-
PDF
- Split View
-
Views
-
Cite
Cite
Anne Derache, Carole L. Wallis, Saran Vardhanabhuti, John Bartlett, Nagalingeswaran Kumarasamy, David Katzenstein, Phenotype, Genotype, and Drug Resistance in Subtype C HIV-1 Infection, The Journal of Infectious Diseases, Volume 213, Issue 2, 15 January 2016, Pages 250–256, https://doi.org/10.1093/infdis/jiv383
Close - Share Icon Share
Abstract
Background. Virologic failure in subtype C is characterized by high resistance to first-line antiretroviral (ARV) drugs, including efavirenz, nevirapine, and lamivudine, with nucleoside resistance including type 2 thymidine analog mutations, K65R, a T69del, and M184V. However, genotypic algorithms predicting resistance are mainly based on subtype B viruses and may under- or overestimate drug resistance in non-B subtypes. To explore potential treatment strategies after first-line failure, we compared genotypic and phenotypic susceptibility of subtype C human immunodeficiency virus 1 (HIV-1) following first-line ARV failure.
Methods. AIDS Clinical Trials Group 5230 evaluated patients failing an initial nonnucleoside reverse-transcriptase inhibitor (NNRTI) regimen in Africa and Asia, comparing the genotypic drug resistance and phenotypic profile from the PhenoSense (Monogram). Site-directed mutagenesis studies of K65R and T69del assessed the phenotypic impact of these mutations.
Results. Genotypic algorithms overestimated resistance to etravirine and rilpivirine, misclassifying 28% and 32%, respectively. Despite K65R with the T69del in 9 samples, tenofovir retained activity in >60%. Reversion of the K65R increased susceptibility to tenofovir and other nucleosides, while reversion of the T69del showed increased resistance to zidovudine, with little impact on other NRTI.
Conclusions. Although genotype and phenotype were largely concordant for first-line drugs, estimates of genotypic resistance to etravirine and rilpivirine may misclassify subtype C isolates compared to phenotype.
Treatment of human immunodeficiency virus 1 (HIV-1) in resource-limited settings currently relies on a nucleoside reverse-transcriptase inhibitor (NRTI) backbone of zidovudine (AZT), stavudine (d4T), abacavir (ABC), or tenofovir (TDF) with lamivudine (3TC) or emtricitabine (FTC). Two nucleosides are combined with a nonnucleoside reverse-transcriptase inhibitor (NNRTI), either nevirapine (NVP) or efavirenz (EFV), as first-line treatment [1]. Currently, more than 12 million individuals have initiated a first-line regimen, the majority of whom are infected with HIV-1 subtype C in India and sub–Saharan Africa and CRF01_AE in South-East Asia [2]. Failure of first-line antiretroviral treatment (ART) among 10%–30% of treated patients may be due to transmitted or acquired drug resistance, drug toxicity, reduced adherence, and treatment interruptions [3–5]. The selection of HIV-1 drug resistance among those failing first-line ART limits second-line and future treatment options.
Of those failing a first-line regimen, most develop 1 or more mutations associated with NNRTI resistance; K103N, Y181C, V106M/A, and G190S. NRTI mutations found after failure of first-line treatment of subtype C include M184V/I and T215F/Y thymidine analog mutations (TAMs) and more complex drug resistance patterns, including K65R and the Q151M complex [6–8]. In contrast to North America and Europe, virus load (VL) monitoring, drug resistance testing, and alternative drug classes and regimens are not widely available in Africa and Asia. However, effective and suppressive regimens for those who have failed first-line are essential for maintaining the benefits of ART and preventing transmitted drug resistance. The optimal strategy for maintaining long-term virologic suppression with limited access to only 2 or at most 3 distinct ART regimens and classes is a challenge to the sustainability of treatment.
A characteristic of first-line failure in subtype C is a higher frequency of the K65R mutation [9–12] sometimes associated with T69del, a single codon deletion that predicts high level of nucleotide and nucleoside resistance [8]. Thus, we selected samples with the T69del and K65R for an experiment in which site-directed mutagenesis and phenotyping were conducted on 3 multidrug-resistant isolates.
Although there was uniform, high-level genotypic and phenotypic resistance to EFV and NVP and most nucleosides, the significance of drug resistance mutations (DRMs) and the activity of second-generation NNRTIs [13–15] and TDF is not well defined. Some studies of etravirine (ETR) and rilpivirine (RPV) suggest that a substantial proportion of NVP and EFV (first-generation NNRTIs) failures may retain susceptibility to these newer “second-generation” NNRTIs [16–18]. To test these hypotheses, genotype and phenotype to NRTI and NNRTI were compared using the Monogram PhenoSense assay and Stanford database drug resistance algorithm among 47 samples, mainly subtype C HIV-1 isolates after first-line failure.
