-
PDF
- Split View
-
Views
-
Cite
Cite
Aravind L, Arun Viswanath S, Ninoo George G, Ranjit Jeyasekharan, Deepak David, Jerin J Ovett, An Interesting Case of Vitamin D-Mediated Severe Hypercalcemia in a Patient With Renal Mass, JCEM Case Reports, Volume 3, Issue 1, January 2025, luae233, https://doi.org/10.1210/jcemcr/luae233
Close - Share Icon Share
Abstract
Hypercalcemia of malignancy (HCM) is the most common cause of hypercalcemia in hospitalized patients. The pathogenesis of HCM is often multifactorial. One of the rare causes of HCM is extra-renal production of 1,25-dihydroxyvitamin D (or calcitriol), which is often seen in patients with lymphoproliferative malignancies. Here we report an interesting case of a 77-year-old female with severe hypercalcemia and renal mass. Initially, she was presumed to have humoral hypercalcemia of malignancy. However, her renal mass turned out to be diffuse large B cell lymphoma upon removal. Her severe hypercalcemia was attributed to a combination of ectopic calcitriol production from the tumor and probable iatrogenic vitamin D intoxication. This case highlights the need to consider multiple concurrent etiologies in patients with severe hypercalcemia.
Introduction
Hypercalcemia of malignancy (HCM) is the most common metabolic complication of malignancies, affecting 2% to 30% of patients with cancer depending on the type and stage of the disease [1]. Common cancers associated with HCM include solid tumors such as breast, lung, and renal cancer, as well as multiple myeloma. The prevalence of HCM in different malignancies is shown in Table 1 [2]. The most common cause of HCM is humoral hypercalcemia of malignancy (HHCM), which refers to a condition in which a malignant tumor produces 1 or more circulating factors that cause hypercalcemia in the absence of evidence for skeletal metastasis. HHCM accounts for HCM in nearly 80% of cases [3]. Less common causes of HCM include tumor-associated local osteolysis, ectopic PTH or 1,25-dihydroxyvitamin D (calcitriol) production. Hypercalcemia in a patient with a renal mass is usually considered HHCM secondary to renal cell carcinoma unless proven otherwise. Here we report a case of an elderly female presenting with severe hypercalcemia and a renal mass with an interesting combination of etiologies.
| Type of cancer . | Prevalence of hypercalcemia (%) . |
|---|---|
| Multiple myeloma | 7.9 |
| Renal malignancy | 3.7 |
| Lung cancer | 3.5 |
| Non-Hodgkin's lymphoma | 2.0 |
| Breast cancer | 1.5 |
| Prostate cancer | 1.5 |
| Colorectal cancer | 1.1 |
| Other malignancies | 1.8 |
| Overall | 2.0 |
| Type of cancer . | Prevalence of hypercalcemia (%) . |
|---|---|
| Multiple myeloma | 7.9 |
| Renal malignancy | 3.7 |
| Lung cancer | 3.5 |
| Non-Hodgkin's lymphoma | 2.0 |
| Breast cancer | 1.5 |
| Prostate cancer | 1.5 |
| Colorectal cancer | 1.1 |
| Other malignancies | 1.8 |
| Overall | 2.0 |
aData from the United States as of 2013 [2].
| Type of cancer . | Prevalence of hypercalcemia (%) . |
|---|---|
| Multiple myeloma | 7.9 |
| Renal malignancy | 3.7 |
| Lung cancer | 3.5 |
| Non-Hodgkin's lymphoma | 2.0 |
| Breast cancer | 1.5 |
| Prostate cancer | 1.5 |
| Colorectal cancer | 1.1 |
| Other malignancies | 1.8 |
| Overall | 2.0 |
| Type of cancer . | Prevalence of hypercalcemia (%) . |
|---|---|
| Multiple myeloma | 7.9 |
| Renal malignancy | 3.7 |
| Lung cancer | 3.5 |
| Non-Hodgkin's lymphoma | 2.0 |
| Breast cancer | 1.5 |
| Prostate cancer | 1.5 |
| Colorectal cancer | 1.1 |
| Other malignancies | 1.8 |
| Overall | 2.0 |
aData from the United States as of 2013 [2].
Case Presentation
A 77-year-old woman presented to our clinic with progressively worsening fatigue, anorexia, and weight loss for more than 6 months. In addition, she was troubled by polyuria and recent-onset constipation. Her past medical history included systemic hypertension for 15 years, which was reasonably controlled on beta-adrenergic blockers. She also had hypothyroidism for 20 years and was on levothyroxine 75 micrograms daily with good biochemical control. One year previously, she was diagnosed with hypertensive nephropathy and stage 3 chronic kidney disease. She was also known to have osteoporosis and had been regularly consuming calcium and vitamin D supplements for 3 years. Additionally, she had received a few undocumented over-the-counter intramuscular injections for body aches in the last year. She had undergone a hysterectomy 25 years previously for a fibroid uterus.
