-
PDF
- Split View
-
Views
-
Cite
Cite
Ryo Takahashi, Tetsuo Shiohara, Yoshiko Mizukawa, Monocyte-Independent and -Dependent Regulation of Regulatory T-Cell Development in Mycoplasma Infection, The Journal of Infectious Diseases, Volume 223, Issue 10, 15 May 2021, Pages 1733–1742, https://doi.org/10.1093/infdis/jiaa590
Close - Share Icon Share
Abstract
Although Mycoplasma pneumoniae (MP) infection has been implicated in the pathogenesis of allergic diseases, the mechanism of this trigger remains unknown. We explored the mechanism for how MP infection could tilt the balance between regulatory T cells (Tregs) and Th17 cells.
We analyzed the frequency, phenotype, and function of Tregs in patients at the different stages of MP and various virus infections over a period of more than 1 year. We examined the effect of monocytes to elucidate signals that can regulate the balance between Treg and Th17 cells.
The functional activity of Tregs was profoundly impaired during the acute stage of MP as well as viral infections. Upon resolution, however, the Treg function remained impaired even 1 year after MP infection. In the resolution stage, the impaired Treg function was associated with an increase in interleukin (IL) 17A+ Tregs and Th17 cells. Development of Th17 cells was dependent on the “aberrant” proinflammatory monocytes (pMOs), characterized by potent ability to produce IL-6 in a Toll-like receptor 2–dependent manner.
Depending on the prevalence of the pMOs, Tregs and Th17 cells could mutually regulate the number and function of the other. The pMOs/IL-6 could be crucial therapeutic targets against MP-induced allergic diseases.
Infections by pathogens, such as viruses and Mycoplasma, are prime candidates for subsequently developing allergic inflammation and autoimmune diseases in susceptible individuals [1]. Available evidence also strongly suggests that viral infections create a favorable milieu for the initiation and progression of adverse drug reactions [2]. However, to maintain or restore a homeostatic environment, there must be mechanisms that protect the host from excessive immune responses to pathogens, which could in itself lead to greater pathological consequences than the invading pathogens themselves. Indeed, several types of regulatory T cells (Tregs) have been described, some of which are induced in response to infections [3, 4]. Evidence is recently accumulating that CD4+Foxp3+ Tregs, both natural and inducible, can inhibit the function of T effector cells (Teffs) at the site of microbial infections, thereby inhibiting severe immunopathology. On the other hand, the Treg response is potentially harmful to the host in terms of infection control, because their activation may secure survival of invading pathogens for an extended period of time, thereby causing chronic infectious diseases. Numbers and function of Treg cells, therefore, should be controlled depending on the stage of infections. During the acute stage of infection, dampening Treg function results in vigorous Teff cell responses that control infections: Several mechanisms acting independently or together can be envisaged [5, 6]. Thus, a time-dependent balanced, rather than biased, Treg responses would be necessary for host protection and the resolution of infection.
Mycoplasma pneumoniae (MP) is one of the most common causes of atypical pneumonia in pediatric and adult populations worldwide, although it causes asymptomatic infection in most humans [7]. Although it can infect multiple organ systems, cutaneous symptoms can be seen in 20%–25% of patients [8], some of which are mild and self-limited; however, more serious complications of MP infection, such as Stevens–Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN), often occur both during and after active infection [7, 9]. It is much less clear, however, whether MP infection plays a role in triggering flares of disease or, alternatively, is merely an innocent bystander that happens to create a favorable milieu for the initiation of adverse drug reactions. These considerations suggest that MP infection may make hosts more susceptible to a potential trigger of disease via transiently altering immune responses to allergens or drugs. In this regard, we demonstrated that Treg function was profoundly impaired during the acute stage of SJS and TEN [4], while the role of Tregs in the context of MP infection has rarely been investigated [10].
