-
PDF
- Split View
-
Views
-
Cite
Cite
Jie Zhao, Ying Liu, Jian-Nan Hu, Min Peng, Ning Dong, Xiao-Mei Zhu, Tao Ma, Yong-Ming Yao, Autocrine Regulation of Interleukin-3 in the Activity of Regulatory T Cells and its Effectiveness in the Pathophysiology of Sepsis, The Journal of Infectious Diseases, Volume 223, Issue 5, 1 March 2021, Pages 893–904, https://doi.org/10.1093/infdis/jiaa441
Close - Share Icon Share
Abstract
Regulatory T cells (Tregs) play a crucial role in modulating the inflammatory response and participated in sepsis-related immune dysfunctions. However, little is known about the regulatory mechanisms by which Tregs are kept in check during immune responses. Here, we verified the simultaneous expression of interleukin-3 (IL-3) and its receptor (IL-3R) in Tregs. Then, by modulation of IL-3 expression via lentiviral transduction-mediated small interfering RNA, we demonstrated that IL-3 negatively regulated Tregs activity via an autocrine mechanism. Furthermore, we found that anti-IL-3 antibody treatment significantly diminished inflammatory cytokines and organ injury, and improved survival in septic mice, which was associated with enhanced Treg percentage and function. Collectively, these results suggest that IL-3 negatively regulates the activity of Tregs in a previously unrecognized autocrine manner, and plays an important role in the excessive inflammatory response in sepsis, which might be utilized as a therapeutic strategy for the treatment of complications in sepsis.
Sepsis is the leading cause of death in intensive care units. It is estimated that there are more than 600 000 cases of sepsis annually with a mortality rate ranging between 30% and 50% in the United States, and the costs of medical care are estimated to exceed $17 billion annually [1]. Moreover, the mortality rate and medical expenses are rising due to an increased number of immunosuppressed patients, exposure to drug-resistant organisms, and a growing elderly population [2]. However, there are no specific therapeutic interventions that are approved for treatment in sepsis. Therefore, there is an urgent need to find novel therapeutic targets, which requires a deeper understanding of the mechanisms of sepsis.
Accumulating evidence now demonstrates that regulatory T cells (Tregs) can actively suppress the immune response and potentially may be involved in septic immune dysfunction [3]. Increased ratios of Tregs have been described early after sepsis and remained elevated in patients who died [4, 5]. Studies in mice revealed that Tregs were detrimental to the proliferation and function of effector T cells (Teffs) in sepsis [6], and this effect could be mitigated by small interfering RNA (siRNA) that inhibited Tregs [7]. Moreover, investigators used antibody against glucocorticoid-induced tumor necrosis factor (TNF)-receptor–related protein to block Tregs, which improved immune function in sepsis and enhanced microbial killing and survival [8]. Thus, it has been suggested that Tregs persist in sepsis, augment immune dysfunction, contribute to poor outcomes, and may serve as targets for immune modulation [9]. Taken together, it is essential to understand the regulatory mechanisms by which Tregs are kept in check during immune responses.
Interleukin-3 (IL-3) belongs to the family of hematopoietic cytokines that regulates the production and function of cells of the hematopoietic and immune system [10–13]. The important role of IL-3 in mediating inflammation and autoimmunity has recently been shown in various disease models [13]. Along with this property, Weber et al reported that IL-3 contributed to the emergency myelopoiesis in sepsis and identified IL-3 as a critical driving force of sepsis [14]. However, until now, little has been known about the relationship between IL-3 and Tregs. Interestingly, Sharma et al has shown that, as a specific CD4+ T-cell subset, Tregs could secrete IL-3 as well [15]. IL-3 receptor (IL-3R) is also expressed at both the gene and protein levels in Tregs [15]. The coexpression of IL-3 and IL-3R on Tregs impelled us to evaluate whether there exists a direct link between this cytokine and the activity of Tregs. In the present study, after verifying the expression of IL-3 and IL-3R simultaneously on Tregs, we documented that IL-3 interacted with its receptor negatively to regulate Treg activity via a previously unrecognized autocrine mechanism. Furthermore, using a mouse abdominal sepsis model, we noted that blocking IL-3 significantly decreased the amount of circulating inflammatory cytokines, ameliorated organ injury, and improved the survival rate, confirming its efficiency in the treatment of sepsis. More importantly, our findings indicated that IL-3, probably through its autocrine action, was capable of downregulating the functional properties of Tregs in vivo, which represents a novel mechanism of IL-3 involvement in the pathogenesis of septic complications.
