-
PDF
- Split View
-
Views
-
Cite
Cite
Anisley Valenciaga, Pamela Brock, Benjamin O’Donnell, Steven W Ing, Diagnosing Hypoparathyroidism, Sensorineural Deafness, and Renal Dysplasia Syndrome and a Novel GATA3 Variant, JCEM Case Reports, Volume 3, Issue 1, January 2025, luae246, https://doi.org/10.1210/jcemcr/luae246
Close - Share Icon Share
Abstract
Hypoparathyroidism (hypoPTH), sensorineural deafness, and renal dysplasia (HDR) syndrome is a rare autosomal dominant condition with approximately 200 cases published. HDR syndrome is caused by variants of GATA binding protein 3 gene (GATA3), which encodes a transcription factor, with multiple types of GATA3 variants reported. We present the case of a 76-year-old woman who was diagnosed with hypoPTH when she was aged 40 years and transferred care to our institution. Further history elucidated presence of deafness at age 1 year and chronic kidney disease with a left atrophic kidney diagnosed in her 60 seconds. Genetic testing identified a novel GATA3 missense variant of unknown significance (c.791G > A, p.Cys264Tyr). There was no family history of hypoPTH, deafness, or renal disease, which might indicate incomplete penetrance or de novo mutation. Advanced modeling of protein sequence and biophysical properties predicts abnormal protein function, suggesting possible pathogenicity. In addition, a likely pathogenic variant in the same amino acid was previously described in a patient with HDR, supporting the in silico prediction of pathogenicity in our patient's variant. Syndromic hypoPTH should be considered in patients even if presenting later in life with presumed chronic isolated conditions. Genetic testing can guide further disease screening and family testing when appropriate.
Introduction
Hypoparathyroidism (hypoPTH), sensorineural deafness, and renal dysplasia (HDR) syndrome, also known as Barakat syndrome, was initially described by Barakat et al in 1977, with the report of two brothers with hypoPTH, nerve deafness, and steroid-resistant nephrosis [1]. The syndrome was later identified as an autosomal dominant condition by Bilous et al in 1992 [2]. Subsequent reports showed the syndrome can be inherited with incomplete penetrance [3]. Although it is defined by the presence of the phenotypic HDR triad, there have been cases reported with variable phenotypic expression. A review of 180 cases found hypoPTH in 93.3%, hearing loss in 96.7%, and renal involvement in 72.2% of the cases [4]. The syndrome can be accompanied by other features including congenital heart disease, retinitis pigmentosa, cognitive disability, and polycystic ovaries [4].
HDR is a rare disease, with approximately 200 cases described in the literature so far [4, 5]. Patients can present with hypocalcemia symptoms due to hypoPTH, including myalgias, neuromuscular irritability, tetany, seizures, and cardiomyopathy. However, with the introduction of newborn hearing screening and prenatal ultrasound, cases are diagnosed more commonly with deafness and renal disease.
The GATA binding protein 3 gene (GATA3) has been identified as responsible for HDR syndrome [6]. GATA3 is located on chromosome 10p (10p14) and encodes a protein that belongs to the zinc-finger family of transcription factors. These zinc-finger domains play an important role in DNA binding and are ultimately involved in vertebrate embryonic development of the parathyroid glands, auditory system, kidney, thymus, and central nervous system [6, 7]. GATA3 variants include frameshift, nonsense, missense, intragenic deletions, and acceptor splice-site mutations [8].
The present report describes the case of a patient diagnosed with HDR syndrome in her seventh decade of life, with a novel missense variant of GATA3.
Case Presentation
A 76-year-old woman with hypoPTH of unknown etiology transferred care to our endocrinology clinic. HypoPTH was diagnosed at age 40 years and had been managed with calcitriol, vitamin D3, and a calcium-rich diet. She could not recall symptoms at initial presentation but reported possible leg swelling and weight gain. She had a history of a remote foot fracture after twisting her ankle, but no nephrolithiasis, tetany, seizures, or other hypoPTH-related symptoms. She lacked history of neck surgery or radiation. Her past medical history was significant for bilateral hearing loss diagnosed at one year of age, as well as chronic kidney disease (CKD) grade 3 along with an atrophic left kidney diagnosed in her 60s. There was no evidence of renal tubular acidosis, any other endocrinopathy, or other diseases related to hearing impairment. Her family history was significant for Alzheimer disease and ovarian cancer in multiple relatives and CKD in a maternal uncle (see family pedigree in Fig. 1). The patient does not have children.
