-
PDF
- Split View
-
Views
-
Cite
Cite
Carol H Wysham, Davida F Kruger, Practical Considerations for Initiating and Utilizing Flash Continuous Glucose Monitoring in Clinical Practice, Journal of the Endocrine Society, Volume 5, Issue 9, September 2021, bvab064, https://doi.org/10.1210/jendso/bvab064
Close - Share Icon Share
Abstract
Use of continuous glucose monitoring (CGM) has been shown to improve clinical outcomes in patients with type 1 diabetes (T1D) and type 2 diabetes (T2D), including improved glycemic control, better treatment adherence, and an increased understanding of their treatment regimens. Retrospective analysis of CGM data allows clinicians and patients to identify glycemic patterns that support and facilitate informed therapy adjustments. There are currently 2 types of CGM systems: real-time CGM (rtCGM) and flash CGM. The FreeStyle Libre 2 (FSL2) is the newest flash CGM system commercially available. Because the FSL2 system was only recently cleared for use in the US, many endocrinologists and diabetes specialists may be unfamiliar with the strengths, limitations, and potential of the FSL2 system. This article focuses on practical approaches and strategies for initiating and using flash CGM in endocrinology and diabetes specialty practices.
The benefits of continuous glucose monitoring (CGM) have been demonstrated in large clinical trials [1-4] and numerous real-world, observational studies [5-15]. On the strength of this growing body of evidence, use of this CGM is recommended for most individuals with diabetes who are treated with intensive insulin regimens [16].
CGM technology first emerged as real-time CGM (rtCGM); however, flash continuous glucose monitoring (flash CGM), another method of CGM, is being adopted by an increasing number of individuals with type 1 diabetes (T1D) and type 2 diabetes (T2D) for their daily self-management.
Large randomized controlled trials using flash CGM have demonstrated significant improvements in hypoglycemia, glycemic variability, and patient satisfaction in individuals with well-controlled T1D [3] and T2D [17] who were treated with intensive insulin therapy. Improvements in glycated hemoglobin A1c (HbA1c) and percentage of time within the target glucose range were observed in T1D [3] but not T2D [17] patients. Moreover, recent real-world observational studies have reported significant reductions in hospital admissions for severe hypoglycemia and/or diabetic ketoacidosis in large cohorts of T1D adults who used flash CGM for 12 months [6, 7]. Decreased hypoglycemia frequency, reduced HbA1c, and improved quality of life among young adults with T1D treated with insulin pump therapy have also been demonstrated [18].
The FreeStyle Libre 2 (FLS2) (Abbott Diabetes Care, Alameda, CA) is the newest flash CGM system commercially available. Unlike earlier versions of the FreeStyle Libre system, which did not offer alarms, the FSL2 offers optional alarms as an added safeguard against severe hypoglycemia and hyperglycemia. Users can select the times and situations when the alarms are operational, which can help counter “alarm fatigue” and support persistence in their system.
Because the FLS2 system was only recently cleared for use in the United States, many clinicians may be unfamiliar with the strengths, limitations, and potential of the FSL2 system in managing T1D and insulin-treated T2D patients who are treated with intensive insulin management regimens. The aim of this article is to provide practical guidance for initiating and utilizing the FSL2 system in endocrinology and diabetes specialty practices.
FSL2 System Overview
The FSL2 system is indicated for use in patients ≥4 years of age. The FSL2 sensor continuously samples and measures interstitial glucose levels, generating a new glucose value each minute. The overall accuracy, as assessed by mean absolute relative difference (MARD), is 9.2%, with 92.4% of glucose values within ± 20 mg/dL [19]. At this time, the sensor is only approved for placement in the upper arm. When users scan their sensors, the glucose data are transmitted to the reader or smartphone. The current glucose concentration, trend arrows, and the most recent 8 hours of sensor glucose readings are displayed in 15-minute intervals. If more than 8 hours lapse between scans, only the last 8 hours of data are reported. Therefore, clinicians should strongly encourage patients to scan frequently in order to protect their data as they maintain vigilance in monitoring their glucose levels.
When the alarms are turned on, the system automatically alerts the user of current low glucose, high glucose, and if the signal between the sensor and reader/smartphone is lost. The user receives a message on the reader/smartphone through sound or vibration (based on the user’s personal preference). Users have an option to turn on “Override Do Not Disturb” in alarm settings that will enable alarms to present, even if the phone is muted or “Do Not Disturb” is on.