Diagnostic Assessment
Her physical examination revealed signs of dehydration; however, her vitals were stable at the time of presentation. Initial laboratory investigations showed elevated serum calcium levels (16.2 mg/dL; 4.0 mmol/L) (reference range: 8.5-10.5 mg/dL; 2.1-2.6 mmol/L) and normal inorganic phosphate levels (3.8 mg/dL; 1.2 mmol/L) (reference range: 2.5-4.5 mg/dL; 0.8-1.5 mmol/L). Her serum creatinine at admission was 2.8 mg/dL (247.5 µmol/L) (reference range: 0.4-1.2 mg/dL; 35.4-106.1 µmol/L) and her estimated glomerular filtration rate was 16.9 mL/min/m2. Other baseline investigations were normal. A workup to identify the cause of hypercalcemia was initiated. Her PTH level was in the low-normal range (11 pg/mL; 1.2 pmol/L) (reference range: 10-55 pg/mL; 1.1-5.8 pmol/L). Therefore, the possibility of PTH-independent hypercalcemia was considered.
Considering her age and the severity of hypercalcemia, we first ruled out multiple myeloma with serum electrophoresis, serum light chain assay, and IL-6 measurements, all of which were normal. Meanwhile, her serum 25-hydroxy vitamin D (25(OH)D) level was found to be in a toxic range (105 ng/mL; 262.1 nmol/L) (normal reference range: 25-80 ng/mL; 62.4-199.7 nmol/L). Therefore, a provisional diagnosis of hypercalcemia due to vitamin D intoxication was made based on 25(OH)D levels above 100 ng/mL and suppressed PTH levels [4].
Treatment
The patient was admitted to the ward for treatment and further evaluation. Her calcium and vitamin D supplements were discontinued. She was initially hydrated intravenously with normal saline, followed by intravenous loop diuretics and calcitonin administered intramuscularly. Her serum calcium levels gradually decreased with symptomatic treatment (11.2 mg/dL; 2.8 mmol/L) as shown in Table 2, and her renal function also improved. The patient was discharged in stable condition with instructions to avoid all over-the-counter injections.
Serum calcium levels and relevant laboratory findings from initial presentation to last follow-up
| Parameters with unit of measurement (SI unit) . | Normal range . | At initial presentation . | At first discharge . | During second admission . | After starting steroid therapy . | In follow-up . | After chemotherapy . |
|---|---|---|---|---|---|---|---|
| Albumin corrected calcium | 8.5-10.5 mg/dL (2.1-2.6 mmol/L) | 16.2 mg/dL (4.0 mmol/L) | 11.2 mg/dL (2.8 mmol/L) | 14.7 mg/dL (3.7 mmol/L) | 9.8 mg/dL (2.4 mmol/L) | 13.5 mg/dL (3.4 mmol/L) | 9.4 mg/dL (2.3 mmol/L) |
| Serum inorganic phosphate | 2.5-4.5 mg/dL (0.8-1.5 mmol/L) | 3.8 mg/dL (1.2 mmol/L) | |||||
| Intact PTH | 10-55 pg/mL (1.1-5.8 pmol/L) | 11 pg/mL (1.2 pmol/L) | |||||
| PTHrP | 37.72 pg/mL (≤ 4 pmol/L) | 20.7 pg/mL (2.2 pmol/L) | |||||
| 25(OH)D | 25-80 ng/mL (60-200 nmol/L) | 105 ng/mL (262 nmol/L) | 34.8 ng/mL (87 nmol/L) | ||||
| 1,25(OH)2D | 19-79 pg/mL (45-190 pmol/L) | 178 pg/mL (427 pmol/L) | 41 pg/mL (98.4 pmol/L) |
| Parameters with unit of measurement (SI unit) . | Normal range . | At initial presentation . | At first discharge . | During second admission . | After starting steroid therapy . | In follow-up . | After chemotherapy . |
|---|---|---|---|---|---|---|---|
| Albumin corrected calcium | 8.5-10.5 mg/dL (2.1-2.6 mmol/L) | 16.2 mg/dL (4.0 mmol/L) | 11.2 mg/dL (2.8 mmol/L) | 14.7 mg/dL (3.7 mmol/L) | 9.8 mg/dL (2.4 mmol/L) | 13.5 mg/dL (3.4 mmol/L) | 9.4 mg/dL (2.3 mmol/L) |
| Serum inorganic phosphate | 2.5-4.5 mg/dL (0.8-1.5 mmol/L) | 3.8 mg/dL (1.2 mmol/L) | |||||
| Intact PTH | 10-55 pg/mL (1.1-5.8 pmol/L) | 11 pg/mL (1.2 pmol/L) | |||||
| PTHrP | 37.72 pg/mL (≤ 4 pmol/L) | 20.7 pg/mL (2.2 pmol/L) | |||||
| 25(OH)D | 25-80 ng/mL (60-200 nmol/L) | 105 ng/mL (262 nmol/L) | 34.8 ng/mL (87 nmol/L) | ||||
| 1,25(OH)2D | 19-79 pg/mL (45-190 pmol/L) | 178 pg/mL (427 pmol/L) | 41 pg/mL (98.4 pmol/L) |
Abnormal values are shown in bold font. Values in parenthesis are International System of Units.