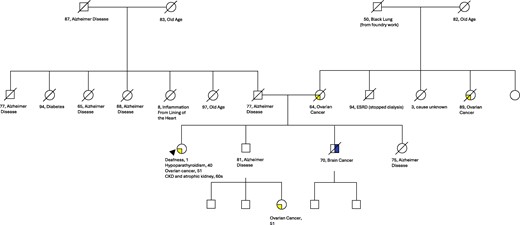
Family pedigree with absent history of hypoparathyroidism, sensorineural deafness, and renal dysplasia (HDR) syndrome phenotypes.
Diagnostic Assessment
On presenting to our clinic, laboratory testing showed undetectable parathyroid hormone (PTH) level, with normal serum calcium (S-Ca), normal 25OH vitamin D (25(OH)D), and low urinary calcium (Table 1) while taking calcitriol 0.25 µg 3 times per week and vitamin D3 2000 units daily. She also had adequate calcium intake in her diet. Test results from her initial hypoPTH diagnosis were unavailable. However, results spanning 5 years before presenting to our clinic showed low S-Ca with consistently low PTH levels (see Table 1). The doses of calcium and calcitriol supplementation at the time of the low S-Ca values are not clear. Bone densitometry was normal. Echocardiogram demonstrated mild tricuspid regurgitation.
Laboratory results on first transition of care visit while on calcitriol and vitamin D3 replacement, and prior to presentation
| Laboratory item . | Reference range . | 2021a . | 2020 . | 2019 . | 2017 . | 2016 . |
|---|---|---|---|---|---|---|
| Serum calcium | 8.6-10.5 mg/dL (2.1-2.6 mmol/L) | 9.7 mg/dL (2.43 mmol/L) | 7.8 mg/dL (L) (2.0 mmol/L) | 8.2 mg/dL (L) (2.1 mmol/L) | 8.1 mg/dL (L) (2.0 mmol/L) | 7.6 mg/dL (L) (1.9 mmol/L) |
| Albumin | 3.5-5.0 g/dL (35-50 g/L) | 4.1 g/dL (41 g/L) | 4.9 g/dL (49 g/L) | 4.1 g/dL (41 g/L) | 4.3 g/dL (43 g/L) | 3.9 g/dL (39 g/L) |
| Magnesium | 1.6-2.6 mg/dL (0.7-1.1 mmol/L) | 1.7 mg/dL (0.7 mmol/L) | ||||
| Phosphorus | 2.8-4.1 mg/dL (0.9-1.3 mmol/L) | 4.4 mg/dL (H) (1.4 mmol/L) | ||||
| 25 Hydroxyvitamin D | 30.0-100.0 ng/mL (74.9-249.6 nmol/L) | 54 ng/mL (134.8 nmol/L) | ||||
| Parathyroid hormone | 14.0-72.0 pg/mL (1.5-7.6 pmol/L) | <6.3 pg/mL (L) (<0.7 pmol/L) | 8 pg/mL (L) (0.8 pmol/L) | 7.8 pg/mL (L) (0.8 pmol/L) | 10.8 pg/mL (L) (1.1 pmol/L) | |
| BUN | 7-22 mg/dL (2.5-7.9 mmol/L) | 35 mg/dL (H) (12.5 mmol/L) | 25 mg/dL (H) (8.9 mmol/L) | |||
| Serum creatinine | 0.50-1.20 mg/dL (44.2-106.1 µmol/L) | 1.68 mg/dL (H) (148.5 µmol/L) | 1.45 mg/dL (H) (128.2 µmol/L) | 1.44 mg/dL (H) (127.2 µmol/L) | ||
| Estimated GFR | ≥60 mL/min/1.73 m2 (≥1 mL/s) | 30 mL/min/1.73 m2 (L) (0.5 mL/s) | 37 mL/min/1.73 m2 (L) (0.6 mL/s) | 37 mL/min/1.73 m2 (L) (0.6 mL/s) | 36 mL/min/1.73 m2 (L) (0.6 mL/s) | |