METHODS
Animals and Procedures
Male BALB/c mice at least 6 to approximately 8 weeks of age were purchased from the Institute of Laboratory Animal Sciences at the Chinese Academy of Medical Sciences. Sepsis was induced by cecal ligation and puncture (CLP) as previously described [16]. Laparotomy was performed under isoflurane anesthesia. After making a 1-cm long incision, cecum was exposed, ligated below the ileocecal valve, and punctured twice with a 22-gauge needle. A small amount of stool was extruded to ensure patency. Mice were subcutaneously injected with 1 mL sterile phosphate-buffered saline (PBS) following abdominal closure. Then mice were administered via tail vein 2 μg/mouse anti-IL-3 antibody (Ab; BD Pharmingen) or its isotype control (BD Pharmingen) at 2, 6, and 12 hours after CLP surgery. Mice were monitored for 5 days to determine survival. All experimental manipulations were undertaken in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals, with the approval of the Scientific Investigation Board of Tianjin Medical University.
Cell Isolation and Culture
Spleens were removed and prepared for single-cell suspension. CD4+ T cells were isolated using a magnetic bead separation kit (Miltenyi Biotec). To isolate CD4+CD25+ T cells (Tregs), purified CD4+ T-cell populations were incubated with phycoerythrin (PE)-labeled anti-CD25 Ab and anti-PE magnetic beads and isolated by magnetic-activated cell sorting separation column. CD4+CD25− T cells, used as Teffs, were isolated by negative selection using anti-CD25 microbeads. The purity of all CD4+CD25+ and CD4+CD25− T cells fractions was greater than 90%. For T-cell receptor (TCR)-dependent stimulation, a combination of anti-CD3 (2 μg/mL) and anti-CD28 (5 μg/mL) monoclonal antibodies (mAbs; Biolegend) was used, and lipopolysaccharide (LPS; 5 μg/mL; Sigma-Aldrich) was used for Toll-like receptor 4 (TLR4)-dependent stimulation.
RNA Extraction, Reverse Transcription PCR, and Real-Time PCR
Total RNA was extracted with TRIzol Reagent (Ambion) and reverse transcribed to cDNA using AMV Reverse Transcription System (Promega). Reverse transcription polymerase chain reaction (RT-PCR) was performed with 2XTaq PCR Master Mix (Tiangen). Real-time PCR was performed with HotStart SYBR Green qPCR Master Mix (ExCell) in a 2-step real-time PCR system. Primers for IL-3, IL-3R, and β-actin are listed in the Supplementary Material.
Lentivirus Packaging and Transduction
Lentiviral transfer vectors containing siRNA targeting IL-3 were purchased from Genechem. A lentiviral vector with a scrambled siRNA sequence was used as the negative control. The procedure for lentiviral vector formation and transduction followed the recommendations of the manufacturer and was performed as described previously [17, 18]. Briefly,1 × 105 isolated Tregs were seeded in 96-well plates and lentivirus (LV) was added to the cells in the presence of 5 μg/mL Polybrene (Genechem) for 10 hours. An inverted fluorescence microscope was used to observe green fluorescent protein (GFP)-positive cells, and the knockdown efficiency of IL-3 expression was determined by real-time PCR and western blot analysis.
Flow Cytometry
Single-cell suspensions were resuspended at a concentration of 105–106/100 μL in PBS. For surface staining, Tregs were labeled in a staining buffer with fluorescein isothiocyanate (FITC)–anti-CD4, allophycocyanin (APC)–anti-CTLA-4, or FITC–anti-IL-3Rα Abs (eBioscience) for 30 minutes. Staining for intracellular IL-3 or Foxp3 with APC–anti-IL-3 (BD Biosciences) or PE-Cy7–anti-Foxp3 Ab (eBioscience) was performed after fixation and permeabilization of the cells. Teffs (1 × 105/well) were stained with CellTrace carboxyfluorescein succinimidyl ester (CFSE; Invitrogen), and incubated with isolated Tregs at a ratio of 4:1. After 96 hours, proliferation (CFSE dilution) was analyzed. All data were analyzed by flow cytometry in a BD Canto II cytometer.
Western Blot
Isolated Tregs were homogenized with a lysis buffer containing protease inhibitor mixture and were centrifuged to obtain lysates. The lysates were electrophoresed in a 10% sodium dodecyl sulfate gel and transferred onto a nylon membrane. The membrane was sequentially reacted with anti-IL-3 mAb (R&D system) and peroxidase-conjugated secondary Ab. The immune complexes were visualized using an enhanced chemiluminescence detection system (Applygen Technologies).