Abbreviations: 1,25(OH)2D, 1,25-dihydroxyvitamin D (calcitriol); 25(OH)D, 25-hydroxycalciferol (cholecalciferol); PTHrP, PTH-related peptide.
Serum calcium levels and relevant laboratory findings from initial presentation to last follow-up
| Parameters with unit of measurement (SI unit) . | Normal range . | At initial presentation . | At first discharge . | During second admission . | After starting steroid therapy . | In follow-up . | After chemotherapy . |
|---|---|---|---|---|---|---|---|
| Albumin corrected calcium | 8.5-10.5 mg/dL (2.1-2.6 mmol/L) | 16.2 mg/dL (4.0 mmol/L) | 11.2 mg/dL (2.8 mmol/L) | 14.7 mg/dL (3.7 mmol/L) | 9.8 mg/dL (2.4 mmol/L) | 13.5 mg/dL (3.4 mmol/L) | 9.4 mg/dL (2.3 mmol/L) |
| Serum inorganic phosphate | 2.5-4.5 mg/dL (0.8-1.5 mmol/L) | 3.8 mg/dL (1.2 mmol/L) | |||||
| Intact PTH | 10-55 pg/mL (1.1-5.8 pmol/L) | 11 pg/mL (1.2 pmol/L) | |||||
| PTHrP | 37.72 pg/mL (≤ 4 pmol/L) | 20.7 pg/mL (2.2 pmol/L) | |||||
| 25(OH)D | 25-80 ng/mL (60-200 nmol/L) | 105 ng/mL (262 nmol/L) | 34.8 ng/mL (87 nmol/L) | ||||
| 1,25(OH)2D | 19-79 pg/mL (45-190 pmol/L) | 178 pg/mL (427 pmol/L) | 41 pg/mL (98.4 pmol/L) |
| Parameters with unit of measurement (SI unit) . | Normal range . | At initial presentation . | At first discharge . | During second admission . | After starting steroid therapy . | In follow-up . | After chemotherapy . |
|---|---|---|---|---|---|---|---|
| Albumin corrected calcium | 8.5-10.5 mg/dL (2.1-2.6 mmol/L) | 16.2 mg/dL (4.0 mmol/L) | 11.2 mg/dL (2.8 mmol/L) | 14.7 mg/dL (3.7 mmol/L) | 9.8 mg/dL (2.4 mmol/L) | 13.5 mg/dL (3.4 mmol/L) | 9.4 mg/dL (2.3 mmol/L) |
| Serum inorganic phosphate | 2.5-4.5 mg/dL (0.8-1.5 mmol/L) | 3.8 mg/dL (1.2 mmol/L) | |||||
| Intact PTH | 10-55 pg/mL (1.1-5.8 pmol/L) | 11 pg/mL (1.2 pmol/L) | |||||
| PTHrP | 37.72 pg/mL (≤ 4 pmol/L) | 20.7 pg/mL (2.2 pmol/L) | |||||
| 25(OH)D | 25-80 ng/mL (60-200 nmol/L) | 105 ng/mL (262 nmol/L) | 34.8 ng/mL (87 nmol/L) | ||||
| 1,25(OH)2D | 19-79 pg/mL (45-190 pmol/L) | 178 pg/mL (427 pmol/L) | 41 pg/mL (98.4 pmol/L) |
Abnormal values are shown in bold font. Values in parenthesis are International System of Units.
Abbreviations: 1,25(OH)2D, 1,25-dihydroxyvitamin D (calcitriol); 25(OH)D, 25-hydroxycalciferol (cholecalciferol); PTHrP, PTH-related peptide.
