-
PDF
- Split View
-
Views
-
Cite
Cite
Christina J. Atchison, Julia Stowe, Nick Andrews, Sarah Collins, David J. Allen, Sameena Nawaz, David Brown, Mary E. Ramsay, Shamez N. Ladhani, Rapid Declines in Age Group–Specific Rotavirus Infection and Acute Gastroenteritis Among Vaccinated and Unvaccinated Individuals Within 1 Year of Rotavirus Vaccine Introduction in England and Wales, The Journal of Infectious Diseases, Volume 213, Issue 2, 15 January 2016, Pages 243–249, https://doi.org/10.1093/infdis/jiv398
Close - Share Icon Share
Abstract
Background. The oral infant rotavirus vaccine, Rotarix, was introduced in England and Wales in July 2013. We estimated the impact on laboratory-confirmed rotavirus infections and hospitalizations for all-cause acute gastroenteritis (AGE) during the first year after introduction.
Methods. We extracted data on laboratory-confirmed rotavirus infections (July 2000 through June 2015) and all-cause AGE–associated hospitalizations (July 2007 through June 2014) for all age groups using national databases (LabBase2 and HES). We determined the ratio of the rate during the 2013–2014 rotavirus season to the rate during the prevaccination era.
Results. In infants, there was a 77% decline (rate ratio [RR], 0.23; 95% confidence interval [CI], .16–.32) in laboratory-confirmed rotavirus infections and a 26% decline (RR, 0.74; 95% CI, .65–.84) in all-cause AGE–associated hospitalizations in 2013–2014, compared with the prevaccination era. Large reductions were also observed in older children, adults, and older adults. We estimated that 10 884 laboratory-confirmed infections and 50 427 all-cause AGE–associated hospital admissions were averted in 2013–2014. Similar reductions have been observed for laboratory-confirmed rotavirus infections during the 2014–2015 season.
Conclusions. The rapid declines in rotavirus infection and AGE in vaccinated and unvaccinated age groups within 1 year of introducing an infant rotavirus vaccination program are far greater than expected and than previously reported by other countries.
Rotavirus is the most common cause of severe gastroenteritis among children aged <5 years worldwide [1]. In England and Wales, prior to routine immunization, rotavirus gastroenteritis placed a huge burden on healthcare resources, being responsible for an estimated 750 000 episodes of diarrhea, 80 000 general practice consultations [2], and 14 300 hospital admissions in children aged <5 years annually [3]. The cost to the National Health Service (NHS) was estimated to be £14.2 million per year [3].
Rotarix (GlaxoSmithKline Biologicals, Rixensart, Belgium), an oral monovalent live-attenuated human rotavirus vaccine, was added to the immunization program in England and Wales on 1 July 2013 as a 2-dose schedule for infants at 2 and 3 months of age. The vaccine strain in Rotarix is derived from the most-common circulating wild-type human rotavirus strain, G1P [4], and is the only rotavirus vaccine used in the United Kingdom [5]. The high protective efficacy (>85%) of Rotarix against severe rotavirus gastroenteritis has been demonstrated in a number of large randomized controlled trials in middle-income and high-income countries [5]. Postlicensure studies for Rotarix from the United States, Latin America, Australia, and Europe have demonstrated its impact in real-world settings, with significant reductions in hospital admissions for rotavirus disease [6]. A 29%–48% reduction in all-cause diarrhea hospitalizations and a 35%–81% reduction in rotavirus-specific hospitalizations has been reported from active surveillance in sentinel centers in Belgium, Austria, Australia, El Salvador, and Brazil [6–8]. Passive surveillance studies in Australia and Latin America have shown an 11%–40% reduction in all-cause diarrhea hospitalizations in children aged <5 years [4, 6, 9]. Similar findings have been reported with RotaTeq (Merck, Whitehouse Station, New Jersey), a pentavalent human-bovine reassortment vaccine, which is the only other licensed rotavirus vaccine available [10]. In many studies, positive impacts were observed not only in the vaccine-eligible cohorts but also in unvaccinated older children and young adults, providing evidence of herd protection [6, 11–13].
This study examined national data on vaccine coverage, hospitalizations for acute gastroenteritis (AGE), and reports of laboratory-confirmed rotavirus to evaluate the impact of the national rotavirus immunization program in England and Wales. Although the United Kingdom is not the first European country to introduce rotavirus vaccines into its national infant immunization program, it is one of the first to use Rotarix exclusively and, therefore, provides a unique opportunity to assess the early impact of this specific vaccine nationally and across all age groups in an industrialized setting with high vaccine coverage.