In this study, we explored the hypothesis that frequencies and function of Tregs could be specifically altered by MP infection depending on the stage of infection. Serial samples were obtained from not only patients with MP infection but also those with varicella zoster virus (VZV), Epstein–Barr virus (EBV), and parvovirus B19 infections, and the frequencies and function of Tregs were followed for 1 year after infection. The functional activity of Tregs was profoundly impaired during acute infection with MP, VZV, EBV, and parvovirus B19. Upon clinical resolution, the impaired functional activity of Tregs had been restored to baseline in patients with these viral infections. In contrast, the suppressive function of Tregs in patients with MP infection remained abrogated even 1 year after clinical resolution. This long-lasting inhibitory effect of MP infection on Treg function after MP infection was dependent on monocyte subsets producing interleukin (IL) 6, which can drive Tregs into a Th17-like phenotype with reduced suppressive function.
MATERIALS AND METHODS
Subjects
Data regarding patients and diagnosis are described in the Supplementary Data. Peripheral blood was obtained on informed consent in accordance with the Declaration of Helsinki under protocols approved by the Institutional Review Board at the Kyorin University School of Medicine.
Antibodies and Reagents
All antibodies (Abs) and reagents were purchased from the companies as described in the Supplementary Data.
T-Cell Proliferation Assay
Fluorescence-activated cell sorter (FACS)–sorted CD4+CD25++ Teffs were co-cultured with FACS-sorted Treg cells in the presence of allogeneic antigen-presenting cells (APCs) obtained from healthy adult volunteers in U-bottom 96-well plates as described previously [11–13] and in the Supplementary Data.
Stimulation of T Cells and Monocytes
Peripheral blood mononuclear cells (PBMCs) were stimulated with XXX (PMA) plus 1 μg/mL ionomycin, or 10 μg/mL of Pam3Cys, or 1 μg/mL of XXX (LPS) for 4 hours in the presence of 10 μg/mL brefeldin A (Sigma-Aldrich). All cells were cultured in RPMI 1640 medium (Sigma-Aldrich) supplemented with 10% fetal calf serum. After stimulation, intracellular staining was done (Supplementary Data).
Flow Cytometric Analysis
Cells were stained with a combination of labeled Abs and then the samples were subjected to flow cytometric analysis (see the Supplementary Data for details).
Identification of Monocyte Subpopulations
PBMCs were gated on the putative monocyte fraction including a portion of the adjacent lymphocytes, as demonstrated previously [12, 14] and in the Supplementary Data.
Induction of Tregs and Th17 Cells by Proinflammatory Monocytes
For induction of Treg and Th17 cell development by monocytes (MOs), CD14dim proinflammatory monocytes (pMOs) were prepared and cultured as described in the Supplementary Data.
Statistical Analysis
Data are expressed as mean ± standard error of the mean. Statistical significance was determined using Mann–Whitney U test. To assess correlations, Spearman rank correlation coefficient test was used to assess correlations.
RESULTS
Frequencies and Phenotypes of Tregs During and After MP or Viral Infections
We initially evaluated the frequencies of various T-cell subsets in total PBMCs and their cytokine production in patients with MP infection and those with viral infections at their acute and resolution stages, respectively. As shown in Supplementary Figure 2A, a significant decrease in the frequencies of CD4+ T cells associated with a significant increase in the frequencies of CD8+ T cells was detected in patients with VZV infection and EBV infection, but not in MP infection. In contrast, the frequencies of interferon-γ–, tumor necrosis factor alpha (TNF-α)–, IL-4–, and IL-17A–producing CD4+ T cells were significantly higher in patients with MP infection, particularly at the resolution stage (Supplementary Figure 2B). Such long-lasting enhancement of cytokine production induced by PMA regardless of cytokine patterns was only observed in patients with MP infection, as far as we examined, and has never been reported in the setting of antecedent infection. These findings suggested that Treg function is likely to be persistently abrogated after infection with MP, although we cannot totally exclude the possibility that subclinical viral reactivations possibly triggered by MP infection could be also involved in the multistep pathological process.
As shown in Figures 1A and 1B, no significant alterations in the mean frequencies of Tregs were found in these patients at all of the time points examined, as compared with those of 34 age-matched healthy controls (HCs), except for those with EBV infection, in which a marked decrease was detected at the acute stage. We next performed a phenotypic characterization using monoclonal antibodies (mAbs) specific for markers associated with Treg phenotype and/or function. The vast majority of Tregs detected in CD4+ T cells from patients with MP infection were found to express CLA, CTLA-4, CD39, CD127dim/–, and HELIOS, as detected in HCs (Figure 1D).