Although daily calibration with fingerstick tests is not required, it is recommended that users perform confirmatory blood glucose testing when: hypoglycemia is detected; a trend arrow indicates rapidly changing glucose values; and symptoms are not reflected by the displayed glucose value. The FSL2 sensor can be worn for up to 14 days, which aligns with current recommendations regarding the number of glucose readings needed to ensure reliable data [20]. Studies have shown that glucose readings from the most recent 14 days correlate strongly with 3 months of mean glucose, time in ranges, and hyperglycemia metrics [20, 21].
For retrospective analysis, glucose data can be downloaded to the LibreView software in the clinician office or downloaded remotely, using the Abbott-approved compatible mobile app, which can be programmed to automatically upload glucose data to the cloud to enable access by clinicians. The app also allows users to share data with family members and friends but not in real time.
Integrating the FSL2 System into Clinical Practice
Integrating FSL2 technology into the office workflow requires consideration of several factors, including patient selection, training/education, data downloading/interpretation, and billing for services. The first step is identifying patients who are most appropriate for FSL2 use.
Patient Selection
Collaboration with patients is vital when helping select the most appropriate CGM device. Treatment satisfaction and patient perceptions about the quality of their care contribute significantly to clinical outcomes [22, 23], and clinicians play a key role in promoting treatment satisfaction through good communication with their patients [24]. Therefore, the selection process should be reflective of each patient’s economic circumstances and lifestyle preferences. However, these considerations must be weighed against each patient’s clinical status (eg, impaired hypoglycemia awareness, frequent/severe hypoglycemia). The following is a list of patients who may benefit from using the FSL2 system:
Treated with intensive insulin regimens (multiple daily injection [MDI] or insulin pump) [1, 2, 5]
Increased risk for hypoglycemia, impaired hypoglycemia awareness, frequent nocturnal hypoglycemia, frequent severe hypoglycemia [25-27].
Pregnant with pre-existing T1D [28, 29], T2D [28], or gestational diabetes [30]
Newly diagnosed T2D (for episodic use as an educational tool) [31]
T2D patients not on intensive insulin regimens who are under good control but may benefit from full-time or episodic CGM as an alternative to self-monitoring of blood glucose [31]
Patients who lack adequate insurance coverage and cannot afford other rtCGM systems but desire improved glycemic control [32].
Patient Education/Training
When initiating the FSL2, it is important that each patient receives thorough instruction in how to use their device (eg, set up, sensor placement) and how to interpret their data, focusing primarily on detecting and treating immediate and/or impending hypoglycemia and hyperglycemia. To this end, patients should be strongly encouraged to scan their sensor frequently. They should also be advised when confirmatory fingerstick testing is required (eg, when symptoms do not match their sensor value). A checklist for patient training, recommendations for scanning frequency, and use of trend arrows and other CGM data were recently published and can accessed online [33]. A recent publication by Kruger et al provides comprehensive recommendations for integrating CGM use in clinical practices [34].
Data Downloading and Interpretation
Connecting patient data to the clinic
The first step in connecting patient data is to access the LibreView website at www.libreview.com to create LibreView and download the device drivers. Step-by-step instructions for setting up these accounts downloading drivers are available at https://pro.libreview.io/articles/qsg/. Once the accounts are set up, clinicians can connect with patients in 2 ways:
The clinician creates the patient’s account in his/her practice under HCP LibreView account and then sends an email to patient to connect. A LibreView link is sent to patient in the email. The patient needs to click on link, go to LibreView and get connected. Thereafter, if patient uploads the data from reader at his home, it will show up in the HCP account as well.
The patient can create a LibreView account and enter the clinician’s practice ID to connect with his/her clinician. The clinician will then accept the request and the account will be linked to the clinician account.
Interpreting CGM data
An international consensus panel recently published recommendations for use of CGM metrics in assessing glycemic status and guiding therapy [35]. Importantly, these recommendations were endorsed by a number of international diabetes organizations, including the American Association of Clinical Endocrinologists, American Diabetes Association, European Association for the Study of Diabetes, International Society for Pediatric and Adolescent Diabetes, Juvenile Diabetes Research Foundation, and the Endocrine Society. Among the 10 metrics identified by the consensus panel, 5 are considered to be the most useful for efficient interpretation of downloaded CGM data. These metrics include mean glucose, the Glucose Management Indicator (GMI), glycemic variability (coefficient of variation [%CV]) or standard deviation (SD); time in range (TIR); time below range (TBR) and time above range (TAR). Table 1 presents the established TIR, TBR, and TAR targets for uncompromised and compromised (older/high-risk) T1D and T2D populations.