METHODS
Data Sources
Vaccine coverage
A sentinel surveillance program was set up to extract data on coverage monthly directly from general practice systems for children reaching the upper age for vaccination (25 weeks) [14]. The ImmForm web-based system has been developed over a number of years, collecting and presenting data in relation to immunization programs in England. The system automatically extracts data from participating general practice clinical systems, stores the data securely, and provides a variety of hierarchical reports to support program performance management at local and national levels. Vaccine coverage data can be reported at the desired frequency, usually monthly, allowing rapid assessment of new vaccination programs. Monthly data on rotavirus vaccine coverage are collected on the number of infants in a general practice who, in the survey month, reach 25 weeks of age (denominator) and the number of infants in the denominator who received (1) a first dose and (2) a second dose of Rotarix from 6 weeks of age up to 24 weeks of age, including vaccinations given by other healthcare providers. General practice participation ranged from 84% to 91% of all general practices in England every month [14]. Coverage data were available from October 2013 through March 2015.
National Laboratory Reports (LabBase2)
Microbiology laboratories across England and Wales receive patients’ stool specimens for testing from clinicians in hospital or primary care settings and electronically report laboratory-confirmed rotavirus infections to Public Health England (PHE) for surveillance purposes. Reporting by diagnostic laboratories is voluntary, but a recent survey in England and Wales indicated that laboratory testing and reporting practices are generally high and consistent year round [15]. We extracted weekly counts (by date of specimen) of laboratory-confirmed rotavirus infections in all age groups between July 2000 and June 2015.
Hospital Episode Statistics (HES)
The HES database holds records on all episodes of NHS hospital care in England. The main reason for admission (primary diagnosis) and up to 19 secondary diagnoses are coded using the International Classification of Diseases, Tenth Revision (ICD-10) [16]. We extracted weekly counts of hospital admissions for all-cause AGE (ICD-10 codes A00–A09, for infectious intestinal diseases, and codes K52.9 and P78.3, for unspecified noninfectious intestinal diseases; Supplementary Table A1) for all age groups for all available years (July 2007 through June 2014). Coding of hospital episodes is based on clinical and microbiological information recorded by clinicians at the time patients are discharged. Our case definition of all-cause AGE was deliberately broad because many patients with infectious AGE will not have routinely undergone microbiological testing or received a specific microbiological diagnosis before discharge. Therefore, more-specific infectious AGE diagnoses are potentially subject to misclassification within HES, and restricting our analysis to rotavirus-specific AGE–associated admissions would substantially underestimate the clinical impact of the rotavirus vaccine on AGE. Codes for unspecified noninfectious intestinal diseases were used because previous studies have shown that these codes are often associated with infectious causes, as they exhibit the same seasonal pattern as codes for infectious intestinal diseases [3].
Data Analysis
We analyzed data by rotavirus epidemiological year, defined as July through June. To calculate rates, we obtained mid-year population estimates for year and age group from the Office of National Statistics [17]. Data were analyzed as a time series of weekly counts of (1) laboratory-confirmed rotavirus infections and (2) hospital admissions for all-cause AGE. We fitted negative binomial models to counts with an offset for the denominator (population estimates by year and age), controlling for secular trends (as a function of year as a linear variable). A variable indicating postvaccination era was used to determine the ratio of the rates in the 2013–2014 rotavirus season to those in the prevaccination era. Separate models were fitted for each of 9 age groups (<1 year, 1 year, 2 years, 3 years, 4 years, 5–14 years, 15–44 years, 45–64 years, and ≥65 years) for comparison with previous studies [4, 8, 10], whereby only children in the <1 year age group were in the vaccinated cohort 1 year into the rotavirus vaccination program. To investigate autocorrelation, we examined the residuals from the models (logged differences between observed and predicted numbers). Although there was some evidence of 1-week and 2-week autocorrelations, models including these lag terms gave almost identical estimates and standard errors for the vaccine indicator variable, so the terms were not included in final models. Averted laboratory-confirmed rotavirus infections and all-cause AGE–associated hospital admissions were estimated by fitting the models to the prevaccination data only (taking into account secular trends) and then calculating the difference between the actual numbers observed and those predicted from these models for the 2013–2014 rotavirus season.
Statistical significance was at the 5% level (2-sided). We analyzed data with Stata IC, version 13.0 (StatCorp, College Station, Texas).
Ethical Approval
Public Health England has approval, under Patient Information Advisory Group Section 60 of the Health and Social Care Act 2001, to process confidential information from patients for the purposes of monitoring the efficacy and safety of vaccination programs.
