisto, J. C. Buckingham, M. Perretti, and R. J. Flower. 2010. Anti-inflammatory role of the murine formyl-peptide receptor 2: ligand-specific effects on leukocyte responses and experimental inflammation. J. Immunol. 184: 2611–2619.
Two papers appeared in the Journal of Immunology in 2010 describing the generation and use of novel transgenic mouse strains to elucidate the regulatory role of the murine, G-protein–coupled receptor mFpr2 in various aspects of the immune response (1, 2).
The paper from our laboratory (1) detailed the role for this receptor in mediating the endogenous anti-inflammatory effects of the protein annexin A1 (Anx-A1). A further paper using a different transgenic strain described experiments suggesting that the same receptor was crucial in facilitating normal innate and adaptive immune responses (2). We now write to clarify the nature of the gene deletion produced using our strategy, in the light of our latest understanding of the mouse Fpr gene family; in addition to deleting the murine Fpr2 gene, we now believe that our targeting construct would also have resulted in the deletion of what is now termed Fpr3.
In our paper, we sought mainly to extend our work on the role of the ALX/FPRL2 receptor as an endogenous counterregulator of the innate response by developing an appropriate transgenic mouse model. Such an approach entailed identification of the appropriate murine receptor, but this proved unexpectedly difficult to pin down. Unlike the human formyl-peptide receptor (FPR) family, which has only three members (FPR1, -2, and -3, formerly termed FPR, FPRL1, and FPRL2), of which FPR2 is the accepted receptor for lipoxin A4 (LXA4) and Anx-A1 (3), the murine Fpr family comprises at least eight genes (4, 5). Confusingly, two highly (82%) homologous receptors formerly termed Fpr-rs1 and Fpr-rs2 were found also to have strong homology with the human ALX receptor (∼74 and 76%, respectively, at both amino acid and RNA level). Both receptors recognized and responded to the ligand LXA4 (6, 7), and so either (or perhaps both) Fpr-rs1 and Fpr-rs2 genes could have coded for the receptor(s) we sought.
Since its initial description, however, the nomenclature and annotation of the murine Fpr family has changed. Fpr-rs2 was renamed Fpr2, but the situation regarding Fpr-rs1 was more complex. On the basis of the information available at the time of our publication, we originally believed this gene to comprise two isoforms, one of which was subsequently officially designated as Fpr3.
Our current understanding, based upon the latest annotations of the murine gene family, however, is that our targeting strategy would also delete Fpr3 itself because this gene also incorporates an exon found in Fpr2, and so insertion of the GFP reporter and the presence of the pgk-neo cassette in the cds (exon 2) of “Fpr-rs2” (as described in our paper) would prevent expression (Fig. 1) of this gene also. The transcription of the Fpr1 gene is unaffected.
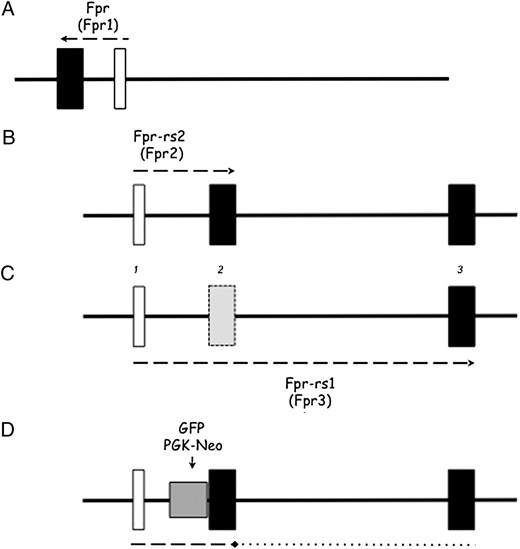
Annotation of the mouse genome in the vicinity of the Fpr2 gene. Open boxes, noncoding exons; black boxes, coding exons. Arrangement of the Fpr gene (now called Fpr1) (A), which is transcribed from a different strand to Fpr-rs2 (now called Fpr2) (B), which is transcribed from exons labeled (for convenience) 1 and 2. C, Fpr-rs1 (now called Fpr3) includes exon 1 from Fpr2 and exon 3, skipping exon 2 (shown with dotted line). D, In the transgenic strain we generated, the PGK-Neo cassette was inserted in reverse orientation into intron 1 of Fpr2 and the GFP fused in-frame (vector shown in gray) with the ATG start codon. This would have prevented transcriptional read-through of the Fpr3 as well as Fpr2 genes.
This supposition was confirmed by the multiplex PCR data shown below in Fig. 2, which is an amendment of Fig. 1 from our original publication (1). In Fig. 1 (1), the lane showing the absence from the null animals of (what was then believed by us to be) one of two isoforms of Fpr-rs1 was omitted.

Expression of Fpr genes in Fpr2 null mice. Multiplex PCR was used to compare the expression of Fpr1 (formerly Fpr) and Fpr2 (formerly Fpr-rs2) in wild-type and Fpr2 null mice as generated in Ref. 1. Primers were compared with the internal control (IC) gene (18S rRNA). In addition to the deletion of the Fpr2 gene, this strategy also deletes Fpr3, which incorporates exon 2 of Fpr2.
If our current interpretation is correct, the results we reported (1) could be interpreted as being in part due to the deletion of this additional Fpr receptor, and the apparent differences between mouse phenotypes in the two papers may therefore have a logical explanation. Because LXA4 failed to produce its antimigratory properties in the mouse colony we generated, and the effect of Anx-A1 and its bioactive N-terminal peptide were also substantially diminished or absent (1), it is certainly the case that we functionally deleted the appropriate receptor(s) for our purpose.
Murine Fpr1, Fpr2, and Fpr3 RNA are all expressed in leukocytes, spleen, and lung (4), but there is little (as yet), published on the potential differences in function between the highly homologous Fpr2 and Fpr3 receptors. Interestingly, the selective human FPR3 protein agonist, F2L, loses efficacy in murine cells lacking Fpr2 (8), highlighting the difficulty in attempting a direct correlation between the function of the human and murine gene families.
Ultimately, only further experimental work will clarify biological function of the many Fpr gene products transcribed from this complex locus.
We thank Dr. Charles Mein for his invaluable help and advice on the organization and annotation of the the murine Fpr gene family.



