-
PDF
- Split View
-
Views
-
Cite
Cite
Lauren L Agoubi, Sandeep P Khot, R Alan Failor, Nicole K Zern, Pheochromocytoma-induced Subarachnoid and Intracerebral Hemorrhage, Journal of the Endocrine Society, Volume 7, Issue 1, January 2023, bvac176, https://doi.org/10.1210/jendso/bvac176
Close - Share Icon Share
Abstract
Pheochromocytomas are rare adrenal tumors that are often diagnosed in workup for endocrine causes of refractory hypertension, as an incidental imaging finding, or in patients with classic symptoms of headache, palpitations, and/or diaphoresis. We describe a case of pheochromocytoma presenting in a 63-year-old woman with spontaneous and multifocal subarachnoid and intracerebral hemorrhage without underlying vasculopathy. The patient previously had no documented episodes of hypertension and took no regular medications. She experienced sudden-onset severe headache and presented with hypertensive crisis. Cranial imaging showed bifrontal and right temporal convexal subarachnoid and intracerebral hemorrhage of unknown etiology. Cranial arterial catheterization showed no vascular malformation underlying the site of hemorrhage. Given concern for potential malignant etiology, cross-sectional body imaging was performed that revealed a 7-cm right adrenal heterogeneous mass. Biochemical workup demonstrated markedly elevated plasma metanephrine and normetanephrine levels, diagnostic of pheochromocytoma. She underwent α- and β-blockade, and evaluation with a multidisciplinary team including repeat intracranial imaging to ensure resolution of the intracranial bleeding before definitive surgical management. She then underwent successful laparoscopic adrenalectomy. This case demonstrates that the workup of cryptogenic intracranial hemorrhage and hypertensive crisis should include evaluation for catecholamine-secreting tumors.
Pheochromocytomas are rare catecholamine-secreting tumors of the adrenal gland that are typically benign. The majority of these tumors occur sporadically, but approximately one-quarter of patients with pheochromocytomas carry germline mutations [1]. Many pheochromocytomas (40%) are detected by symptoms of excess catecholamine secretion, including headache, sweating, and palpitations, though this triad is present less commonly than often assumed (15%-24%) [2]. Hypertension is also seen in approximately 50% of patients; however, only 0.2% to 0.4% of hypertensive patients have an underlying pheochromocytoma. Many other patients are asymptomatic, harboring a pheochromocytoma that is detected as an incidental finding on cross-sectional imaging. This case highlights a rare presentation of pheochromocytoma causing spontaneous bifrontal subarachnoid hemorrhage without underlying vasculopathy.
Case Presentation
We present a 63-year-old woman with no relevant past medical history. She provided a family history of hypertension, and reports that her blood pressure had been elevated on several occasions, thought to be “white-coat hypertension,” but carried no formal diagnosis. She developed an abrupt-onset, unprovoked, severe headache and presented to local urgent care where blood pressure was 207/108 mm Hg. Despite initial treatment with lisinopril, she continued to have an unresolved, worsening headache accompanied by nausea and vomiting leading to a repeat evaluation 24 hours later. Systolic blood pressure was again more than 200 mm Hg. On examination, she did not have any focal neurological deficits. A computed tomography (CT) angiogram of the head demonstrated bilateral frontotemporal convexal subarachnoid hemorrhage, 2 focal areas of intracerebral hemorrhage involving the right frontal and temporal lobes, and no underlying vascular abnormality. Contrast-enhanced brain magnetic resonance imaging (MRI) revealed no underlying mass lesions and MR angiogram did not demonstrate evidence of aneurysm or vasculitis. She was placed on a nicardipine infusion and transferred to our institution.
On admission to our institution, the patient’s blood pressure was well controlled on the nicardipine infusion, and she was then transitioned to oral metoprolol and captopril. She subsequently had a self-limited episode of atrial fibrillation with transient troponin elevation. A cerebral digital subtraction catheter angiogram (DSA) was obtained 2 days after her symptoms began and demonstrated no aneurysms, malformations, or occlusions (Fig. 1A). Interval CT scan of the head showed no progression of the multifocal hematomas (Fig. 1B) and she continued to exhibit no neurologic symptomatology aside from headache, which improved as blood pressure normalized with treatment. Given unclear etiology of the multifocal intracranial hemorrhage, workup for metastatic disease was pursued. CT scan of the chest/abdomen/pelvis revealed a complex right adrenal mass measuring 75 × 69 × 77 mm (Fig. 2). There were several cystic areas of the mass with internal septations, and thus the Hounsfield units were quite low in some areas and elevated in others. Further assessment with MRI of the abdomen demonstrated T1 hyperintensity and moderate heterogeneous T2 hyperintensity without evidence of macroscopic or intracellular fat, thus not consistent with an adenoma.