A week later, the patient returned with rebound hypercalcemia (serum calcium—14.7 mg/dL; 3.7 mmol/L). Meanwhile, an abdominal ultrasonogram was done to assess renal morphology in view of deranged renal function. It revealed a solid mass lesion measuring 7 × 5 centimeters in the lateral cortex of the lower pole of the left kidney. Since the severity of her hypercalcemia did not correlate with 25(OH)D levels, we suspected HHM due to renal mass, and relevant investigations were ordered. Her PTH-related peptide (PTHrP) levels were found to be normal (2.2 pmol/L) (reference range: ≤ 4.2 pmol/L), but her calcitriol levels were quite high (178 pg/mL; 427.2 pmol/L) (normal reference range: 19-79 pg/mL; 45.6-189.6 pmol/L). Therefore, a final diagnosis of hypercalcemia due to combined 25(OH)D intoxication and calcitriol-mediated HCM due to renal mass was made. In addition to hydration with IV fluids, we started her on IV hydrocortisone (200 mg/day) and zoledronate (4 mg IV over 60 minutes) after stabilization of renal function, leading to effective control of hypercalcemia over the next 5 days (see Table 2).
Outcome and Follow-up
Since no other lesions were found on computed tomography imaging of the chest and abdomen, we proceeded with an open left radical nephrectomy (Fig. 1A). The histopathology (Fig. 1B) and immunohistochemistry revealed the renal lesion to be diffuse large B cell lymphoma. Postoperatively, she was started on the R-CHOP chemotherapy regimen (rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisolone). After 10 months of follow-up, the patient is doing well in remission after 5 cycles of chemotherapy with normal calcium levels (9.4 mg/dL; 2.3 mmol/L), 25(OH)D (34.8 ng/mL; 87 nmol/L), and calcitriol levels (41 pg/mL; 98.4 nmol/L) as shown in Table 2.
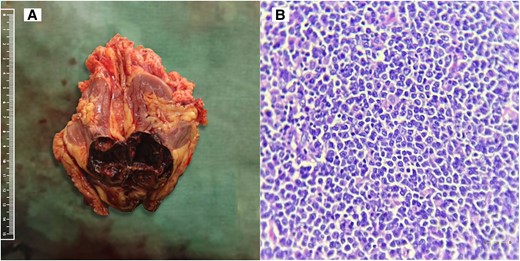
(A) Nephrectomy specimen showing macroscopic tumor appearance. (B) High-power view with 400× magnification (scale bar = 100 µm) showing small to medium-sized lymphoid cells arranged in a nested pattern with amphophilic cytoplasm, irregular nuclear membrane, vesicular nuclear chromatin, prominent nucleoli, and mitotic figures.
Discussion
Here we present an intriguing case of an elderly female with severe hypercalcemia initially believed to be due to vitamin D intoxication. However, it was later discovered that calcitriol-mediated hypercalcemia from renal lymphoma was also contributing to her condition. This case underscores the importance of remaining vigilant when the severity of hypercalcemia does not align with measured 25(OH)D levels in suspected cases of vitamin D intoxication, especially in elderly patients where HCM is common. Figure 2 depicts a simplified diagnostic approach to hypercalcemia.
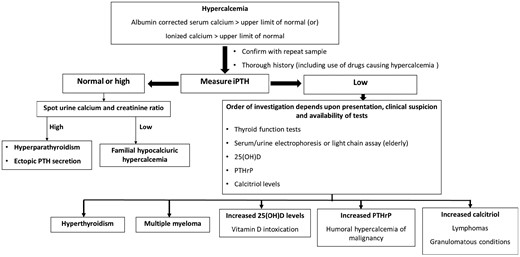
Simplified diagnostic approach to hypercalcemia.
Abbreviations: 25(OH)D, 25-hydroxycalciferol (cholecalciferol); iPTH, intact parathyroid hormone; PTHrP, parathyroid related peptide.
Although vitamin D intoxication is a common cause of hypercalcemia in our setting, we were unable to definitively document vitamin D abuse in our patient. It was only presumed based on 25(OH)D levels and a history of frequent over-the-counter injections. Hypercalcemia due to vitamin D intoxication is typically not seen at levels less than 150 ng/mL (375 nmol/L) [5]. However, a previously reported prospective study from India [4] found that 25(OH)D levels in cases of vitamin D intoxication can be as low as 105 ng/mL (262.5 nmol/L).
While a correlation between measured 25(OH)D levels and the severity of hypercalcemia has not been established, we believed our patient had inappropriately severe hypercalcemia for her 25(OH)D levels. This prompted further investigation in our patient.