METHODS
Study Design and Participants
AIDS Clinical Trials Group (ACTG) 5230 is a single-arm, open-label, multicenter pilot study to evaluate the safety and efficacy associated with lopinavir/ritonavir monotherapy in protease inhibitor(PI)–naive patients failing an initial NNRTI-containing regimen in Thailand (Chiang Mai University ACTG CRS), South Africa (University of the Witwatersrand HIV CRS), India (Y. R. Gaitonde Centre for AIDS Research and Education, VHS CRS), Malawi (Kamuzu Central Hospital, University of North Carolina Lilongwe CRS), and Tanzania (Kilimanjaro Christian Medical Centre CRS). Consenting subjects had received at least 1 year of ART and were screened for virologic failure, defined as 2 VL > 1000 copies/mL within 30 days of study entry. CD4 lymphocyte cell counts and HIV-1 VL were available at screening, as well as the composition and duration of first-line ART. Among patients screened for this study, 47 samples were selected for phenotypic testing; 38 with subtype C infection and 9 (6C, 1 subtype D, and 2 CRF01_AE) were selected for the presence of the T69 deletion.
HIV-1 Drug Resistance Testing and Subtyping
Population-based HIV drug resistance testing was performed retrospectively using ViroSeq HIV-1 Genotyping System (v.2.0), as per manufacturer's instructions (Celera Diagnostics, Alameda, California). Sequences were analyzed using the ViroSeq v2.7 or Sequencher v4.8 software (when insertions or deletions were present), and drug resistance patterns in protease and reverse-transcriptase were determined using the Stanford Algorithm [14].
Phenotype
Phenotypic profile was assessed with the Monogram Biosciences PhenoSense assay on 47 plasma samples. PhenoSense is a recombinant virus phenotyping assay that provides an in vitro measure of drug susceptibility to NRTI: ABC, AZT, d4T, TDF, FTC, 3TC, didanosine (ddI), and NNRTI (NVP, EFV, ETR, and RPV) [19].
Genotype-Phenotype Comparisons
Stanford HIV Database estimates antiretroviral (ARV) drug resistance by summing expert knowledge and the scores of each drug based on the mutations associated with resistance, numerically scored as: susceptible 0–9, potential low-level resistance 10–14, low-level resistance 15–29, intermediate resistance 30–59, and high resistance ≥60 [14].
The Monogram PhenoSense assay classifies susceptibility as the fold change in resistance relative to a wild-type control, with sample values categorized as sensitive, partially sensitive, and resistant, according to lower and upper cut-points for each ARV [19].
Classification of genotypic and phenotypic resistance equated sensitive phenotypes with susceptible and low-level genotypic resistance, numerical scores of 0–29 and numerical scores for genotype ≥30 were correlated with partially and highly resistant phenotype.
Statistical Analysis
Fisher's exact test (P value <.05) was considered significant for associations between drug regimens and genotypic mutations, and between drug resistance classified by phenotype and genotype.
Recombinant Virus Construction
Three samples with both the K65R and the T69del were selected for investigation of phenotype after reversion of either the K65R or the T69del by site-directed mutagenesis. The pol gene was reamplified to obtain a polymerase chain reaction fragment encompassing the reverse transcriptase (RT) gene flanked by enzyme restriction sites. The fragment was cloned into the pNLPFB vector after enzymatic digestion. Site-directed mutagenesis was performed using the QuickChange II XL Site-Directed Mutagenesis Agilent kit to revert the T69del to T69 (K65R alone) and to revert the K65R to K65 (T69del alone). Molecular clones have been transfected into C8166 cells line, virus stocks were generated after infection of SupT1 cells, and the resulting virus analyzed by Monogram PhenoSense assays.
RESULTS
Characteristics of Study Subjects
Genotype and phenotype results were available for 47 subjects; however, the study enrollment characteristics, summarized in Table 1, are shown for 44 (3 subjects, although screened, were not enrolled in the study).
| Characteristics . | Overall (n = 44) . | Malawi (n = 30) . | South Africa (n = 8) . | Tanzania (n = 5) . | Thailand (n = 1) . |
|---|---|---|---|---|---|
| Age, median | 39 | 38.5 | 40.5 | 39 | 44 |
| Female, % | 66.0 | 74.0 | 62.5 | 40 | 0.0 |
| VL median, log copies/mL | 4.75 | 5.01 | 4.26 | 5.01 | 4.29 |
| CD4 median, cells/µL | 115 | 56 | 209 | 172 | 6 |
| First-line regimen | |||||
| d4T 3TC EFV | 5 | 0 | 5 | 0 | 0 |
| d4T 3TC NVP | 25 | 17 | 2 | 5 | 1 |
| TDF 3TC NVP | 3 | 3 | 0 | 0 | 0 |
| AZT 3TC EFV | 1 | 0 | 1 | 0 | 0 |
| AZT 3TC NVP | 10 | 10 | 0 | 0 | 0 |
| Time median on first-line, months | 36 | 39 | 22 | 54 | 32 |
| Characteristics . | Overall (n = 44) . | Malawi (n = 30) . | South Africa (n = 8) . | Tanzania (n = 5) . | Thailand (n = 1) . |
|---|---|---|---|---|---|
| Age, median | 39 | 38.5 | 40.5 | 39 | 44 |
| Female, % | 66.0 | 74.0 | 62.5 | 40 | 0.0 |
| VL median, log copies/mL | 4.75 | 5.01 | 4.26 | 5.01 | 4.29 |
| CD4 median, cells/µL | 115 | 56 | 209 | 172 | 6 |
| First-line regimen | |||||
| d4T 3TC EFV | 5 | 0 | 5 | 0 | 0 |
| d4T 3TC NVP | 25 | 17 | 2 | 5 | 1 |
| TDF 3TC NVP | 3 | 3 | 0 | 0 | 0 |
| AZT 3TC EFV | 1 | 0 | 1 | 0 | 0 |
| AZT 3TC NVP | 10 | 10 | 0 | 0 | 0 |
| Time median on first-line, months | 36 | 39 | 22 | 54 | 32 |
Abbreviations: 3TC, lamivudine; ACTG, AIDS Clinical Trials Group; AZT, zidovudine; d4T, stavudine; EFV, efavirenz; NVP, nevirapine.