Targets for assessment of glycemic control: T1D/T2D and older/high-risk individuals [19]
| Diabetes group . | Time in range . | . | Time below range . | . | Time above range . | . |
|---|---|---|---|---|---|---|
| . | Within target range . | % of readings time/day . | Below target level . | % of readings time/day . | Above target level . | % of readings time/day . |
| T1Da/T2D | 70-180 mg/dL | >70% >16 hr, 48 min | <70 mg/dL | <4% <1 hr | >180 mg/dL | <25% <6 hr |
| <54 mg/dL | <1% <15 min | >250 mg/dL | <5% <1 hr, 12 min | |||
| Older/high-risk T1D/T2D | 70-180 mg/dL | >50% >12 hr | <70 mg/dL | <1% <15 min | >250 mg/dL | <10% <2 hr, 24 min |
| Diabetes group . | Time in range . | . | Time below range . | . | Time above range . | . |
|---|---|---|---|---|---|---|
| . | Within target range . | % of readings time/day . | Below target level . | % of readings time/day . | Above target level . | % of readings time/day . |
| T1Da/T2D | 70-180 mg/dL | >70% >16 hr, 48 min | <70 mg/dL | <4% <1 hr | >180 mg/dL | <25% <6 hr |
| <54 mg/dL | <1% <15 min | >250 mg/dL | <5% <1 hr, 12 min | |||
| Older/high-risk T1D/T2D | 70-180 mg/dL | >50% >12 hr | <70 mg/dL | <1% <15 min | >250 mg/dL | <10% <2 hr, 24 min |
Abbreviations: T1D, type 1 diabetes; T2D, type 2 diabetes.
aFor age <25 years, if the HbA1c goal is 7.5%, then set time in range target to approximately 60%.
Targets for assessment of glycemic control: T1D/T2D and older/high-risk individuals [19]
| Diabetes group . | Time in range . | . | Time below range . | . | Time above range . | . |
|---|---|---|---|---|---|---|
| . | Within target range . | % of readings time/day . | Below target level . | % of readings time/day . | Above target level . | % of readings time/day . |
| T1Da/T2D | 70-180 mg/dL | >70% >16 hr, 48 min | <70 mg/dL | <4% <1 hr | >180 mg/dL | <25% <6 hr |
| <54 mg/dL | <1% <15 min | >250 mg/dL | <5% <1 hr, 12 min | |||
| Older/high-risk T1D/T2D | 70-180 mg/dL | >50% >12 hr | <70 mg/dL | <1% <15 min | >250 mg/dL | <10% <2 hr, 24 min |
| Diabetes group . | Time in range . | . | Time below range . | . | Time above range . | . |
|---|---|---|---|---|---|---|
| . | Within target range . | % of readings time/day . | Below target level . | % of readings time/day . | Above target level . | % of readings time/day . |
| T1Da/T2D | 70-180 mg/dL | >70% >16 hr, 48 min | <70 mg/dL | <4% <1 hr | >180 mg/dL | <25% <6 hr |
| <54 mg/dL | <1% <15 min | >250 mg/dL | <5% <1 hr, 12 min | |||
| Older/high-risk T1D/T2D | 70-180 mg/dL | >50% >12 hr | <70 mg/dL | <1% <15 min | >250 mg/dL | <10% <2 hr, 24 min |
Abbreviations: T1D, type 1 diabetes; T2D, type 2 diabetes.
aFor age <25 years, if the HbA1c goal is 7.5%, then set time in range target to approximately 60%.