RESULTS
A total of 206 750 laboratory-confirmed rotavirus infections were reported in England and Wales between July 2000 and June 2015 (a 15-year period). Between July 2007 and June 2014 (a 7-year period), there were 2 251 424 hospital admissions for all-cause AGE, of which 0.7% were coded as rotavirus-specific AGE. Most infectious AGE in HES was coded as A09, “diarrhea and gastroenteritis of presumed infectious origin” (53% of all infectious AGE HES diagnoses; Supplementary Table A2).
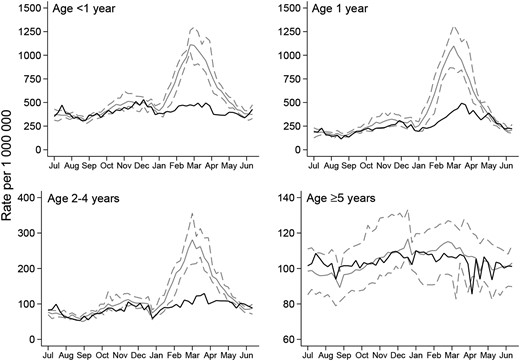
Figure 1A shows that the annual rate of laboratory-confirmed rotavirus infections was relatively stable during the prevaccination period from July 2000 through June 2013 and declined after vaccination was introduced in July 2013. This decline coincided with the rapid attainment and maintenance of a national oral rotavirus vaccine coverage level of 93% for a full vaccine course (2 doses) by 25 weeks of age. There was a secular increase in the annual rate of all-cause AGE–associated hospital admissions during the prevaccination period from July 2007 through June 2013, followed by a decline between July 2013 and June 2014 (Figure 1B). Substantial attenuation in laboratory-confirmed rotavirus infections and all-cause AGE–associated hospital admissions was seen across all age groups among children <5 years old but was most marked in infants aged <1 year, the age group that included children in the vaccinated cohort 1 year into the rotavirus vaccination program (Figures 2 and 3).
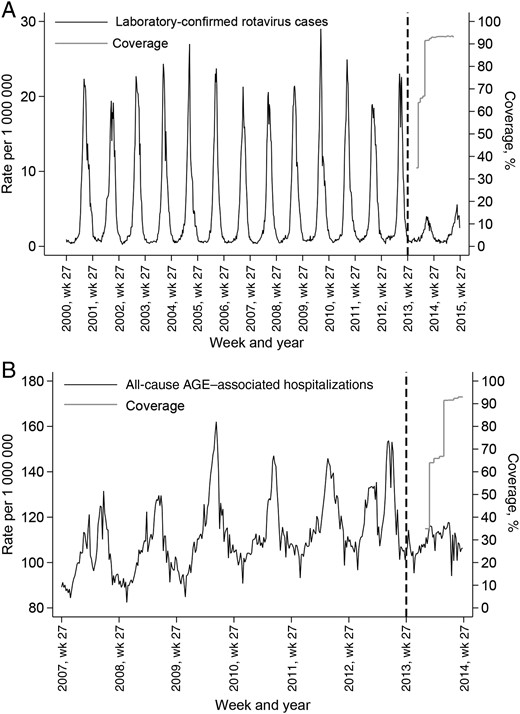
Infant rotavirus vaccine coverage and weekly rate (per 1 000 000) of laboratory-confirmed rotavirus infections reported (A) and all-cause acute gastroenteritis (AGE)–associated hospital admissions (B) in England and Wales, July 2000 through June 2015. Dashed black lines represent the start of a national universal rotavirus immunization program with Rotarix (1 July 2013). Coverage assessment was for a full vaccine course (2 doses) by 25 weeks of age.

Weekly rate (per 1 000 000), by age group, of laboratory-confirmed rotavirus infections reported in England and Wales, July 2000 through June 2015. Solid gray lines represent the mean weekly rate for the prevaccination era. Dashed gray lines represent the maximum and minimum weekly rate for the prevaccination era. Solid black lines represent the weekly rate for the postvaccination rotavirus season (July 2013 through June 2014). Dashed black lines represent the weekly rate for the postvaccination rotavirus season (July 2014 through June 2015).
Weekly rate (per 1 000 000), by age group, of all-cause acute gastroenteritis–associated hospital admissions in England and Wales, July 2007 through June 2014. Solid gray lines represent the mean weekly rate for the prevaccination era. Dashed gray lines represent the maximum and minimum weekly rate for the prevaccination era. Solid black lines represent the weekly rate for the postvaccination rotavirus season (July 2013 through June 2014).