| Characteristics . | Overall (n = 44) . | Malawi (n = 30) . | South Africa (n = 8) . | Tanzania (n = 5) . | Thailand (n = 1) . |
|---|---|---|---|---|---|
| Age, median | 39 | 38.5 | 40.5 | 39 | 44 |
| Female, % | 66.0 | 74.0 | 62.5 | 40 | 0.0 |
| VL median, log copies/mL | 4.75 | 5.01 | 4.26 | 5.01 | 4.29 |
| CD4 median, cells/µL | 115 | 56 | 209 | 172 | 6 |
| First-line regimen | |||||
| d4T 3TC EFV | 5 | 0 | 5 | 0 | 0 |
| d4T 3TC NVP | 25 | 17 | 2 | 5 | 1 |
| TDF 3TC NVP | 3 | 3 | 0 | 0 | 0 |
| AZT 3TC EFV | 1 | 0 | 1 | 0 | 0 |
| AZT 3TC NVP | 10 | 10 | 0 | 0 | 0 |
| Time median on first-line, months | 36 | 39 | 22 | 54 | 32 |
| Characteristics . | Overall (n = 44) . | Malawi (n = 30) . | South Africa (n = 8) . | Tanzania (n = 5) . | Thailand (n = 1) . |
|---|---|---|---|---|---|
| Age, median | 39 | 38.5 | 40.5 | 39 | 44 |
| Female, % | 66.0 | 74.0 | 62.5 | 40 | 0.0 |
| VL median, log copies/mL | 4.75 | 5.01 | 4.26 | 5.01 | 4.29 |
| CD4 median, cells/µL | 115 | 56 | 209 | 172 | 6 |
| First-line regimen | |||||
| d4T 3TC EFV | 5 | 0 | 5 | 0 | 0 |
| d4T 3TC NVP | 25 | 17 | 2 | 5 | 1 |
| TDF 3TC NVP | 3 | 3 | 0 | 0 | 0 |
| AZT 3TC EFV | 1 | 0 | 1 | 0 | 0 |
| AZT 3TC NVP | 10 | 10 | 0 | 0 | 0 |
| Time median on first-line, months | 36 | 39 | 22 | 54 | 32 |
Abbreviations: 3TC, lamivudine; ACTG, AIDS Clinical Trials Group; AZT, zidovudine; d4T, stavudine; EFV, efavirenz; NVP, nevirapine.
Among the 44 subjects with available data, the median age was 39 years and 34% were male. They were from different clinical sites: Malawi (n = 30), South Africa (n = 8), Tanzania (n = 5), and Thailand (n = 1). The overall median VL at baseline was 4.75 log copies/mL (interquartile range [IQR] = 0.85). Among the different clinical sites, the viral loads were distributed as followed: 5.01 log copies/mL in Tanzania and Malawi (IQRs = 0.96 and 0.74, respectively), and 4.26 log copies/mL in South Africa (IQR = 0.46). The median CD4 was 115 cells/µL and was distributed as followed among the different sites: 172 cells/µL in Tanzania, 56 cells/µL in Malawi, and 209 cells/µL in South Africa.
The first-line NNRTI-based treatment regimens included 30 on d4T+3TC+NNRTI (5 on EFV and 25 on NVP), 12 on AZT+3TC+NNRTI (1 on EFV and 11 on NVP), and 3 on TDF+3TC+NVP. The median duration on prior regimen before study entry was 36 months (range, 2–100 months): 54 months in Tanzania (range, 12–72 months), 39 months in Malawi (range, 2–110 months), and 22 months in South Africa (range, 20–53 months).
Genotypic Resistance
All 47 subjects had NNRTI resistance mutations with the exception of 1 with a wild-type virus. NNRTI-specific mutations were: Y181C 60% (n = 28), G190A/S 32% (n = 15), K103N 28% (n = 13), K101E 21% (n = 10), V106M/A 11% (n = 5), Y188L 2% (n = 1), and M230L 2% (n = 1). The Y181C mutation was significantly associated with NVP-based (25/38) compared to EFV-based regimens (0/6). Regarding NRTI resistance mutations, 32 subjects harbored TAMs and 14 had 3 or more TAMs. The TAM-2 pathway (D67N, K70R, T215F, K219Q/R/E) in 20/47 subjects was more common than the TAM-1 pathway (M41L, L210W, T215Y) in 6/47, and 6/47 had mutations from both pathways. Resistance to 3TC/FTC (M184V/I) was present in 38/47 of the subjects. The absence of TAMs was more common among subjects treated with d4T (13/30) than AZT (1/11) (P = .04). The K65R mutation was found in 12 samples, and was associated with T69del in 9 cases. Nineteen isolates (40%) were classified as intermediate- or high-level resistance to TDF, and 9 harbored K65R associated with a T69 amino acid deletion, predominantly among those treated with d4T compared with AZT (7/30 vs 0/11) (P = .09). The 10 samples with intermediate- and high-level resistance to TDF, without K65R or T69d, harbored multiple TAMs (mean = 3.6) and M184V: 7 samples had mutations associated with the TAM-1 pathway, 5 of them with mutations also associated with the TAM-2 pathway (mainly D67N). Three others displayed only TAM-2 mutations.