Frequencies and phenotypic analysis of regulatory T cells (Tregs) in peripheral blood mononuclear cells from healthy controls (HCs) and patients in the acute and resolution stages of infections using flow cytometry. A, Representative flow cytometry dot plots showing Tregs. B, Mean frequency of Tregs in the patients and HCs. C, Mean frequency of Tregs in patients with Mycoplasma pneumoniae (MP) at onset according to 3 age groups (<25 years, n = 6; 25 to 35 years, n = 9; ≥35 years, n = 8). D, Phenotypes of Tregs. E, Representative FACS dot plots showing each Treg fraction. F, Mean frequency of each Treg fraction. Each value represents mean ± standard error of the mean. MP patients, n = 12; varicella zoster virus (VZV) patients, n = 7; parvovirus B19 (PB19) patients, n = 6; Epstein–Barr virus (EBV) patients, n = 5; controls, n = 24.
A recent study has demonstrated that Foxp3+ Tregs could be classified into functionally distinct subpopulations, CD45RA+Foxp3+ resting/natural occurring Tregs (Fr.I) and CD45RA–Foxp3++ activated/induced Tregs (Fr.II), both of which have potent suppressive function, and CD45RA–Foxp3+ nonsuppressive Tregs (Fr.III) [15]. We therefore investigated which subpopulations could be altered in MP infection. As shown in Figure 1E and 1F, the most remarkable increase was found in the non-Treg fraction (Fr.III) at the acute and resolution stages whereas the induced Treg (iTreg) (Fr.II) fraction was decreased: these alterations again suggested the long-lasting defects of Treg function after MP infection.
Treg Function During and After MP Infection and Viral Infections
As shown in Figure 2, Tregs obtained from patients with these infections at the acute stage, either MP or viruses, exhibited a significantly impaired capacity to suppress CD3-driven Teff proliferation, as compared with those from HCs. The degree of the functional defects in patients with acute infections, such as MP and VZV, was comparable to that in patients with TEN, which was previously demonstrated by us [4].
![Functional analysis of regulatory T cells (Tregs) at the different stages of infection. A, Percentage proliferation of T effector cells (Teffs). CD4+CD25− Teffs from healthy controls (HCs) yielded a baseline, mean proliferative response of 5613 ± 323 count per minute (mean ± standard error of the mean [SEM]). B, In vitro suppression assay by coculture of Tregs (Mycoplasma pneumoniae [MP] A or MP-R) and Teffs (MP-A or MP-R) (B) and Tregs (MP-R or HCs) and Teffs (MP-R or HCs) (C). D, Mean frequency of Tregs in MP infection and HCs. E, Percentage proliferation of Teffs. Each value represents mean ± SEM. MP patients, n = 9; varicella zoster virus (VZV) patients, n = 6; parvovirus B19 (PB19) patients, n = 6; Epstein–Barr virus (EBV) patients, n = 3; HCs, n = 14.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/jid/223/10/10.1093_infdis_jiaa590/1/m_jiaa590f0002.jpeg?Expires=1747870165&Signature=F9s57WBOaHDgBeV3So0oHgWSpeS9pu9xxYGfoIsyyCWt8~MacbJ01Gzc9J4HdYJ2-Wd0QfEMaeiQyDu2cWMoShZXbWkSl1MFGHEUYwM1sBx8LZugvlTPJYBSr9qsva0S6d-gPFnGSv6ds8tIqtSOapceYZDTN3HsUtSXLm5EF~Mm7GKSp-xoHj6KaZE8tSnyEoi5Okk1WALg4vRaLGuD~AlH1c1G1VNNOYxwhZweqXTuOEbTYROq9REGR3sG53GmAcGUJpf851MYVwv8dQQdgLbyq2pMzcgStZNXqpa05mXny5FoYWob5nAIRaDlUXCAC~TNhKatJDbpDHNdUD862g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Functional analysis of regulatory T cells (Tregs) at the different stages of infection. A, Percentage proliferation of T effector cells (Teffs). CD4+CD25− Teffs from healthy controls (HCs) yielded a baseline, mean proliferative response of 5613 ± 323 count per minute (mean ± standard error of the mean [SEM]). B, In vitro suppression assay by coculture of Tregs (Mycoplasma pneumoniae [MP] A or MP-R) and Teffs (MP-A or MP-R) (B) and Tregs (MP-R or HCs) and Teffs (MP-R or HCs) (C). D, Mean frequency of Tregs in MP infection and HCs. E, Percentage proliferation of Teffs. Each value represents mean ± SEM. MP patients, n = 9; varicella zoster virus (VZV) patients, n = 6; parvovirus B19 (PB19) patients, n = 6; Epstein–Barr virus (EBV) patients, n = 3; HCs, n = 14.