The consensus panel also endorsed use of the Ambulatory Glucose Profile (AGP) as the preferred template for reporting CGM data. The Ambulatory Glucose Profile report presents the metrics in a standardized report that includes statistical information (eg, average glucose, GMI, TBR/TIR/TAR, and glycemic variability [SD; CV]) and a graphical depiction of the 24-hour glucose (eg, glucose profile). Most of these elements were incorporated into the current version of the LibreView Summary Report. (Fig. 1)
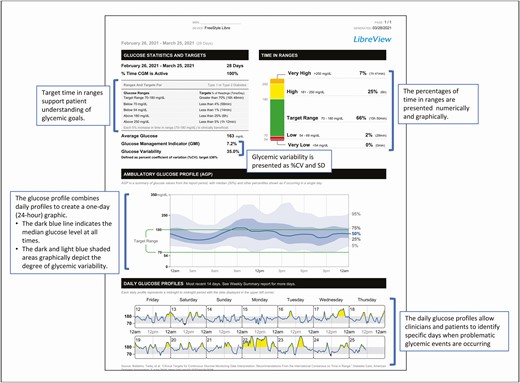
The FreeStyle LibreView download software features a number of reports that can be used to analyze various aspects of patient status. However, we have found that sufficient data can often be obtained from the Summary Report, using a 4-step process for interpretation.
Step 1. Check the overall glycemic status. The average glucose number provides a “first glance” at the overall glycemic status of the patient. An average glucose level of 150 mg/dL generally reflects a HbA1c of 7.0%. The %CV and SD metrics provide an immediate indication of the degree of glycemic variability. Excessive variability can be addressed by simultaneously reducing the %TBR and increasing %TAR.
Step 2. Check the TBR, TIR, and TAR statistics, focusing on hypoglycemia (TBR) first.
If the TBR statistics are above the cutpoints (>4% less than 70 mg/dL; >1% less than 54 mg/dL), the visit should focus on this issue. Otherwise, move on to the TIR and TAR statistics. An appropriate goal for older and/or high-risk patients is <1% less than 70 mg/dL.
Step 3. Review the 24-hour glucose profile to identify the time part(s) and magnitude(s) of the problem identified. Again, the first priority is to address hypoglycemia.
Because the profile is compilation of all the data obtained, it may be necessary to review multiple days to identify any particular day(s) when the patterns are most notable (eg, weekdays vs weekends).
Step 4. Review the treatment regimen.
For patients treated with intensive insulin regimens, we advise checking the appropriateness of the basal rate. It also important to determine how/when the doses, meals, and correction doses are calculated and administered.
For patients treated with less intensive regimens (eg, insulin, oral medications, noninsulin injectables), the data can be used to guide changes in dosage(s) and addition or discontinuation of medications.
The following case example illustrates how this approach can be applied to an 80-year-old female with a 30-year history of T1D and treated with MDI therapy during the past 25 years. The patient lives alone, is very active, and does not count carbohydrates when dosing her insulin. In this example, the average glucose and GMI are in range for an older adult; however, her glycemic variability is in the upper range for %CV. (Fig. 2A) This suggests that some additional follow-up on this may be warranted. Although her %TIR, %TAR, and %TBR are within acceptable levels, (Fig. 2B) the glucose profile indicates a pattern of low glucose before lunch and after supper. (Fig. 2C) Importantly, review of the daily glucose profiles shows no clear pattern of the days when low glucose is occurring. (Fig. 2D)
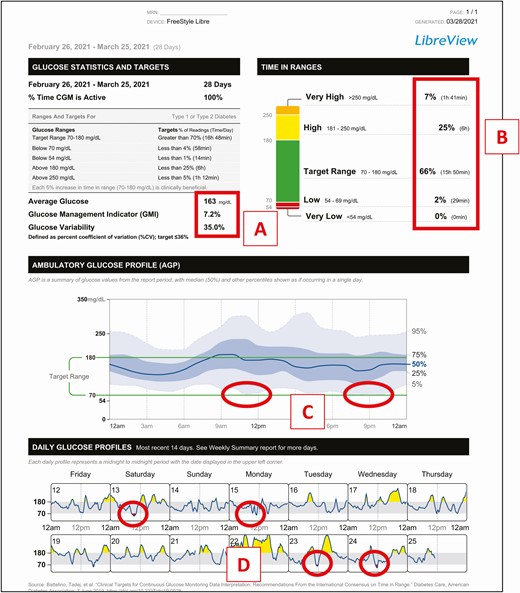
Case example. Patient history: 80-year-old female with T1D treated with MDI therapy.
Although the data do not show occurrences of severe hypoglycemia, the pattern does indicate an increased risk for hypoglycemia. Discussion with patient reveals that her physical activity level varies from day to day, and that most of her lows are related to activity. She agrees to pay more attention to glucose trends and to treat before engaging in activity if her glucose level is <180 mg/dL and is trending down. Additionally, although the patient is unwilling to use carbohydrate counting, which is contributing to her glycemic variability, she does agree to reduce her insulin dosages when eating lower carbohydrate. As demonstrated in this example, the data presented in the Summary Report can be quickly and interpreted, facilitating more informed and targeted therapy decisions.