Phenotypic Resistance
Among the 47 samples assessed for phenotypic susceptibility, most were highly resistant to 3TC/FTC (96%) and first-generation NNRTI (NVP 98%, EFV 94%). Resistance to thymidine analogs d4T and AZT was found for 30% and 53% of the samples, respectively. Among the samples, 64% and 83% had partial or high-level resistance to ABC and ddI, respectively. Only 3 samples (6%) were highly resistant to TDF, 16 were partially resistant (34%), 50% were susceptible, and 10% hypersusceptible. Despite the presence of NNRTI-associated resistance mutations, 43% and 51% were fully susceptible to RPV and ETR, respectively.
Genotype and Phenotype
Concordance between genotype and phenotype for ABC, 3TC/FTC, AZT, EFV, and NVP ranged from 91% to 100%. Stavudine demonstrated only 57% concordance between genotype and phenotype, due mainly to sensitive phenotypes categorized as intermediate/high-resistance genotypes (20/33). Tenofovir and ddI had 81% and 72% concordance, respectively. Genotypic algorithms overestimated resistance to ETR and RPV, misclassifying 28% and 32%, respectively (Figure 1). Discordant findings are identified as those susceptible (fold changes <2 for RPV and <2.9 for ETR) by phenotypic assay despite predicted genotypic resistance. High-level resistance to second-generation NNRTI was significantly associated with prior NVP versus EFV use (P < .02) and with Y181C, the mutation most closely associated with phenotypic resistance to ETR and RPV (P < .0001 and P < .0005, respectively), while other mutations were not significantly associated with second-generation NNRTI resistance (Table 2). The K101E mutation showed a trend toward increased RPV susceptibility (P < .05). Phenotypic testing demonstrated a disproportionate susceptibility to TDF, ETR, and RPV despite high levels of genotypic resistance (Figure 1). However, phenotypic resistance despite genotypic susceptibility was rarely observed.
Association Between Known RT DRMs and Phenotype Susceptibility for Second-Generation NNRTI ETR and RPV, With Corresponding Fisher Test P Values
| . | ETR . | RVP . | ||||
|---|---|---|---|---|---|---|
| Sensitive (n = 24) . | Resistant (n = 23) . | P Value . | Sensitive (n = 20) . | Resistant (n = 27) . | P Value . | |
| A98G | 4 | 6 | .33 | 2 | 7 | .16 |
| K101E | 7 | 3 | .16 | 7 | 3 | .05 |
| K103N | 9 | 4 | .11 | 8 | 5 | .1 |
| V106A/M | 3 | 2 | .52 | 2 | 3 | .64 |
| V108I | 2 | 4 | .31 | 1 | 5 | .18 |
| E138AQG | 6 | 1 | .05 | 4 | 3 | .33 |
| V179D | 0 | 3 | .11 | 0 | 3 | .18 |
| Y181C | 6 | 22 | <.0001 | 6 | 22 | <.0005 |
| Y188L | 1 | 0 | .51 | 0 | 1 | .57 |
| G190AS | 9 | 6 | .3 | 7 | 6 | .26 |
| H221Y | 4 | 12 | .01 | 4 | 12 | .07 |
| F227L | 3 | 0 | .12 | 2 | 1 | .38 |
| M230L | 0 | 1 | .5 | 0 | 1 | .57 |
| . | ETR . | RVP . | ||||
|---|---|---|---|---|---|---|
| Sensitive (n = 24) . | Resistant (n = 23) . | P Value . | Sensitive (n = 20) . | Resistant (n = 27) . | P Value . | |
| A98G | 4 | 6 | .33 | 2 | 7 | .16 |
| K101E | 7 | 3 | .16 | 7 | 3 | .05 |
| K103N | 9 | 4 | .11 | 8 | 5 | .1 |
| V106A/M | 3 | 2 | .52 | 2 | 3 | .64 |
| V108I | 2 | 4 | .31 | 1 | 5 | .18 |
| E138AQG | 6 | 1 | .05 | 4 | 3 | .33 |
| V179D | 0 | 3 | .11 | 0 | 3 | .18 |
| Y181C | 6 | 22 | <.0001 | 6 | 22 | <.0005 |
| Y188L | 1 | 0 | .51 | 0 | 1 | .57 |
| G190AS | 9 | 6 | .3 | 7 | 6 | .26 |
| H221Y | 4 | 12 | .01 | 4 | 12 | .07 |
| F227L | 3 | 0 | .12 | 2 | 1 | .38 |
| M230L | 0 | 1 | .5 | 0 | 1 | .57 |
Abbreviations: DRMs, drug resistance mutations; ETR, etravirine; NNRTI, nonnucleoside reverse-transcriptase inhibitor; RT, reverse-transcriptase; RPV, rilpivirine.