| Random urine calcium | mg/dL (mmol/L) | 9 mg/dL (2.25 mmol/L) | ||||
| Random urine creatinine | mg/dL (µmol/L) | 89 mg/dL (7867 µmol/L) | ||||
| 24-h urine calcium | 100.0-300.0 mg/24 h (2.5-7.5 mmol/24 h) | 93.1 mg/24 h (L) (2.3 mmol/24 h) | ||||
| 24-h urine creatinine | 0.60-1.80 g/24 h (5.3-15.9 mmol/24 h) | 0.92 g/24 h (8.1 mmol/24 h) | ||||
| 24-h urine volume | (800-2000 mL/24 h) | 1034 mL/24 h |
| Laboratory item . | Reference range . | 2021a . | 2020 . | 2019 . | 2017 . | 2016 . |
|---|---|---|---|---|---|---|
| Serum calcium | 8.6-10.5 mg/dL (2.1-2.6 mmol/L) | 9.7 mg/dL (2.43 mmol/L) | 7.8 mg/dL (L) (2.0 mmol/L) | 8.2 mg/dL (L) (2.1 mmol/L) | 8.1 mg/dL (L) (2.0 mmol/L) | 7.6 mg/dL (L) (1.9 mmol/L) |
| Albumin | 3.5-5.0 g/dL (35-50 g/L) | 4.1 g/dL (41 g/L) | 4.9 g/dL (49 g/L) | 4.1 g/dL (41 g/L) | 4.3 g/dL (43 g/L) | 3.9 g/dL (39 g/L) |
| Magnesium | 1.6-2.6 mg/dL (0.7-1.1 mmol/L) | 1.7 mg/dL (0.7 mmol/L) | ||||
| Phosphorus | 2.8-4.1 mg/dL (0.9-1.3 mmol/L) | 4.4 mg/dL (H) (1.4 mmol/L) | ||||
| 25 Hydroxyvitamin D | 30.0-100.0 ng/mL (74.9-249.6 nmol/L) | 54 ng/mL (134.8 nmol/L) | ||||
| Parathyroid hormone | 14.0-72.0 pg/mL (1.5-7.6 pmol/L) | <6.3 pg/mL (L) (<0.7 pmol/L) | 8 pg/mL (L) (0.8 pmol/L) | 7.8 pg/mL (L) (0.8 pmol/L) | 10.8 pg/mL (L) (1.1 pmol/L) | |
| BUN | 7-22 mg/dL (2.5-7.9 mmol/L) | 35 mg/dL (H) (12.5 mmol/L) | 25 mg/dL (H) (8.9 mmol/L) | |||
| Serum creatinine | 0.50-1.20 mg/dL (44.2-106.1 µmol/L) | 1.68 mg/dL (H) (148.5 µmol/L) | 1.45 mg/dL (H) (128.2 µmol/L) | 1.44 mg/dL (H) (127.2 µmol/L) | ||
| Estimated GFR | ≥60 mL/min/1.73 m2 (≥1 mL/s) | 30 mL/min/1.73 m2 (L) (0.5 mL/s) | 37 mL/min/1.73 m2 (L) (0.6 mL/s) | 37 mL/min/1.73 m2 (L) (0.6 mL/s) | 36 mL/min/1.73 m2 (L) (0.6 mL/s) | |
| Random urine calcium | mg/dL (mmol/L) | 9 mg/dL (2.25 mmol/L) | ||||
| Random urine creatinine | mg/dL (µmol/L) | 89 mg/dL (7867 µmol/L) | ||||
| 24-h urine calcium | 100.0-300.0 mg/24 h (2.5-7.5 mmol/24 h) | 93.1 mg/24 h (L) (2.3 mmol/24 h) | ||||
| 24-h urine creatinine | 0.60-1.80 g/24 h (5.3-15.9 mmol/24 h) | 0.92 g/24 h (8.1 mmol/24 h) | ||||
| 24-h urine volume | (800-2000 mL/24 h) | 1034 mL/24 h |
Values in parenthesis are International System of Units (SI).