A solid renal mass in the elderly is most commonly renal cell carcinoma [6]. The HCM associated with renal cell carcinoma is often HHCM mediated by PTHrP. Since PTHrP levels were normal in our patient, we measured other non-PTHrP humoral factors that can lead to hypercalcemia in renal masses, such as IL-6 and calcitriol [7]. We found normal IL-6 levels and high calcitriol levels in our patient. The increased calcitriol is likely mediated by excess 1-alpha hydroxylase activity within the renal tumor [7]. In cases of vitamin D intoxication, calcitriol levels are expected to be low due to suppression of PTH by hypercalcemia, leading to a loss of PTH-dependent stimulation of CYP27B1 (1α-hydroxylase) activity responsible for converting 25(OH)D to calcitriol [8]. The documentation of high calcitriol in our case carried a 2-fold significance. First, it confirmed that the source of hypercalcemia in our patient was due to both HCM mediated by calcitriol from the renal mass and vitamin D intoxication. Second, the documentation of high calcitriol levels carried therapeutic significance by facilitating the use of glucocorticoids, which specifically act by inhibiting 1α-hydroxylase activity, leading to prompt control of hypercalcemia in our patient, as shown in Table 2 [9].
We came across a similar case report in the literature [10] where an elderly male was found to have severe PTH-independent hypercalcemia with a renal mass and elevated 25(OH)D and calcitriol levels. Like our case, the authors could not clearly demonstrate the iatrogenic overdosage of 25(OH)D in that patient. Following Occam's razor, we explored whether an increase in both 25(OH)D and calcitriol could be explained by 1 etiology alone. Direct production of 25(OH)D from a renal mass seems improbable. However, a renal mass, especially lymphomas, can cause monoclonal gammopathy, which can interfere with 25(OH)D immunoassays and result in spuriously high 25(OH)D levels [11]. But serum electrophoresis did not reveal any evidence of monoclonal gammopathy in our case. Similarly, higher serum levels of 25(OH)D and its metabolite in vitamin D intoxication can interfere with immunoassays of calcitriol [12]. However, we believe that the 25(OH)D levels in our patient were not high enough to cause such interference in immunoassays. Both concerns about assay interference could have been negated by measuring both 25(OH)D and calcitriol levels with the help of liquid chromatography coupled with tandem mass spectrometry [13]. Unfortunately, this could not be done in our patient due to the nonavailability of liquid chromatography-tandem mass spectrometry in our facility.
The normalization of 25(OH)D and calcitriol levels with the remission of renal lymphoma could support the possibility of a renal lesion contributing to an increase in both 25(OH)D and calcitriol levels. However, this normalization occurred over a period of 6 months from the time of the initial presentation in our patient. Hence the possibility of vitamin D intoxication-mediated hypercalcemia could not definitively be ruled out since calcium levels in vitamin D intoxication also normalize over a period ranging from 4 to 18 months in previous studies [4]. The origin of calcitriol from the tumor could have been proved by immunostaining of the tumor for CYP27B1 [7]. Unfortunately, due to nonavailability, we could not do the same.
Learning Points
Severe hypercalcemia in elderly subjects should be considered as HCM unless proven otherwise, and a meticulous search for mass lesions should be conducted.
It is important to explore alternative causes of hypercalcemia when the severity of hypercalcemia does not align with measured 25(OH)D levels in cases of vitamin D intoxication.
Measurement of PTHrP should be considered in all cases of suspected HCM. If PTHrP levels are normal, it is advisable to investigate other potential causes of HCM, including measuring cytokines and calcitriol levels.
If available, calcitriol levels should be measured in all cases of HCM, as it provides the therapeutic benefit of a preferential response of hypercalcemia to glucocorticoid therapy.
Acknowledgments
We would like to acknowledge the role of Dr. Jithesh P K, Department of Pathology, Dr. Jeyasekharan Medical Trust Hospital & Nursing Home, Kottar-Parvathipuram Road, Nagercoil, Tamilnadu, India, 629003.
Contributors
All authors made individual contributions to authorship. A.L., A.V.S., N.G.G., and R.J. were involved in the initial diagnosis and management of the patient. D.D. was responsible for the patient's surgery. J.J.O. is currently managing the patient from the oncology side. A.L. and A.V.S. were responsible for manuscript preparation and final submission. N.G.G. and R.J. were responsible for proof correction and finalizing the manuscript. All authors reviewed and approved the final draft.
Funding
No funding received for this work.
Disclosures
None declared.
Informed Patient Consent for Publication
Signed informed consent was obtained from the patient.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.