Association Between Known RT DRMs and Phenotype Susceptibility for Second-Generation NNRTI ETR and RPV, With Corresponding Fisher Test P Values
| . | ETR . | RVP . | ||||
|---|---|---|---|---|---|---|
| Sensitive (n = 24) . | Resistant (n = 23) . | P Value . | Sensitive (n = 20) . | Resistant (n = 27) . | P Value . | |
| A98G | 4 | 6 | .33 | 2 | 7 | .16 |
| K101E | 7 | 3 | .16 | 7 | 3 | .05 |
| K103N | 9 | 4 | .11 | 8 | 5 | .1 |
| V106A/M | 3 | 2 | .52 | 2 | 3 | .64 |
| V108I | 2 | 4 | .31 | 1 | 5 | .18 |
| E138AQG | 6 | 1 | .05 | 4 | 3 | .33 |
| V179D | 0 | 3 | .11 | 0 | 3 | .18 |
| Y181C | 6 | 22 | <.0001 | 6 | 22 | <.0005 |
| Y188L | 1 | 0 | .51 | 0 | 1 | .57 |
| G190AS | 9 | 6 | .3 | 7 | 6 | .26 |
| H221Y | 4 | 12 | .01 | 4 | 12 | .07 |
| F227L | 3 | 0 | .12 | 2 | 1 | .38 |
| M230L | 0 | 1 | .5 | 0 | 1 | .57 |
| . | ETR . | RVP . | ||||
|---|---|---|---|---|---|---|
| Sensitive (n = 24) . | Resistant (n = 23) . | P Value . | Sensitive (n = 20) . | Resistant (n = 27) . | P Value . | |
| A98G | 4 | 6 | .33 | 2 | 7 | .16 |
| K101E | 7 | 3 | .16 | 7 | 3 | .05 |
| K103N | 9 | 4 | .11 | 8 | 5 | .1 |
| V106A/M | 3 | 2 | .52 | 2 | 3 | .64 |
| V108I | 2 | 4 | .31 | 1 | 5 | .18 |
| E138AQG | 6 | 1 | .05 | 4 | 3 | .33 |
| V179D | 0 | 3 | .11 | 0 | 3 | .18 |
| Y181C | 6 | 22 | <.0001 | 6 | 22 | <.0005 |
| Y188L | 1 | 0 | .51 | 0 | 1 | .57 |
| G190AS | 9 | 6 | .3 | 7 | 6 | .26 |
| H221Y | 4 | 12 | .01 | 4 | 12 | .07 |
| F227L | 3 | 0 | .12 | 2 | 1 | .38 |
| M230L | 0 | 1 | .5 | 0 | 1 | .57 |
Abbreviations: DRMs, drug resistance mutations; ETR, etravirine; NNRTI, nonnucleoside reverse-transcriptase inhibitor; RT, reverse-transcriptase; RPV, rilpivirine.
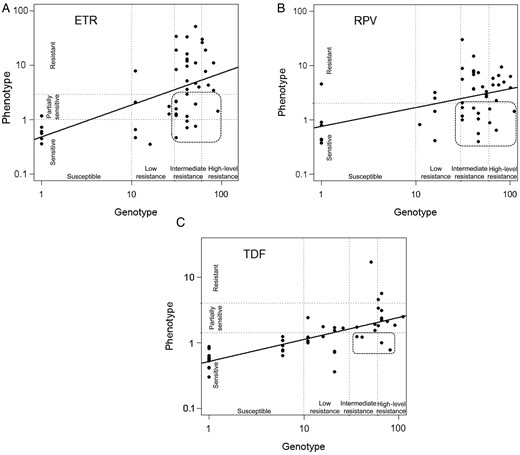
Log values of Stanford genotype scores plotted to log values of Monogram phenotypic fold change, illustrating genotypic and phenotypic correlations for ETR (A), RPV (B), and TDF (C). The circled areas indicate samples that were misclassified as genotypically resistant, although they displayed susceptibility to TDF, ETR, and RPV upon phenotyping. Abbreviations: ETR, etravirine; RPV, rilpivirine; TDF, tenofovir.
Site-Directed Mutagenesis Reversion of K65R and T69del
Reversion of the K65R mutation increased phenotypic susceptibility to TDF, 3TC, and FTC, and modestly reduced d4T and AZT resistance. In contrast, reversion of the T69del increased resistance to AZT with little impact on other NRTIs. Interestingly, there were modest (2-fold) increases in susceptibility to EFV, ETR, and RPV following reversion of the T69del in subject 521, as shown in Table 3.