Billing for Services
Current Procedural Terminology Codes
There are currently 2 Current Procedural Terminology (CPT) codes that cover CGM initiation, 95249 and 95250, which cover placement, hook-up, calibration of monitor, patient training, and printout of recording; 95249 is used when the device is owned by the patient, 95250 is used when the device is owned by the clinic. A third CPT code, 95251, is used and reported to insurers when clinicians perform analysis, interpretation, and report on a minimum of 72 hours of CGM data. This may be conducted with data from patient-owned or clinician-owned CGM device. This report is distinct from an evaluation and management service and does not include an assessment of the patient or indicate a plan of care for the patient.
An Evaluation/Management (E/M) CPT code (99201–99205, 99211–99215, 99241–99245) may be reported with the CGM codes if documentation supports the medical necessity of a significant and separately identifiable E/M service performed the same date as the CGM service(s). Clinicians must bill the E/M code with modifier “-25” and submit the 95251 billing on the same day for the same patient if the E/M was a significant and distinct identifiable service that was “above and beyond” the services associated with CPT 95251. Table 2 presents a summary of the CPT codes and associated requirements. A comprehensive description of the CPT codes and their use can be accessed at the American Association of Clinical Endocrinologists website [36].
| Code . | Description/requirements for billing . | Personnel who can perform and bill for service . |
|---|---|---|
| 95249 | APPLICATION: • When CGM system is patient-owned SERVICES COVERED: • Sensor placement, hook-up, calibration of monitor, patient training, and printout of recording REQUIREMENTS: • ≥72 hours of CGM data are available for interpretation. • Patient must bring the data receiver into the healthcare provider office where the entire process is performed. • This code should only be reported once during the time the patient owns the device. • Obtaining a new sensor and/or transmitter without a change in the receiver does not warrant reporting subsequent times. • The code should not be reported in conjunction with 99091 and/or 0446T. • The correct date of service is the date the CGM recording is printed in the office. • If a separate and significant evaluation and management (E/M) service is performed on the same date, a modifier 25 may be required to be added to the E/M code. | • Trained RN, PharmD/RPh, RD, CDE, or MA can perform service if within scope of practice. • Billing can be done by supervising physician, advanced practitioner, or hospital outpatient department. |
| 95250 | Ambulatory CGM of interstitial tissue fluid via a subcutaneous sensor with a minimum of 72 hours; clinician-owned equipment, sensor placement, hook-up, calibration of monitor, patient training, removal of sensor, and printout of recording. • The code can only be reported one time per month and should not be reported in conjunction with 99091 and/or 0446T. • All elements described in the code description must be performed to appropriately report the code to insurers. • The correct date of service is the date that the CGM recording is printed in the office. • If a separate and significant evaluation and management (E/M) service is performed on the same date, a modifier 25 may be required to be added to the E/M code. | • Trained RN, PharmD/RPh, RD, CDE, or MA can perform service if within scope of practice. • Billing can be done by supervising physician, advanced practitioner, or hospital outpatient department. |
| 95251 | This code is used and reported to insurers when clinicians perform an analysis, interpretation, and report on a minimum of 72 hours of CGM data. The analysis, interpretation, and report may be done with data from patient-owned or clinician-owned CGM device. Importantly, the analysis, interpretation and report is distinct from an evaluation and management service and does not include an assessment of the patient or indicate a plan of care for the patient. | • Only MD/DO, NP, PA, and CNS can perform and bill for services associated with this code. |
| Code . | Description/requirements for billing . | Personnel who can perform and bill for service . |
|---|---|---|
| 95249 | APPLICATION: • When CGM system is patient-owned SERVICES COVERED: • Sensor placement, hook-up, calibration of monitor, patient training, and printout of recording REQUIREMENTS: • ≥72 hours of CGM data are available for interpretation. • Patient must bring the data receiver into the healthcare provider office where the entire process is performed. • This code should only be reported once during the time the patient owns the device. • Obtaining a new sensor and/or transmitter without a change in the receiver does not warrant reporting subsequent times. • The code should not be reported in conjunction with 99091 and/or 0446T. • The correct date of service is the date the CGM recording is printed in the office. • If a separate and significant evaluation and management (E/M) service is performed on the same date, a modifier 25 may be required to be added to the E/M code. | • Trained RN, PharmD/RPh, RD, CDE, or MA can perform service if within scope of practice. • Billing can be done by supervising physician, advanced practitioner, or hospital outpatient department. |