Abbreviations: BUN, blood urea nitrogen; GFR, glomerular filtration rate; H, data are abnormally high; L, data are abnormally low.
aTransition of care visit.
Laboratory results on first transition of care visit while on calcitriol and vitamin D3 replacement, and prior to presentation
| Laboratory item . | Reference range . | 2021a . | 2020 . | 2019 . | 2017 . | 2016 . |
|---|---|---|---|---|---|---|
| Serum calcium | 8.6-10.5 mg/dL (2.1-2.6 mmol/L) | 9.7 mg/dL (2.43 mmol/L) | 7.8 mg/dL (L) (2.0 mmol/L) | 8.2 mg/dL (L) (2.1 mmol/L) | 8.1 mg/dL (L) (2.0 mmol/L) | 7.6 mg/dL (L) (1.9 mmol/L) |
| Albumin | 3.5-5.0 g/dL (35-50 g/L) | 4.1 g/dL (41 g/L) | 4.9 g/dL (49 g/L) | 4.1 g/dL (41 g/L) | 4.3 g/dL (43 g/L) | 3.9 g/dL (39 g/L) |
| Magnesium | 1.6-2.6 mg/dL (0.7-1.1 mmol/L) | 1.7 mg/dL (0.7 mmol/L) | ||||
| Phosphorus | 2.8-4.1 mg/dL (0.9-1.3 mmol/L) | 4.4 mg/dL (H) (1.4 mmol/L) | ||||
| 25 Hydroxyvitamin D | 30.0-100.0 ng/mL (74.9-249.6 nmol/L) | 54 ng/mL (134.8 nmol/L) | ||||
| Parathyroid hormone | 14.0-72.0 pg/mL (1.5-7.6 pmol/L) | <6.3 pg/mL (L) (<0.7 pmol/L) | 8 pg/mL (L) (0.8 pmol/L) | 7.8 pg/mL (L) (0.8 pmol/L) | 10.8 pg/mL (L) (1.1 pmol/L) | |
| BUN | 7-22 mg/dL (2.5-7.9 mmol/L) | 35 mg/dL (H) (12.5 mmol/L) | 25 mg/dL (H) (8.9 mmol/L) | |||
| Serum creatinine | 0.50-1.20 mg/dL (44.2-106.1 µmol/L) | 1.68 mg/dL (H) (148.5 µmol/L) | 1.45 mg/dL (H) (128.2 µmol/L) | 1.44 mg/dL (H) (127.2 µmol/L) | ||
| Estimated GFR | ≥60 mL/min/1.73 m2 (≥1 mL/s) | 30 mL/min/1.73 m2 (L) (0.5 mL/s) | 37 mL/min/1.73 m2 (L) (0.6 mL/s) | 37 mL/min/1.73 m2 (L) (0.6 mL/s) | 36 mL/min/1.73 m2 (L) (0.6 mL/s) | |
| Random urine calcium | mg/dL (mmol/L) | 9 mg/dL (2.25 mmol/L) | ||||
| Random urine creatinine | mg/dL (µmol/L) | 89 mg/dL (7867 µmol/L) | ||||
| 24-h urine calcium | 100.0-300.0 mg/24 h (2.5-7.5 mmol/24 h) | 93.1 mg/24 h (L) (2.3 mmol/24 h) | ||||
| 24-h urine creatinine | 0.60-1.80 g/24 h (5.3-15.9 mmol/24 h) | 0.92 g/24 h (8.1 mmol/24 h) | ||||
| 24-h urine volume | (800-2000 mL/24 h) | 1034 mL/24 h |
| Laboratory item . | Reference range . | 2021a . | 2020 . | 2019 . | 2017 . | 2016 . |