The impaired capacity of Tregs at the acute stage of viral infections, however, had returned to a presumed baseline, which was indistinguishable from that of HCs, upon clinical resolution (Figure 2A). In contrast, functional activity of Tregs obtained from MP patients remained defective even after clinical resolution. To determine whether this defect was due to increased resistance of Teff obtained from MP patients to Treg-mediated suppression, we performed criss-cross experiments using the Teff fraction from the acute stage and the Treg fraction from the resolution stage, and vice versa. We found that functional activity of the Treg fraction from either the acute or resolution stage was similarly impaired (Figure 2B) and that the CD25– Teff fraction from the resolution stage of MP infection was not resistant to suppression by Tregs from HCs (Figure 2D). These results suggest that the functional defect induced by MP infection resides fundamentally in the Tregs but not in Teffs.
We then examined how long the impairment in suppressive function of Tregs persisted following MP infection. The frequencies and function of Tregs were sequentially examined at 3 different time points: <3 months, 3 months to 1 year, or >1 year after MP infection. No significant difference was found in the frequency of Tregs between these different time points (Figure 2D). Because the mean age of MP patients was significantly younger than that of other groups with viral infections, it was possible that long-lasting defects of Treg function by MP infection is due to the uncertain effect of age. However, this possibility is unlikely, because age had no significant effects on Treg frequency in MP patients (Figure 1C) and Treg function in either MP patients or HCs. Surprisingly, the impairment in suppressive function of Tregs associated with a reduction of iTregs remained detected even 1 year after MP infection, although the magnitude of the impairment became gradually less apparent (Figure 2E).
Increased Frequencies of IL-17A+ Tregs and Th17 Cells
We next asked whether such defects in Treg function observed at the resolution stage of MP infection could be intrinsic or secondary to the paucity of signals necessary for Treg development or to the abundance of signals that can tip the balance of Tregs and Th17 cells toward Treg impairment: The relative balance of Tregs and Th17 cells would determine the outcome of the host response to several infectious pathogens. We therefore analyzed the expression of IL-17A+ in Tregs at either the acute or resolution stage. As shown in Figure 3A and 3B, IL-17A+ cells in Treg cells were significantly increased in frequency at the acute and resolution stages, although IL-17A–producing CD4+ T cells (Th17 cells) were not increased at the acute stage (Supplementary Figure 2B). Particularly, iTregs with a Th17-like phenotype were markedly increased at the acute and resolution stage (Figure 3C). The increase in the frequency of IL-17A+ Tregs was associated with the impairment of the functional capacity of Tregs.