We found statistically significant reductions in laboratory-confirmed rotavirus infections and all-cause AGE–associated hospital admissions during 2013–2014, compared with the prevaccination era, in all age groups (Table 1). The largest reductions were seen in the youngest age groups. In infants <1 year of age, there was a 77% decline (rate ratio [RR], 0.23; 95% confidence interval [CI], .16–.32; P < .0001) in laboratory-confirmed rotavirus infections. We also observed large reductions in laboratory-confirmed rotavirus infections in older unvaccinated age groups. In individuals aged ≥5 years, there was a 50% decline (RR, 0.50; 95% CI, .37–.67; P < .0001) in laboratory-confirmed rotavirus infections (Table 1). The decline in all-cause AGE–associated hospitalizations in infants <1 year of age was 26% (RR, 0.74; 95% CI, .65–.84; P < .0001), resulting in 5256 hospital admissions averted in this age group during 2013–2014. The largest decline in all-cause AGE–associated hospital admissions (33%) was seen in children aged 1 year (RR, 0.67; 95% CI, .54–.82; P < .0001), resulting in 4648 hospitalizations averted in this age group during 2013–2014. In total, we estimated that 10 884 laboratory-confirmed rotavirus infections and 50 427 all-cause AGE–associated hospital admissions across all age groups were averted during 2013–2014 (Table 1). Of note, 90% of all averted all-cause AGE–associated hospital admissions (45 171 of 50 427) were in unvaccinated age groups; 42% alone (21 368 of 50 427) were in adults aged ≥65 years. Restricting our analysis to rotavirus-specific AGE admissions in children aged <5 years, we observed an 80% reduction (RR, 0.20; 95% CI, .14–.30; P < .0001) in infants <1 year of age (970 hospitalizations averted) and a 63% reduction (RR, 0.37; 95% CI, .22–.64; P < .0001) in children aged 1–4 years (834 hospitalizations averted).
Laboratory-Confirmed Rotavirus Infections and All-Cause Acute Gastroenteritis–Associated Hospital Admissions Before and After Vaccine Introduction in England and Wales, Including Estimated Numbers of Events Averted by the Rotavirus Vaccination Program
| Event, Age . | Events Before Introduction, No., Mean (Minimum) . | Events AfterIntroduction, No. . | RR (95% CI) . | P Values . | Events Averted, No. . | |
|---|---|---|---|---|---|---|
| Actual . | Predicteda . | |||||
| Laboratory-confirmed infection | ||||||
| <1 y | 6041 (5310) | 1402 | 6212 | 0.23 (.16–.32) | <.0001 | 4810 |
| 1 y | 5417 (4895) | 2083 | 6109 | 0.34 (.23–.50) | <.0001 | 4026 |
| 2 y | 1696 (1402) | 604 | 1691 | 0.36 (.24–.52) | <.0001 | 1087 |
| 3 y | 617 (534) | 207 | 612 | 0.34 (.23–.50) | <.0001 | 405 |
| 4 y | 316 (257) | 112 | 322 | 0.35 (.23–.52) | <.0001 | 210 |
| ≥5 yb | 788 (652) | 343 | 689 | 0.50 (.37–.67) | <.0001 | 346 |
| Overall | … | … | … | … | … | 10 884 |
| All-cause acute gastroenteritis admission | ||||||
| <1 y | 20 663 (20 131) | 15 101 | 20 357 | 0.74 (.65–.84) | <.0001 | 5256 |
| 1 y | 14 678 (14 019) | 10 078 | 14 726 | 0.67 (.54–.82) | <.0001 | 4648 |
| 2 y | 6108 (5760) | 4524 | 6089 | 0.73 (.61–.88) | <.0001 | 1565 |
| 3 y | 3490 (3074) | 2986 | 3696 | 0.81 (.69–.94) | <.0001 | 710 |
| 4 y | 2490 (2197) | 2402 | 2735 | 0.88 (.77–1.00) | <.006 | 333 |
| 5–14 y | 11 426 (10 446) | 11 782 | 12 802 | 0.92 (.87–.98) | <.0001 | 1020 |
| 15–44 y | 64 381 (56 408) | 70 781 | 76 824 | 0.92 (.90–.94) | <.0001 | 6043 |