|---|---|---|---|---|---|---|
| Serum calcium | 8.6-10.5 mg/dL (2.1-2.6 mmol/L) | 9.7 mg/dL (2.43 mmol/L) | 7.8 mg/dL (L) (2.0 mmol/L) | 8.2 mg/dL (L) (2.1 mmol/L) | 8.1 mg/dL (L) (2.0 mmol/L) | 7.6 mg/dL (L) (1.9 mmol/L) |
| Albumin | 3.5-5.0 g/dL (35-50 g/L) | 4.1 g/dL (41 g/L) | 4.9 g/dL (49 g/L) | 4.1 g/dL (41 g/L) | 4.3 g/dL (43 g/L) | 3.9 g/dL (39 g/L) |
| Magnesium | 1.6-2.6 mg/dL (0.7-1.1 mmol/L) | 1.7 mg/dL (0.7 mmol/L) | ||||
| Phosphorus | 2.8-4.1 mg/dL (0.9-1.3 mmol/L) | 4.4 mg/dL (H) (1.4 mmol/L) | ||||
| 25 Hydroxyvitamin D | 30.0-100.0 ng/mL (74.9-249.6 nmol/L) | 54 ng/mL (134.8 nmol/L) | ||||
| Parathyroid hormone | 14.0-72.0 pg/mL (1.5-7.6 pmol/L) | <6.3 pg/mL (L) (<0.7 pmol/L) | 8 pg/mL (L) (0.8 pmol/L) | 7.8 pg/mL (L) (0.8 pmol/L) | 10.8 pg/mL (L) (1.1 pmol/L) | |
| BUN | 7-22 mg/dL (2.5-7.9 mmol/L) | 35 mg/dL (H) (12.5 mmol/L) | 25 mg/dL (H) (8.9 mmol/L) | |||
| Serum creatinine | 0.50-1.20 mg/dL (44.2-106.1 µmol/L) | 1.68 mg/dL (H) (148.5 µmol/L) | 1.45 mg/dL (H) (128.2 µmol/L) | 1.44 mg/dL (H) (127.2 µmol/L) | ||
| Estimated GFR | ≥60 mL/min/1.73 m2 (≥1 mL/s) | 30 mL/min/1.73 m2 (L) (0.5 mL/s) | 37 mL/min/1.73 m2 (L) (0.6 mL/s) | 37 mL/min/1.73 m2 (L) (0.6 mL/s) | 36 mL/min/1.73 m2 (L) (0.6 mL/s) | |
| Random urine calcium | mg/dL (mmol/L) | 9 mg/dL (2.25 mmol/L) | ||||
| Random urine creatinine | mg/dL (µmol/L) | 89 mg/dL (7867 µmol/L) | ||||
| 24-h urine calcium | 100.0-300.0 mg/24 h (2.5-7.5 mmol/24 h) | 93.1 mg/24 h (L) (2.3 mmol/24 h) | ||||
| 24-h urine creatinine | 0.60-1.80 g/24 h (5.3-15.9 mmol/24 h) | 0.92 g/24 h (8.1 mmol/24 h) | ||||
| 24-h urine volume | (800-2000 mL/24 h) | 1034 mL/24 h |
Values in parenthesis are International System of Units (SI).
Abbreviations: BUN, blood urea nitrogen; GFR, glomerular filtration rate; H, data are abnormally high; L, data are abnormally low.
aTransition of care visit.
While there was no family history consistent with HDR, her medical history was concerning for the syndrome. After providing informed consent, she underwent germline testing (Invitae hypoparathyroidism panel, including 18 genes, Table 2).