Assessment of Cytokine Levels
Plasma and cell culture supernatant samples were collected to determine the levels of murine cytokines using commercial enzyme-linked immunosorbent assay (ELISA) kits (IL-3, OSD Biotechnology; IL-10, transforming growth factor-β [TGF-β], TNF-α, and interferon-γ [IFN-γ], ExCell Biotech).
Statistical Analysis
The mean and SEM were calculated for all parameters determined in this study. Statistical significance was determined by 2-tailed Student t tests or 1-way ANOVA with Tukey multiple comparison test. The survival curves by the Kaplan–Meier procedure were analyzed by a log-rank test. P < .05 was accepted as statistically significant.
RESULTS
IL-3 Secretion by CD4+CD25+ Tregs Following Activation
Recent articles have identified activated CD4+ T cells as a major source of IL-3, and Sharma et al reported that, as a specific CD4+ T-cell subset, Tregs could also secrete IL-3 [15]. In the current study, we tried to determine whether IL-3 could be induced in Tregs following activation. Tregs were isolated from splenocytes. After verifying the purity of isolated Tregs, stimulation with anti-CD3/CD28 mAbs, which represents the prototypical activation via TCR-dependent mechanism, or LPS, which indicates innate immune stimulation upon binding with TLR4, were performed. Figure 1 shows that no detectable levels of IL-3 mRNA or protein were found in resting Tregs, whereas activation with anti-CD3/CD28 Abs or LPS induced substantial expression of IL-3 mRNA and secretion of IL-3 protein. At 24 hours, IL-3 mRNA expression peaked and averaged >40-fold after activation above the level seen without stimulation. In addition, approximately 150 pg/mL IL-3 was measured in cell-free supernatant after incubating for 24 hours and remained sustained >200 pg/mL during incubation. These data thus validate that activated Tregs can secrete IL-3, and both TCR-dependent and TLR-dependent activation are responsible for the induction of IL-3 in Tregs.

IL-3 expression by Tregs in response to TCR or TLR4-dependent stimulation. Tregs isolated from splenocytes were stimulated with anti-CD3 (2 μg/mL) and anti-CD28 (5 μg/mL) mAbs for TCR-dependent stimulation or LPS (5 μg/mL) for TLR4-dependent stimulation. At indicated intervals, IL-3 mRNA and protein expression were measured by quantitative RT-PCR and ELISA. Time course for the induction of IL-3 mRNA in response to anti-CD3 and anti-CD28 mAb or LPS is shown as fold changes in (A) and (C), respectively. Time course for the concentration of IL-3 protein in culture supernatants upon stimulation with anti-CD3 and anti-CD28 mAb or LPS is shown as mean ± SEM in (B) and (D), respectively. Abbreviations: ELISA, enzyme-linked immunosorbent assay; IL-3, interleukin-3; LPS, lipopolysaccharide; mAb, monoclonal antibody; RT-PCR, reverse transcription polymerase chain reaction; SEM, standard error of the mean; TCR, T-cell receptor; TLR4, Toll-like receptor 4; Treg, T regulatory cell.
IL-3R Expression on CD4+CD25+ Tregs
IL-3 exerts its biological activity by binding to the specific high-affinity receptor. Therefore the expression of IL-3Rα, also known as CD123 (cluster of differentiation 123), was measured at the mRNA level by RT-PCR. It was noted that isolated Tregs showed expression of IL-3Rα, which was further elevated after activation with anti-CD3/CD28 mAbs (Figure 2A). The expression of IL-3R was also analyzed by flow cytometry by surface labeling for IL-3Rα. As shown in Figure 2B, about 28% of freshly isolated Tregs were positive for IL-3Rα expression, whereas after activation nearly 60% of Tregs expressed IL-3Rα. Furthermore, the membrane IL-3Rα and intracytoplasmic IL-3 were labeled simultaneously and examined via flow cytometry. We showed that IL-3 and IL-3R were coexpressed on isolated Tregs, and their expressions were further elevated upon stimulation, as shown in Figure 2C. These results indicate that Tregs express IL-3R at both the gene and protein levels, and it can be modulated by the activation status of these cells.