| 95250 | Ambulatory CGM of interstitial tissue fluid via a subcutaneous sensor with a minimum of 72 hours; clinician-owned equipment, sensor placement, hook-up, calibration of monitor, patient training, removal of sensor, and printout of recording. • The code can only be reported one time per month and should not be reported in conjunction with 99091 and/or 0446T. • All elements described in the code description must be performed to appropriately report the code to insurers. • The correct date of service is the date that the CGM recording is printed in the office. • If a separate and significant evaluation and management (E/M) service is performed on the same date, a modifier 25 may be required to be added to the E/M code. | • Trained RN, PharmD/RPh, RD, CDE, or MA can perform service if within scope of practice. • Billing can be done by supervising physician, advanced practitioner, or hospital outpatient department. |
| 95251 | This code is used and reported to insurers when clinicians perform an analysis, interpretation, and report on a minimum of 72 hours of CGM data. The analysis, interpretation, and report may be done with data from patient-owned or clinician-owned CGM device. Importantly, the analysis, interpretation and report is distinct from an evaluation and management service and does not include an assessment of the patient or indicate a plan of care for the patient. | • Only MD/DO, NP, PA, and CNS can perform and bill for services associated with this code. |
| Code . | Description/requirements for billing . | Personnel who can perform and bill for service . |
|---|---|---|
| 95249 | APPLICATION: • When CGM system is patient-owned SERVICES COVERED: • Sensor placement, hook-up, calibration of monitor, patient training, and printout of recording REQUIREMENTS: • ≥72 hours of CGM data are available for interpretation. • Patient must bring the data receiver into the healthcare provider office where the entire process is performed. • This code should only be reported once during the time the patient owns the device. • Obtaining a new sensor and/or transmitter without a change in the receiver does not warrant reporting subsequent times. • The code should not be reported in conjunction with 99091 and/or 0446T. • The correct date of service is the date the CGM recording is printed in the office. • If a separate and significant evaluation and management (E/M) service is performed on the same date, a modifier 25 may be required to be added to the E/M code. | • Trained RN, PharmD/RPh, RD, CDE, or MA can perform service if within scope of practice. • Billing can be done by supervising physician, advanced practitioner, or hospital outpatient department. |
| 95250 | Ambulatory CGM of interstitial tissue fluid via a subcutaneous sensor with a minimum of 72 hours; clinician-owned equipment, sensor placement, hook-up, calibration of monitor, patient training, removal of sensor, and printout of recording. • The code can only be reported one time per month and should not be reported in conjunction with 99091 and/or 0446T. • All elements described in the code description must be performed to appropriately report the code to insurers. • The correct date of service is the date that the CGM recording is printed in the office. • If a separate and significant evaluation and management (E/M) service is performed on the same date, a modifier 25 may be required to be added to the E/M code. | • Trained RN, PharmD/RPh, RD, CDE, or MA can perform service if within scope of practice. • Billing can be done by supervising physician, advanced practitioner, or hospital outpatient department. |
| 95251 | This code is used and reported to insurers when clinicians perform an analysis, interpretation, and report on a minimum of 72 hours of CGM data. The analysis, interpretation, and report may be done with data from patient-owned or clinician-owned CGM device. Importantly, the analysis, interpretation and report is distinct from an evaluation and management service and does not include an assessment of the patient or indicate a plan of care for the patient. | • Only MD/DO, NP, PA, and CNS can perform and bill for services associated with this code. |
| Code . | Description/requirements for billing . | Personnel who can perform and bill for service . |
|---|---|---|
| 95249 | APPLICATION: • When CGM system is patient-owned SERVICES COVERED: • Sensor placement, hook-up, calibration of monitor, patient training, and printout of recording REQUIREMENTS: • ≥72 hours of CGM data are available for interpretation. • Patient must bring the data receiver into the healthcare provider office where the entire process is performed. • This code should only be reported once during the time the patient owns the device. • Obtaining a new sensor and/or transmitter without a change in the receiver does not warrant reporting subsequent times. • The code should not be reported in conjunction with 99091 and/or 0446T. • The correct date of service is the date the CGM recording is printed in the office. • If a separate and significant evaluation and management (E/M) service is performed on the same date, a modifier 25 may be required to be added to the E/M code. | • Trained RN, PharmD/RPh, RD, CDE, or MA can perform service if within scope of practice. • Billing can be done by supervising physician, advanced practitioner, or hospital outpatient department. |