| Gene . | Transcript . |
|---|---|
| AIRE | NM_000383.3 |
| CASR | NM_000388.3 |
| CHD7 | NM_017780.3 |
| CYP24A1 | NM_000782.4 |
| FAM111A | NM_022074.3 |
| GATA3 | NM_001002295.1 |
| GCM2 | NM_004752.3 |
| GNA11 | NM_002067.4 |
| GNAS | NM_000516.5 |
| HADHA | NM_000182.4 |
| HADHB | NM_000183.2 |
| PDE4D | NM_001104631.1 |
| PTH | NM_000315.3 |
| PTH1R | NM_000316.2 |
| SLC34A1 | NM_003052.4 |
| STX16 | NM_001001433.2 |
| TBCE | NM_003193.4 |
| TBX1 | NM_080647.1 |
| Gene . | Transcript . |
|---|---|
| AIRE | NM_000383.3 |
| CASR | NM_000388.3 |
| CHD7 | NM_017780.3 |
| CYP24A1 | NM_000782.4 |
| FAM111A | NM_022074.3 |
| GATA3 | NM_001002295.1 |
| GCM2 | NM_004752.3 |
| GNA11 | NM_002067.4 |
| GNAS | NM_000516.5 |
| HADHA | NM_000182.4 |
| HADHB | NM_000183.2 |
| PDE4D | NM_001104631.1 |
| PTH | NM_000315.3 |
| PTH1R | NM_000316.2 |
| SLC34A1 | NM_003052.4 |
| STX16 | NM_001001433.2 |
| TBCE | NM_003193.4 |
| TBX1 | NM_080647.1 |
| Gene . | Transcript . |
|---|---|
| AIRE | NM_000383.3 |
| CASR | NM_000388.3 |
| CHD7 | NM_017780.3 |
| CYP24A1 | NM_000782.4 |
| FAM111A | NM_022074.3 |
| GATA3 | NM_001002295.1 |
| GCM2 | NM_004752.3 |
| GNA11 | NM_002067.4 |
| GNAS | NM_000516.5 |
| HADHA | NM_000182.4 |
| HADHB | NM_000183.2 |
| PDE4D | NM_001104631.1 |
| PTH | NM_000315.3 |
| PTH1R | NM_000316.2 |
| SLC34A1 | NM_003052.4 |
| STX16 | NM_001001433.2 |
| TBCE | NM_003193.4 |
| TBX1 | NM_080647.1 |
| Gene . | Transcript . |
|---|---|
| AIRE | NM_000383.3 |
| CASR | NM_000388.3 |
| CHD7 | NM_017780.3 |
| CYP24A1 | NM_000782.4 |
| FAM111A | NM_022074.3 |
| GATA3 | NM_001002295.1 |
| GCM2 | NM_004752.3 |
| GNA11 | NM_002067.4 |
| GNAS | NM_000516.5 |
| HADHA | NM_000182.4 |
| HADHB | NM_000183.2 |
| PDE4D | NM_001104631.1 |
| PTH | NM_000315.3 |
| PTH1R | NM_000316.2 |
| SLC34A1 | NM_003052.4 |
| STX16 | NM_001001433.2 |
| TBCE | NM_003193.4 |
| TBX1 | NM_080647.1 |
She did not have any previously described pathogenic variant in the genes tested; however, a variant of unknown significance (VUS) in GATA3 (c.791G > A, p.Cys264Tyr) and a VUS in TBX1 (c.1474T > A, p.Tyr492Asn) was identified. The variants were classified using criteria from the American College of Medical Genetics and Genomics. To our knowledge, the specific GATA3 variant seen in the patient has not been previously reported in the literature and does not appear in the genome aggregation database (gnomAD) or the human gene mutation database (HGMD) [9, 10]. There is one entry for this variant in ClinVar, a public archive of reports of human variations, submitted by Invitae (clinical condition was not provided) [11]. Advanced modeling of protein sequence and biophysical properties such as structural, functional, and spatial information, amino acid conservation, physicochemical variation, residue mobility, and thermodynamic stability, performed at Invitae indicated that this missense variant is expected to disrupt GATA3 protein function. In addition, the VarSome germline variant classifier, which employs multiple predictor engines, recognizes it as potentially pathogenic [12]. Of note, a likely pathogenic de novo missense variant in the same GATA3 amino acid was described by Jayasinghe et al in a male patient with clinical features of HDR [13]. Regarding the TBX1 variant, it is present in population databases at a frequency higher than what would be expected for a pathogenic variant (gnomAD 0.001%), it has not been reported in the literature in individuals with TBX1-related conditions, and the prediction algorithms provide mixed results on pathogenic potential. Of note, the patient also underwent clinical germline genetic testing due to her personal and family history of ovarian cancer, but no pathogenic variants were identified in cancer predisposition genes.
Treatment
The patient has continued supplementation with calcitriol 0.25 µg 3 times a week and vitamin D3 1000 units daily.
Outcome and Follow-up
Her S-Ca and 25(OH)D levels have remained in the normal range. She will continue bone densitometry screening as well as follow up with nephrology and audiology. Since the patient has no biological children and no family history of hypoPTH, familial testing was deferred in her remaining living relatives.