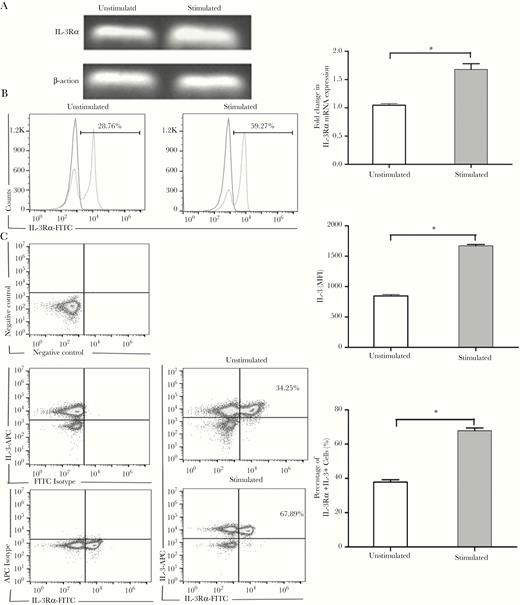
Expression of IL-3Rα on Tregs. Tregs isolated from splenocytes were stimulated with anti-CD3 (2 μg/mL) and anti-CD28 (5 μg/mL) mAbs for 72 hours. IL-3Rα (CD123) gene expression in isolated Tregs was examined by RT-PCR. Representative results from 3 independent experiments and the calculated ratios of IL-3Rα to β-actin are shown in (A). The expression of IL-3Rα on isolated Tregs was analyzed by flow cytometry with surface labeling for IL-3Rα. Representative results and calculated MFI of IL-3Rα expression are shown in (B). Coexpression of surface IL-3Rα and intracytoplasmic IL-3 was examined. Representative results are shown in (C). All values are mean ± SEM. * P < .05 for stimulated vs unstimulated group. Abbreviations: APC, allophycocyanin; IL-3Rα, interleukin-3 receptor α; mAb, monoclonal antibody; FITC, fluorescein isothiocyanate; MFI, mean fluorescence intensity; RT-PCR, reverse transcription polymerase chain reaction; SEM, standard error of the mean; Tregs, T regulatory cell.
Silencing of IL-3 Negatively Regulates Tregs In Vitro Upon Activation
As IL-3 and IL-3R are expressed on Tregs simultaneously, we next addressed whether the secretion of such cytokines following activation may mediate autocrine activity that affects Tregs. To test this hypothesis, Tregs were transfected with a LV-IL-3 siRNA to target IL-3 mRNA or LV-control siRNA to target nonspecific mRNA. GFP was detected by inverted fluorescence microscopy after transfection, revealing that more than 80% of transduced cells expressed the GFP marker (Figure 3A). As shown in Figure 3B, the relative expression of IL-3 mRNA in the experimental group (LV-IL-3 siRNA) was significantly lower than that in the control group (LV-control siRNA) or in the blank group. Similarly, western blot analysis showed that the IL-3 protein level in the experimental group was lower than that in the control group or the blank group (Figure 3C).
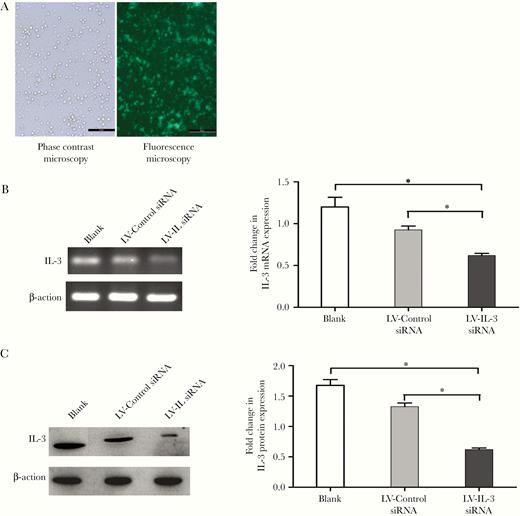
Knockdown of IL-3 via lentivirus transduction. Isolated Tregs were transduced by LV-IL-3 siRNA or LV-control siRNA. After transduction, cells were collected and analyzed for GFP expression by inverted fluorescence microscope (A, 100×). The knockdown efficiency was confirmed by measuring IL-3 expression using RT-PCR and immunoblot analysis; representative RT-PCR results and the ratios of IL-3 to β-actin (B); and representative immunoblot results and band intensities (C) are shown. All values are mean ± SEM. *P < .05 for LV-IL-3 siRNA vs control group. Isolated Tregs without transduction was used as “blank” group. Abbreviations: GFP, green fluorescent protein; IL-3, interleukin-3; LV, lentivirus; RT-PCR, reverse transcription polymerase chain reaction; SEM, standard error of the mean; siRNA, small interfering RNA; Treg, T regulatory cell.