PhenoSense (Monogram) Results for 3 Samples With Both K65R and T69del (BL), T69del Alone (65rev), and K65R Alone (69rev), After Site-Directed Mutagenesis
| . | Sample 410 . | Sample 426 . | Sample 521 . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| K65R, T69del, L74V, K219R, Y181C, G190A . | K65R, T69del, Q151M, K103N, Y181C . | K65R, T69del, K70R, V75I, F77L, Q151M, V179D, Y181C, H221Y . | |||||||
| Genotype at Study Entry Failing Regimen . | d4T 3TC NVP . | d4T 3TC NVP . | d4T 3TC NVP . | ||||||
| BL . | 65rev . | 69rev . | BL . | 65rev . | 69rev . | BL . | 65rev . | 69rev . | |
| ABC | 16 | 4.35 | 20 | 13 | 5.93 | 9.79 | 13 | 4.7 | 8.01 |
| ddI | 3.92 | 1.65 | 4.6 | 9.19 | 3.86 | 8.13 | 19 | 4.89 | 13 |
| FTC | max | 3.96 | 34 | max | 6.52 | max | max | 1.67 | 49 |
| 3TC | max | 3.61 | 52 | max | 4.75 | max | max | 1.44 | max |
| d4T | 16 | 2.83 | 23 | 10 | 4.41 | 7.23 | 21 | 5.58 | 11 |
| TDF | 16 | 3.22 | 21 | 2.26 | 0.6 | 1.99 | 2.64 | 0.52 | 3.62 |
| AZT | 139 | 9.76 | max | 1.54 | 1.61 | 4.46 | 29 | 11 | 106 |
| EFV | 31 | 33 | 38 | 20 | 20 | 18 | 10 | 9.23 | 5.3 |
| ETR | 3.47 | 3.55 | 3.69 | 6.44 | 4.32 | 5.55 | 44 | 43 | 24 |
| NVP | max | max | max | max | max | max | max | max | max |
| RPV | 2.92 | 2.96 | 3 | 4.3 | 2.47 | 3.26 | 4.67 | 2.78 | 2.01 |
| . | Sample 410 . | Sample 426 . | Sample 521 . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| K65R, T69del, L74V, K219R, Y181C, G190A . | K65R, T69del, Q151M, K103N, Y181C . | K65R, T69del, K70R, V75I, F77L, Q151M, V179D, Y181C, H221Y . | |||||||
| Genotype at Study Entry Failing Regimen . | d4T 3TC NVP . | d4T 3TC NVP . | d4T 3TC NVP . | ||||||
| BL . | 65rev . | 69rev . | BL . | 65rev . | 69rev . | BL . | 65rev . | 69rev . | |
| ABC | 16 | 4.35 | 20 | 13 | 5.93 | 9.79 | 13 | 4.7 | 8.01 |
| ddI | 3.92 | 1.65 | 4.6 | 9.19 | 3.86 | 8.13 | 19 | 4.89 | 13 |
| FTC | max | 3.96 | 34 | max | 6.52 | max | max | 1.67 | 49 |
| 3TC | max | 3.61 | 52 | max | 4.75 | max | max | 1.44 | max |
| d4T | 16 | 2.83 | 23 | 10 | 4.41 | 7.23 | 21 | 5.58 | 11 |
| TDF | 16 | 3.22 | 21 | 2.26 | 0.6 | 1.99 | 2.64 | 0.52 | 3.62 |
| AZT | 139 | 9.76 | max | 1.54 | 1.61 | 4.46 | 29 | 11 | 106 |
| EFV | 31 | 33 | 38 | 20 | 20 | 18 | 10 | 9.23 | 5.3 |
| ETR | 3.47 | 3.55 | 3.69 | 6.44 | 4.32 | 5.55 | 44 | 43 | 24 |
| NVP | max | max | max | max | max | max | max | max | max |
| RPV | 2.92 | 2.96 | 3 | 4.3 | 2.47 | 3.26 | 4.67 | 2.78 | 2.01 |
Numbers represent fold change of susceptibility to each ARV, compared to the Monogram reference (without any DRMs).
Abbreviations: 3TC, lamivudine; ABC, abacavir; ARV, antiretroviral; AZT, zidovudine; d4T, stavudine; ddI, didanosine; DRMs, drug resistance mutations; EFV, efavirenz; ETR, etravirine; FTC, emtricitabine; NVP, nevirapine; RPV, rilpivirine, TDF, tenofovir.