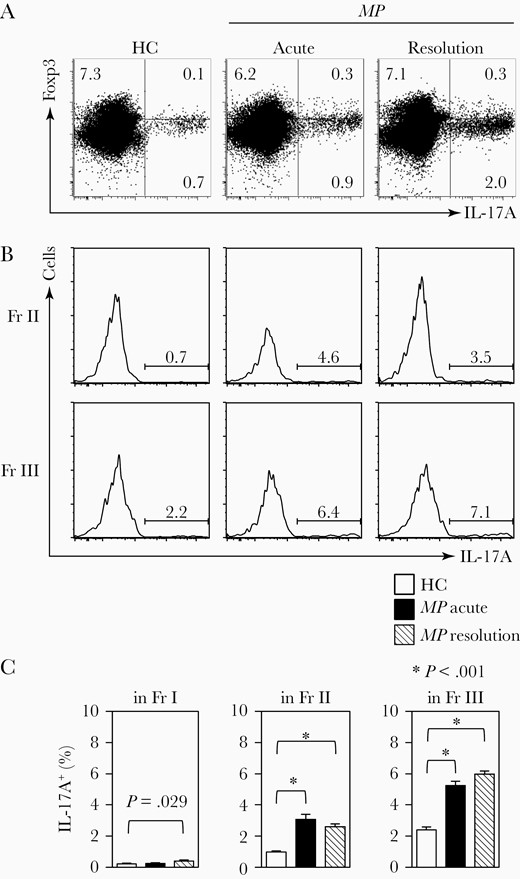
Increased frequencies of interleukin 17A–positive (IL-17A+) regulatory T cells (Tregs) and Th17 cells in patients with Mycoplasma pneumoniae (MP) at the acute and resolution stages. A, Representative flow cytometry dot plots showing IL-17A+Foxp 3+ cells and IL-17A+Foxp3– (Th17) cells. Numbers in each quadrant indicate the frequency of each fraction (Fr.). B, Representative flow cytometry histograms showing IL-17A+ in Treg fraction. C, The mean frequency of IL-17A+ cells in each Treg fraction. Each value represents mean ± standard error of the mean. MP patients, n = 16; healthy controls (HCs), n = 17.
Potent Ability of Monocyte Subsets to Impair Treg Function
On the basis of available evidence to suggest a close interaction between Tregs and monocytes [16, 17], we sought to characterize monocyte populations in patients with MP infection. Human blood monocytes are heterogeneous populations and can be separated into distinct subsets: CD14+CD16– classical monocytes (cMOs) and CD14dimCD16+ pMOs (Figure 4A). We therefore investigated whether these MO subsets would be numerically or functionally altered upon MP infection. No significant alterations in the frequencies of these subsets were detected in Figure 4B, as compared with those in HCs. Given the ability of MP to stimulate Toll-like receptor 2 (TLR2) signaling pathway [18] and taking into consideration of previous reports demonstrating the function of TLR2 ligation to abrogate Treg suppressive function [19, 20], we sought to investigate the effect of MOs on the functional impairment of Tregs in the setting of MP infection. As shown in Figure 5A, pMOs obtained from MP patients at the resolution stage, but not the acute stage, had significantly increased ability to produce IL-6, but not IL-10, upon TLR2 ligation by synthetic ligands (Pam3cys), but not TLR4 ligation (LPS). pMOs obtained from the acute stage of MP infection had significantly decreased ability to produce IL-1β upon TLR2 ligation and TNF-α upon TLR4 ligation, respectively (Supplementary Figure 3). There was also an inverse relationship between IL-6 production by pMOs and the frequency of iTregs (Fr.II) in Foxp3+ T cells while the IL-6 production was positively correlated with the frequency of non-Tregs (Fr.III) in the resolution stage of MP patients (Figure 5B).

Frequencies of monocyte (MO) subsets in the different stages of Mycoplasma pneumoniae (MP) infection. A, Representative flow cytometry dot plots showing the proinflammatory monocyte (pMO) and classical monocyte (cMO) populations. B, Mean frequencies of MOs, pMOs, and cMOs in peripheral blood mononuclear cells (PBMCs) from patients with MP infection (n = 16) and healthy controls (HCs, n = 11). Each value represents mean ± standard error of the mean.