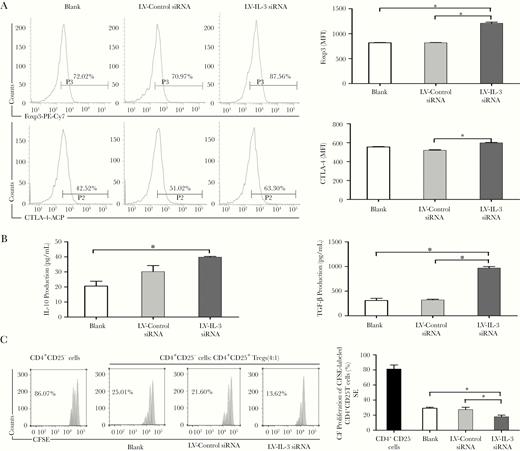
We then sought to evaluate the impact of the downregulation of IL-3 on the activity of Tregs. The expression of Foxp3, which is a master transcription factor for Tregs, and CTLA-4, which is a crucial functional marker of Tregs, were analyzed by flow cytometry. We found that knockdown of IL-3 significantly upregulated the expression of Foxp3 and CTLA-4 as compared with the control siRNA following anti-CD3/CD28 Abs or LPS treatment (Figure 4A and Figure 5A). In addition, IL-10 and TGF-β levels in culture supernatants were markedly higher in the LV-IL-3 siRNA group than that in the control group (Figure 4B and Figure 5B). To test the suppressive activity, Tregs were isolated and cocultured with CFSE-labeled Teffs upon stimulation. It was found that Tregs treated with LV-IL-3 siRNA suppressed Teff proliferation with greater potency than vehicle-treated Tregs (Figure 4C and Figure 5C). Together, these results potentially suggest that IL-3 possesses a critical autocrine and negative regulatory effect on Tregs.

Downregulation of IL-3 modulates the phenotype and function of Tregs in vitro upon TCR stimulation. After lentivirus transduction, Tregs were stimulated with anti-CD3(2 μg/mL) and anti-CD28(5 μg/mL) mAbs for 72 hours. Foxp3 and CTLA-4 expressions in Tregs were determined by flow cytometry. Representative histograms and MFI of Foxp3 and CTLA-4 expression are shown in (A). Culture supernatants were collected to determine IL-10 and TGF-β levels by ELISA (B). For analysis of suppression, Tregs were cocultured with Teffs stained with CellTrace CFSE at ratio 1:4. Proliferation of Teffs was analyzed by FACS for CFSE dilution. Representative histograms and calculated suppression of Teff proliferation by Tregs is shown in (C). All values represent mean ± SEM. Isolated Tregs without transduction was used as “blank” group. *P < .05. Abbreviations: APC, allophycocyanin; CFSE, carboxyfluorescein succinimidyl ester; CTLA-4, cytotoxic T-lymphocyte-associated protein 4; ELISA, enzyme-linked immunosorbent assay; FACS, fluorescence-activated cell sorting; IL-3, interleukin-3; mAb, monoclonal antibody; MFI, mean fluorescence intensity; PE, phycoerythrin; SEM, standard error of the mean; TCR, T-cell receptor; Teff, effector T cell; TGF-β, transforming growth factor-β; Treg, T regulatory cell.
Downregulation of IL-3 modulates the phenotype and function of Tregs in vitro upon TLR4 stimulation. Isolated Tregs were transduced by lentivirus-mediated LV-IL-3 siRNA or LV-control siRNA. Cells were then stimulated with LPS (5 μg/mL) for 48 hours. Foxp3 and CTLA-4 expression in Tregs were determined by flow cytometry. Representative histogram and MFI of Foxp3 and CTLA-4 expression are shown in (A). Culture supernatants were collected to determine IL-10 and TGF-β levels by ELISA (B). For analysis of suppression, Tregs were cocultured with Teffs stained with CFSE at ratio 1:4. Proliferation of Teffs was analyzed by FACS for CFSE dilution. Representative histograms from 3 independent experiments and calculated suppression of Teffs proliferation by Tregs are shown in (C). All values are mean ± SEM. Isolated Tregs without transduction was used as “blank” group. *P < .05. Abbreviations: APC, allophycocyanin; CFSE, carboxyfluorescein succinimidyl ester; CTLA-4, cytotoxic T-lymphocyte-associated protein 4; ELISA, enzyme-linked immunosorbent assay; FACS, fluorescence-activated cell sorting; IL-3, interleukin-3; LPS, lipopolysaccharide; LV, lentivirus; MFI, mean fluorescence intensity; PE, phycoerythrin; SEM, standard error of the mean; siRNA, small interfering RNA; Teff, effector T cell; TGF-β, transforming growth factor-β; TLR4, Toll-like receptor 4; Tregs, T regulatory cell.