A (left), Sagittal view of the cerebral digital subtraction cerebral angiogram obtained 2 days after headache onset shows no evidence of vasculopathy or vascular malformation. B (right), Axial noncontrast head computed tomography scan with frontal lobe right-sided intracerebral hemorrhage (black arrow) and left-sided subarachnoid hemorrhage (white arrow).
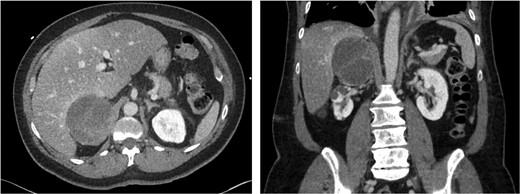
Computed tomography scan images: axial and coronal views of the right adrenal tumor.
A biochemical workup (Table 1) was performed. The patient’s laboratory work demonstrated markedly elevated plasma-free normetanephrine (926.5 pg/mL) and metanephrine (1938 pg/mL) diagnostic of pheochromocytoma. Serum electrolytes, dexamethasone suppression test, dehydroepiandrosterone sulfate, and testosterone were all within normal limits.
Initial laboratory evaluation of patient presenting with spontaneous subarachnoid hemorrhage and adrenal mass
| Laboratory test, units . | Result (normal range) . |
|---|---|
| Serum potassium, mEq/L | 3.5 (3.6-5.2) |
| Serum glucose, mg/dL | 95 (62-125) |
| Serum magnesium, mg/dL | 1.9 (1.8-2.4) |
| Plasma-free metanephrines, pg/mL | 1938 (0-88) |
| Plasma-free normetanephrines, pg/mL | 926.5 (0-191.8) |
| Serum 8 Am cortisol after low-dose dexamethasone suppression, μg/dL | |
| Serum testosterone, ng/dL | 1.3 (< 1.8) |
| Serum DHEA-sulfate, μg/dL | 21.3 (7-40) |
| Serum glucose, mg/dL | 32.7 (29.4-220.5) |
| Laboratory test, units . | Result (normal range) . |
|---|---|
| Serum potassium, mEq/L | 3.5 (3.6-5.2) |
| Serum glucose, mg/dL | 95 (62-125) |
| Serum magnesium, mg/dL | 1.9 (1.8-2.4) |
| Plasma-free metanephrines, pg/mL | 1938 (0-88) |
| Plasma-free normetanephrines, pg/mL | 926.5 (0-191.8) |
| Serum 8 Am cortisol after low-dose dexamethasone suppression, μg/dL | |
| Serum testosterone, ng/dL | 1.3 (< 1.8) |
| Serum DHEA-sulfate, μg/dL | 21.3 (7-40) |
| Serum glucose, mg/dL | 32.7 (29.4-220.5) |
Abbreviation: DHEA, dehydroepiandrosterone.
Initial laboratory evaluation of patient presenting with spontaneous subarachnoid hemorrhage and adrenal mass
| Laboratory test, units . | Result (normal range) . |
|---|---|
| Serum potassium, mEq/L | 3.5 (3.6-5.2) |
| Serum glucose, mg/dL | 95 (62-125) |
| Serum magnesium, mg/dL | 1.9 (1.8-2.4) |
| Plasma-free metanephrines, pg/mL | 1938 (0-88) |
| Plasma-free normetanephrines, pg/mL | 926.5 (0-191.8) |
| Serum 8 Am cortisol after low-dose dexamethasone suppression, μg/dL | |
| Serum testosterone, ng/dL | 1.3 (< 1.8) |
| Serum DHEA-sulfate, μg/dL | 21.3 (7-40) |
| Serum glucose, mg/dL | 32.7 (29.4-220.5) |
| Laboratory test, units . | Result (normal range) . |
|---|---|
| Serum potassium, mEq/L | 3.5 (3.6-5.2) |
| Serum glucose, mg/dL | 95 (62-125) |
| Serum magnesium, mg/dL | 1.9 (1.8-2.4) |
| Plasma-free metanephrines, pg/mL | 1938 (0-88) |
| Plasma-free normetanephrines, pg/mL | 926.5 (0-191.8) |
| Serum 8 Am cortisol after low-dose dexamethasone suppression, μg/dL | |
| Serum testosterone, ng/dL | 1.3 (< 1.8) |
| Serum DHEA-sulfate, μg/dL | 21.3 (7-40) |
| Serum glucose, mg/dL | 32.7 (29.4-220.5) |
Abbreviation: DHEA, dehydroepiandrosterone.