| 45–64 y | 64 734 (53 920) | 71 082 | 80 566 | 0.88 (.86–.90) | <.0001 | 9484 |
| ≥65 y | 133 468 (121 978) | 134 079 | 155 447 | 0.86 (.83–.90) | <.0001 | 21 368 |
| Overall | … | … | … | … | … | 50 427 |
| Event, Age . | Events Before Introduction, No., Mean (Minimum) . | Events AfterIntroduction, No. . | RR (95% CI) . | P Values . | Events Averted, No. . | |
|---|---|---|---|---|---|---|
| Actual . | Predicteda . | |||||
| Laboratory-confirmed infection | ||||||
| <1 y | 6041 (5310) | 1402 | 6212 | 0.23 (.16–.32) | <.0001 | 4810 |
| 1 y | 5417 (4895) | 2083 | 6109 | 0.34 (.23–.50) | <.0001 | 4026 |
| 2 y | 1696 (1402) | 604 | 1691 | 0.36 (.24–.52) | <.0001 | 1087 |
| 3 y | 617 (534) | 207 | 612 | 0.34 (.23–.50) | <.0001 | 405 |
| 4 y | 316 (257) | 112 | 322 | 0.35 (.23–.52) | <.0001 | 210 |
| ≥5 yb | 788 (652) | 343 | 689 | 0.50 (.37–.67) | <.0001 | 346 |
| Overall | … | … | … | … | … | 10 884 |
| All-cause acute gastroenteritis admission | ||||||
| <1 y | 20 663 (20 131) | 15 101 | 20 357 | 0.74 (.65–.84) | <.0001 | 5256 |
| 1 y | 14 678 (14 019) | 10 078 | 14 726 | 0.67 (.54–.82) | <.0001 | 4648 |
| 2 y | 6108 (5760) | 4524 | 6089 | 0.73 (.61–.88) | <.0001 | 1565 |
| 3 y | 3490 (3074) | 2986 | 3696 | 0.81 (.69–.94) | <.0001 | 710 |
| 4 y | 2490 (2197) | 2402 | 2735 | 0.88 (.77–1.00) | <.006 | 333 |
| 5–14 y | 11 426 (10 446) | 11 782 | 12 802 | 0.92 (.87–.98) | <.0001 | 1020 |
| 15–44 y | 64 381 (56 408) | 70 781 | 76 824 | 0.92 (.90–.94) | <.0001 | 6043 |
| 45–64 y | 64 734 (53 920) | 71 082 | 80 566 | 0.88 (.86–.90) | <.0001 | 9484 |
| ≥65 y | 133 468 (121 978) | 134 079 | 155 447 | 0.86 (.83–.90) | <.0001 | 21 368 |
| Overall | … | … | … | … | … | 50 427 |
Data obtained before vaccine introduction are from 2000–2001 to 2012–2013 (for laboratory-confirmed infections) and from 2007–2008 to 2012–2013 (for all-cause acute gastroenteritis admissions). Data from after vaccine introduction are for 2013–2014.
Abbreviations: CI, confidence interval; RR, rate ratio.
a Predicted cases based on extrapolation of the trend in incidence before 2013–2014, using negative binomial regression.
b We hypothesized that indirect protection may be afforded to adults of child-bearing age, so smaller age groups were initially considered. However, because the number of reported laboratory-confirmed rotavirus infections in older children and adult age groups was small, the population aged ≥5 years was combined to maximize statistical power.
Laboratory-Confirmed Rotavirus Infections and All-Cause Acute Gastroenteritis–Associated Hospital Admissions Before and After Vaccine Introduction in England and Wales, Including Estimated Numbers of Events Averted by the Rotavirus Vaccination Program
| Event, Age . | Events Before Introduction, No., Mean (Minimum) . | Events AfterIntroduction, No. . | RR (95% CI) . | P Values . | Events Averted, No. . | |
|---|---|---|---|---|---|---|
| Actual . | Predicteda . | |||||
| Laboratory-confirmed infection | ||||||
| <1 y | 6041 (5310) | 1402 | 6212 | 0.23 (.16–.32) | <.0001 | 4810 |
| 1 y | 5417 (4895) | 2083 | 6109 | 0.34 (.23–.50) | <.0001 | 4026 |
| 2 y | 1696 (1402) | 604 | 1691 | 0.36 (.24–.52) | <.0001 | 1087 |
| 3 y | 617 (534) | 207 | 612 | 0.34 (.23–.50) | <.0001 | 405 |
| 4 y | 316 (257) | 112 | 322 | 0.35 (.23–.52) | <.0001 | 210 |
| ≥5 yb | 788 (652) | 343 | 689 | 0.50 (.37–.67) | <.0001 | 346 |
| Overall | … | … | … | … | … | 10 884 |