Anti-IL-3 Treatment Inhibits Excessive Inflammatory Response and Improves Survival Rate in Sepsis
Previous studies have demonstrated the potential role of IL-3 as a septic diagnostic tool and prognosis biomarker, and knockout of IL-3 or treatment with anti-IL-3R could partially protect against sepsis lethality [15]. Herein, anti-IL-3 Ab was used to evaluate its efficiency in the treatment for sepsis. We performed CLP, a clinically relevant animal model for human sepsis, and anti-IL-3 Ab was injected via the tail vein in septic mice. Remarkably, we noted that >70% (19 of 26) of the septic mice treated with anti-IL-3 Ab survived compared with <50% PBS-treated mice (13 of 27) or isotype control group (12 of 25) (Figure 6A), indicating a significant increase in the survival rate following treatment with anti-IL-3 Ab. Plasma levels of TNF-α and IFN-γ were increased in the setting of sepsis and, as expected, anti-IL-3 treatment inhibited the increases in these proinflammatory cytokines at 48 hours (Figure 6B). By contrast, plasma levels of the prototype anti-inflammatory cytokine IL-10 were elevated after anti-IL-3 treatment at 48 hours (Figure 6B). These results suggest that anti-IL-3 treatment has the potential to inhibit the production of proinflammatory cytokines and induce anti-inflammatory cytokines. Histological sections of lung from septic mice showed signs of injury, including thickening of the alveolar septa and interstitial edema, and in liver sections, swelling of hepatocytes and infiltrating neutrophils were observed. As shown in Figure 6C, both of these septic-related organ injuries were attenuated by anti-IL-3 treatment.
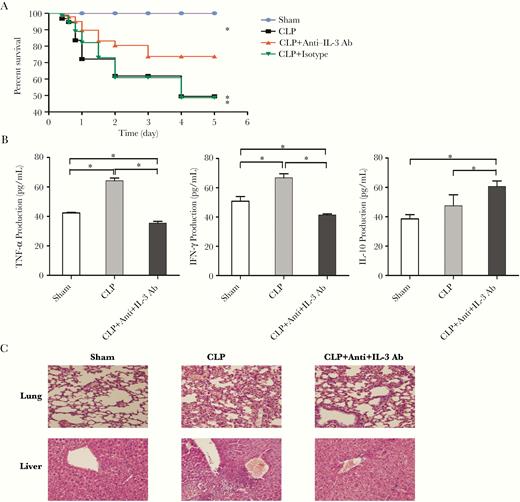
Anti-IL-3 treatment mitigates inflammatory response, alleviates histopathology damage, and improves the survival rate in septic mice. Mice were injected with anti-IL-3 Ab or isotype control via tail vein at 2, 6, and 12 hours after CLP surgery. To determine survival, mice were monitored every 12 hours for 5 consecutive days as shown in (A). Serum TNF-α, IFN-γ, and IL-10 levels were measured using ELISA kits (B). Pulmonary and liver samples were obtained and stained for H&E histopathology (original magnification × 200) (C). All values represent mean ± SEM. Sham operation was performed by isolating the cecum without ligation and puncture. *P < .05. Abbreviations: Ab, antibody; CLP, cecal ligation and puncture; ELISA, enzyme-linked immunosorbent assay; IFN-γ, interferon-γ; IL-3, interleukin-3; SEM, standard error of the mean; TNF-α, tumor necrosis factor-α.
Anti-IL-3 Treatment Modulates the Frequency and Function of Tregs In Vivo
To elucidate the protective mechanism, we examined the effects of anti-IL-3 treatment on the activity of Tregs in the septic model. CLP-induced sepsis resulted in an increasing percentage of Tregs in septic mice compared with sham-treated mice, which was further augmented by anti-IL-3 Ab (Figure 7A). Foxp3 expression was markedly upregulated in Tregs from the CLP mice, and it was further enhanced in anti-IL-3–treated mice (Figure 7B). A similar increase in CTLA-4 expression was found. Tregs from anti-IL-3–treated mice presented significant inhibition of Teffs proliferation compared with those from mice without treatment (Figure 7C). Collectively, these results suggest that the frequency and function of Tregs are both regulated by IL-3 in vivo in the pathophysiological process of sepsis.