After multidisciplinary evaluation, she was diagnosed with pheochromocytoma and initiated on α-blockade in addition to calcium channel blockade. Rebound tachycardia ensued and β-blockade was subsequently reinitiated as well. On outpatient evaluation following the acute management of hypertensive crisis and intracranial hemorrhage, she recalled several mild preceding symptoms consistent with an undiagnosed pheochromocytoma. She reported several months of low-grade intermittent headaches, as well as occasional, self-limited episodes of heart palpitations, lasting several minutes. She also endorsed episodes of mild panic attacks. She attributed these symptoms to stress related to the COVID-19 pandemic and her work. She had no recent history of weight loss, and physical examination showed no cushingoid features or virilization. She had no family history of pheochromocytoma although she did endorse her mother having been treated for some type of aneurysm.
Prior to proceeding with surgical treatment of the pheochromocytoma, a repeat head CT scan and CT angiogram was obtained about 7 weeks following her initial presentation that showed resolution of the subarachnoid and intracerebral hemorrhage with no evidence of cerebral vasoconstriction and she was cleared for surgery. The etiology of the multifocal intracranial hemorrhages was presumed to be related to reversible cerebral vasoconstriction syndrome (RCVS), a neurologic disorder characterized by thunderclap or severe headache with associated reversible constriction of cerebral arteries that is typically precipitated by vasoactive triggers (eg, stimulant drugs, strenuous activity, eclampsia, migraine headaches). Traditionally RCVS produces repetitive headaches rather than a single isolated event as in our case.
The patient then underwent surgical resection via uncomplicated laparoscopic right adrenalectomy. The large, 7-cm mass was found to be well encapsulated without gross invasion or adherence to adjacent structures. She was admitted to the surgical intensive care unit postoperatively for hemodynamic and glycemic monitoring per our pheochromocytoma pathway. She had labile blood pressures on her first postoperative night that were managed with fluid resuscitation. On postoperative day 1, she was transferred to floor care after her blood pressure normalized without need for vasopressor agents. She was discharged on postoperative day 3 and remained normotensive without medication.
Pathology revealed a well-differentiated pheochromocytoma without vascular or capsular invasion and with uninvolved margins. The tumor measured 6.9 cm and weighed 102 g. Immunohistochemistry studies demonstrated 2.5% Ki-67–positive cells and retention of succinate dehydrogenase subunit B. The modified grading of adrenal pheochromocytoma and paraganglioma (m-GAPP) score was 2 [3].
After discharge, she was seen in follow-up after 3 weeks. She had stable blood pressure measuring 140 mm Hg systolic and was started on an antihypertensive agent by her primary care provider. She reported mild intermittent headaches without neurological symptoms. She additionally noted feeling much less anxiety than prior. Postoperative catecholamines were normal (plasma metanephrine 11.8 pg/mL, plasma normetanephrine 77.7 pg/mL). She will be followed yearly with plasma fractionated metanephrine levels to monitor for recurrence. Referral for genetic evaluation was placed; however, unfortunately the patient has not completed genetic testing to date. This case highlights the importance of including adrenal etiology when evaluating spontaneous intracranial hemorrhage associated with hypertensive crisis.