| All-cause acute gastroenteritis admission | ||||||
| <1 y | 20 663 (20 131) | 15 101 | 20 357 | 0.74 (.65–.84) | <.0001 | 5256 |
| 1 y | 14 678 (14 019) | 10 078 | 14 726 | 0.67 (.54–.82) | <.0001 | 4648 |
| 2 y | 6108 (5760) | 4524 | 6089 | 0.73 (.61–.88) | <.0001 | 1565 |
| 3 y | 3490 (3074) | 2986 | 3696 | 0.81 (.69–.94) | <.0001 | 710 |
| 4 y | 2490 (2197) | 2402 | 2735 | 0.88 (.77–1.00) | <.006 | 333 |
| 5–14 y | 11 426 (10 446) | 11 782 | 12 802 | 0.92 (.87–.98) | <.0001 | 1020 |
| 15–44 y | 64 381 (56 408) | 70 781 | 76 824 | 0.92 (.90–.94) | <.0001 | 6043 |
| 45–64 y | 64 734 (53 920) | 71 082 | 80 566 | 0.88 (.86–.90) | <.0001 | 9484 |
| ≥65 y | 133 468 (121 978) | 134 079 | 155 447 | 0.86 (.83–.90) | <.0001 | 21 368 |
| Overall | … | … | … | … | … | 50 427 |
| Event, Age . | Events Before Introduction, No., Mean (Minimum) . | Events AfterIntroduction, No. . | RR (95% CI) . | P Values . | Events Averted, No. . | |
|---|---|---|---|---|---|---|
| Actual . | Predicteda . | |||||
| Laboratory-confirmed infection | ||||||
| <1 y | 6041 (5310) | 1402 | 6212 | 0.23 (.16–.32) | <.0001 | 4810 |
| 1 y | 5417 (4895) | 2083 | 6109 | 0.34 (.23–.50) | <.0001 | 4026 |
| 2 y | 1696 (1402) | 604 | 1691 | 0.36 (.24–.52) | <.0001 | 1087 |
| 3 y | 617 (534) | 207 | 612 | 0.34 (.23–.50) | <.0001 | 405 |
| 4 y | 316 (257) | 112 | 322 | 0.35 (.23–.52) | <.0001 | 210 |
| ≥5 yb | 788 (652) | 343 | 689 | 0.50 (.37–.67) | <.0001 | 346 |
| Overall | … | … | … | … | … | 10 884 |
| All-cause acute gastroenteritis admission | ||||||
| <1 y | 20 663 (20 131) | 15 101 | 20 357 | 0.74 (.65–.84) | <.0001 | 5256 |
| 1 y | 14 678 (14 019) | 10 078 | 14 726 | 0.67 (.54–.82) | <.0001 | 4648 |
| 2 y | 6108 (5760) | 4524 | 6089 | 0.73 (.61–.88) | <.0001 | 1565 |
| 3 y | 3490 (3074) | 2986 | 3696 | 0.81 (.69–.94) | <.0001 | 710 |
| 4 y | 2490 (2197) | 2402 | 2735 | 0.88 (.77–1.00) | <.006 | 333 |
| 5–14 y | 11 426 (10 446) | 11 782 | 12 802 | 0.92 (.87–.98) | <.0001 | 1020 |
| 15–44 y | 64 381 (56 408) | 70 781 | 76 824 | 0.92 (.90–.94) | <.0001 | 6043 |
| 45–64 y | 64 734 (53 920) | 71 082 | 80 566 | 0.88 (.86–.90) | <.0001 | 9484 |
| ≥65 y | 133 468 (121 978) | 134 079 | 155 447 | 0.86 (.83–.90) | <.0001 | 21 368 |
| Overall | … | … | … | … | … | 50 427 |
Data obtained before vaccine introduction are from 2000–2001 to 2012–2013 (for laboratory-confirmed infections) and from 2007–2008 to 2012–2013 (for all-cause acute gastroenteritis admissions). Data from after vaccine introduction are for 2013–2014.
Abbreviations: CI, confidence interval; RR, rate ratio.
a Predicted cases based on extrapolation of the trend in incidence before 2013–2014, using negative binomial regression.
b We hypothesized that indirect protection may be afforded to adults of child-bearing age, so smaller age groups were initially considered. However, because the number of reported laboratory-confirmed rotavirus infections in older children and adult age groups was small, the population aged ≥5 years was combined to maximize statistical power.
Laboratory data on rotavirus infections were also available for the 2014–2015 season, from July 2014 through June 2015. Weekly reports of laboratory-confirmed rotavirus infections in 2014–2015 remained at low levels, particular in vaccinated cohorts, indicating sustained reduction in rotavirus infections during the second year of the rotavirus immunization program (Figures 1A and 2).