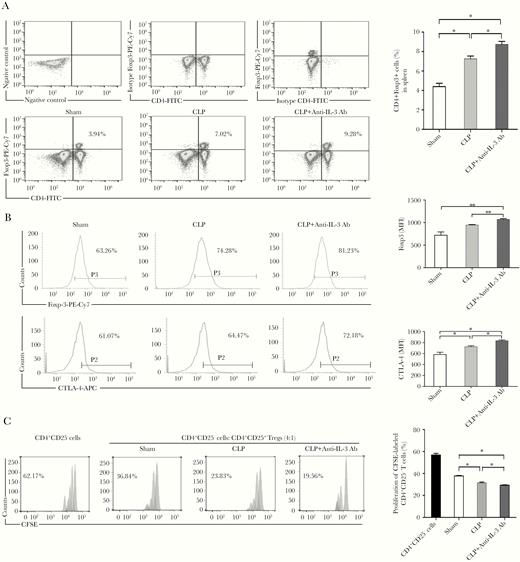
The effects of anti-IL-3 treatment on Tregs in septic mice. Mice were sacrificed and spleens were collected at 48 hours after CLP surgery. Percentages of CD4+Foxp3+ Tregs were assessed by flow cytometry. Representative dot plots and percentages of CD4+Foxp3+ Tregs in the CD4+ T-cell subpopulation are shown in (A). Tregs were isolated and the expression of Foxp3 and CTLA-4 on isolated Tregs were determined by flow cytometry. Representative histograms and MFI of Foxp3 and CTLA-4 expression are shown in (B). For analysis of suppression, Teffs were stained with 5 μM CFSE and cultured with Tregs at ratio 4:1. Proliferation of Teffs was analyzed by FACS for CFSE dilution. Representative histogram from 3 independent experiments and calculated suppression of Teffs proliferation by Tregs are shown in (C). All values represent mean ± SEM. Sham operation was performed by isolating the cecum without ligation and puncture. *P < .05, **P < .01. Abbreviations: Ab, antibody; CFSE, carboxyfluorescein succinimidyl ester; CLP, cecal ligation and puncture; CTLA-4, cytotoxic T-lymphocyte-associated protein 4; FACS, fluorescence-activated cell sorting; FITC, fluorescein isothiocyanate; IL-3, interleukin-3; MFI, mean fluorescence intensity; PE, phycoerythrin; Teff, effector T cell; Tregs, T regulatory cell.
DISCUSSION
IL-3 belongs to the family of hematopoietic cytokines with 4 short α-helices that includes granulocyte-macrophage colony-stimulating factor and IL-5. All 3 cytokines bind to a specific α-subunit but use a common β-receptor subunit for signal transduction [19]. As a classical cytokine that regulates the production and function of cells of the hematopoietic and immune system, IL-3 induces activation and/or increases the survival of various target cells, including mast cells, basophils, monocytes, B cells, T cells, and endothelial cells [20, 21]. Recently, an important role of IL-3 in inflammation and autoimmunity has gained considerable attention, and IL-3 is regarded as a classical proinflammatory cytokine and a crucial early inductor of inflammation as well. Primarily, IL-3 is produced by activated T cells [22] but can also be expressed by innate response B cells [14], basophils, neurons, and microglial cells [23–26]. IL-3 released from T cells has been reported to be acting as a growth factor responsible for enhanced basophils production following parasite infection, and contributing to the development of lupus nephritis by its action on immune cells [27]. In this study, our data indicate that, as a subset of T cells, Tregs show IL-3 expression at both the gene and protein level upon TCR-dependent and TLR-dependent activation. In accordance with our data, Sharma et al identified Tregs as a major source of IL-3 and demonstrated the direct effects of Tregs on basophil activation via an IL-3–dependent pattern [15]. Importantly, as shown in the present study and data published by Srivastava et al [28], Tregs also expressed the high-affinity IL-3R α-subunit (CD123) on their surface. Thus, it seems appropriate to infer that IL-3 produced by Tregs may act on themselves in an autocrine manner via the interaction with coexpressed IL-3R.
Tregs represent an important mechanism in the maintenance of immune homeostasis [29–31], and Foxp3 is the most reliable marker and determines the development and function of Tregs [32]. Although regulatory mechanisms by which Tregs suppress different immune cell subsets have been extensively studied, how Tregs are regulated is still not well understood. From our data, the expression of IL-3 and IL-3R on the same cells strongly suggests that Tregs might produce IL-3 for themselves. Many cytokines act in an autocrine function. For IL-3, its production by basophils has been reported to be responsible for priming of these cells, which represents a key mechanism behind the hyperactive nature of basophils in allergic disease [26]. In the current study, we investigated the effects of endogenous IL-3 on function and phenotype of Tregs by modulation of IL-3 expression via lentiviral transduction-mediated siRNA. Consequently, in response to CD3/CD28 activation, we noted that downregulation of IL-3 markedly enhanced Foxp3 expression, diminished IL-10 and TGF-β production, and significantly decreased proliferation of Teffs. Moreover, we and others have previously reported that Tregs with direct engagement of TLR4 by LPS exerted enhanced function in vitro and remained suppressive in vivo without the need for TCR ligation [33–35]. We therefore considered that this mode of innate immune stimulation might also induce Tregs to produce IL-3 that would then mediate autocrine effects. As expected, the expression of Foxp3 and CTLA-4, production of IL-10 and TGFβ, and suppressive activity of Tregs were significantly augmented by siRNA-mediated silencing of IL-3. Based on these findings, it is fair to conclude that Tregs produce IL-3 for themselves upon both TCR-dependent and TLR-dependent activation as an autocrine mechanism, and this negative autocrine action is also novel considering its outcome, that is the function of the Tregs.