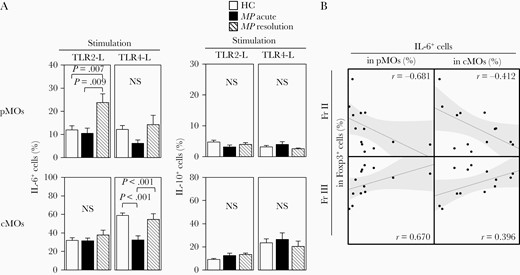
Increased production of interleukin (IL) 6 by proinflammatory monocytes (pMOs) obtained from the resolution stage of Mycoplasma pneumoniae (MP) infection, upon Toll-like receptor (TLR) 2-L stimulation. A, Mean frequencies of IL-6+ and IL-10+ cells in pMOs or classical monocytes (cMOs), upon TLR2-L or TLR4-L stimulation. B, There was an inverse relationship between IL-6 production by pMOs and the frequency of fraction II (iTregs) in Foxp3+ T cells, whereas IL-6 production was positively correlated with the frequency of fraction III (non-Tregs) in the resolution stage of MP patients. Each value represents mean ± standard error of the mean. MP patients, n = 7; healthy controls (HCs), n = 13.
Interactions Between Tregs and pMOs
To demonstrate interactions between Tregs and MOs, we performed coculture using freshly purified T cells from HCs and purified allogeneic MO subpopulations obtained from either HCs or MP patients at the acute/resolution stages. Cells were stimulated with anti-CD3 plus CD28 mAbs for 7 days. As shown in Figure 6A and 6B, CD3-driven, IL-17A+ iTreg (Fr.II) proliferation as assessed by an increase in the frequency of CFSElowIL-17A+CD4+ T cells and IL-17+ cells in iTreg (Fr.II) fraction was significantly increased when pMOs derived from MP patients at the resolution stage were used as MOs, compared with that when pMOs derived from HCs or acute stage of MP infection were used. The IL-17A+CD4+ T cells and the iTreg (Fr.II) proliferation was inhibited by the addition of anti–IL-6 mAb at the start of cocultures, indicating that IL-17A+ iTregs can be expanded by IL-6 derived from pMOs. Interestingly, the ability to expand IL-17A+ iTregs or Th17 cells was not detected in pMOs obtained from MP patients at the acute stage. These results indicate that pMOs present at the resolution stage of MP infection, but not at the acute stage, have the potent ability to drive the development of iTregs toward a Th17 phenotype, thereby inhibiting Treg suppressive functions via the release of IL-6 (Figure 6C).
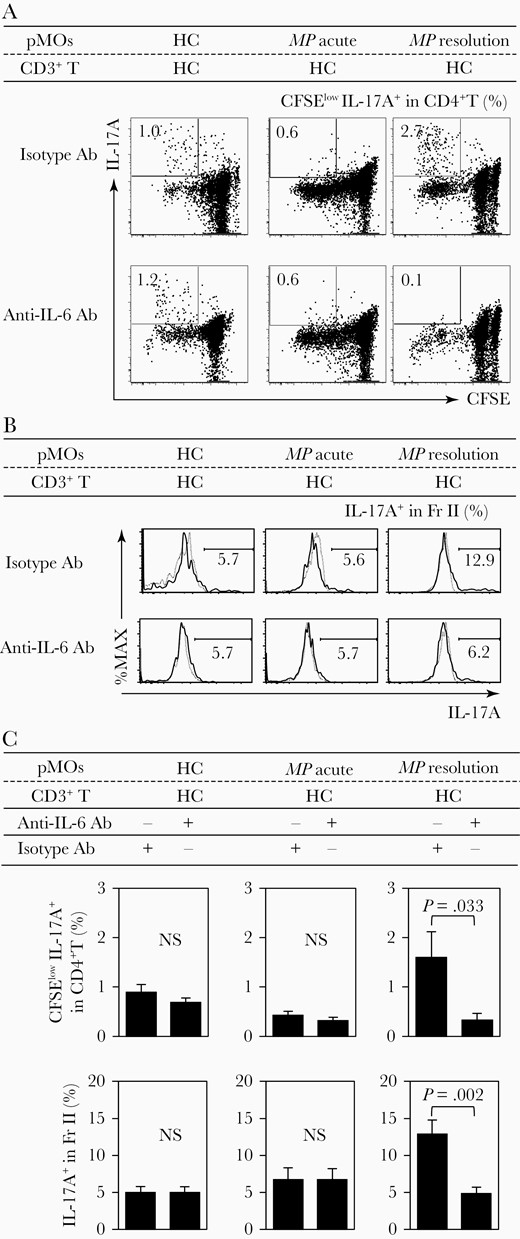
Increased Th17 cells and interleukin (IL) 17A+ induced regulatory T cells (iTregs) by IL-6 from proinflammatory monocytes (pMOs) of patients with Mycoplasma pneumoniae (resolution stage). A, Representative dot plots showing the frequencies of 5-(and -6)-Carboxyfluorescein diacetate succinimidyl ester (CFSE)low Th17 cells. B, Representative histograms showing IL-17A+ cells in iTregs. C, Mean frequency of CFSElow Th17 cells and IL-17A+ cells in iTreg in cocultures of healthy control (HC) T cells and HC-pMOs or MP infection pMOs (acute/resolution) under the presence/absence of anti–IL-6 antibody (Ab). Each value represents mean ± standard error of the mean. MP patients, n = 8; HCs, n = 13.