PhenoSense (Monogram) Results for 3 Samples With Both K65R and T69del (BL), T69del Alone (65rev), and K65R Alone (69rev), After Site-Directed Mutagenesis
| . | Sample 410 . | Sample 426 . | Sample 521 . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| K65R, T69del, L74V, K219R, Y181C, G190A . | K65R, T69del, Q151M, K103N, Y181C . | K65R, T69del, K70R, V75I, F77L, Q151M, V179D, Y181C, H221Y . | |||||||
| Genotype at Study Entry Failing Regimen . | d4T 3TC NVP . | d4T 3TC NVP . | d4T 3TC NVP . | ||||||
| BL . | 65rev . | 69rev . | BL . | 65rev . | 69rev . | BL . | 65rev . | 69rev . | |
| ABC | 16 | 4.35 | 20 | 13 | 5.93 | 9.79 | 13 | 4.7 | 8.01 |
| ddI | 3.92 | 1.65 | 4.6 | 9.19 | 3.86 | 8.13 | 19 | 4.89 | 13 |
| FTC | max | 3.96 | 34 | max | 6.52 | max | max | 1.67 | 49 |
| 3TC | max | 3.61 | 52 | max | 4.75 | max | max | 1.44 | max |
| d4T | 16 | 2.83 | 23 | 10 | 4.41 | 7.23 | 21 | 5.58 | 11 |
| TDF | 16 | 3.22 | 21 | 2.26 | 0.6 | 1.99 | 2.64 | 0.52 | 3.62 |
| AZT | 139 | 9.76 | max | 1.54 | 1.61 | 4.46 | 29 | 11 | 106 |
| EFV | 31 | 33 | 38 | 20 | 20 | 18 | 10 | 9.23 | 5.3 |
| ETR | 3.47 | 3.55 | 3.69 | 6.44 | 4.32 | 5.55 | 44 | 43 | 24 |
| NVP | max | max | max | max | max | max | max | max | max |
| RPV | 2.92 | 2.96 | 3 | 4.3 | 2.47 | 3.26 | 4.67 | 2.78 | 2.01 |
| . | Sample 410 . | Sample 426 . | Sample 521 . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| K65R, T69del, L74V, K219R, Y181C, G190A . | K65R, T69del, Q151M, K103N, Y181C . | K65R, T69del, K70R, V75I, F77L, Q151M, V179D, Y181C, H221Y . | |||||||
| Genotype at Study Entry Failing Regimen . | d4T 3TC NVP . | d4T 3TC NVP . | d4T 3TC NVP . | ||||||
| BL . | 65rev . | 69rev . | BL . | 65rev . | 69rev . | BL . | 65rev . | 69rev . | |
| ABC | 16 | 4.35 | 20 | 13 | 5.93 | 9.79 | 13 | 4.7 | 8.01 |
| ddI | 3.92 | 1.65 | 4.6 | 9.19 | 3.86 | 8.13 | 19 | 4.89 | 13 |
| FTC | max | 3.96 | 34 | max | 6.52 | max | max | 1.67 | 49 |
| 3TC | max | 3.61 | 52 | max | 4.75 | max | max | 1.44 | max |
| d4T | 16 | 2.83 | 23 | 10 | 4.41 | 7.23 | 21 | 5.58 | 11 |
| TDF | 16 | 3.22 | 21 | 2.26 | 0.6 | 1.99 | 2.64 | 0.52 | 3.62 |
| AZT | 139 | 9.76 | max | 1.54 | 1.61 | 4.46 | 29 | 11 | 106 |
| EFV | 31 | 33 | 38 | 20 | 20 | 18 | 10 | 9.23 | 5.3 |
| ETR | 3.47 | 3.55 | 3.69 | 6.44 | 4.32 | 5.55 | 44 | 43 | 24 |
| NVP | max | max | max | max | max | max | max | max | max |
| RPV | 2.92 | 2.96 | 3 | 4.3 | 2.47 | 3.26 | 4.67 | 2.78 | 2.01 |
Numbers represent fold change of susceptibility to each ARV, compared to the Monogram reference (without any DRMs).
Abbreviations: 3TC, lamivudine; ABC, abacavir; ARV, antiretroviral; AZT, zidovudine; d4T, stavudine; ddI, didanosine; DRMs, drug resistance mutations; EFV, efavirenz; ETR, etravirine; FTC, emtricitabine; NVP, nevirapine; RPV, rilpivirine, TDF, tenofovir.
DISCUSSION
The widespread implementation of first-line regimens, with limited agents and monitoring, may lead to the extensive accumulation of DRMs. Here, we found multiple NRTI and NNRTI mutations, including relatively unusual NRTI mutations: K65R, TAM2, and T69del. The NNRTI resistance mutations observed mirror those reported in similar populations with subtype C [20–24]. Comparison of genotype and phenotype of these isolates demonstrated differences between the predicted genotype and measured phenotypic resistance, which may have implications for third-line options and future treatment strategies.
Although there was clear high-level resistance to EFV and NVP among the first-line failures, 51% and 43% of subjects were phenotypically susceptible to second-generation NNRTIs, ETR, and RPV, despite predicted genotypic resistance. Receipt of NVP, and the selection of Y181C and G190A mutations, have been associated with reduced ETR susceptibility [25–27]. In this subtype C comparison, K103N and K101E mutations were associated with ETR and RPV susceptibility by phenotype, consistent with recent observations in South Africa [28] where only 10% of first-line failures were fully resistant to ETR [29]. Constructs from subtype C isolates with common NNRTI resistance mutations, such as K103N, V106M, and G190A, demonstrate in vitro ETR and RPV susceptibility [30]. Nonetheless, PhenoSense drug susceptibility assays do not include the RT connection and RNase H domains, which may be potential mechanisms for RT inhibitor resistance [31].
In this study, the most frequent resistance mutation observed was M184V/I, followed by TAMs, K65R, the T69del, and the Q151M complex, which confer resistance to most NRTIs, including TDF. Treatment of nonsubtype B viruses with first-line ART includes the selection of M184V/I and K65R among subtype C and CRF02_AG samples [10, 32, 33]. The β3-β4 deletion at position T69 has been reported in combination with multiple TAMs [34–36], K65R and/or Q151M [37]. K65R alone conveys reduced susceptibility to all NRTIs except AZT [38, 39], although this may come at a cost of reduced fitness [40], and the T69del may confer both AZT hypersusceptibility and 3TC resistance [38]. Here, 12/47 (26%) had the K65R and/or the T69del, which predicted TDF resistance, but only 6% had high-level phenotypic resistance to TDF. This is similar to the findings in a query of the Stanford database (http://hivdb.stanford.edu/cgi-bin/RT_Phenotype.cgi), which yielded 66 subtype B isolates with K65R with a median phenotypic fold-resistance by PhenoSense of 1.7, and only 7/69 (10%) exhibited more than 5-fold resistance to TDF. Finally, there was a difference between phenotypic resistance to d4T and AZT (30% and 53%, respectively) associated with the K70R mutation, usually following an AZT-based regimens [41]; although 9 participants had this mutation, 6/9 of them were on a d4T-based regimen.