Evidence is accumulating that IL-3 plays a critical role in autoimmunity and inflammation [36]. For example, in mice with collagen-induced arthritis, the upregulation of IL-3 preceded the increases in classical proinflammatory cytokines in the synovium and blockade of IL-3 significantly reduced immune cell infiltration and proinflammatory cytokine expression [37]. Administration of IL-3 aggravated lupus nephritis with significantly higher renal activity scores, more renal fibrosis, and more glomerular leukocyte infiltration and IgG deposition [27]. In a model of sepsis, Weber et al observed that IL-3 caused proliferation and mobilization of myeloid cells that generated excessive proinflammatory cytokines, thereby fueling systemic inflammation, organ injury, and even death [14]. In the present study, we investigated the contribution of IL-3 to the development of sepsis by using a blocking antibody. Compared with the control group, the treatment of mice with anti-IL-3 antibody improved the survival in this widely used and clinically relevant CLP model of sepsis. Anti-IL-3 antibody intervention also ameliorated histopathological lesion in the lungs and livers. Accordingly, high serum levels of proinflammatory cytokines, which are biomarkers and causative agents of poor prognosis in sepsis, were reversed by the administration of the anti-IL-3 antibody. Thus, we confirm the effectiveness of the anti-IL-3 blocking strategy and support the therapeutic potential of IL-3 in sepsis. To better understand the mechanisms of how IL-3 affects the development of sepsis, we performed a further investigation to analyze the potential impact of IL-3 blocking on the function and phenotype of Tregs. As compared with the control mice, both percentages of Tregs and the expression of Foxp3 and CTLA-4 were dramatically increased in the anti-IL-3 antibody treatment group. Moreover, Tregs from anti-IL-3–treated mice showed an enhanced suppressive response in comparison to those from the control mice. Taken together, our data provide evidence linking IL-3 with the activity of Tregs in vivo and reveal an unexpected mechanism that IL-3 exerted on Tregs in the pathophysiology of sepsis.
Upon infectious challenge to the host, establishing a balance between inflammatory and anti-inflammatory responses is critical in the process of sepsis. The protective roles and the detrimental potential of the immune response thus have to be delicately balanced [2]. At the center of this balance lies Tregs, as proposed by Sakaguchi et al nearly 2 decades ago and since established experimentally [35]. Beyond doubt, equilibrium both in the number and function of Tregs is important to ensure pathogen clearance and protection from immunopathology. Our current observations are novel and significant because we described, for the first time to our knowledge, an autocrine IL-3–mediated feedback loop restricting the phenotype and function of Tregs both in vitro and in vivo. This has important implications for understanding how Tregs are modulated to shape the immune response during sepsis. In the pathogenesis of septic complications, IL-3–mediated proinflammatory responses are needed to restrict bacterial growth and, ultimately, to clear microbes [38]. Coincidental upregulation of Tregs may be beneficial by limiting damage due to systemic inflammation but may hamper effective anti-infective immunity, promote bacterial growth, and exacerbate disease [39]. In favor of host survival to acute massive inflammations, timing in combating pathogens could be more demanding. Therefore, IL-3 appears to be harnessed for negative feedback regulation of function and phenotype of Tregs to ensure effective bacterial clearance in a threatening infection. Further studies are needed to elucidate the precise signaling pathways of IL-3–mediated activity of Tregs.
In summary, data presented here not only highlight that IL-3 negatively regulates the activity of Tregs, functioning in an autocrine manner by binding with IL-3R in vitro and in vivo, but also support the concept that, in addition to its proinflammatory action, IL-3 plays a crucial role in septic inflammatory response by negatively regulating immune function of Tregs. Our work strongly indicates that IL-3 might be utilized as an important therapeutic strategy for the treatment of sepsis and should be given more attention in future research.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank Yu-Jie Qui and Hong-Yue Li for technical assistance.
Financial support. This work was supported by the National Natural Science Foundation of China (grants 81871546 and 81471841 to T.M; grant 81730057 to YM. Y), and the National Key Research and Development Program of China (grant 2017YFC1103302 to YM.Y).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.