Discussion
Pheochromocytoma associated with hypertension was first reported in 1886 by Dr Felix Frankel, who described a young woman with multiple attacks of palpitations, headaches, anxiety, and dizziness, resulting in what Frankel called “generalized vasoactive disease.” [4] The most common presenting symptoms of pheochromocytoma are paroxysmal episodes of headaches, sweating, palpitations, and hypertension, though sequelae of catecholamine surge can also lead to presentation (heart failure, etc), or patients may be completely asymptomatic as well [2]. A review of the disease process published in 1960 by Dr David Hume cites the first report of subarachnoid hemorrhage in a patient with pheochromocytoma as occurring in 1933. In this case, the tumor as well as intracranial bleeding were revealed by autopsy [5]. Additionally, intracerebral hemorrhage in and of itself has been shown to cause an endogenous catecholamine release, bringing attention to the need to distinguish between pseudopheochromocytoma and pheochromocytoma by evaluating for an adrenal mass [6, 7]. Other cases of intracerebral hemorrhage as the presenting sign of pheochromocytoma have been reviewed in the literature, as presented by Pekic et al in 2019 [8]. This review of 12 cases primarily occurred in children/young adults with the mean age being 25.4 ± 4.0, with the oldest patient aged 51 years. The majority of patients in this review had preceding symptoms, some for years before the intracerebral hemorrhage. It certainly seems more common to encounter symptoms without a clear diagnosis of etiology. Togha et al [2] describe a woman with repeated attacks of thunderclap headache and episodic hypertension for whom a diagnosis of recurrent RCVS was made over a 2-year period based on serial MR angiography. In this case, the recurrence of RCVS over years in the setting of severe hypertension and intracerebral hemorrhage ultimately led to the diagnosis of pheochromocytoma. Our patient's clinical course is an outlier from these previously described cases; her presentation was more fulminant with symptoms progressing over 2 days and with multifocal hematomas involving both parenchymal and subarachnoid areas. At age 63 years, she is older than previous pheochromocytoma patients described presenting in this manner.
Although a presumed diagnosis of RCVS was made in our case, there was no evidence of vasoconstriction on DSA, the gold-standard test for RCVS, though this was presumed to be related to the short 2-day period between headache onset and the DSA. In a series of 30 patients with RCVS after intracranial hemorrhage, a third of patients had normal initial vessel imaging, including DSA, though were found to have evidence of RCVS on repeat imaging. Further supporting that RCVS is a dynamic arteriopathy, another study of serial MR angiography after thunderclap headache onset among patients diagnosed with RCVS revealed the peak of cerebral vasoconstriction occurred around 16 days after headache, around the time of headache resolution, and the majority of patients demonstrated worsening of vasoconstriction on repeat imaging despite improvement in symptoms [9]. In our patient, the presumptive diagnosis of RCVS was based on the clinical presentation and repeat DSA was not obtained during the period of presumed maximal vasoconstriction given the lack of any clinical utility, though CT angiogram obtained after 7 weeks revealed no evidence of vasoconstriction.
The paroxysmal nature of pheochromocytoma symptoms, if present, can make diagnosis difficult in many cases. In this case, the patient’s prior episodes of “white coat hypertension” may represent missed opportunities to intervene at an earlier stage, demonstrating the importance of timely recognition and treatment of hypertension. In any patient presenting with hypertensive emergency, a thorough history elucidating episodic symptoms should raise suspicion for hypertension secondary to a catecholamine-secreting tumor. Biochemical testing and/or cross-sectional imaging should be pursued for any suspicion of pheochromocytoma, especially in the context of paroxysmal symptomatology. β-Blocker therapy is commonly used as an initial treatment for hypertensive urgency and RCVS; however, in patients with undiagnosed pheochromocytoma this can lead to unopposed α-stimulation of the vasculature and precipitate worsening of the clinical condition. In our patient, this manifested as atrial fibrillation. On diagnosis of pheochromocytoma, α-blockade should be initiated promptly to complement existing β-blocker therapy if already given. As an alternative to α-blockade, calcium channel blockers have also been shown to be safe for minimizing the morbidity of perioperative hemodynamic changes in patients with pheochromocytoma [10]. Calcium channel blockers including the nicardipine infusion used for our patient are also useful in hypertensive crisis and may be the best choice of initial medical therapy given the risk of β-blockade for patients with undiagnosed pheochromocytoma.
Conclusion
This case illustrates a unique presentation of pheochromocytoma and demonstrates the importance of including catecholamine-secreting adrenal tumors on the differential for hypertensive crisis and intracerebral hemorrhage of unknown etiology for patients of any age. Prompt diagnosis is critical to appropriate medical treatment and ultimately definitive surgical management.
Acknowledgments
Dr Wendy Suhre, University of Washington Department of Anesthesiology, contributed to this patient's care in preoperative evaluation and coordination for surgery as well as review of the manuscript.
Financial Support
The authors received no financial support for the research, authorship, and/or publication of this article.
Disclosures
The authors have nothing to disclose.
Data Availability
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
References
Abbreviations
- CT
computed tomography
- DSA
digital subtraction catheter angiogram
- MRI
magnetic resonance imaging
- RCVS
reversible cerebral vasoconstriction syndrome