DISCUSSION
The introduction of the oral rotavirus vaccination program in July 2013 led to rapid attainment of high levels of vaccine coverage in England and Wales and was associated with rapid and significant reductions in both laboratory-confirmed rotavirus infections and all-cause AGE–associated hospital admissions, which has continued into the second year of the program. The greatest impact was observed in infants <1 year of age, who were eligible to receive the vaccine as part of the national program. What makes our results more impressive is that, although high vaccine coverage was rapidly achieved in vaccine-eligible children, overall vaccine coverage for infants <1 year of age would have been lower. Even if every eligible birth was associated with vaccination (100% coverage), the mean coverage among infants <1 year of age in the first year of the program would still be only 50%, since, at the beginning of the year, 0% would have been vaccinated and, at the end of the year, 100% would have been vaccinated (50% overall). Significant reductions were also seen in unvaccinated older infants, children, and adults, which may be in part due to indirect effects of introducing the infant program.
Exploring the impact of rotavirus vaccination on all-cause AGE admissions can be considered equivalent to a vaccine-probe study, where the prevented unspecific disease burden can be assumed to be caused by the agent that the vaccine is targeted against. Before routine vaccination, rotavirus gastroenteritis was estimated to be responsible for 14 300 hospital admissions in children aged <5 years annually in England and Wales [3]. Consistent with these findings, we estimated that 12 512 all-cause AGE–associated hospital admissions in children aged <5 years were averted during 2013–2014. This reduction in hospitalizations is unexpectedly high considering that only infants <1 year of age were eligible for vaccination, and it suggests that previous studies may have underestimated the number of rotavirus-attributable hospital admissions in England and Wales [3].
The direct impact we observed in the vaccine-eligible cohort (ie, infants <1 year of age) was similar to that in reports from other countries with national rotavirus immunization programs. In Europe, countries that have implemented a universal infant rotavirus immunization program have observed 65%–84% reductions in rotavirus-specific hospitalizations in vaccine-eligible children following vaccine introduction [7, 8, 18–20]. In Belgium, where coverage is estimated to be at least 90% and where both Rotarix and RotaTeq are in use, a 50%–61% decline in laboratory-confirmed rotavirus infections was observed across all pediatric age groups in postvaccination years, compared to the prevaccination era [21]. In Australia, in the first postvaccination year (during which Rotarix and RotaTeq were used), rotavirus-specific hospitalizations among infants <1 year of age decreased by 50% [12]. In the United States, a 81% reduction in rotavirus-coded hospitalizations and a 24% reduction in diarrhea-associated hospitalizations were observed in vaccine-eligible children in the first year following the introduction of rotavirus vaccine (RotaTeq) [13].
Reductions have also been observed among unvaccinated older infants, children, and young adults [11, 12], providing evidence of indirect protection. In Australia, following introduction of rotavirus vaccination in 2007, reductions in rotavirus-specific and all-cause AGE–associated hospitalizations were observed in older children (5–19 years of age) during the first 3 years after vaccination, including a 48% reduction in rotavirus-specific hospitalizations in the first year [12]. Rates in adult age groups remained unchanged [12]. In Queensland, Australia, the introduction of Rotateq was associated with a 50%–60% reduction in rotavirus hospitalizations among individuals aged <20 years and a 60% reduction in hospitalizations due to nonrotavirus–associated AGE among children aged <5 years by 2008, with no reduction observed in any other age group [22]. In the United States, where Rotateq was introduced in 2006, rotavirus-attributable and cause-unspecified gastroenteritis–associated discharges significantly decreased in 2008 among individuals aged 0–4 years, 5–14 years, and 15–24 years [11]. The authors estimated that 15% of 66 000 averted hospitalizations attributable to the vaccination program were among unvaccinated people 5–24 years old. However, annual rotavirus-attributable and cause-unspecified gastroenteritis–associated discharges were not significant reduced in ≥25 year olds [11]. Indirect protection in adults was also inferred from a 48% reduction in rotavirus positivity among stool samples sent for testing that were obtained from inpatients and outpatients at one hospital in Chicago [23]. Our findings support these recent studies highlighting a larger than previously recognized burden of rotavirus disease in older children and young adults. These results highlight infants as key mediators for sustaining rotavirus transmission within households and in the wider community.