DISCUSSION
In this study, we provide a cellular and molecular explanation for why MP infection could predispose individuals to persistent and defective Treg responses. We for the first time demonstrate that Treg function becomes defective not only in the acute stage of MP as well as viral infections but also in the resolution stage of MP infection. To elucidate the mechanism behind the Treg dysfunction, we have focused on monocytes and Treg/Th17 cell interplay, given that their interaction might be important for the initiation and shaping of subsequent immune responses in the setting of infection. Treg dysfunction in the acute stage of MP infection was found to be an MO-independent, relatively common mechanism that can temporarily limit Treg function in the acute stage of infection, because similar, transient Treg dysfunction can be also observed in the setting of many viral infections. In contrast, Treg dysfunction specifically observed in the resolution stage of MP infection was quite unique, in that it cannot be observed in the setting of viral infection; and it was totally dependent on “aberrant” pMO. Thus, MP infection could trigger not only development of allergic diseases but also lead to a true autoimmune disease in susceptible individuals [21]. In support of this view, previous clinical reports described that patients with MP-associated SJS displayed polysensitivity to multiple drugs with different structures that cannot be easily explained by drug antigen-driven T-cell activation [22]. Tregs also have been shown to suppress unrelated immune responses in a non-antigen-specific manner either through cell-to-cell contact or through the regulatory cytokines they produce, a mechanism known as bystander suppression [3]. Thus, their decreasing Treg function in patients with MP infection would serve to lower the activation threshold of drug-specific or allergen-specific T cells, thus facilitating the development of drug eruptions or asthma.
Many microbes, especially commensal bacteria, have evolved immunomodulatory mechanisms that subvert a host’s immune system to establish chronic infections. In general, microbial products enhance rather than abrogate Treg cell functions [23]. If so, how does MP persistently abrogate Treg functions even after clinical resolution whereas, in other viral infections, such as VZV, parvovirus B19, and EBV, a loss of Treg function is transient and the defective Tregs regain their functional competence upon clinical resolution? TLR2 ligation on mouse Tregs [18] and human Tregs [24] have been shown to abrogate suppressive Treg function: many recent studies have shown that ligation of TLR2 by synthetic ligands results in expansion of Treg cells but temporarily abrogates their suppressive function. Given the ability of MP to stimulate TLR2 signaling pathway, Tregs at the acute stage of MP infection are likely to lose their suppressive capacity in response to engagement of TLRs without the need for interactions with MOs. A prolonged loss of Treg function specifically observed at the resolution stage of MP infection, but not in other viral infections, suggested an alternative mechanism by which Treg function could be persistently abrogated. In this regard, this study clearly demonstrates that “aberrant” pMOs at the resolution stage of MP infection can drive Tregs to differentiate further to a Th17 phenotype through their ability to abundantly produce IL-6, thereby limiting the suppression by Tregs. In view of the crucial role of pMOs for mediating innate host defense responses [25], MOs, either pMOs or cMOs, could be endowed with the capacity to fine-tune Treg functions as positive and negative regulators based on their unique cytokine production profile: Generally cMOs would serve to maintain Treg function by producing IL-10 [16]; however, this effect in the setting of MP infection would be observed antagonistically by the direct action of MP on the TLR2 signaling pathway, which may allow the persistence of this pathogen. In the resolution stage, “aberrant” pMOs would deliver a negative signal, IL-6, upon interaction with Tregs, hence tempering the function of Tregs through the conversion to a Th17-like phenotype. A similar shift to a “aberrant” pMOs with the capacity to drive Tregs to a Th17 phenotype was also observed in a certain type of severe drug eruptions, termed drug-induced hypersensitivity syndrome/drug reaction with eosinophilia and systemic symptoms (DiHS/DRESS), in which Treg functions became impaired after clinical resolution and their defects were associated with the appearance of autoimmune disease [16, 26–28]. These “aberrant” pMOs, in DiHS/DRESS, which can drive Tregs to differentiate further to a Th17 phenotype through the release of IL-6 [17], share some characteristics with those observed at the resolution stage of MP infection and may contribute to the persistent loss of Treg function. Thus, our results suggest a previously unrecognized mechanism that could explain the persistent loss of Treg function in the setting of MP infections.