We investigated the impact of the K65R and T69del through site-directed mutagenesis in 3 isolates. The findings, illustrated in Table 3, show a resensitization to all nucleosides with reversion of K65R, though only a modest increase in AZT resistance associated with the restoration of the T69del. Phenotypic resistance to AZT, d4T, and TDF in 1 sample (410) may be due to L74V, suggesting that further study of this mutation is warranted.
The patterns of NRTI mutations in non-B and particularly subtype C viruses included a predominance of TAM-2, Q151M, K65R, and M184V at the sites that do not routinely use VL monitoring. DRMs in subtype C have been linked to continuing failing drugs [8–13, 32]. Subjects not monitored by VL with more prolonged failure have lower CD4 cell counts, higher VL, and more complex resistance patterns [42]. The implementation of VL monitoring in resource-limited settings will facilitate faster switching with a decrease in NRTI- and NNRTI-associated mutations [33].
Although phenotypic susceptibility would suggest that subjects failing a first-line regimen including EFV might benefit from the inclusion of ETR or RPV in a salvage regimen, these preliminary observations in a small number of subtype C samples should be interpreted with caution. Based on cost effectiveness [43], the ACTG has planned a third-line study (A5288) of ETR and other new ARVs in salvage treatment regimens in resource-limited settings. If ETR and RPV are introduced into third-line or salvage regimens, it may be preferable to use EFV rather than NVP in first-line regimens. Genotypic resistance estimates were determined for ETR using the scoring system from the DUET I and II studies [44], predominately based on HIV-1 subtype B. Although there was a high level of ETR- and RPV-associated mutations, subtype-specific polymorphisms or changes in the RNase H and connection domains of these samples were not examined. A recent study [45] found samples phenotypically resistant without ETR resistance-associated mutations. This might be due to minor variants not detected by bulk sequencing. But minor variants, while detected by newer sequencing technologies, may not pose significant risk for virologic failure [46, 47]. The findings here provide an additional rationale for considering ETR and RPV in salvage and for pre- and postexposure prophylaxis (PREP or PEP) as EFV is increasingly used in first-line treatment.
In conclusion, inexpensive RT inhibitors–based ART regimens have achieved unprecedented access to care in resource-limited settings. However, in the absence of VL monitoring, the accumulation of DRMs may limit future treatment options. The resistance mutations in subtype C and other non-B viruses are not dissimilar from those observed in subtype B. Some distinct features of subtype C include an increased prevalence of V106M, a high frequency of the K65R and the T69del, and a greater frequency of TAM-2 (D67N, K70R, T215F, K219Q/E/R) versus TAM-1 (M41L, L210W, T215Y). However, despite uniform genotypic resistance to first-line NNRTIs, more than 40% were phenotypically susceptible to second-generation NNRTIs, ETR, and RPV, and 60% retained full and 34% partial susceptibility to TDF. Studies of genotype and phenotype from first-line failure, as TDF and EFV replace d4T and NVP, can inform future treatment strategies in Africa and Asia.
Notes
Acknowledgments. The authors thank all ACTG A5230 study individuals for their participation. In addition, the authors thank the clinical research site members for their participation and for their supporting grants at the 5 sites that conducted ACTG A5230: Drs Agnes Moses and Albert Mwafongo, University of North Carolina (UNC) Project Lilongwe; Drs Venance Maro and John Crump, Kilimanjaro Christian Medical Centre (KCMC); Professor Thira Sirisanthana and Patchanee Samutarlai, Chiang Mai University; Petronilla Borain and Vuyokazi Jezile, University of Witswatersrand; and Dr Poongulali and Beulah Faith, Y. R. Gaitonde Centre for AIDS Research and Education (YRG CARE).
Financial support. This work was supported by an award (U01AI068636) from the National Institute for Allergy and Infectious Diseases and was further supported by the National Institute of Mental Health and the National Institute of Dental and Craniofacial Research. The reagents were supplied by Abbott/Abbvie. Drug supplies were provided by Abbott/Abbvie and Gilead Sciences Inc. Site-supporting grants included the following: UNC Project Lilongwe, U01AI069518; Duke University and KCMC, U01069484; University of Witswatersrand, U01AI38858; YRG CARE, U01AI069432; and Statistical Data Analysis Center grant (AI068634). A. D. and D. K received research support from Gilead Sciences, and laboratory work at Stanford University was supported by the National Institutes of Health (NIH) 1 R01 AI066922-01A2.
Potential conflicts of interest. A. D. declares financial supports from Gilead Sciences. D. K. declares research supports from Gilead Sciences and Roche Molecular Systems. C. L. W. discloses personal fees from Abbvie, MDS, and Celera. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
Author notes
Presented in part: 20th Conference on Retroviruses and Opportunistic Infections, Atlanta, Georgia, 3–7 March 2013.