However, in England and Wales, the reduction in both laboratory-confirmed rotavirus infections and all-cause AGE–associated hospitalizations in adults within 12 months of the introduction of the rotavirus vaccination program, particularly among older adults, who contributed the highest number of averted all-cause AGE–associated hospitalizations in our study, has not been previously reported. The greater than expected reduction in disease burden that we observed in unvaccinated age groups could, in part, be explained by indirect vaccine effects through the rapid attainment and maintenance of high vaccination coverage among infants, especially when compared to the United States, for example, where coverage with ≥1 Rotateq dose by January 2008 was 57% among infants <1 year old, 17% among those 1 year old, and negligible among older children [11].
Our study has strengths and limitations. The use of large, long-term, single-source, national data sets allowed rapid assessment of both the microbiological and clinical impact of rotavirus vaccination. The analysis, however, was descriptive and ecological, and, therefore, the effects measured may be due to other factors not related to immunization. Rotavirus testing practices, for example, could have changed in the postvaccination period. However, PHE sentinel surveillance across a number of NHS hospital microbiology laboratories in England indicates no change in diagnostic guidelines over this period and no change in the number of stool samples being tested for rotavirus in any age group during the 2013–2014 rotavirus season, compared with the previous year [24]. Also, the accuracy of HES diagnosis codes depends on the clinical diagnoses recorded in patients' medical records. Coding practices could change over time, but our case definition of all-cause AGE was deliberately broad to allow for potential variation in misclassification of more-specific AGE diagnoses. Moreover, discharge diagnoses recorded in HES are regularly validated for consistency and undergo internal quality control [25]. As many patients with AGE (especially older children and adults) are not routinely tested to obtain a specific microbiological diagnosis [26], analysis of laboratory-confirmed rotavirus infections alone would substantially underestimate the disease burden and the clinical impact of the rotavirus immunization program. Indeed, as expected, our analysis showed a much larger reduction in rotavirus-specific hospitalizations, compared with all-cause AGE–associated hospitalizations, among infants <1 year of age (80% vs 26%). However, the number of admissions averted among infants <1 year of age was 5 times that for all-cause AGE–associated admissions, compared with rotavirus-specific admissions (5256 vs 970), providing further support for using all-cause AGE–associated hospitalizations in the main analysis. Finally, there is natural year-to-year variability in the size of the rotavirus season. It is possible that at least some of the observed decrease may be due to a less active rotavirus year, independent of vaccination effect. In the Netherlands, for example, a low-burden 2013–2014 rotavirus season was reported in the absence of rotavirus vaccination [27]. The authors speculated that the mild winter, the relatively high rotavirus season in the previous year, the low birth rate, and, in neighboring countries, rotavirus immunization programs were possible contributing mechanisms [27]. The continued low incidence of laboratory-confirmed rotavirus infections during the second year of the program in England and Wales and the return to normal levels of rotavirus activity reported from the Netherlands for 2014–2015 (Dr Susan Hahné, National Institute for Public Health and the Environment, the Netherlands, personal communication) support the hypothesis that the infant immunization program was the main reason for the observed reductions during 2013–2014 in the United Kingdom.
Our study has shown a substantial reduction in rotavirus disease within a few months of vaccine introduction in infants, and this has continued into the second year of the program. It will, however, be important to continue monitoring these trends over time to confirm the potential herd immunity effects suggested by our study and the longer-term impact of rotavirus vaccination. The number of averted AGE hospitalizations we estimated for unvaccinated age groups could represent large healthcare cost savings attributable to the vaccination program and should be considered in future cost-effectiveness studies, which have so far only considered cost benefits afforded by direct protection of children [28, 29]. In clinical trials, rotavirus vaccines have been shown to be more efficacious against severe rotavirus disease [5]. Therefore, the impact on milder disease presenting in the community may be less marked than on hospitalizations; this merits further study.
Notes
Acknowledgments. We thank John Harris and Natalie Adams, Gastrointestinal Emerging and Zoonotic Infections Department, Public Health England, for extracting the LabBase2 data; and Joanne White and Pauline Waight, Immunisation, Hepatitis, and Blood Safety Department, Public Health England, for providing vaccine coverage data and data management assistance, respectively.
C. J. A., D. B., S. N. L., and M. E. R. developed the study protocol. S. C., D. J. A., and S. N. monitored data collection. Data were cleaned and analysed by C. J. A. and J. S. with help from N. A., who also helped write the statistical analysis plan. C. J. A. drafted and revised the manuscript. All authors contributed to subsequent revisions and approved the final version.
Disclaimer. The views expressed are those of the author(s) and not necessarily those of the National Health Service, the National Institute for Health Research, the Department of Health, or Public Health England.
Financial support. This work was supported by the National Institute for Health Research Health Protection Research Unit in Immunisation at the London School of Hygiene and Tropical Medicine, in partnership with Public Health England.
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References