Our results indicate that pMOs uniquely detected in patients with MP infection in the resolution stage might have pathologic role for driving Tregs to a Th17-like phenotype owing to their preferential production of IL-6 in response to TLR2 ligand stimulation and that cytokines produced by the pMOs are key to determining the balance between Tregs and Th17 cells. Given the possibility that a large percentage of adults may be latently infected with MP, if even a portion of the effect we observed in this study could extend to healthy individuals, MP could prove to be playing a role in many cases of severe drug eruptions. Thus, pMOs present in the resolution stage of MP infection have unique functional properties, such as preferential production of IL-6, different from those observed in HCs and other viral infections. Pathological events uniquely observed in MP infection preceding severe drug eruption and asthma can be attributed to the effect of IL-6 produced by “aberrant” pMOs.
Because iTregs and Th17 cells have a higher lineage plasticity than have been recognized thus far [29–32], IL-17A+ iTregs we identified at the resolution stage of MP infection and among in vitro cocultures may exist as a transient population that differentiates into either iTreg or Th17 cells, as demonstrated by Zhou et al [31]. In view of our finding that Th17 cells were increased at the resolution stage but not in the acute stage of MP infection, while IL-17A+ iTregs were increased in MP infection regardless of the stage, we suggest that iTregs can differentiate into IL-17A+ iTregs and eventually to Th17 cells in the presence of IL-6–producing pMOs in the setting of MP infection.
CONCLUSIONS
Our results suggest that preceding or underlying MP infection, either symptomatic or asymptomatic, and perhaps other viral infections (in case of acute stage), may alter one’s susceptibility to developing severe drug eruptions and asthma by persistently abrogating Treg function directly (acute stage) or indirectly through the interaction with “aberrant” pMOs producing IL-6 (resolution stage). Because such aberrant pMOs were not detected in many viral infection even at the resolution stage (authors’ unpublished data), Treg dysfunction by generation of aberrant pMOs would represent a monocyte-dependent, unique mechanism by which Treg function becomes defective during the resolution stage of MP infection; in contrast, Treg dysfunction observed at the acute stage would be a monocyte-independent, relatively common mechanism that can temporarily limit Treg function during the acute stage of various infections. We propose that patients with MP infection should be carefully followed up even after clinical resolution because they are at greater risk of eventually developing allergic diseases and autoimmune diseases. Our new insights highlight the possibility that pMOs/IL-6 in MP infection could act as negative modulators of Treg responses in vivo and thus considered as critical therapeutic targets against MP-induced allergic diseases, such as SJS/TEN.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Financial support. This work was supported in part by the Ministry of Education, Culture, Sports, Science and Technology of Japan (grant number 24390276 to T. S.); the Ministry of Health, Labor and Welfare of Japan (to T. S.); the Japanese Research Committee on Severe Cutaneous Adverse Reaction (H26-nanchi(nan)-ippan-081 to T. S.); and the Japan Society for the Promotion of Science Grant-in-Aid for Scientific Research (C) (grant number 15K09781 to R. T.).
Potential conflicts of interest. All authors: No reported conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.