| 95250 | Ambulatory CGM of interstitial tissue fluid via a subcutaneous sensor with a minimum of 72 hours; clinician-owned equipment, sensor placement, hook-up, calibration of monitor, patient training, removal of sensor, and printout of recording. • The code can only be reported one time per month and should not be reported in conjunction with 99091 and/or 0446T. • All elements described in the code description must be performed to appropriately report the code to insurers. • The correct date of service is the date that the CGM recording is printed in the office. • If a separate and significant evaluation and management (E/M) service is performed on the same date, a modifier 25 may be required to be added to the E/M code. | • Trained RN, PharmD/RPh, RD, CDE, or MA can perform service if within scope of practice. • Billing can be done by supervising physician, advanced practitioner, or hospital outpatient department. |
| 95251 | This code is used and reported to insurers when clinicians perform an analysis, interpretation, and report on a minimum of 72 hours of CGM data. The analysis, interpretation, and report may be done with data from patient-owned or clinician-owned CGM device. Importantly, the analysis, interpretation and report is distinct from an evaluation and management service and does not include an assessment of the patient or indicate a plan of care for the patient. | • Only MD/DO, NP, PA, and CNS can perform and bill for services associated with this code. |
Documentation
When billing for the CGM data interpretation visit (CPT 95251), all that is needed is a simple statement confirming that at least 72 hours of CGM data were available for interpretation and that the findings and recommendations for therapy adjustments were shared with the patient. Clinicians may also want to include a brief description of the recommendations made to the patient. For example: “I have reviewed and interpreted the data, shared the information with the patient, and we have agreed to the following changes: (describe changes).” A list of the recommended changes and a screen shot of the Summary Report and can then be entered into the electronic medical record.
Because each private insurer determines the coverage that they will provide, it is important that clinicians obtain copies of their current insurers’ published CGM coverage decisions and policies. If the policy is inadequate, clinicians may need to provide a rationale to the plan’s medical team to help advance the policy.
Summary
Recent studies have demonstrated the clinical value of FSL2 use in T1D [3, 37] and insulin-treated T2D [4]. In this article, we have outlined the basic issues that must be considered as a starting point for initiating use of the FSL2 system in clinical practice. Successful integration requires practices to develop a formal plan that clearly identifies the roles and responsibilities of each clinic staff member in providing training/education, facilitating data downloading, and interpreting the glucose data. Therefore, all members of the health care team must become knowledgeable and skilled in integrating FSL2 use into their practices and in teaching their patients to use CGM safely and effectively.
Abbreviations
- CGM
continuous glucose monitoring
- CPT
Current Procedural Terminology
- CV
coefficient of variation
- FLS2
FreeStyle Libre 2
- HbA1c
glycated hemoglobin A1c
- GMI
Glucose Management Indicator
- MDI
multiple daily injection
- rtCGM
real-time continuous glucose monitoring
- T1D
type 1 diabetes
- T2D
type 2 diabetes
- TAR
time above range
- TBR
time below range
- TIR
time in range
Acknowledgments
Funding for the development of this manuscript was provided by Abbott Diabetes Care, Alameda, CA. The authors received no honoraria but wish to thank Christopher G. Parkin, MS, CGParkin Communications, Inc., and Anne L. Peters, MD, for their assistance in developing this manuscript. C.H.W. and D.F.K. developed the content for the manuscript and contributed equally to manuscript writing and revisions.
Financial Support: Abbott Diabetes Care provided financial support for editorial assistance in developing this manuscript. The authors received no compensation.
Additional Information
Disclosures: C.W. has received research funding from Abbott Diabetes Care, Allergan, Gilead, Eli Lilly, and Company, Mylan, and Novo Nordisk, and has served as a speaker/advisor for Abbott Diabetes Care, Astra Zeneca, Janssen, Novo Nordisk, and Sanofi. D.F.K. has served on advisory boards and/or speaker bureaus for Dexcom and Abbott Diabetes Care, and her institution has received research support from Dexcom and Abbott Diabetes Care.
Data Availability
Availability of data is not applicable to this article.