Discussion
HDR, a rare syndrome with about described 200 cases, is diagnosed early in life via newborn hearing screening and prenatal ultrasound. A minority of cases are diagnosed later in life as phenotypic penetrance varies or as the patients’ medical and family histories become available [14]. This patient was diagnosed with deafness at age 1 year, developed hypoPTH at age 40 years, and CKD with an atrophic left kidney was recognized in her 60s. Her comprehensive clinical presentation was not considered to be syndromic until she was in her 70s. This case highlights the importance of gathering detailed medical history even when managing a known chronic condition, as this information can elucidate a potential syndrome or inherited condition with wide repercussions.
Genetic testing for germline variants in genes linked to hypoPTH identified an interesting finding of a VUS in GATA3 (c.791G > A, p.Cys264Tyr). GATA3 variants have been linked to HDR syndrome [6]. However, to our knowledge, the missense variant found in our patient has not been previously described and is not listed in GnomAD and HGMD databases [9, 10]. There is one entry for this variant in ClinVar, although clinical condition is not specified [11]. Given the lack of prior data on this variant, prediction algorithms were used to determine its likelihood for pathogenic potential. Advanced modeling tools do predict that the variant would disrupt GATA3 protein function, and other variants involving the same amino acid are classified as pathogenic. Multiple prediction tools on VarSome predict this variant to be suspicious [12]. While this variant has not been previously described, a de novo missense likely pathogenic variant involving the same amino acid in a person with clinical features of HDR was described in 2021 [13]. This evidence supports the in silico predictions of a potentially pathogenic classification in the variant described in our patient.
Recognizing syndromic and familial hypoPTH is crucial to identify those patients and families who would benefit from genetic testing and further genetic counseling. Our patient deferred further family testing given the lack of hypoPTH phenotype in her family members and because she does not have any children. It is worth mentioning that she had one relative, now diseased, with CKD (see Fig. 1), but given the multitude of etiologies for CKD and no other history available from that relative, association with potential HDR is unclear. The absence of hypoPTH, deafness, and renal disease in her family is interesting, since the disease is inherited in an autosomal dominant fashion. Therefore, it is possible that in her case, the GATA3 variant developed de novo or has incomplete penetrance.
Reporting HDR cases is important to build on the current literature and identify previously unknown genetic variants, whose classification may be updated with additional data. This process helps guide providers and families on further disease screening and genetic testing for relatives. When a VUS is reported, clinicians can use the available prediction models to discuss further family testing with patients when appropriate. It is also crucial to report untraditional cases such as this one, where no strong family history of HDR phenotypes is present, as they might elucidate different modes of variant generation or inheritance. This case, to our knowledge, is the first in describing this particular variant in an HDR patient, and the second to point out a potentially pathogenic variant in this GATA3 amino acid in patients with HDR. More reports are needed to reclassify the pathogenicity of this VUS.
Learning Points
The HDR phenotype can be present for years but not recognized as a syndrome until later in life.
Thorough past medical and family histories in patients with hypoPTH can highlight patients and families who would benefit from genetic testing and further disease screenings.
New genetic variants should be modeled through available predictor engines to assess potential for pathogenicity if such data are not available clinically.
Contributors
All authors made individual contributions to authorship. A.V., B.O., and S.I. were involved in the diagnosis and management of the patient. P. B. was involved in genetic counseling for the patient and genetic test interpretation. All authors reviewed and approved the final draft.
Funding
No public or commercial funding.
Disclosures
None declared.
Informed Patient Consent for Publication
Signed informed consent obtained directly from the patient.
Data Availability Statement
Original data generated and analyzed during this study are included in this published article.
References
Abbreviations
- 25(OH)D
25OH vitamin D
- BUN
blood urea nitrogen
- CKD
chronic kidney disease
- GATA3
GATA binding protein 3 gene
- GFR
glomerular filtration rate
- HDR
hypoparathyroidism sensorineural deafness and renal dysplasia
- hypoPTH
hypoparathyroidism
- PTH
parathyroid hormone
- S-Ca
serum calcium
- VUS
variant of unknown significance



